Abstract
Purpose
It is difficult to substantiate the clinical diagnosis of postoperative delirium with objective parameters in intensive care units (ICU). The purpose of this study was to analyze (1) whether the bilateral bispectral (BIS) index, (2) cortisol as a stress marker, and (3) interleukin-6 as a marker of inflammation were different in delirious patients as compared to nondelirious ones after cardiac surgery.
Methods
On the first postoperative day, delirium was analyzed in 114 patients by using the confusion assessment method for ICU (CAM-ICU). Bilateral BIS data were determined; immediately thereafter plasma samples were drawn to analyze patients’ blood characteristics. The current ICU medication, hemodynamic characteristics, SOFA and APACHE II scores, and artificial ventilation were noted.
Results
Delirium was detected at 19.1 ± 4.8 h after the end of surgery in 32 of 114 patients (28%). Delirious patients were significantly older than nondelirious ones and were artificially ventilated 4.7-fold more often during the testing. In delirious patients, plasma cortisol and interleukin-6 levels were higher (p = 0.01). The mean BIS index was significantly lower in delirious patients (72.6 (69.6–89.1); median [interquartile range (IQR), 25th–75th percentiles] than in nondelirious patients, 84.8 (76.8–89.9). BIS EEG raw data analysis detected significant lower relative alpha and higher theta power. A significant correlation was found between plasma cortisol levels and BIS index.
Conclusions
Early postoperative delirium after cardiac surgery was characterized by increased stress levels and inflammatory reaction. BIS index measurements showed lower cortical activity in delirious patients with a low sensitivity (27%) and high specificity (96%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early postoperative delirium is a common complication of cardiac surgery, occurring on average in 20–40% of patients and showing a notable peak in the elderly [1]. Postoperative delirium was associated with increased mortality and postoperative complications, functional decline, and increased cost [2]. Despite the high prevalence and negative sequelae of postoperative delirium, its pathophysiology is not known in detail and seems to be multifactorial [1, 3]. Nevertheless, it was proposed that systemic inflammation may actually contribute to the pathogenesis of delirium by compromising blood–brain barrier (BBB) integrity [4]. Inflammatory markers peaked 6–24 h postoperatively and returned to baseline levels over 2–4 days [5]. In a recent study, a possible relationship between interleukin-6 and impaired cognition, including delirium, was suggested [6].
In parallel to inflammation, steroid hormones such as glucocorticoids can influence nervous system function [7]. Thus, a pronounced stress response characterized by sustained high glucocorticoid levels was thought to be associated with the progression of CNS diseases such as Alzheimer’s disease [8]. For ICU delirium, however, the role of serum biomarkers including glucocorticoids in the pathogenesis of delirium is controversial [9] and the role of increased cortisol in ICU delirium after cardiac surgery is the subject of debate [10] and has not been clarified yet.
Decreased cortical brain activity is one characteristic of delirious patients as has been shown in elderly individuals who have not undergone surgery [11]. Our previous study on the surgical ICU [12] detected decreased cerebral cortical activity in patients during delirium, measured by a time-consuming 16-channel EEG system. Furthermore, investigating the role of monolateral BIS EEG (ASPECT Medical System) in detecting dementia or delirium produced controversial results [13, 14]. Up to now, no studies have used the newly available bilateral BIS EEG VISTA® to investigate postoperative ICU delirium.
Therefore, we hypothesized that bilateral four-channel BIS EEG would be able to detect significant differences between delirious and nondelirious patients after cardiac surgery in whom consciousness, arousal, and delirium are assessed with CAM-ICU, including Richmond agitation and sedation scale (RASS). Furthermore, the relationship between BIS EEG and plasma markers such as cortisol and interleukin-6 will be studied in these patients, too. Until now, to our knowledge no studies were undertaken examining the previously addressed questions.
Materials and methods
Patients
The study was set in a 16-bed cardiac surgical intensive care unit (ICU) of a 1,400-bed university teaching hospital (University of Heidelberg, Germany). Patients or their legal representatives were provided with information regarding informed consent by the study tutor on the first postoperative day. Verbal and written informed consent was obtained first from their legal representatives and after recovery by the patients themselves. This human study was approved by the appropriate local Ethics Committee of the University of Heidelberg and was performed in accordance with the ethical standards of the Declaration of Helsinki. The present study was registered at http://www.clinicaltrials.gov.
