Abstract
Zinc and cadmium concentrations in rat (Rattus norvegicus var. alba) tissues were analyzed by inductively coupled plasma optical emission spectrometry. Rats were fed the zinc and cadmium hyperaccumulating plant, Arabidopsis halleri. When compared to the control group, a Cd increase in all tissues (liver, kidneys, small intestine, spleen, testes, muscle), with the exception of bone tissue was observed. In comparison to the control group, the kidneys, liver and small intestine contained 375, 162, and 80 times more Cd, respectively. Differences between zinc concentrations in rats fed with A. halleri and those of the control group were significant only in the small intestine and kidney tissues. Results suggest using the hyperaccumulating plant A. halleri as a feed stresses the consumer organism not through its Zn content, but through its Cd content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metal contamination has become a worldwide problem (Dadar et al. 2014; Jankovská et al. 2010; Magdaleno et al. 2014; Petrović et al. 2013). Cadmium (Cd) is an environmental pollutant ranked eighth among the top 20 hazardous substances priority list (Klaassen et al. 2009), and human activity has markedly increased the distribution of Cd in the global environment. Zinc (Zn) is an essential element for all organisms. However, it is toxic when taken in excess (Johnson et al. 2001).
Metal concentrations in agricultural soils can become elevated due to anthropogenic emissions as well as from geological origins of the soil (Garrett 2000; McLaughlin et al. 1999). Some metals can easily transfer to aerial parts of plants and thus enter the food chain (Meyer et al. 2010; Zhao et al. 2006). Phytoextraction refers to the uptake of contaminants from soil or water by plant roots and their translocation to any harvestable plant part. Phytoextraction has the potential to remove contaminants and promote long-term cleanup of soil or wastewater. Arabidopsis halleri (syn. Cardaminopsis halleri) is a model plant investigated for Zn and Cd hyperaccumulation; (Meyer et al. 2010; Zhao et al. 2006). However, Huguet et al. (2012) determined that mechanisms of Cd storage and detoxification in A. halleri differ from those that were previously found for Zn.
One of the main ways humans or animals are exposed to contaminants is through diet (Raad et al. 2014). Excessive intake of metals through diet can lead to their bioaccumulation in consumer tissues and numerous toxicities, e.g. hepatic and renal damage (Bulat et al. 2008; Järup et al. 1998; Nordberg 2004). The transfer of metals along food chains constitutes an important route of exposure that must be taken into account for ecotoxicological risk assessment (Hispard et al. 2008).The laboratory rat (Rattus norvegicus var. alba) is a common model organism used in toxicology. Numerous studies have used this model organism to assess the health risk of various substances (Brzóska et al. 2001; Cadková et al. 2013; Kumara et al. 2015). The aim of this work was to determine whether or not the hyperaccumulating plant A. halleri can affect element accumulation in the organism of laboratory rats (Rattus norvegicus var. alba) when added to feed.
Materials and Methods
This experiment was conducted during a 6 weeks period on 12 Wistar rats, each with an initial body weight of 150 g. During the experiment, each animal was placed in a metabolic cage (one animal per cage). The room housing the cages was equipped with air-conditioning. A constant temperature (22–24°C) and humidity level (approx. 70 %) were maintained at a constant day/night cycle (08:00 am–08:00 pm). Animals were given unlimited access to water. A. halleri aboveground biomass was sampled in the flowering stage in Pb, Cd, and Zn contaminated areas in the vicinity of Příbram city (Czech Republic), dried at laboratory temperature and homogenized.
Rats were randomly divided in two groups (six animals per group). Animals in the control group (C) were fed a commercially manufactured feed (ST-1) and the experimental group (P) was fed a mixture of ST-1 (60 %) and A. halleri (40 %). Trace element contents in the feed (ST-1) and mixture (60 % ST-1 + 40 % A. halleri) are presented in Table 1. The amount of added A. halleri (group P) was calculated in such a way that 25 g of feed contained 20 mg of zinc. Each individual was given 150 g of feed (6 × 25 g) per week, i.e. 120 mg Zn per week, i.e. 720 mg Zn per 6 weeks.
