Abstract
Purpose
Intraoperative fluoroscopy has been proposed as a feasible method to improve the accuracy of anatomical tunnel positioning. However, it has so far not been determined, whether this technique reduces the variability of tunnel positioning in a clinical set-up. Therefore, the purpose of this study was to determine the variability of tunnel positions applying intraoperative fluoroscopy.
Methods
Femoral and tibial tunnel positions of 112 fluoroscopic ACL reconstruction cases were determined according to validated radiological measurement methods. Mean positions, standard deviations and ranges were calculated to determine the variability of the tunnel positions. Subgroup variability analysis was performed to analyse cases in which tunnel positions were corrected.
Results
Applying intraoperative fluoroscopy, the variability of tunnel positions was found to be 3 % at the femur (range 15.4 %) and 2.3 % at the tibia (9.7 %). In 34 cases (30.0 %), non-satisfactory tunnel positions were identified and could be corrected achieving more accurate positions regarding to radiological parameters (14× femur, 16× tibia, 4× femur and tibia).
Conclusions
The results of the presented study indicate that intraoperative fluoroscopy allows to identify non-accurate tunnel positions regarding to radiological criteria. The determined low variability indicates that fluoroscopic-based ACL reconstruction can be recommended as a feasible, easy and effective adjunct that enables surgeons to create more consistent and reliable tunnel positions in ACL reconstruction.
Level of evidence
IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The goal of ACL reconstruction is to restore physiological joint biomechanics in order to restore of knee joint stability with full range of motion as well as prevention of secondary cartilage and meniscal lesions. Despite greater knowledge of the knee anatomy, biomechanics and improvements in operative ACL reconstruction techniques, till date considerable rates of graft failure exist [3, 8, 9, 46]. In addition to factors such as patient selection, surgeon’s experience, graft choice, graft fixation or rehabilitation that are discussed to affect the outcome of ACL reconstructions, there is general agreement that correct anatomical tunnel positioning is fundamental to successful ACL reconstruction and long-term stability [2, 16, 22, 25, 29, 48]. However, the importance of anatomical tunnel positioning in ACL reconstruction is well accepted, the implementation of this knowledge into intraoperative tunnel positioning seems to remain difficult since reliable landmarks, allowing correct tunnel identification, do not consistently exist [12, 17, 31, 32, 34, 36, 38, 44]. Radiological studies indicate that approximately 10–40 % of the tunnel positions in primary ACL reconstructions have been placed non-anatomically [1, 13, 41, 43].
Recently, McConkey et al. [25] analysed the variability of tunnel positioning in conventional ACL reconstruction techniques in a cadaver study. The results showed a significant variability of tunnel positions among surgeons as well as it was reported that surgeons are significantly more likely to assess their tunnel positions to be ideal than independent surgeons.
Since different radiological measurement methods exist that allow to identify the anatomical insertion areas of the ACL in radiographs [2, 6, 10, 19], image-assisted tunnel position techniques have proposed to guide tunnel positioning and to improve accuracy and reliability [17, 20, 21, 36]. Among these, intraoperative fluoroscopy has been proposed as a feasible method to improve the accuracy of anatomical tunnel positioning [7, 14, 28, 39] by identification and fluoroscopic guidance of tunnel positioning related to radiographic recommendations.
However, it has so far not been determined, whether intraoperative fluoroscopy reduces the variability of tunnel positioning in a clinical set-up. Therefore, the purpose of this study was (1) to determine the variability of tunnel positions applying intraoperative fluoroscopy as well as (2) to determine whether more consistent results can be achieved by identification and correction of tunnel positions that were fluoroscopically assessed to be unsatisfactory. The hypothesis of this study was that intraoperative fluoroscopic-assisted tunnel positioning is a feasible method that allows for more accurate anatomical tunnel positioning in ACL reconstruction by reducing the variability of femoral and tibial tunnel positions.
Materials and methods
One hundred and twelve consecutive cases of ACL reconstructions, performed by three experienced ACL surgeons, were analysed in a cohort study, providing intraoperative fluoroscopic images of femoral and tibial tunnel positions. The quality of fluoroscopic images allowed in all cases to determine the tunnel positions precisely. The mean age at time of surgery was 27 years (range 13–47 years), showing skeletally mature radiographs with closed physes in all cases. No osteoarthrosis higher than Kellgren–Lawrence grade A was seen in the radiographs. Sex distribution showed 41 female and 71 male cases.
