Abstract
Purpose
The purpose of the current study was to investigate the potential effect of intraoperative fluoroscopy on the accuracy of femoral tunnel placement in anatomic ACL reconstruction, using an ideal anatomic point as reference and evaluating postoperative tunnel placement based on 3D CT.
Methods
An experienced ACL surgeon, using the anatomic approach for femoral tunnel placement, relying on intraarticular landmarks and remnants of the torn ACL—and novel to the fluoroscopic assist—was introduced to its use. A prospective series of patients was included where group 1 (without fluoroscopy) and group 2 (with fluoroscopy) both had postoperative CT scans so that femoral tunnel position could be evaluated and compared to an ideal tunnel centre based on anatomic studies by using the Bernard and Hertel grid.
Results
Group 2, where fluoroscopy was used, had a mean femoral tunnel that was closer to the ideal anatomic centre than group 1. In the Bernard and Hertel grid, the distance in the high-low axis (y-axis) was found significantly closer (P = 0.001), whilst the deep-shallow axis (x-axis) and a total absolute distance were not significantly closer to the ideal described anatomic centre.
Conclusions
Intraoperative fluoroscopy was found effective as an aid for placing the femoral tunnel in a more accurate position, as compared to a desired anatomic centre. Although the concept of the “one-size-fits-all” approach for tunnel placement is debatable, the avoidance of grossly misplaced tunnels is the benefit of using fluoroscopy during ACL reconstruction. The authors hold that fluoroscopy is readily available, safe and easy to use and therefore a good aid in the anatomic approach for graft tunnel placement, for example, in a learning situation, in revision cases and when performing low volumes of such surgery.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In surgical reconstruction of the anterior cruciate ligament (ACL), correct and accurate graft tunnel placement is a prerequisite for successful postoperative results. Erroneous tunnel placement can have detrimental effects on the function of the knee and is by some authors described as the most common cause of failure after the primary surgery [25, 37]. Such misplacements will fail to restore native knee kinematics and inevitably result in postoperative residual laxity of the knee or restricted range of motion, predisposing the knee for secondary failure [2, 3].
In the “anatomic approach” for ACL reconstruction, femoral tunnel placement is based on identification of the native insertion sites of the torn ligament. Intraarticular landmarks, known as “lateral intercondylar ridge” and “lateral bifurcate ridge”, demarcate the femoral attachment of the native ACL and can therefore give guidance to where the graft tunnels should be placed in order to perform an “anatomic” reconstruction. Although these landmarks are well described, they can have a somewhat variable presence and visibility [11, 33, 35]. The arthroscopic identification of the ridges can therefore be challenging to surgeons novel to this technique. Some recent papers also suggest that even experienced knee surgeons can misinterpret where to place the femoral tunnel [4, 16, 24].
Several aids have been proposed to ensure correct tunnel placement in ACL reconstruction [1, 6, 16, 24, 32]. Computer-assisted surgery (CAS), or navigated surgery, is a well-known principle utilized in a variety of surgical fields [8, 26, 36]. In ACL reconstructions, they have been found to give consistent and accurate tunnel placement [10, 14, 19, 32]. Along with the price of such equipment, morbidity to the patient (due to invasive fixation of the rig) and complexity of the systems are downsides perhaps hindering a widespread use [19]. Other authors have demonstrated how intraoperative use of fluoroscopy—available in most orthopaedic departments—can aid tunnel placement [13, 15, 31]. Although the clinical impact of intraoperative fluoroscopy has yet to be established, accuracy of tunnel placement—and avoidance of unwanted tunnel variability—can itself act as an intermediary outcome after surgery [16, 24].
