Abstract
A total of 84 European hares collected from eleven Serbian regions investigated upon cadmium (Cd) and zinc (Zn) presence. Strong statistically significant correlations between Cd concentrations in kidney and liver were registered in animals older than 12 months. Significant differences between Zn concentrations in liver in comparison to kidney were found within every single age group with exception of the oldest. Negative correlation (Ps—Pearson’s correlation) between Zn and Cd concentrations were found in liver samples within the age group of 12 months (Ps = −0.67, p = 0.004).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd) and zinc (Zn) are elements having similar geochemical and environmental properties (Atafar et al. 2010; Moustakas et al. 2011) Toxicity in wildlife from metals exposures is generally poorly understood and is rarely quantified in field settings. Animal tissue levels can provide important data regarding the fate and bioavailability of heavy metals within natural ecosystems (Wren 1986; Lazarus et al. 2008; Bilandžić et al. 2010). In general, the gastrointestinal tract and the liver regulate the uptake and transfer of Zn. Interactions between essential and non-essential metals are very common (e.g., Cd uptake can mimic that of Zn).
The objectives of this study were to evaluate the environmental Cd and Zn concentrations in European hare from different Serbian regions. The tissue samples acquired bring up a concept of using hares as promising Cd and Zn biomonitors as well as to investigate how the different age distribution within hare population affects comparison between Cd and Zn levels among sampling regions and age groups. The present study was also projected to estimate bioaccumulation trends of Cd and Zn during life time of European hares and interactions between Cd and Zn in hare organs if any exists and try to model the dependency of liver Cd and Zn concentrations.
Materials and Methods
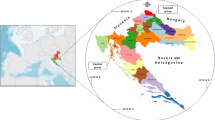
A total of 168 tissue samples (84 kidneys and 84 livers) obtained from all hunted free-range hares—Lepus europaeus were investigated upon Cd and Zn presence. The hares were acquired in a eleven regions from western, central and south part of Serbia during regular hunting season 2010/2011. The geographical locations from where samples had been collected are shown in Fig. 1.
After extraction, eye samples were placed in marked plastic bags with zipper open/close system, and immediately transported to the laboratory. The eye lenses were fixed in 5 % formalin for 72 h and then dried in a thermostat at 37°C for 96 h, under normal pressure. After they were dried, they were weighed on a precise analytical scale (Mettler AE 200) to 1 mg precision. For further data analysis, hares were sub-divided according to their age into five groups: 3–6 months old (100–200 mg), 12 months old (200–280 mg), 12–24 months old (280–310 mg); 24–36 months old (310–370 mg) and older than 36 months (≥370 mg).
The whole liver and kidney were sampled from each animal. Until analyzed the liver and kidney samples were stored at −20°C. Samples (1 g) after the process of homogenisation were digested with 8 mL of HNO3 (65 % v/v, Analytical grade, JT Baker, Center Valley, USA) and 2 mL of H2O2 (30 %, Analytical grade, Kemika, Zagreb, Croatia) using the method of acid microwave digestion. Samples were destroyed in the microwave digestion unit (Milestone TC, EVISA, EU) with temperature control. The digestion program began at a potency of 1,000 W, then was ramped for 10 min to 200°C, after which samples were held at 1,000 W and a temperature of 180°C for 20 min. Calibrations were prepared from commercial solutions in HNO3 (0.2 %) with 1,000 mg/L of each element (JT Baker, Center Valley, USA). All results are expressed on wet weight basis (w/w).
Cadmium content was determinated using graphite furnace atomic absorbtion spectrometry (GFAAS) technique using an atomic absorption spectrophotometer-Varian SpectrAA 220, equipped with GTA 110 furnace with constant temperature zone. Cadmium was measured at 228.8 nm, LOD (0.005 mg/kg).
Zinc was measured using an atomic absorption spectrophotometer-Varian SpectrAA 220 at 213.9 nm, LOD (0.2 mg/kg). The trueness of the method was tested with the standard reference material (BCR No.186—Community Bureau of Reference) and by carrying out a recovery check using spiked (fortified) samples. The reference material data was ±10 % from the certified mean values. The recoveries in different sample materials were 95 %–97 % in liver and 98 %–102 % in kidney for cadmium; 96–101 % in liver and 98 %–102 % in kidney for zinc, and the variation coefficient was between 4 % and 9 %.