According to preoperative MRI examinations, that showed ACL rupture in all cases, preoperative clinical assessment of all patients showed an increased anterior tibia translation in Lachman’s examination as well as a rotational instability in pivot-shift examination. In all cases, full weight bearing and free range of motion were achieved at the time of surgery.
In 96 cases, primary single-bundle ACL reconstruction was performed using hamstrings autograft. In nine cases, revision ACL reconstruction (5× ipsilateral patellar tendons, 1× ipsilateral quadriceps tendons, 3× contralateral hamstrings tendons) and in seven cases ACL reconstruction (4× ipsilateral quadriceps tendons, 3× contralateral hamstrings tendons) were combined with an additional knee ligament reconstruction.
Surgical procedure
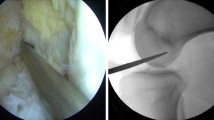
After positioning of the patients, standard arthroscopy portals were created and cartilage as well as meniscal therapy was performed if required. For identifying arthroscopic landmarks required for tunnel positioning, first the femoral tunnel was created. Depending on the knee size and the graft diameters, standard femoral 6–7 mm offset aiming devices (Accufex, Smith&Nephew, Andover, MA, USA) were used to create femoral tunnels. In all cases, the femoral aiming devices were introduced through an additional deep anteromedial portal. Identifying the anatomical femoral ACL insertion area, the over the top position as well as the clockwise notch orientation, the aiming devices were positioned within the centre of the anatomical insertion area. A 2.4-mm drill guide (Smith&Nephew, Andover, MA, USA) was passed through the femoral aiming device into the lateral condyle with the knee in at least 110° flexion until the tip of the drill guide perforated the skin on the lateral aspect of the femur. The drill guide was then passed through the knee, until the end of the drill guide ended flush with the inner bony wall of the lateral condyle. In 90° of flexion, the entrance point of the femoral tunnel was evaluated and again it was checked, whether the end of the drill guide and the bone surface of the inner lateral notch wall ended flush (Fig. 1a). Then, a tibial aiming device (Accufex, Smith&Nephew, Andover, MA, USA) was introduced into the knee over the anteromedial portal. After central and lengthwise incision of the ACL stump, the intraarticular tip of the tibial aiming device was positioned within the centre of the ACL stump, so that the drill guide would pass the intraarticular tibial cortex on height of the anterior border of the anterior horn of the lateral meniscus. Then, a second 2.4-mm drill guide was passed through the tibial aiming device into the knee. After removing the tibial aiming device, the position of the drill guide was checked (Fig. 1b) and considered correctly placed when its exit point was continuous with a line marking the posterior edge of the lateral meniscus and posterior to the medial tibial spine. Then, the drill guide was drilled backward, unless the tip ended flush with the tibial cortical surface, which was controlled visually as well as with a palpation hook (Fig. 1c). In cases of unsatisfactory tunnel positions, the drill guides were removed and new positions were created unless anatomical positions were achieved. Since flush ending of the tibial drill guide tip as well as of the end of the femoral drill guide was essential to determine the correct positions in the radiographs, this was carefully controlled before fluoroscopic image acquisition. Once satisfactory drill guide positions were achieved regarding the drill guide positions in relation to the tibial and femoral ACL footprint, a standard fluoroscope was positioned to acquire lateral radiographs of the knee. Two lateral fluoroscopic images—one of the femur and one of the tibia—were acquired, showing overlapping femoral condyles for femoral images and a plane joint view and rotation at the tibia. Tunnel positions were then assessed according to the radiological measurement methods of Bernard and Hertel [5] for the femur and Staubli [40] for the tibia.
If tunnel positions were judged to be correct, images of the femoral and tibial drill guide positions were printed for documentation of the tunnel positions. Then, following conventional ACL reconstruction techniques, the femoral and tibial tunnels were established using cannulated reamers and the grafts were passed through the tibial into the femoral tunnels and fixed with bioresorbable screws (BioRCI, Smith&Nephew, Andover, MA, USA) at 10° flexion.