Some studies have suggested that there might be a significant learning curve when placing the femoral tunnel based on the “anatomic approach” utilizing remnants of the torn ACL and intraarticular bony landmarks [16, 29]. In the light of these findings, the aim of the current study was to examine the effect of intraoperative fluoroscopy on the accuracy of femoral tunnel placement, using the anatomic approach for femoral tunnel placement, in a surgeon novel to the fluoroscopic assist. By performing postoperative 3D CT, femoral tunnel placement could be evaluated and compared to an “ideal anatomic centre” to examine whether any improvement could be found. The hypothesis was that the intraoperative fluoroscopy would provide a femoral tunnel placement that was closer to the anatomic centre than before its introduction.
Materials and methods
Eighty-one consecutive patients undergoing anterior cruciate ligament reconstruction at TBA Hospital from February 2013 to March 2014 were prospectively included in the study. A single experienced surgeon, performing more than 100 ACL reconstructions annually, did all the surgeries. Patients who underwent revision surgery were excluded from the study. Postoperative CT scans were performed at 4–8 weeks after surgery to evaluate femoral tunnel placement of the treated knee.
Surgical technique
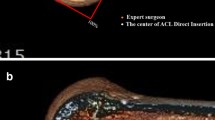
A uniform single-bundle anatomic technique, using double-loop semitendinosus and gracilis (DLSTG) autograft, was performed in all patients. Independent femoral tunnel reaming was performed with the use of an accessory anteromedial portal [7]. A microfracture awl was used to localize and demarcate the centre of the femoral ACL footprint, guided by the remnants of the native ACL and intraarticular bony landmarks [7, 34]. The aim was to perform a centre-to-centre reconstruction. A guide wire was inserted in the desired location, and a femoral tunnel was made using a cannulated reamer.
The first 48 patients (group 1) of the study were included as a control group before initialization of fluoroscopy. The surgeon was provided postoperative feedback on the femoral tunnel placement, as measured on 3D CT and compared to an optimal anatomic centre by means of the Bernard and Hertel (B&H) grid (also known as the quadrant method) [5, 16, 30]. The feedback enabled the surgeon to correct possible systematic misplacements.
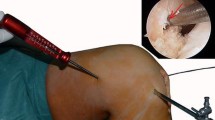
In the next 33 patients (group 2), intraoperative fluoroscopy (Fluoroscan InSight, Hologic Inc., Bedford, USA) was introduced to the surgeon. A generic template displaying the optimal anatomic centre (to be explained) in a B&H grid was made available so that all fluoroscopic images could be visually compared to that at the time of surgery [6, 16]. A true lateral sagittal projection, with completely overlapping femoral condyles, was to be obtained before the tunnel placement could be assessed (Fig. 1) [12]. Any misplacement, as compared to the ideal centre, was corrected before the femoral tunnel was reamed.
3D CT measurements
The postoperative CT was performed on an extended knee. A GE LightSpeed VCT 64-slice CT scanner was used at 100 kV, mAs 80 in standard and bone algorithm. The slice thicknesses of the images were 0.625. For the volume rendering, the GE AW Volume Share 2 software was used. 3D reconstructions were made positioning the knee in a true lateral view and removing the medial femoral condyle to visualize the inside of the lateral femoral condyle.
All postoperative measurements were performed in Mdesk 3.4.2.2 (RSA BioMedical, Umea, Sweden) using the B&H grid [5, 30]. The technique for assessment of the femoral tunnel is similar to one previously published [16], and the coordinates of the tunnel centre were then compared to an empirical optimal anatomic position (27 % along the deep-shallow axis and 34 % along the high-low axis), as introduced by Bird et al. [6], based on average empirical femoral footprint localizations from cadaver studies. An absolute value of the distance in the B&H grid (sum of the absolute distance from the ideal point in x-axis and y-axis) was calculated. This measure, formerly used for similar comparisons, is a method for assessing the variance of tunnel placement, eliminating the effect of differing prefix (positive or negative) when comparing different tunnel positions along the deep-shallow or high-low axes [17].