After exposure, rats were sedated and euthanized with a T-61 Euthanasia Solution injection (S: Embutramidum 200 mg, Mebezonii iodidum 50 mg, Tetracaini hydrochloridum 5 mg in 1 mL. PL: Dimethylformamidum, Aqua pro inj., Merck, Canada). Individual autopsies were carried out with Teflon instruments in order to obtain appropriate sample soft tissue (liver, kidney, testes, muscle, spleen and bone) for analyses. All tissues were weighed, properly washed in double distilled water, placed into Petri dishes and stored at −20°C until chemical analysis. Frozen tissues samples were dried by LYOVAC GT 2 (LEYBOLD-HERAEUS, GmbH, Germany) and microwave digested by lyophilisation using the mixture of hydrogen peroxide and nitric acid using MWS-3 + (Berghof Products + Instruments, Germany) as described in detail by Jankovská et al. (2011).
Concentrations of Cd in the digests were measured by Electrothermal Atomic Absorption Spectrometry technique using a Varian AA 280Z (Varian, Australia) with graphite tube atomizer GTA 120 and PSD 120 programmable sample dispenser. Concentrations of Cu, Fe, Mn and Zn in digests were determined by optical emission spectroscopy with inductively coupled plasma (ICP-OES) with axial plasma configuration using a Varian VistaPro, equipped with autosampler SPS-5 (Australia). Measurement conditions for all lines were power 1.2 kW, plasma flow 15.0 L min−1, auxillary flow 0.75 L min−1, and nebulizer flow 0.9 L min−1.
The quality of analytical data was assessed by simultaneous analysis of certified reference material CRM 12-02-01 (Bovine Liver) (4 % of all the samples). Analytical data obtained for all determined elements were within in the confidence interval given by the producer. The background of the trace element laboratory was monitored by analysis of 15 % blanks prepared under the same conditions, but without samples, and experimental data were corrected by mean concentration of the elements in blanks, and compared with detection limits (mean ± 3 SD of blanks) which were 0.08 ng mL−1 for Cd, 0.7 ng mL−1 for Cu, 10.1 ng mL−1 for Fe, 2.7 ng mL−1 for Mn, and 7.5 ng mL−1 for Zn. Data of elements (Cd, Cu, Fe, Mn and Zn) tissue concentrations were analyzed with the two-sample t test for unpaired data. The differences were considered significant if p < 0.05. All computations used the program Statistica version 10. (Statsoft, USA).
Results and Discussion
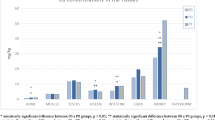
Significant differences between Cd concentrations in the control (C) and A. halleri treated (P) groups were found in all tissues with the exception of bone tissue (Table 2). Differences between Zn concentrations in group P and C were significant only in the small intestine and kidney tissues (Table 2). The highest concentrations of Cd in the A. halleri treated group (P) were found in rat kidneys (23.013 µg g−1) followed by the liver (3.455 µg g−1). Remaining organs had the following concentrations in descending order: intestine, spleen, testis, bone and muscle. In the control group, Cd concentrations ranged from 0.099 µg g−1 in bone to 0.007 µg g−1 in the testes (Table 2).
Iron (Fe) concentrations in our experiment were highest in the spleen (Table 2); manganese (Mn) concentrations were highest in the liver (Table 2), and Zn levels were highest in the bones and testes (Table 2). Differences between other element concentrations in tissues of treated group P and those of control group C were significant only in the intestinal tissue for Cu and Fe (Table 2), in the kidney for Mn, Fe and Cu (Table 2) and in the spleen tissue for Mn (Table 2).