Statistical analysis was performed by the MINITAB software package, version 16.0. Concentrations were expressed as median values and range of minimum to maximum. The Kruskal-Wallis test and the post hoc Man–Whitney non parametric test were used to examine statistical differences of heavy metal concentrations among groups. The Wilcoxon signed rank test was used to examine differences between Cd and Zn concentrations in kidney and liver within age groups. The significance of correlations between Cd and Zn levels were calculated using Pearson’s correlation (Ps). The differences were considered statistically significant when the p value was <0.05.
Results and Discussion
The concentrations of Cd and Zn in brown hare organs in relation to sampling regions are listed in Table 1.
Looking at all sampling regions (Fig. 1) significant differences of median values were noted in hares regarding to Cd levels in kidney and liver (p = 0.001 and 0.007, respectively).
Differences between Cd content in kidney in comparison to liver were significant (p = 0.001). Significant statistical differences (p = 0.001) were registered between Cd content in kidney and also in liver (p = 0.001) of hares among all represented age groups. Age trends of Cd and Zn concentrations in various organs of European hare are shown in Table 2.
For Zn, within the whole hare population (n = 84) higher concentrations expressed as median values (mg/kg w.w) were found in liver (25.4) and slightly lower Zn concentrations (21.4) were found in kidney samples. These differences were statistically significant (p = 0.001).
Based on non-parametric analysis between sampling localities, we found significant differences of Zn concentrations in kidney (p = 0.001) while in liver these differences were not statistically siginificant (p = 0.155). Zinc concentrations in liver, between all age groups, did not differ significantly (p = 0.512) but in kidney these differences were statistically significant (p = 0.001). Significant differences are registered between Zn concentrations in liver and kidney (pairwise differences) within every single age group (3–6 m: n = 11, p = 0.001; 12 m: n = 16, p = 0.002; 12–24 m: n = 17, p = 0.015; 24–36 m: n = 28 p = 0.002) with exception of the oldest (≥36 m: n = 12, p = 0.926).
The significant correlation between Cd and Zn concentrations in kidney within the whole hare population was found (Ps = 0.57, p = 0.001; r2 = 0.32, p = 0.001, r2-coefficient of determination), and also in different tissue samples (kidney–liver);(ZnK-CdL, Ps = 0.52, p = 0.001; r2 = 0.27, p = 0,001) and (CdK-CdL: Ps = 0.83, p = 0.001; r2 = 0.70, p = 0,001). Strong statistically significant correlations between Cd concentrations in kidney and liver are found in three groups older than 12 months (12–24 months: Ps = 0.81, p = 0.001; 24–36 months: Ps = 0.78, p = 0.001; ≥36 months: Ps = 0.79, p = 0.004). Negative correlation ZnL-CdL was found in liver within the age group of 12 months (Ps = −0.67, p = 0.004; r2 = 0.46, p = 0.004).
Accumulation of toxic and essential elements in hare organs was studied by a number of authors (Venalainen et al. 1996; Eiraa et al. 2005; Kramarova et al. 2005; Myslek and Kalisinska 2006; Pedersen and Lierhagen 2006; Kolesarova et al. 2008). Considering values obtained by sampling regions (Table 1) we noticed that the mean values, of both the metals, are probably affected by random individual variations, age structure of collected animals and the sample size from the particular sampling region. However, changes in the slope constant Zn/Cd for the liver samples, sorted by age, may reflect environmental Cd exposure during the individual development of European hares (Fig. 2).
Looking at the slope constants Zn/Cd among age groups presented in Fig. 2, in a form of linear regression equation: YZnL = kXCdL + b (Zn L—zinc concentration in liver; Cd L—cadmium concentration in liver; b-intercept value with Y axis) we registered a sharp decline of the regression line (k = −30.1) in age group of 12 months. This trend is also supported by taking into account the strong negative correlation found within this age group. It seems that Cd amplifies Zn deficiency in yearlings but also reduces or delays toxic effects of Cd at presented levels. From obtained results, it can also be substantial taking into account the significant correlations of Cd concentration in different tissue (CdK-CdL) which are registered in age groups older than 12 months (12–24 months: Ps = 0.81, p = 0.010; 24–36 months: Ps = 0.78, p = 0.001; ≥ 36 months: Ps = 0.79, p = 0.004). These correlations were not registered in age groups 3–6 and 12 months (Ps = 0.142; p = 0.552 and Ps = 0.06; p = 0.826, respectively).