Two independent examiners, not involved in the treatment of the patients, examined the tunnel placement on all postoperative CT scans. Repeated measurements were performed with a minimum of 3 months apart to establish the intrarater reliability. The study was reviewed and approved by the regional ethical committee (REK Helse Vest ID: 2014:264), and all patients gave their informed consent prior to their inclusion in the study.
Statistical analysis
An a priori significance level of 0.05 was chosen. All data handling and statistical analysis were performed in SPSS 22.0 (SPSS Inc., Chicago, IL, USA). For descriptive analysis, Chi-square statistics were used to test for differences in frequencies. Interrater and intrarater reliability was measured using the intraclass correlation coefficients (ICC) utilizing Cronbach’s alpha statistics. Also, the agreement between observers was plotted and visually controlled in Bland–Altman plots. Between-group differences in tunnel placements were examined with the Student’s t test. To examine for any continued “learning effect” from postoperative feedback from CT scans, group 1 was further split into group A (N = 24) and B (N = 24), and t tests were performed to investigate the differences in outcome variables. Based on a former study using the same CT technology, a group size of 50 patients would provide a statistical power of 84 % given a significance level of 0.05 [16]. This calculation was based on a minimal detectable difference of 5 % in the x-axis and with a SD of 8.
Results
Eighty-one patients (48 in group 1 and 33 in group 2) were included. Sixty-six (81 %) underwent the postoperative CT, so that tunnel placement could be evaluated. Of the included patients, 44 % were men, and in 41 % of the cases, the right knee was treated. Mean age at surgery was 31.5 (SD 11.1). There were no significant demographic differences between the fluoroscopy-assisted group (group 2) and the non-fluoroscopy-assisted group (group 1) (n.s.). At surgery, 63 patients were found to have a graft size of 8 mm, whilst 28 patients had a graft size of 9 mm as measured by a graft sizing tube. The distribution of graft sizes between the two groups was not found to differ (n.s.).
Interobserver reliability, represented by the intraclass correlation coefficient (ICC), was measured for both the high-low and the deep-shallow measurements. The ICC was found to be 0.923 and 0.974, respectively—both considered very good. The ICCs for intraobserver reliability for measurement at two points in time were 0.952 and 0.918, respectively. Bland–Altman plots for the high-low and deep-shallow measurements are presented in Figs. 4 and 5.
Tunnel placement
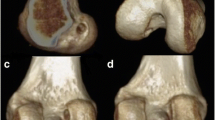
The mean tunnel placement and the mean absolute tunnel placement for groups 1 and 2 are presented in Table 1. All tunnels of the two groups are indicated separately on two template knees (Fig. 2). When testing for differences in the mean of the absolute distances between groups 1 and 2 (12.5 vs. 9.8), these were not found to differ (n.s.). When testing for differences in the high-low and deep-shallow tunnel placement between groups, there was a significant difference in the high-low direction (P = 0.001) with a mean of 38.3 in the fluoroscopy-assisted group and a mean of 28.5 in the non-fluoroscopy group. There was, however, no difference in the deep-shallow direction (n.s.) between the groups. When comparing tunnel placement in groups A and B (i.e. first and second half of patients in group 1 based on chronological order of surgeries), none of the mean high-low, mean deep-shallow or mean absolute distances were found to differ significantly (n.s.).
Template CT showing femoral tunnel placement in Bernard–Hertel grid as compared to an ideal anatomic centre before and after fluoroscopy. a White dot represents anatomic reference of 27 % in deep-shallow and 34 % in high-low directions [6]. b Purple dots before fluoroscopy and green dots after fluoroscopy
Discussion
The main finding of the current study is the improvement of femoral tunnel placement, as compared to an ideal centre based on a mean from anatomic studies, after introduction of intraoperative fluoroscopy in a knee surgeon novel to its use (as an aid in tunnel positioning using the accessory anteromedial portal). Based on measurements made on postoperative 3D CT scans in a B&H grid, the tunnel position was improved significantly in the high-low direction (P = 0.001), but not so in the deep-shallow direction (n.s.) nor in a constructed absolute distance from the ideal centre (n.s.).