If the radiographic control of the tunnel positions was judged to be non-anatomical regarding radiological criteria, tunnel positions were corrected as described before and new fluoroscopic images were acquired to control the new tunnel positions. Images were printed and it was documented, whether the tibial or femoral tunnel had to be corrected. After achieving satisfactory anatomical tunnel positions, tunnel reaming, graft passage and fixation followed as described before.
In all cases, the three surgeons applied the above-described fluoroscopic ACL reconstruction technique, as it was the standard procedure of the reporting centre.
Radiological measurement of tunnel positions
All acquired and printed fluoroscopic images, identifying the femoral and tibial tunnel positions, were scanned and digitally transferred to a professional CAD drawing and measurement software (Canvas 9.0, ACD Systems, Seattle, USA). To determine the femoral and tibial tunnel positions as well as to measure deviations between tunnel positions, in cases of drill guide correction, reliable radiological measurement methods were used.
At the femur, Bernard and Hertel’s quadrant method [6] was used to determine the tunnel position. Therefore, a quadrant was projected along Blumensaat’s line and its size was limited by the sagittal depth and height of the overlapping condyles. For femoral evaluation, the depth and height of the drill guides positions were measured in relation to a projected quadrant (Fig. 2).
Tibial drill guides positions were measured in the sagittal plane according to Staubli’s [40] measurement method. In the lateral radiographs, a line was drawn parallel to the sagittal tibial joint line. The length of the line is limited ventrally by the tibial cortex and dorsally by the descending eminentia intercondylaris. Then, the different positions were measured along the tibial line (Fig. 3).
Statistical analysis
Femoral height and depth positions as well as tibial sagittal tunnel positions were analysed using a statistical analysis software (SPSS, version 13.0, Chicago, IL, USA). Mean positions, standard deviations and ranges were calculated to determine the variability of the tunnel positions. Correlation analysis was performed to compare whether in cases of tunnel position correction a higher accuracy could be achieved.
Reliability of radiological measurements
To ensure the reliability of the tunnel position measurements, two independent observers measured the tibial and femoral tunnel positions in all radiographs and interclass correlation (ICC) were calculated. Since the measurements were—according to Landis and Koch [23] —assessed to be highly reliable (ICC: femur depth 0.973, femur height 0.981, tibia 0.936), further measurement data of observer 2 (WS) were analysed.
Results
Variability analysis (Table 1)
As shown in Table 1, applying intraoperative fluoroscopy mean femoral tunnel positions were found to be at 28.1 % (depth and height) and mean tibial tunnel positions were found to be at 42.1 %. The variability for femoral tunnel positioning was 3 % at the femur and 2.3 % at the tibia. However, ranges of 15.4 % at the femur and 9.7 % at the tibia were investigated. Figure 4 shows the variability of femoral and tibial tunnel positions in a plot graph analysis.
Variability analysis drill guide corrections
Out of 112 cases of fluoroscopic-assisted femoral tunnel positions, corrections were necessary in 12 cases (10.7 %). At the tibia 16 (14.2 %), drill guide positions had to be corrected and in four cases (3.6 %) femoral and tibial drill guide positions had to be corrected. In all cases, accuracy improvements of the tunnel positions could be achieved by repositioning of the drill guides.
Analysis of femoral cases in which drill guide corrections were performed shows that the femoral depth positions were changed in three cases to a more posterior and in nine cases to a more anterior position. In four cases, drill guide reposition led only to changes of the femoral height, whereas the femoral depth position did not change (Fig. 5a). Femoral height positions of all cases were corrected from an anterior (19.7 %) towards a more posterior (26.1 %) tunnel position (Fig. 5b). Figure 5c shows the femoral height and depth deviations between the initial drill guide positions and the corrected positions.
As shown in Fig. 6a at the tibia, 13 corrected tunnel positions were initially assessed too far posterior (44–48 %) and in seven cases too far anterior (31–39 %). Figure 6b shows the deviations between the initial drill guide positions and the corrected positions at the tibia.
Above-described corrections of the drill guide positions led to more consistent femoral and tibial tunnel positions, improving variability and ranges in the subgroup analysis (femur depth/height: SD −1.7/0.6; tibia: SD −3.8) as well as in the cohort (femur depth/height: SD −0.5/1.1; tibia: SD −0.8).