Inclusion criteria were: age greater than 18 years, elective coronary artery bypass grafting (CABG) with or without aortic valve replacement, and the signed informed consent. These patients represented a relatively homogenous group with a standardized postoperative medication protocol. We did not include patients suffering from psychiatric or neurological disorders such as dementia, depression, or schizophrenia and stroke patients or those with a history of alcohol abuse. Furthermore, patients with early (less than 24 h) postoperative complications in ICU such as drastic hypotension or arterial fibrillation were not included in the study. We excluded patients with pronounced hearing and/or visual impairment problems and non-German-speaking people because the neuropsychological test was in German. In all, four patients were excluded from the present study. All tests were performed on the first postoperative day (about 19 h after the end of the surgery) in ICU to analyze early changes in patients’ cognitive function. Up to 24 h after surgery, the effect of narcotics/sedatives and artificial ventilation is greater than at 48 h. However, our patients receive a standardized medication regime on our cardiac ICU (see below). Therefore the effect of medication is minimized. Furthermore, at later time points after surgery some of the included patients were still in the ICU, whereas some had already been transported to another station; therefore, to exclude different conditions and places of investigation, the postsurgical time of about 19 h was chosen for this study.
Characterization of the patients
For patient evaluation, a structural preoperative study tool was employed that included the patients’ age, gender, body weight and height, and American Society of Anesthesiologists (ASA) classification. Additionally, the lengths of hospital stay, the time of cardiac surgery, and hospital mortality were recorded. On the first postoperative day, blood analysis was performed. Furthermore, delirium testing scores, including the RASS score, acute physiology and chronic health evaluation (APACHE II), and sequential organ failure assessment (SOFA) scores, and body temperature were determined. Artificial ventilation and ICU medications (over the last 12 h until analysis) were noted.
Delirium assessment
The investigator involved in delirium testing (P.F.) was trained by a psychologist in scoring procedures and a further detailed briefing from a psychiatrist. Postoperative delirium was assessed in ICU patients every time by the same investigator (P.F.), once on the first postoperative day from 8 to 9 a.m. using the German translation of the confusion assessment method for intensive care unit (CAM-ICU) [15, 16]. In the CAM-ICU tests, patients’ levels of agitation or sedation were also determined by using RASS.
Bilateral bispectral (BIS) index
For monitoring patients’ cortical function, the BIS VISTA monitoring system (BISx4™) with bihemispheric capabilities (bilateral BIS Quattro®) was used (ASPECT Medical Systems, Norwood, MA, USA). Bilateral BIS EEG measurements were done immediately after CAM-ICU testing. Therefore, after briefly preparing the skin with dry gauze and isopropyl alcohol, the BIS Quattro sensors were applied on the right and left sides of the forehead according to the manufacturer’s instruction to give a four-channel bilateral reference frontotemporal montage. The sensors were connected to a digital signal converter and then to the portable BIS VISTA monitor, which displays both a raw EEG waveform and a numerical BIS value ranging from 0 (deeply unconscious with isoelectric EEG) to 100 (alert). Impedances were measured to ensure they were 5 kΩ or less. Raw EEG data were sampled at 128 samples/s and recorded continuously for 15–20 min in real time; this prolonged data recording interval was used to establish a standardized and stable period of EEG recording that is most free of artifacts. Processed variables were downloaded live and recorded to the computer every 5 s. All BIS data were reviewed and analyzed offline. Thirty-seven raw EEG datasets were additionally analyzed in relation to alpha and theta frequencies by an investigator without knowledge of the patients’ characteristics and clinical diagnosis.
All drugs, including sedative and analgesic medications and medication for pain treatment that were given on the first postoperative day were noted. The following hemodynamic parameters were analyzed: mean arterial blood pressure, heart frequency to characterize cardiac function, and Horowitz quotient (paO2/FiO2) to characterize lung function.