With the use of a fluoroscopic overlay, much alike that in the current study, Moloney et al. [24] investigated femoral tunnel placements in simulated ACL reconstructions of cadaver knees in 20 surgeons (with variable experience of ACL surgery). Their finding—that more than half of all surgeons initially had a femoral tunnel placement more than 2.5 mm away from the native femoral footprint centre—emphasizes why the use of sole intraarticular landmarks for ACL reconstruction can be hazardous in ACL reconstruction. With their introduction of a fluoroscopic overlay—utilizing an ideal anatomic centre of 29 and 37 % (as compared to 27 and 34 % in the current study) in the deep-shallow and high-low axes of the B&H grid, respectively—a significant improvement was seen in the intended tibial and femoral tunnel placements. Since these reconstructions were all simulated, no actual tunnel evaluations were performed in that study. Also the use of only the bony femoral notch—where all other soft tissue landmarks were dissected off—made the experiment less realistic as compared to live surgery.
The improvement of tunnel placement in the current study was only found in the high-low axis, but the results of our expert surgeon must, however, be seen in the light of a former study performed by utilizing feedback from postoperative 3D CT as aid for tunnel placement [16]. In that study, following our surgeon changing from a transtibial to an anatomic approach for femoral tunnel placement, a significant improvement of femoral tunnel placement was found in the high-low axis, the deep-shallow axis and also in the absolute distance from the ideal tunnel centre when introducing feedback from postoperative CT. Thus, in the current work, fluoroscopy is not the only “aid” being used for tunnel placement, and the postoperative results in group 1 (without fluoroscopy) are therefore somewhat biased as compared to tunnel placement in a surgeon where only the intraarticular bony landmarks have been used as aid for tunnel placement. We did, however, examine whether there was still a learning effect of the sole postoperative feedback, but no differences were found between the former and the latter half of the procedures in group 1 (without fluoroscopy). For the sake of comparison, a plot of the first 50 patients surgically reconstructed with only guidance of intraarticular landmarks—formerly included in another study—has been attached, although not included in the current analyses (Fig. 3).
Although utilizing a different approach for aiding tunnel placement, results from several clinical studies involving computer-aided surgery (CAS) could be considered relevant. Chouteau et al. [10] evaluated a series of 73 ACL reconstructed patients where about half of the patients had surgery after a CAS system (utilizing fluoroscopy) was introduced. Although they found no clinical effect at a review 2 years after surgery, the tunnel accuracy—as measured on postoperative radiographs—was significantly improved. Sagittal radiographs as the ones used in that study does, however, have clear limitations in evaluating femoral tunnel placement. Another study, using a somewhat different system of navigation, evaluated the mismatch between tibial tunnel placement and the extension of Blumensaat’s line (as seen on sagittal radiographs) [14]. Given that a tibial tunnel that respected the extension of the Blumensaat line would be a more desirable placement (keeping in mind that a too anterior tibial tunnel placement would predispose the graft for roof impingement), they found a significant better match in the navigated group.
Kawakami et al. [19] used a similar approach as in the current study for placing femoral and tibial tunnels. By the means of fluoroscopically assisted CAS, they used the femoral B&H grid to place tunnels related to an ideal point on a navigated overlay. When evaluating postoperative tunnel placement in a group where CAS had been used, as compared to one where no aid for tunnel placement was used, they found the CAS group to have a femoral tunnel significantly closer to the “ideal” anatomic point (P = 0.001). Tunnel position was, however, only evaluated in the deep-shallow plane of the B&H grid for single-bundle ACL reconstruction. Clearly navigated surgery seems advantageous when it comes to securing correct tunnel placement in ACL surgery. Complexity of the systems in terms of their both set-up and usage, invasiveness (due to rigid fixation to bone) and the expense of such equipment are downsides that will, perhaps, hinder the widespread availability of CAS in ACL surgery.