Discussion
According to the hypothesis, the most important finding of the presented study was that intraoperative fluoroscopic-assisted tunnel positioning is a feasible method that allows for more accurate anatomical tunnel positioning in ACL reconstruction by reducing the variability of femoral and tibial tunnel positions. In the presented study, tunnel positions were evaluated after positioning of the drill guides and corrected when necessary using intraoperative fluoroscopy. Therefore, the results on the one hand describe the variability of fluoroscopic-assisted tunnel positions in ACL reconstruction, and on the other hand, the correction potential to determine tunnel positions that are identified to be not satisfactory according to radiological criteria.
There is general agreement that correct anatomical tunnel positioning is fundamental for successful ACL reconstruction since different biomechanical and clinical studies report better results in anatomical than in non-anatomical tunnel positioning [29, 30, 33, 35]. However, since inconsistencies in tunnel positioning as well as reasonable rates of tunnel misplacement exist, tunnel positioning in ACL reconstruction remains difficult.
Among other studies that correlate clinical results with tunnel positions [8, 9, 33, 35], Khalfayan et al. [16] prospectively studied 128 ACL reconstructions, emphasizing not only the strong correlation between correct tunnel positions and good clinical results, it was also shown, that inconsistencies of tunnel positions exist, ranging between 35–99 % at the femur and 13–39 % at the tibia. As wide ranges of tunnel positions as well as tunnel misplacement rates of up to 88 % in revision surgery exist [4, 24, 47], it can be therefore assumed that there is a floating transition between variability of tunnel positioning and tunnel misplacement. Moloney et al. [28] as well as Meuffels et al. [27] showed that even experienced surgeons had difficulties to reliably identify ACL insertions with arthroscopic techniques alone.
Pinzewski et al. [33] analysed the variability of tunnel positions in post-operative radiographs of 200 ACL reconstructions. Limited by the quality of the radiographs, the variability of the tunnel positions was found to be 5 %.
Also, the MOON group [45] recently reported on the variability of tunnel positions in ACL reconstruction analysing 78 ACL reconstructions of 8 surgeons using 3D CT scans in a comparable study set-up as the presented study. They presented variability (femur: 8.7/7 % height/depth; tibia: 6 %) as well as the presented ranges (femur: 22/19 % depth/height; tibia: 16 %) were assessed to be relatively consistent.
To achieve more consistent results in ACL tunnel positioning, different techniques have been proposed to reduce the variability of tunnel positioning and tunnel misplacement [15, 33, 36, 37, 39, 49]. Among these, fluoroscopic-assisted tunnel positioning has been recommended by many authors to increase the accuracy of anatomical tunnel positions in ACL reconstruction [16, 19, 26, 28, 42] since it is reported that intraoperative fluoroscopy enables to control tunnel positions according to radiographic identification of the ACL insertions. Thus, till date only a few publications exist, reporting data on the benefits of fluoroscopy in ACL reconstruction and no study so far has determined, whether intraoperative fluoroscopy leads to more consistent results in tunnel positioning.
Analysing the interobserver variability of graft position measurements Klos et al. [18] investigated 17 cases of fluoroscopic-assisted ACL reconstructions. Determining a high intraclass correlation coefficient, variability and ranges of the tunnel positions were not determined. Mehta et al. [26] investigated in a retrospective study 407 ACL reconstructions, evaluating the influence of intraoperative fluoroscopy on decision making, whether tunnel positions were required to be changed. Regardless of the surgeon’s experience, tibial and femoral drill guide’s positions were changed in 15 %, which is close to the reported changing rate of 14.3 % femoral and 17.8 % tibial tunnel positions of the presented study.
However, the presented study indicates that fluoroscopic-assisted ACL reconstruction allows to achieve more consistent tunnel positions compared to conventional ACL reconstruction techniques, intraoperative fluoroscopy alone is limited to the assessment of tunnel positions after positioning of drill guides. Fluoroscopy therefore only enables surgeons to roughly estimate whether the chosen tunnel positions match with recommended radiographic tunnel positions. In order to achieve even more consistent results in ACL tunnel positioning, different other fluoroscopic-assisted methods, like fluoroscopic overlay technique or fluoroscopic navigation, were developed to increase accuracy by planning and guiding of tunnel positions [28, 36]. Though different, the basic principles of both methods—fluoroscopic overlay and fluoroscopic navigation—are comparable: projecting target points onto fluoroscopic images that can be achieved under either radiological or navigated guidance. Among other publications [7, 11], Klos et al. [17] showed that adding navigation to fluoroscopy reduced variability and ranges of tunnel positions in ACL reconstruction.