ICU blood collection and analysis
The venous blood samples (1 ml) were taken immediately after BIS measurement for determination of ICU blood parameters (leukocytes, C-reactive protein, hemoglobin, hematocrit, erythrocytes, urea, creatinine, glucose, lactate). The blood was analyzed directly by using standard laboratory techniques. In addition, 2-ml samples were taken and centrifuged at 7,000 rpm for 10 min to determine plasma levels of cortisol and interleukin-6. Thereafter, the supernatant was taken and stored for a maximum of 1 month at −80°C until biochemical parameters were determined. An investigator blinded to all clinical data conducted these tests. The plasma samples were analyzed by applying a commercial cortisol EIA kit, which operates on the basis of competition between a horseradish peroxidase (HRP)–cortisol conjugate and the cortisol in the sample (Oxford Biomedical Research, Oxford, MI, USA). Interleukin-6 was assayed by a quantitative sandwich ELISA technique (Quantikine Human IL-6, R&D Systems, Abingdon, UK). Respective intra-assay and inter-assay coefficients of variation were 7.1 and 3.4% and 7.3 and 3.1% for cortisol and interleukin-6, respectively.
Data analysis and statistics
Power analysis, assuming a clinically important difference of 4 mean BIS levels [13] between the two groups (nondelirious and delirious), suggested that 114 patients were required for the study (alpha = 0.05; 1-beta = 0.8).
All data were analyzed by a person who was not familiar with the results of CAM-ICU testing. The data for descriptive variables were analyzed by chi-squared test. The Mann–Whitney U test or Fisher’s exact tests were used as appropriate to determine whether baseline features and postoperative data, including BIS EEG results, differed between those with and without delirium if data were not normally distributed. The results of blood analyses were normally distributed and compared for statistical significance using ANOVA followed by the post Tukey test. Bivariate correlation analysis was done according to Pearson or Spearman.
Statistical analyses were performed on SPSS 16.0 version (SPSS Inc., Chicago, IL, USA). Logistic regression analysis was used to assess BIS EEG as a predictor of delirium. A p value of less than 0.05 was considered to be statistically significant.
Results
Patient characteristics and hemodynamic and blood ICU data
ICU delirium was identified on the first postoperative day in 32 of the 114 patients (28%). The ratio of male to female patients was 89:25. All patients were classified as ASA III or IV. Concerning preoperative risk factors (diabetes mellitus, hypertension, peripheral occlusive vascular disease) no significant differences between delirious and nondelirious patients were observed. Furthermore, although patients with an additional aortic valve replacement have a greater risk of developing postoperative delirium because of air embolism and longer surgery time, the results of the present study showed that CABG patients with aortic replacement surgery developed more often early postoperative delirium as compared to patients without aortic replacement surgery (31 vs. 25%); however, this difference failed to reach the significance level.
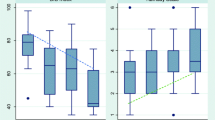
Patient characteristics are presented in Table 1. No significant differences between delirious and nondelirious patients were observed except for patients’ age. Significantly more patients with the diagnosis ‘delirium’ required continuous positive airway pressure (CPAP) ventilation on the first postoperative day. Hemodynamic and blood ICU data were not significantly different between the groups (Table 1). In contrast, interleukin-6 and cortisol concentrations were significantly higher in delirious patients (Fig. 1).
Intraoperative data including time of cardiopulmonary arrest or type of surgery (aortocoronary venous bypass without or with aortic valve replacement) were not significantly different between delirious and nondelirious patients (data not shown).
Nearly all (except three) patients received furosemide (5–20 mg), piritramide (Dipidolor®, 2–9 mg), and propofol (Disoprivan®, <2 mg/kg/h) on the first postoperative day as a standardized postoperative intravenous medication regime on cardiac ICU. The above-mentioned three patients received metamizole (Novalgin® 500 mg) or acetaminophen (Benuron® 500 mg) additionally. No other sedative or analgetic medication was administered. To exclude any effect of piritramide on delirium, the dosage of this sedative was compared between the groups. The mean piritramide dosage was 4.5 ± 2.3 mg on the first postoperative day. No significant difference was observed between nondelirious (4.3 ± 2.2 mg) and delirious (4.7 ± 2.5 mg) patients.
BIS EEG analysis
The levels of suppressed (isoelectric) EEG were less than 3% at all RASS levels. To acquire high-quality data, only BIS EEG values with a signal quality index greater than 65 and EMG less than 50 were included in the present analysis. Therefore and because of technical problems or artifacts (bad signal quality or increased patient muscle activity) the BIS EEG was analyzed in n = 102 patients; 12 EEGs were excluded.