Zn is one of the most important trace elements in the biological system (Hambidge 2000). Approximately 85 % of Zn in the body is located in muscle and bone, and 11 % is found in the skin and liver (Jackson 1989). In both groups (P and C), bone tissues contained one of the highest concentrations of Zn; however, muscle tissues contained the lowest Zn levels. As an essential element, Zn is regulated through homeostatic mechanisms, and therefore, its content in the body is relatively stable (Welshe et al. 1994). King et al. (2000) reported that their study with laboratory rats proved the effect of homeostatic mechanisms, even at various Zn doses (10–100 mg kg−1). This is partially supported by current results. Although there was a 12-fold increase in Zn levels in rat feed (group P) in the current study, Zn concentrations in the body tissues of both groups were very similar. Zn homeostasis can be disrupted by other elements, such as Cd (Ince et al. 1999). Both metals bind preferentially to the same proteins–albumin in the bloodstream and metallothionein (MT) and other proteins in tissues (Brzóska and Moniuszko-Jakoniuk 2001). Excessive intake of Zn and Cd stimulates the synthesis of cysteine rich MTs in the mucosa of the small intestine (Kägi 1991). Binding to MTs reduces the toxic effects of these two metals (Kelly et al. 1996). The higher affinity of Cd for MTs increases the concentration of free Zn2+ ions, which in turn stimulates the synthesis of other MTs (Funk et al. 1987). Swiergosz-Kowalewska (2001) reported that a Zn increase in the liver and kidneys is caused by Cd intoxication, and the author attributed this phenomenon to the higher synthesis of MTs, which, in the Cd5Zn2MT form, are passed from blood plasma to the liver and kidneys. A Zn increase in the liver and kidneys after Cd exposure was also described by Jihen et al. (2010). Furthermore, Chmielnicka et al. (1989) claimed that increased Zn content in the liver and kidneys can serve as indicators of ultrastructural damage caused by Cd intoxication. All of these findings are in agreement with current results. Higher Cd and Zn levels in group P feed resulted in higher concentrations of these metals in the small intestine and kidneys. The increase in Zn and Cd levels in these organs can be explained through the reduction of the toxic effects of Cd and Zn homeostasis (Brzóska et al. 2001; Liu et al. 1992). Although a determination of MTs was not made in the current work, it is believed that the greater portion of both metals was bound to these proteins. Brzóska and Moniuszko-Jakoniuk (2001) summarize that the simultaneous administration of Cd and Zn reduces intestinal absorption of total Cd. Since the group C diet did not contain any Cd, this fact can be neither confirmed nor excluded.
In general, most of the dietary fiber comes from the cell walls of aerial plant parts (Selvendran 1984), and high fiber diet increases dietary cadmium intake (Järup et al. 1998). Since group C feed (ST-1) did not contain any Cd, A. halleri was the main source of Cd in this experiment. Furthermore, one of the most important metabolic parameters for Cd uptake is a lack of Fe. Low levels of Fe in the intestinal tract can increase Cd intake by up to 6 % (Flanagan et al. 1978). As described below, group P diet contained less Fe than did group C. After absorption in the gastrointestinal tract, the majority of Cd in the body is bound to MTs (Nordberg 2004). The kidneys are the primary target organ for chronic Cd exposure (Järup et al. 1998). A large portion of absorbed Cd is also stored in the liver and intestinal mucosa (Cooke and Johnson 1996). This is in accordance with current results, wherein rat kidneys from group P contained the highest Cd concentrations. The next two highest concentrations of Cd were measured in the liver and small intestine. The increase in Cd content in the small intestine, liver and kidneys can be attributed to an increase in the synthesis of MTs, which is stimulated by an increase in both Zn and Cd intake through feed. Furthermore, MTs have a strong binding affinity for Cu, which is even higher than that of Cd and Zn ions (Sabolic et al. 2010); it is believed that, despite the lower Cu status in the group P diet, the Cu increase in the kidneys and small intestine could be caused by an increase in MT synthesis.