Several limitations are seen in this study. First, no control group was investigated, analysing the variability in conventional ACL reconstruction techniques. Since intraoperative fluoroscopy was standard procedure in the reporting department, the author’s felt it is inappropriate to withhold fluoroscopy to a control group. Reporting on the variability of tunnel positions in conventional ACL reconstruction, comparable study set-ups were chosen before [45]. Further, the precision could not be determined, since intraoperative fluoroscopy was used in the presented study alone. Therefore, planning of target points as well as measurement of the absolute deviations of the drill guide’s positions in relation to the target points (precision) was not possible. However, we could investigate the accuracy of fluoroscopic-assisted tunnel positioning by measuring deviations from the calculated mean positions. Experienced ACL surgeons, who were familiar with radiographic determination of tunnel positions, have performed all cases of ACL reconstruction. Therefore, a certain bias generating better results can be estimated. Last, the presented study did not take clinical correlations into account.
Conclusion
As a main result, the presented study shows that intraoperative fluoroscopy enables surgeons to create more consistent and reliable tunnel positions in ACL reconstruction. Since there is till date no common agreement on optimal tunnel positioning, the described method can be recommended as a feasible, easy and effective method that allows for precise determination and comparison of tunnel positions in order to investigate on optimal tunnel positioning in ACL reconstruction.
References
Ahn JH, Lee YS, Chang MJ, Yim HS (2011) Analysis of revision anterior cruciate ligament reconstruction according to the combined injury, degenerative change, and MRI findings. Knee 18(6):382–386
Amis AA, Beynnon B, Blankevoort L, Chambat P, Christel P, Durselen L, Friederich N, Grood E, Hertel P, Jakob R et al (1994) Proceedings of the ESSKA scientific workshop on reconstruction of the anterior and posterior cruciate ligaments. Knee Surg Sports Traumatol Arthrosc 2(3):124–132
Barrett AM, Craft JA, Replogle WH, Hydrick JM, Barrett GR (2011) Anterior cruciate ligament graft failure: a comparison of graft type based on age and Tegner activity level. Am J Sports Med 39(10):2194–2198
Behrend H, Stutz G, Kessler MA, Rukavina A, Giesinger K, Kuster MS (2006) Tunnel placement in anterior cruciate ligament (ACL) reconstruction: quality control in a teaching hospital. Knee Surg Sports Traumatol Arthrosc 14(11):1159–1165
Bernard M, Hertel P (1996) Intraoperative and postoperative insertion control of anterior cruciate ligament-plasty. A radiologic measuring method (quadrant method). Unfallchirurg 99(5):332–340
Bernard M, Hertel P, Hornung H, Cierpinski T (1997) Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 10(1):14–21
Chouteau J, Benareau I, Testa R, Fessy MH, Lerat JL, Moyen B (2008) Comparative study of knee anterior cruciate ligament reconstruction with or without fluoroscopic assistance: a prospective study of 73 cases. Arch Orthop Trauma Surg 128(9):945–950
Group M (2013) Radiographic findings in revision anterior cruciate ligament reconstructions from the Mars cohort. J Knee Surg 26(4):239–247
Group M, Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK, Allen CR, Cooper DE, DeBerardino TM, Lantz BB, Mann BJ, Stuart MJ (2010) Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med 38(10):1979–1986
Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R (1994) Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy 10(5):502–512
Hiraoka H, Kuribayashi S, Fukuda A, Fukui N, Nakamura K (2006) Endoscopic anterior cruciate ligament reconstruction using a computer-assisted fluoroscopic navigation system. J Orthop Sci 11(2):159–166
Howell SM (1998) Principles for placing the tibial tunnel and avoiding roof impingement during reconstruction of a torn anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 6(Suppl 1):S49–S55