Five minutes of artifact-free BIS EEG was analyzed in each patient separately for the right and left sensors. Because no significant differences were found between the BIS EEG of the right (80.1 ± 12.8) and left (79.8 ± 12.9) hemispheres and a high correlation coefficient (r = 0.977, p < 0.001) was detected, the means were taken for further analysis.
RASS assessment from all patients and the distribution of RASS between nondelirious and delirious individuals are shown in Fig. 2a, b. BIS EEG data including all RASS are illustrated in Fig. 2c. In delirious patients [72.6 (69.6–89.1)] the BIS EEG measurements [median (interquartile range IQR, 25th–75th percentiles)] were significantly lower than in nondelirious patients [84.8 (76.8–89.9)]. In parallel to BIS, the spectral edge frequency (SEF), signal quality index (SQI), and electromyogram (EMG) were determined in the respective 5-min artefact-free processed EEG data. Thus, changes in BIS index in delirious patients are not associated with changes in SQI or EMG. The SEF was significantly lower in delirious than in nondelirious patients in parallel to BIS EEG data (Table 2).
BIS EEG data. Median (IQR 25th–75th percentiles). a Represents the BIS index vs. patients’ RASS score (n = 102). Significant differences (*p < 0.05) were found (Mann–Whitney U test). b The RASS dependency is illustrated for delirious and nondelirious patients. As a result of small patient numbers in the RASS −2 and RASS +1 groups of nondelirious patients, no statistical analysis was performed. c Represents a significant (*p = 0.01, Mann–Whitney U test) reduction in the median (±IQR) of BIS index in delirious as compared to nondelirious patients
The relative theta power increase determined after power spectral analysis of raw EEG data was paralleled by significant decrease in alpha power in delirious patients (n = 17, Table 2) as compared to nondelirious patients (n = 20). Therefore, the theta/alpha ratio was significantly higher in delirious patients.
Correlation analysis
BIS EEG data showed a linear positive and significant (p < 0.001) correlation in relation to RASS (r = 0.36), SEF (r = 0.51), and BIS EMG (r = 0.58). In relation to patients’ blood parameters, BIS EEG correlated negatively (r = −0.36, p = 0.022) with cortisol. No significant relationship was obtained between BIS EEG and interleukin-6 nor between cortisol and interleukin-6. The results of the logistic regression analysis for bilateral BIS index as a predictor of ICU delirium showed a 27% sensitivity and 96% specificity with an overall accuracy of 76% (r 2 Nagelkerke = 0.137).
Discussion
BIS EEG
The objective monitoring of consciousness and responsiveness in sedated and critically ill patients in the ICU is difficult but important [17, 18]. It is conducted primarily by using clinical bedside instruments such as sedation scales [19, 20]. However, these scales are often not routinely applied, or the routine performance in ICU is time-consuming [21–23]. In addition, scoring systems provide only an intermittent assessment of critically ill patients’ brain function [24]. Long-term neuromonitoring such as continuous full-spectrum EEG is often not a practical solution in ICU because of extensive technical setup, expensive equipment, and training required for skilled interpretation. Processed EEG techniques obtained from the frontal cerebral cortex may be used instead of full-spectrum EEG [25]. Therefore, the BIS index represents an emerging technology that, in addition to scales and score systems, which might be useful for both intermittent and continuous monitoring of ICU patients.
The results of the present study show that the BIS index (as mean of right and left hemispheres) was significantly lower in delirious patients than in patients without delirium. As is well known BIS values depend on the patients’ sedation score [14]. Indeed, in agreement with previous studies [14], a lower RASS was associated with a lower BIS index in this study. The lower BIS EEG in delirious patients obtained in our present study confirm previous findings using standard and spectral EEG [11, 26, 27], our previous results using a 16-channel EEG [12], and also in part the results of Ely et al. [14] using a monolateral BIS EEG technique.
A delirious patient’s raw EEG has long been known to demonstrate classic findings of alpha slowing and delta/theta activity increasing [28]. These raw EEG findings have also been shown to correlate with clinical severity of brain dysfunction regardless of the underlying medical condition [11]. Our present results from a raw data EEG analysis demonstrate that under analogous medical conditions isolated alpha frequency bands were significantly reduced in delirious patients in parallel to increased theta activity as compared to nondelirious patients. Thus, this raw BIS EEG data analysis suggests that the BIS index summarizing patients’ cortical brain activity into two sensors might adequately reflect patients’ cortical brain activity in the ICU; however, this was not the question addressed in the present study.