Cd accumulation in testes was described for both inorganic and organic Cd forms (Bench et al. 1999; Haouema et al. 2008). In current experiments, Cd concentrations in the testes of A. halleri treated rats were 37 times higher than in unaffected rats. Previous studies indicated a reduction of testicular Zn (Bonda et al. 2004) and an elevation of testicular Fe (Maitani and Suzuki 1986) after Cd exposure. According to Bonda et al. (2004), Zn protects the testes against damage caused by Cd; however, in the case of an excessive amount of Cd in the body, the majority of Zn is bound to Cd-induced renal and hepatic MTs. Current results indicated a minor decrease in Zn and an increase in Fe between groups C and P. Nevertheless, current results did not detect any significant differences. The spleen is not a target organ for Cd toxicity; however, it accumulates Cd and suppresses both T cell and innate immunity (Demenesku et al. 2014). In the exposed group P, an increase in Cd spleen concentrations was noted. Even though an elevated concentration of Zn in the bone tissue was not observed, Kido et al. (1990) indicates that Cd intoxication can have a direct or indirect cause on bone damage. According to Chmielnicka et al. (1989), Cd accumulation in bone tissue can cause a decrease in Zn levels. The current study did not observe this trend. On the contrary, concentrations of Zn were higher in group P. Current results therefore confirm that Zn is an essential element for bones, and it can protect from Cd-induced bone damage when consumed in large amounts (Bulat et al. 2008).
Aside from Zn and Cd, the current study also measured Cu, Fe and Mn content. Stable tissue levels of these essential metals are maintained mainly through tight homeostatic control of intestinal absorption and biliary excretion (Hurley and Keen 1987; Kumara et al. 2015). However, Cd is known to impair these mechanisms (Chmielnicka et al. 1989; Noël et al. 2004; Schroeder and Nason 1974). Differences in Cu, Fe and Mn concentrations in the feed of both tested groups prevented determination of the influence of Cd and Zn on the metabolism of these biometals. In contrast to the feed dosage for control group C, the feed dosage for group P contained lower levels of Cu, Mn and Fe. With the exception of Mn, current results did not reflect this decrease. Determined concentrations were, in fact, higher in certain organs. Despite differences in Cu, Fe, and Mn feed concentrations, it is assumed that the increase in Cu and Fe levels in the kidneys and small intestine could be caused by an increased intake of Cd and Zn in the group P diet (Schroeder and Nason 1974). Nevertheless, it is essential to modify the experimental design in order to better understand the influence of A. halleri on Cu, Fe and Mn metabolism.
The aim of this work was to determine whether or not the hyperaccumulating plant A. halleri can affect element accumulation in the organisms of laboratory rats (Rattus norvegicus var. alba). In A. halleri leaves, Zn is bound mainly to malate or other organic acids (Sarret et al. 2009); Cd is also bound to organic acids, cell wall components and, to a lesser amount, thiol-containing molecules (Huguet et al. 2012). Earlier published papers indicated that plant-incorporated metals are often absorbed in greater amounts in comparison to their inorganic forms, which are artificially added to an animal diet (Cadková et al. 2013; Chan et al. 2004). To our knowledge, this is the first time A. halleri has been used in a feeding study. Current results suggest that using the hyperaccumulating plant A. halleri as a feed stresses the consumer organism not through its Zn content, but through its Cd content.
References
Bench G, Corzett MH, Martinelli R, Balhorn R (1999) Cadmium concentrations in the testes, sperm, and spermatids of mice subjected to long-term cadmium chloride exposure. Cytometry 35:30–36
Bonda E, Włostowski T, Krasowska A (2004) Testicular toxicity induced by dietary cadmium is associated with decreased testicular zinc and increased hepatic and renal metallothionein and zinc in the bank vole (Clethrionomys glareolus). Biometals 17:615–624