Jaureguito JW, Paulos LE (1996) Why grafts fail. Clin Orthop Relat Res 325:25–41
Kasten P, Szczodry M, Irrgang J, Kropf E, Costello J, Fu FH (2010) What is the role of intra-operative fluoroscopic measurements to determine tibial tunnel placement in anatomical anterior cruciate ligament reconstruction? Knee Surg Sports Traumatol Arthrosc 18(9):1169–1175
Kawakami Y, Hiranaka T, Matsumoto T, Hida Y, Fukui T, Uemoto H, Doita M, Tsuji M, Kurosaka M, Kuroda R (2012) The accuracy of bone tunnel position using fluoroscopic-based navigation system in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 20(8):1503–1510
Khalfayan EE, Sharkey PF, Alexander AH, Bruckner JD, Bynum EB (1996) The relationship between tunnel placement and clinical results after anterior cruciate ligament reconstruction. Am J Sports Med 24(3):335–341
Klos TV, Habets RJ, Banks AZ, Banks SA, Devilee RJ, Cook FF (1998) Computer assistance in arthroscopic anterior cruciate ligament reconstruction. Clin Orthop Relat Res 354:65–69
Klos TV, Harman MK, Devilee RJ, Banks SA, Cook FF (1999) Patellar tendon graft position after anterior cruciate ligament reconstruction. Interobserver variability on lateral radiographs. Acta Orthop Scand 70(2):180–184
Klos TV, Harman MK, Habets RJ, Devilee RJ, Banks SA (2000) Locating femoral graft placement from lateral radiographs in anterior cruciate ligament reconstruction: a comparison of 3 methods of measuring radiographic images. Arthroscopy 16(5):499–504
Kodali P, Yang S, Koh J (2008) Computer-assisted surgery for anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev 16(2):67–76
Koh J, Koo SS, Leonard J, Kodali P (2006) Anterior cruciate ligament (ACL) tunnel placement: a radiographic comparison between navigated versus manual ACL reconstruction. Orthopedics 29(10 Suppl):S122–S124
Kopf S, Forsythe B, Wong AK, Tashman S, Irrgang JJ, Fu FH (2012) Transtibial ACL reconstruction technique fails to position drill tunnels anatomically in vivo 3D CT study. Knee Surg Sports Traumatol Arthrosc 20(11):2200–2207
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33(2):363–374
Marchant BG, Noyes FR, Barber-Westin SD, Fleckenstein C (2010) Prevalence of nonanatomical graft placement in a series of failed anterior cruciate ligament reconstructions. Am J Sports Med 38(10):1987–1996
McConkey MO, Amendola A, Ramme AJ, Dunn WR, Flanigan DC, Britton CL, Group MK, Wolf BR, Spindler KP, Carey JL, Cox CL, Kaeding CC, Wright RW, Matava MJ, Brophy RH, Smith MV, McCarty EC, Vida AF, Wolcott M, Marx RG, Parker RD, Andrish JF, Jones MH (2012) Arthroscopic agreement among surgeons on anterior cruciate ligament tunnel placement. Am J Sports Med 40(12):2737–2746
Mehta VM, Paxton EW, Fithian DC (2009) Does the use of fluoroscopy and isometry during anterior cruciate ligament reconstruction affect surgical decision making? Clin J Sport Med 19(1):46–48
Meuffels DE, Reijman M, Verhaar JA (2012) Computer-assisted surgery is not more accurate or precise than conventional arthroscopic ACL reconstruction: a prospective randomized clinical trial. J Bone Joint Surg Am 94(17):1538–1545
Moloney G, Araujo P, Rabuck S, Carey R, Rincon G, Zhang X, Harner C (2013) Use of a fluoroscopic overlay to assist arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med 41(8):1794–1800
Musahl V, Burkart A, Debski RE, Van Scyoc A, Fu FH, Woo SL (2003) Anterior cruciate ligament tunnel placement: comparison of insertion site anatomy with the guidelines of a computer-assisted surgical system. Arthroscopy 19(2):154–160
Musahl V, Plakseychuk A, VanScyoc A, Sasaki T, Debski RE, McMahon PJ, Fu FH (2005) Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med 33(5):712–718
Picard F, DiGioia AM, Moody J, Martinek V, Fu FH, Rytel M, Nikou C, LaBarca RS, Jaramaz B (2001) Accuracy in tunnel placement for ACL reconstruction. Comparison of traditional arthroscopic and computer-assisted navigation techniques. Comput Aided Surg 6(5):279–289
Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH (2012) Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy 28(6):872–881