In agreement with Renna et al. [13], we found that the BIS index could predict delirium with a low sensitivity, a high specificity, and an overall good accuracy (Fig. 3). The decreased number of accurate positively detected delirious patients might possibly be related to artificial continuous positive pressure (CNAP) ventilation (this number was significantly higher in delirious than in nondelirious patients) which can increase the number of technical artifacts. However, EMG activity did not change markedly between the two groups. Postoperative medication as a reason for low sensitivity is not likely because nearly all patients received the same analgesics and sedatives. Medication for the treatment of postoperative cognitive dysfunctions such as haloperidol or physostigmine had no influence on BIS EEG data because it was given only after the completion of the study investigations. In addition, the low sensitivity might also be related to the unequal distribution of RASS between the two groups; however, we did not investigate this in detail as this is intended to be a subject for further investigation. In contrast to the low sensitivity, the specificity of the BIS index was high. Based on these and Renna et al.’s data [13], the overall accuracy of bilateral BIS as a screening tool for postoperative delirium is similar to conventional, quantitative EEG [29].
Cortisol and interleukin-6
We demonstrated that increased plasma cortisol levels are related to postoperative delirium after open-heart surgery, which is in line with previous studies [30, 31]. Changes are not likely related to open-heart bypass surgery because the intraoperative data such as time for cardiopulmonary bypass, time of surgery, and the time between plasma analysis and the end of surgery were not different between delirious and nondelirious patients. Therefore, other mechanisms seem to play a role in the activation of the hypothalamic–pituitary–adrenal (HPA) axis in delirious patients under postoperative conditions. We know that changes in biomarkers such as alterations in the HPA axis frequently accompany aging [32, 33]. Furthermore, pronounced aging is one of the preoperative risk factors for postoperative cognitive dysfunctions [34]. Indeed, our delirious patients were significantly older than the nondelirious ones and this might therefore in part affect our results. However, we cannot exclude the possibility that additional ICU-related factors might be associated with increased plasma levels of cortisol, which we did not investigate in this study.
An interaction between cortisol secretion and cytokines has been reported [35, 36]. Thus, this also could indicate dysregulation of the immune system in postoperative delirium; however, more than one interleukin and more than one time point should be measured in further studies to confirm this notion. A transient postoperative increase in levels of circulating inflammatory markers has been hypothesized to result from tissue damage, cardiopulmonary bypass, and/or anesthesia or from an adrenal stress response. In recent studies, a possible relationship between interleukin-6 and impaired cognition, including delirium, was reported [4–6, 37, 38]. Furthermore, it was proposed that systemic inflammation may actually contribute to the pathogenesis of delirium by compromising blood–brain barrier integrity [4–6]. We found an increase (p = 0.01) in plasma interleukin-6 concentration in delirious ICU patients. Thus, increased postoperative interleukin-6 concentration appears to play an important role in the development of postoperative confusion; the underlying mechanism, however, remains elusive.
Differences between delirious and nondelirious patients in cortisol and interleukin-6 levels are not related to the medication given in the ICU because a similar medication regimen was administered to nearly all ICU patients. Interestingly, apart from higher interleukin-6 and cortisol concentrations, no significant correlation between cortisol and interleukin-6 was observed on the first postoperative day. The stimulatory effect of interleukin-6 on cortisol secretion seems to be time-dependent. Thus, high endogenous cortisol concentrations may induce a negative feedback on interleukin-6 secretion following the immediate postoperative period to reduce excessive cytokine production and to protect the brain postoperatively [39]. However, we only measured interleukin-6 and cortisol at one particular time point and therefore the time-dependent relationship between cortisol and interleukin-6 was not studied in detail. Nevertheless, we did not find a correlation between interleukin-6 and BIS EEG but the relation between plasma cortisol and BIS index was statistically significant. Using uni- and multivariate analyses to define the relationship between BIS index, interleukin-6, and cortisol is the aim of further studies to assess its role as a predictor of postoperative delirium.