Brzóska MM, Moniuszko-Jakoniuk J (2001) Interactions between cadmium and zinc in the organism. Food Chem Toxicol 39:967–980
Brzóska MM, Moniuszko-Jakoniuk J, Jurczuk M, Gałażyn-Sidorczuk M, Rogalska J (2001) The effect of zinc supply on cadmium-induced changes in the tibia of rats. Food Chem Toxicol 39:729–737
Bulat ZP, Djukić-Cosić D, Malicević Z, Bulat P, Matović V (2008) Zinc or magnesium supplementation modulates Cd intoxication in blood, kidney, spleen, and bone of rabbits. Biol Trace Elem Res 124:110–117
Cadková Z, Száková J, Miholová D et al (2013) Faecal excretion dynamic during subacute oral exposure to different Pb species in Rattus norvegicus. Biol Trace Elem Res 152:225–232
Chan DY, Fry N, Waisberg M, Black WD, Hale BA (2004) Accumulation of dietary cadmium (Cd) in rabbit tissues and excretions: a comparison of lettuce amended with soluble Cd salt and lettuce with plant-incorporated Cd. J Toxicol Environ Health A 67:397–411
Chmielnicka J, Hałatek T, Jedlińska U (1989) Correlation of cadmium-induced nephropathy and the metabolism of endogenous copper and zinc in rats. Ecotoxicol Environ Saf 18(3):268–276
Cooke JA, Johnson MS (1996) Cadmium in mammals. In: Beyer WN, Heinz G, Redmon-Norwood AW (eds) Environmental contaminants in wildlife. SETAC special publication series, Lewis, Boca Raton, pp 377–388
Dadar M, Peyghan R, Memari HR (2014) Evaluation of the bioaccumulation of heavy metals in white shrimp (Litopenaeus vannamei) along the Persian Gulf coast. Bull Environ Contam Toxicol 93(3):339–343
Demenesku J, Mirkova I, Ninkova M et al (2014) Acute cadmium administration to rats exerts both immunosuppressive and proinflammatory effects in spleen. Toxicology 326:96–108
Flanagan PR, McLellan JS, Haist J et al (1978) Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology 74(5):841–846
Funk AE, Day FA, Brady FO (1987) Displacement of zinc and copper from copper-induced metallothionein by cadmium and by mercury: in vivo and ex vivo studies. Comp Biochem Phys C 86:1–6
Garrett RG (2000) Natural sources of metals in the environment. Hum Ecol Risk Assess 6(6):954–963
Hambidge M (2000) Human zinc deficiency. J Nutr 130(5):1344S–1349S
Haouema S, Najjarb MF, El Hania A, Sakly R (2008) Accumulation of cadmium and its effects on testis function in rats given diet containing cadmium-polluted radish bulb. Exp Toxicol Pathol 59:307–311
Hispard F, de Vaufleurya A, Cossonb RP et al (2008) Proceedings of the 1st conference of the UK network on persistent organic pollutants (POPs) 29th and 30th March 2006, University of Birmingham, UK. Environ Int 34:381–389
Huguet S, Bert V, Laboudigue A, Barthes V, Isaure MP, Llorens I, Schat H, Sarret G (2012) Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ Exp Bot 82:54–65
Hurley LS, Keen CL (1987) Manganese. In: Mertz W (ed) Trace elements in human and animal nutrition, vol 1. Academic Press, London, pp 185–223
Ince NH, Dirilgen N, Apikyan IG, Tezcanli G, Ustun B (1999) Assessment of toxic interactions of heavy metals in binary mixtures: a statistical approach. Arch Environ Contam Toxicol 36:365–372
Jackson MJ (1989) Physiology of zinc: general aspects. In: Mills CF (ed) Zinc in human biology. Springer, London, pp 1–14
Jankovská I, Miholová D, Bejček V et al (2010) Influence of parasitism on trace element contents in tissues of red fox (Vulpes vulpes) and its parasites Mesocestoides spp. (Cestoda) and Toxascaris leonina (Nematoda). Arch Environ Contam Toxicol 58:469–477
Jankovská I, Miholová D, Petrtýl M et al (2011) Intestinal parasite Acanthocephalus lucii (Acanthocephala) from European perch (Perca fluviatilis) as a bioindicator for lead pollution in the stream “Jevanský potok” near Prague, Czech Republic. Bull Environ Contam Toxicol 86:342–346
Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M (1998) Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand J Work Environ Health 24:1–51
Jihen EH, Fatimaa H, Nouhaa A et al (2010) Cadmium retention increase: a probable key mechanism of the protective effect of zinc on cadmium-induced toxicity in the kidney. Toxicol Lett 196:104–109