Pinczewski LA, Salmon LJ, Jackson WF, von Bormann RB, Haslam PG, Tashiro S (2008) Radiological landmarks for placement of the tunnels in single-bundle reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br 90(2):172–179
Plaweski S, Cazal J, Rosell P, Merloz P (2006) Anterior cruciate ligament reconstruction using navigation: a comparative study on 60 patients. Am J Sports Med 34(4):542–552
Sadoghi P, Kropfl A, Jansson V, Muller PE, Pietschmann MF, Fischmeister MF (2011) Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthroscopy 27(3):355–364
Shafizadeh S, Balke M, Wegener S, Tjardes T, Bouillon B, Hoeher J, Baethis H (2011) Precision of tunnel positioning in navigated anterior cruciate ligament reconstruction. Arthroscopy 27(9):1268–1274
Siebold R (2011) The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 19(5):699–706
Siebold R, Ellert T, Metz S, Metz J (2008) Femoral insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: morphometry and arthroscopic orientation models for double-bundle bone tunnel placement—a cadaver study. Arthroscopy 24(5):585–592
Singh AP, Singh BK (2011) The use of intra-operative image intensifier control for the ACL surgeon. Knee 18(6):379–381
Staubli HU, Rauschning W (1994) Tibial attachment area of the anterior cruciate ligament in the extended knee position. Anatomy and cryosections in vitro complemented by magnetic resonance arthrography in vivo. Knee Surg Sports Traumatol Arthrosc 2(3):138–146
Sullivan JP, Matava MJ, Flanigan DC, Gao Y, Britton CL, Amendola A, Group M, Wolf BR (2012) Reliability of tunnel measurements and the quadrant method using fluoroscopic radiographs after anterior cruciate ligament reconstruction. Am J Sports Med 40(10):2236–2241
Taketomi S, Inui H, Nakamura K, Hirota J, Takei S, Takeda H, Tanaka S, Nakagawa T (2012) Three-dimensional fluoroscopic navigation guidance for femoral tunnel creation in revision anterior cruciate ligament reconstruction. Arthrosc Tech 1(1):e95–e99
Trojani C, Sbihi A, Djian P, Potel JF, Hulet C, Jouve F, Bussiere C, Ehkirch FP, Burdin G, Dubrana F, Beaufils P, Franceschi JP, Chassaing V, Colombet P, Neyret P (2011) Causes for failure of ACL reconstruction and influence of meniscectomies after revision. Knee Surg Sports Traumatol Arthrosc 19(2):196–201
Tsuda E, Ishibashi Y, Fukuda A, Yamamoto Y, Tsukada H, Ono S (2010) Tunnel position and relationship to postoperative knee laxity after double-bundle anterior cruciate ligament reconstruction with a transtibial technique. Am J Sports Med 38(4):698–706
Wolf BR, Ramme AJ, Wright RW, Brophy RH, McCarty EC, Vidal AR, Parker RD, Andrish JT, Amendola A, Group MK (2013) Variability in ACL tunnel placement: observational clinical study of surgeon ACL tunnel variability. Am J Sports Med 41(6):1265–1273
Wright R, Spindler K, Huston L, Amendola A, Andrish J, Brophy R, Carey J, Cox C, Flanigan D, Jones M, Kaeding C, Marx R, Matava M, McCarty E, Parker R, Vidal A, Wolcott M, Wolf B, Dunn W (2011) Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg 24(4):289–294
Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK, Allen CR, Cooper DE, DeBerardino TM, Lantz BB, Mann BJ, Stuart MJ (2010) Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med 38(10):1979–1986
Zantop T, Wellmann M, Fu FH, Petersen W (2008) Tunnel positioning of anteromedial and posterolateral bundles in anatomic anterior cruciate ligament reconstruction: anatomic and radiographic findings. Am J Sports Med 36(1):65–72
Zhu W, Lu W, Han Y, Hui S, Ou Y, Peng L, Fen W, Wang D, Zhang L, Zeng Y (2013) Application of a computerised navigation technique to assist arthroscopic anterior cruciate ligament reconstruction. Int Orthop 37(2):233–238
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sven, S., Maurice, B., Hoeher, J. et al. Variability of tunnel positioning in fluoroscopic-assisted ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 23, 2269–2277 (2015). https://doi.org/10.1007/s00167-014-3029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3029-y