Conclusion
We conclude from the present study that the bilateral BIS index was significantly lower in delirious patients compared to nondelirious, and showed a low sensitivity and high specificity as a predictor for early postoperative delirium after cardiac surgery. A lower BIS index was associated with a higher cortisol level. The association of ICU biomarkers with bilateral BIS EEG and the role of BIS neuromonitoring on more agitated (greater than RASS 1) or deeper sedated (less than RASS-2) patients should be investigated in further prospective studies.
References
Sockalingam S, Parekh N, Bogoch II, Sun J, Mahtani R, Beach C, Bollegalla N, Turzanski S, Seto E, Kim J, Dulay P, Scarrow S, Bhalerao S (2005) Delirium in the postoperative cardiac patient: a review. J Card Surg 20:560–567
Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y (2007) Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 33:66–73
Flacker JM, Lipsitz LA (1999) Neural mechanisms of delirium: current hypotheses and evolving concepts. J Gerontol A Biol Sci Med Sci 54:B239–246 (Erratum in: J Gerontol A Biol Sci Med Sci 1999, 54:B275)
Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, Khabbaz K, Levkoff SE, Marcantonio ER (2008) Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci 63:184–189
Holmes JH, Connolly NC, Paull DL, Hill ME, Guyton SW, Ziegler SF, Hall RA (2002) Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res 51:579–586
de Rooij SE, van Munster BC, Korevaar JC, Levi M (2007) Cytokines and acute phase response in delirium. J Psychosom Res 62:521–525
Melcangi RC, Panzica G (2003) Steroids and the nervous system. Introduction. Ann N Y Acad Sci 1007:1–5
de Quervain DJ, Poirier R, Wollmer MA, Grimaldi LM, Tsolaki M, Streffer JR, Hock C, Nitsch RM, Mohajeri MH, Papassotiropoulos A (2004) Glucocorticoid-related genetic susceptibility for Alzheimer’s disease. Hum Mol Genet 13:47–52
Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK (2006) Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci 61:1281–1286
van der Mast RC, Roest FHJ (1996) Delirium after cardiac surgery: a critical review. J Psychosom Res 41:13–30
Jacobson SA, Leuchter AF, Walter DO (1993) Conventional and quantitative EEG in the diagnosis of delirium among the elderly. J Neurol Neurosurg Psychiatry 56:153–158
Plaschke K, Hill H, Engelhardt R, Thomas C, von Haken R, Scholz M, Kopitz J, Bardenheuer HJ, Weisbrod M, Weigand MA (2007) EEG changes and serum anticholinergic activity measured in patients with delirium in the intensive care unit. Anaesthesia 62:1217–1223
Renna M, Handy J, Shah A (2003) Low baseline bispectral index of the electroencephalogram in patients with dementia. Anesth Analg 96:1380–1385
Ely EW, Truman B, Manzi DJ, Sigl JC, Shintani A, Bernard GR (2004) Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med 30:1537–1543
Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK (2001) Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med 29:1370–1379
Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, Putensen C (2009) Validity and reliability of the CAM-ICU flowsheet to diagnose delirium in surgical ICU patients. J Crit Care 25(1):144–151
Cheung CZ, Alibhai SM, Robinson M, Tomlinson G, Chittock D, Drover J, Skrobik Y (2008) Recognition and labeling of delirium symptoms by intensivists: does it matter? Intensive Care Med 34:437–446
Morandi A, Pandharipande P, Trabucchi M, Rozzini R, Mistraletti G, Trompeo AC, Gregoretti C, Gattinoni L, Ranieri MV, Brochard L, Annane D, Putensen C, Guenther U, Fuentes P, Tobar E, Anzueto AR, Esteban A, Skrobik Y, Salluh JI, Soares M, Granja C, Stubhaug A, de Rooij SE, Ely EW (2008) Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med 34:1907–1915
De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H (2000) Using and understanding sedation scoring systems: a systematic review. Intensive Care Med 26:275–285
Ostermann ME, Keenan SP, Seiferling RA, Sibbald W (2000) Sedation in the intensive care unit. JAMA 283:1451–1459
Bair N, Bobek MB, Hoffman-Hogg L, Mion LC, Slomka J, Arroliga AC (2000) Introduction of sedative, analgesic, and neuromuscular blocking agent guidelines in a medical intensive care unit: physician and nurse adherence. Crit Care Med 28:707–713