Johnson FO, Gilbreath ET, Ogden L, Graham TC, Gorham S (2001) Reproductive and developmental toxicities of zinc supplemented rats. Reprod Toxicol 31:134–143
Kägi JHR (1991) Overview of metallothionein. Methods Enzymol 205:613–626
Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD (1996) Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr 126:1782–1790
Kido T, Nogawa K, Honda R, Tsuritani M, Ishizaki M, Yamada Y, Nakagawa H (1990) The association between renal dysfunction and osteopenia in environmental cadmium-exposed subject. Environ Res 51:71–82
King JC, Shames DM, Woodhouse LR (2000) Zinc homeostasis in humans. J Nutr 130:1360S–1366S
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238(3):215–220
Kumara V, Kalitaa J, Misraa UK, Borab HK (2015) A study of dose response and organ susceptibility of copper toxicity in a rat model. J Trace Elem Med Biol 29:269–274
Liu XY, Jin TY, Nordberg GF, Rännar S, Sjöström M, Zhou Y (1992) A multivariate study of protective effects of Zn and Cu against nephrotoxicity induced by cadmium-metallothionein in rats. Toxicol Appl Pharm 114:239–245
Magdaleno A, Vélez CG, Wenzel MT, Tell G (2014) Effects of cadmium, copper and zinc on growth of four isolated algae from a highly polluted Argentina river. Bull Environ Contam Toxicol 92(2):202–207
Maitani T, Suzuki KT (1986) Essential metal contents and metallothionein-like protein in testes of mice after cadmium administration. Toxicology 40:1–12
McLaughlin MJ, Parker DR, Clarke JM (1999) Metals and micronutrients—food safety issues. Field Crops Res 60:143–163
Meyer CL, Kostecka AA, Saumitou-Laprad P et al (2010) Variability of zinc tolerance among and within populations of the pseudometallophyte species Arabidopsis halleri and possible role of directional selection. New Phytol 185:130–142
Noël L, Guérin T, Kolf-Clauw M (2004) Subchronic dietary exposure of rats to cadmium alters the metabolism of metals essential to bone health. Food Chem Toxicol 42(8):1203–1210
Nordberg GF (2004) Cadmium and health in the 21st Century—historical remarks and trends for the future. Biometals 17(5):485–489
Petrović Z, Teodorović V, Dimitrijević M et al (2013) Environmental Cd and Zn concentrations in liver and kidney of European hare from different Serbian regions: age and tissue differences. Bull Environ Contam Toxicol 90(2):203–207
Raad F, Nasreddine L, Hilan C, Bartosik M, Parent-Massin D (2014) Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem Toxicol 73:35–43
Sabolic I, Breljak D, Skarica M, Carol M, Kramberger H (2010) Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 23:97–926
Sarret G, Willems G, Isaure MP et al (2009) Zinc distribution and speciation in Arabidopsis halleri × Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol 184:581–595
Schroeder HA, Nason AP (1974) Interactions of trace metals in rat tissues. Cadmium and nickel with zinc, chromium, copper, manganese. J Nutr 104:167–178
Selvendran RR (1984) The plant cell wall as a source of dietary fiber: chemistry and structure. Am J Clin Nutr 39:320–337
Swiergosz-Kowalewska R (2001) Cadmium distribution and toxicity in tissues of small rodents. Microsc Res Tech 55:208–222
Welshe CT, Sandstead HH, Prasad AS (1994) Zinc: health effects and research priorities for 1990s. Environ Health Perspect 102(2):5–49
Zhao FJ, Jiang RF, Dunham SJ, McGrath SP (2006) Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytol 172:646–654
Acknowledgments
The authors gratefully acknowledge Brian Kavalir (Ontario, Canada) for his proofreading services. This study was supported by the Grant Agency of Czech Republic (GACR) Project No. 13-18154S and the University-wide internal Grant Agency of the Czech University of Life Sciences Prague (CIGA) Project No. 20142053.
Conflict of interest
None.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Válek, P., Sloup, V., Jankovská, I. et al. Can the Hyperaccumulating Plant Arabidopsis halleri in Feed Influence a Given Consumer Organism (Rattus norvegicus var. alba)?. Bull Environ Contam Toxicol 95, 116–121 (2015). https://doi.org/10.1007/s00128-015-1555-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1555-z