Although fluoroscopy for use in tunnel placement could be further sophisticated (e.g. by on-screen comparison of actual tunnel placement to any preferred reference point instead of simple eyeballing), the simple use of a generic template displaying an ideal tunnel placement, as in the current study, was found to have an effect on accuracy of tunnel placement. Other advantages include its straightforward application and the availability in any orthopaedic department performing trauma surgery. Some concerns should, however, be addressed. The exposure to radiation during surgery, to both surgeon and patient, has been discussed by several authors [9, 23]. In a study of 58 patients undergoing fluoroscopy-assisted ACL reconstruction, Chitavis et al. investigated the radiation doses to determine the long-term risk of cancer. With a mean effective dose (an indicator of long-term cancer risk) of 0.001 mSv per patient, the lifetime risk of developing cancer is due to the intraoperative fluoroscopy of <0.0001 %. Another concern, as with any new addition to surgical techniques, is the time under anaesthesia for patients (and therefore potential cost of prolonged surgery). The current study made no comparison of surgical time. Other authors have recorded the additional time in the OR, displaying a range of a mean 1–5 min [15, 22, 23].
An important issue to address is the limitation of using such a “one-size-fits-all” ideal anatomic point during reconstructions. Clearly, as found in surgeons new to the anatomic technique, such a method can help reduce unwanted variation in femoral tunnel position that might compromise the outcome after surgery [16, 24]. It is, however, a trade-off since most knees display a certain intraindividual variability [18, 21]. Reconstructing using the anatomic technique heavily relies on visualizing intraarticular landmarks and remnants of the ACL. In addition to situations where the anatomic technique is novel to the surgeon, or in those performing only a limited annual number of reconstructions, chronic case reconstructions and revision reconstructions could possibly also benefit from the use of fluoroscopy. The latter cases will both have distorted anatomy, and therefore, other strategies than relying on intraarticular remnants and bony landmarks should be considered. Strengths of the current study include the prospective and systematic registration of data and a follow-up rate above 80 % of patients who had the primary surgery.
The main purpose of the current work was to purvey a simple means to reduce the most unwanted and clear-cut erroneous tunnel placement for certain surgical situations. As suggested by Behrend et al. [4], a certain variation as such is probably well tolerated; it is, however, crucial to determine the “safe zones” of femoral tunnel placement. Further work should therefore investigate the clinical impact of stratified femoral tunnel positions. Although easily measureable, graft tunnel placement should only be considered an intermediary outcome until more clinical studies have established their relation to patient outcomes [16, 27]. Some such studies are present, but they are few and heterogeneous [1, 17, 20, 28]. With the increasing popularity of postoperative CT, there is reason to believe that more such data will be available in near future [6, 27, 32, 38].
Conclusions
In conclusion, the current study found a significant improvement of femoral tunnel placement when introducing intraoperative fluoroscopy to a surgeon who was placing femoral graft tunnels based on the anatomic technique—using intraarticular landmarks only. The authors hold that when performing anatomic ACL surgery in a learning situation, in revision cases or with low annual volumes, the fluoroscopic assist is a sensible way of aiding femoral tunnel placement and avoiding grossly misplaced graft tunnels.