Weinert CR, Chlan L, Gross C (2001) Sedating critically ill patients: factors affecting nurses’ delivery of sedative therapy. Am J Crit Care 10:156–167
Ely EW, Stephens RK, Jackson JC, Thomason J, Truman B, Bernard G, Dittus R (2003) Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 Healthcare professionals. Crit Care Med 31:106–112
Olson DM, Thoyre SM, Auyong DB (2007) Perspectives on sedation assessment in critical care. AACN Adv Crit Care 18:380–395
Sigl JC, Chamoun NG (1994) An introduction to bispectral analysis for the electroencephalogram. J Clin Monit 10:392–404
Koponen H, Partanen J, Paakkonen A, Mattila E, Riekkinen PJ (1989) EEG spectral analysis in delirium. J Neurol Neurosurg Psychiatry 52:980–985
Reischies FM, Neuhaus AH, Hansen ML, Mientus S, Mulert C, Gallinat J (2005) Electrophysiological and neuropsychological analysis of a delirious state: the role of the anterior cingulate gyrus. Psychiatry Res 138:171–181
Romano J, Engel GL (1944) Delirium: electroencephalographic data. Arch Neurol Psychiatry 51:356–377
Rodriguez G, Nobili F, Rocca G, De Carli F, Gianelli MV, Rosadini G (1998) Quantitative electroencephalography and regional blood flow: discriminant analysis between Alzheimer’s patients and healthy controls. Dement Geriatr Cogn Disord 9:274–283
Gustafson Y, Brannstrom B, Berggren D, Ragnarsson JI, Sigaard J, Bucht G, Reiz S, Norberg A, Winblad B (1991) A geriatric anesthesiologic program to reduce acute confusional states in elderly patients treated from femoral neck fractures. J Am Geriatr Soc 39:655–662
O’Keefe ST, Devlin JG (1994) Delirium and the dexamethasone suppression test in the elderly. Neuropsychobiology 30:153–156
Crimmins E, Vasunilashorn S, Kim JK, Alley D (2008) Biomarkers related to aging in human populations. Adv Clin Chem 46:161–216
Gerritsen L, Geerlings MI, Beekman AT, Deeg DJ, Penninx BW, Comijs HC (2009) Early and late life events and salivary cortisol in older persons. Psychol Med 26:1–10
Ramaiah R, Lam AM (2009) Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin 27:485–496
Weber MM, Michl P, Auernhammer CJ, Engelhardt D (1997) Interleukin-3 and interleukin-6 stimulate cortisol secretion from adult human adrenocortical cells. Endocrinology 138:2207–2210
Roth-Isigkeit A, Dibbelt L, Schmucker P, Seyfarth M (2000) The immune-endocrine interaction varies with duration of inflammatory process in cardiac surgery patients. J Neuroendocrinol 12:546–552
Sosman JA, Aronson FR, Sznol M, Atkins MB, Dutcher JP, Weiss GR, Isaacs RE, Margolin KA, Fisher RI, Ernest ML, Mier J, Oleksowicz L, Eckhardt JR, Levitt D, Doroshow JH (1997) Concurrent phase I trials of intravenous interleukin 6 in solid tumor patients: reversible dose-limiting neurological toxicity. Clin Cancer Res 3:39–46
Muller N, Ackenheil M (1998) Psychoneuroimmunology and the cytokine action in the CNS: Implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 22:1–33
Crofford LJ, Kalogeras KT, Mastorakos G, Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP, Wilder RL (1997) Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL6 to cause sustained hypercortisolism in patients with untreated rheumatoid arthritis. J Clin Endocrinol Metab 82:1279–1283
Acknowledgments
The Aspect Medical Systems employees analyzed the BIS EEG raw data. We want to thank Mrs. G. Sennholz from Aspect Medical Systems for her kind support in getting the bilateral BIS EEG as a plant hire and for sponsoring the bilateral sensors. We want to thank Sigrun Himmelsbach for her technical support in the biochemical analyses. No employees of Aspect Medical Systems were involved in conceptual study design, BIS monitoring, data collection, data processing, or statistical analysis or in writing of the manuscript. The study was exclusively designed and conducted by employees of the University of Heidelberg, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plaschke, K., Fichtenkamm, P., Schramm, C. et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 36, 2081–2089 (2010). https://doi.org/10.1007/s00134-010-2004-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2004-4