References
Aglietti P, Buzzi R, Giron F, Simeone AJ, Zaccherotti G (1997) Arthroscopic-assisted anterior cruciate ligament reconstruction with the central third patellar tendon. A 5–8-year follow-up. Knee Surg Sports Traumatol Arthrosc 5:138–144
Amis AA (2012) The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc 20:613–620
Amis AA, Bull AMJ, Lie DTT (2005) Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Oper Tech Orthop 15:29–35
Behrend H, Stutz G, Kessler MA, Rukavina A, Giesinger K, Kuster MS (2006) Tunnel placement in anterior cruciate ligament (ACL) reconstruction: quality control in a teaching hospital. Knee Surg Sports Traumatol Arthrosc 14:1159–1165
Bernard M, Hertel P, Hornung H, Cierpinski T (1997) Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 10:14–21
Bird JH, Carmont MR, Dhillon M, Smith N, Brown C, Thompson P, Spalding T (2011) Validation of a new technique to determine midbundle femoral tunnel position in anterior cruciate ligament reconstruction using 3-dimensional computed tomography analysis. Arthroscopy 27:1259–1267
Brown CH, Spalding T, Robb C (2013) Medial portal technique for single-bundle anatomical anterior cruciate ligament (ACL) reconstruction. Int Orthop 37:253–269
Cheng I, Shen R, Moreau R (2014) An augmented reality framework for optimization of computer assisted navigation in endovascular surgery. In: Conference Proceedings of the IEEE Engineering in Medicine and Biology Society pp 5647–5650
Chitnavis JP, Karthikesaligam A, Macdonald A, Brown C (2010) Radiation risk from fluoroscopically-assisted anterior cruciate ligament reconstruction. Ann R Coll Surg Engl 92:330–334
Chouteau J, Benareau I, Testa R, Fessy MH, Lerat JL, Moyen B (2007) Comparative study of knee anterior cruciate ligament reconstruction with or without fluoroscopic assistance: a prospective study of 73 cases. Arch Orthop Trauma Surg 128:945–950
Ferretti M, Ekdahl M, Shen W, Fu FH (2007) Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy 23:1218–1225
Haasper C, Kopf S, Lorenz S, Middleton KK, Tashman S, Fu FH (2015) Influence of tibial rotation on tibial tunnel position measurements using lateral fluoroscopy in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 23:649–654
Halbrecht J, Levy IM (1993) Fluoroscopic assist in anterior cruciate ligament reconstruction. Arthroscopy 9:533–535
Hiraoka H, Kuribayashi S, Fukuda A, Fukui N, Nakamura K (2006) Endoscopic anterior cruciate ligament reconstruction using a computer-assisted fluoroscopic navigation system. J Orthop Sci 11:159–166
Hughes AW, Dwyer AJ, Govindaswamy R, Lankester B (2012) The use of intra-operative fluoroscopy for tibial tunnel placement in anterior cruciate ligament reconstruction. Bone Joint Res 1:234–237
Inderhaug E, Larsen A, Strand T, Waaler PA, Solheim E (2014) The effect of feedback from post-operative 3D CT on placement of femoral tunnels in single-bundle anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-3355-0
Inderhaug E, Strand T, Fischer-Bredenbeck C, Solheim E (2015) Effect of a too posterior placement of the tibial tunnel on the outcome 10–12 years after anterior cruciate ligament reconstruction using the 70-degree tibial guide. Knee Surg Sports Traumatol Arthrosc 22:1182–1189
Kasten P, Szczodry M, Irrgang J, Kropf E, Costello J, Fu FH (2010) What is the role of intra-operative fluoroscopic measurements to determine tibial tunnel placement in anatomical anterior cruciate ligament reconstruction? Knee Surg Sports Traumatol Arthrosc 18:1169–1175
Kawakami Y, Hiranaka T, Matsumoto T, Hida Y, Fukui T, Uemoto H, Doita M, Tsuji M, Kurosaka M, Kuroda R (2011) The accuracy of bone tunnel position using fluoroscopic-based navigation system in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 20:1503–1510
Khalfayan EE, Sharkey PF, Alexander AH, Bruckner JD, Bynum EB (1996) The relationship between tunnel placement and clinical results after anterior cruciate ligament reconstruction. Am J Sports Med 24:335–341
Klos TV, Banks SA, Habets RJ, Cook FF (2000) Sagittal plane imaging parameters for computer-assisted fluoroscopic anterior cruciate ligament reconstruction. Comput Aided Surg 5:28–34
Larson BJ, DeLange NP (2008) Fluoroscopically-assisted hamstring ACL reconstruction. Orthopedics 31:657–662
Larson BJ, Egbert J, Goble EM (1995) Radiation exposure during fluoroarthroscopically assisted anterior cruciate reconstruction. Am J Sports Med 23:462–464
Moloney G, Araujo P, Rabuck S, Carey R, Rincon G, Zhang X, Harner C (2013) Use of a fluoroscopic overlay to assist arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med 41:1794–1800
Morgan J, Dahm D, Levy B, Stuart M, The MARS Study Group (2012) Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg 25:361–368
Newe A, Becker L, Schenk A (2014) Application and evaluation of interactive 3D PDF for presenting and sharing planning results for liver surgery in clinical routine. PLoS ONE. doi:10.1371/journal.pone.0115697
Owens BD (2013) Location, location, location. Am J Sports Med 41:2481–2483
Pinczewski LA, Salmon LJ, Jackson WFM, von Bormann RBP, Haslam PG, Tashiro S (2008) Radiological landmarks for placement of the tunnels in single-bundle reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br 90:172–179
Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC (2013) Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy 29:98–105
Sullivan JP, Matava MJ, Flanigan DC, Gao Y, Britton CL, Amendola A, MOON Group, Wolf BR, Spindler KS, Dunn WR, Carey JL, Cox CL, Andrish JT, Parker RD, Jones MH, Marx RG, McCarty EC, Vidal AF, Wolcott M, Wright RW, Brophy RH, Smith MV, Kaeding CC (2012) Reliability of tunnel measurements and the quadrant method using fluoroscopic radiographs after anterior cruciate ligament reconstruction. Am J Sports Med 40:2236–2241
Sven S, Maurice B, Hoeher J, Marc B (2015) Variability of tunnel positioning in fluoroscopic-assisted ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 23:2269–2277
Taketomi S, Inui H, Nakamura K, Hirota J, Sanada T, Masuda H, Takeda H, Tanaka S, Nakagawa T (2013) Clinical outcome of anatomic double-bundle ACL reconstruction and 3D CT model-based validation of femoral socket aperture position. Knee Surg Sports Traumatol Arthrosc 22:2194–2201
Tsukada S, Fujishiro H, Watanabe K, Nimura A, Mochizuki T, Mahakkanukrauh P, Yasuda K, Akita K (2014) Anatomic variations of the lateral intercondylar ridge: relationship to the anterior margin of the anterior cruciate ligament. Am J Sports Med 42:1110–1117
van Eck CF, Schreiber VM, Mejia HA, Samuelsson K, van Dijk CN, Karlsson J, Fu FH (2010) “Anatomic” anterior cruciate ligament reconstruction: a systematic review of surgical techniques and reporting of surgical data. Arthroscopy 26:S2–S12
van Eck CF, Kopf S, Irrgang JJ, Blankevoort L, Bhandari M, Fu FH, Poolman RW (2012) Single-bundle versus double-bundle reconstruction for anterior cruciate ligament rupture: a meta-analysis—does anatomy matter? Arthroscopy 28:405–424
Venne G, Rashquinha BJ, Pichora D, Ellis RD, Bicknell R (2014) Comparing conventional and computer-assisted surgery baseplate and screw placement in reverse shoulder arthroplasty. J Shoulder Elb Surg 24(7):1112–1119
Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS (1996) Revision anterior cruciate ligament reconstruction. Oper Tech Orthop 6:181–189
Youm Y-S, Cho S-D, Eo J, Lee K-J, Jung K-H, Cha J-R (2013) 3D CT analysis of femoral and tibial tunnel positions after modified transtibial single bundle ACL reconstruction with varus and internal rotation of the tibia. Knee 20:272–276
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Inderhaug, E., Larsen, A., Waaler, P.A. et al. The effect of intraoperative fluoroscopy on the accuracy of femoral tunnel placement in single-bundle anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 25, 1211–1218 (2017). https://doi.org/10.1007/s00167-015-3858-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3858-3