Further, the slope constants Zn/Cd given in Fig. 2, arise in subsequent age groups in order: −5.33; −1.6 and 3.1. Such difference between bioaccumulation rates of Zn and Cd in the liver can be used as an indicator of Cd exposure (Honda and Tatsukawa 1983). In principle, there has been a distinct increase of bioaccumulation of Cd in hare organs during subsequent stages of life (Table 2). There is registered a somewhat higher increase of hepatic Zn related to Cd in the oldest age group (≥36 months). It can be interpreted that Zn as an essential element has a homeostatic mechanism that maintains optimum tissue levels over a range of exposure to environmental Cd. It can also be speculated, considering the obtained results of hepatic and renal Zn concentrations in yearlings, that Cd is simply transferred to MT according to their binding affinity with subsequent displacement of Zn (Onosaka et al. 1984).
Intercepts calculated form equations (Fig. 2) related to hepatic Zn–Cd correlations by age groups are: 24.9; 26.7; 25.9: 20.6 and 25.1 respectively. It can be stated that these values may correspond to the physiological concentrations in the liver of the hares studied. Registered background tissue levels of Zn refers to those concentrations of metals that derive from natural as well as anthropogenic sources that are not the focus of the risk assessment. Distinct age trends of Zn concentration in liver of European hare have not been established. It is probably because of the native liver MTs of most animals predominantly contain Zn bounded tightly, and are less able to be substituted by Cd.
Summing up, it can be noted that the biological role of Zn metabolism, during development and growth of European hare, is very important.
Considering the concentrations of Cd, during the individual development of European Hare, it should be stated that there is a distinct increase of bioaccumulation of Cd during subsequent stages of life. Observing the relationship between Cd and Zn levels within various age groups, it can be concluded that the bioaccumulation process comes after the first year of life. It may be important information if the hare organs are intended to be used for environmental biomonitoring of Cd. Furthermore, age distribution suggests that the samples can be limited by age to include only those of animals with an exposure period of 2 or 3 years, given they are from the same region, and then compare the obtained results with yearlings. Generally, the renal and hepatic levels of Cd in the present study are almost equivalent to the Slovakian study of European hare (Kramarova et al. 2005). In respect to other studies, these values are close to those registered in hares collected from unpolluted areas of Finland and are, approximately, two times lower than those from Finnish industrial regions (Venalainen et al. 1996). The values are also, approximately, two times lower in comparison to the Cd levels in hare tissue registered in Poland (Myslek and Kalisinska 2006). At the same time the Cd levels are two times higher than those reported in wild rabbits from Portugal (Eiraa et al. 2005). Recorded zinc concentrations in kidney and liver samples from our study (21.4; 25.4), were at a lower level than those from Finland (28.8; 38.1) and Poland (26.5; 31.3).
References
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi H (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Bilandžić N, Sedak M, Đokić M, Šimić B (2010) Wild boar tissue levels of cadmium, lead and mercury in seven regions of continental Croatia. Bull Environ Contam Toxicol 84:738–743
Eiraa C, Torresa J, Vingadab J, Miquela J (2005) Concentration of some toxic elements in Oryctolagus cuniculus and in its intestinal cestode Mosgovoyia ctenoides, in Dunas de Mira (Portugal). Sci Tot Environ 346:81–86
Honda K, Tatsukawa R (1983) Distribution of cadmium and zinc in tissues and organs, and their age-related changes in striped dolphins, Stenella coeruleoalba. Arch Environ Contam Toxicol 12:543–550
Kolesarova A, Slamecka J, Jurcik R et al (2008) Environmental levels of cadmium, lead and mercury in brown hares and their relation to blood metabolic parameters. J Environ Sci Heal A 43:646–650
Kramarova M, Massanyi P, Jancova A et al (2005) Concentration of cadmium in the liver and kidneys of some wild and farm animals. Bull Vet Inst Pulawy 49:465–469
Lazarus M, Orct T, Blanuša M, Vicković I, Sostaric B (2008) Toxic and essential metal concentrations in four tissues of red deer (Cervus elaphus) from Baranja, Croatia. Food Addit Contam 25:270–283
Moustakas NK, Akoumianaki-Ioannidou A, Barouchas PE (2011) The effects of cadmium and zinc interactions on the concentration of cadmium and zinc in pot marigold (Calendula officinalis L.). Aust J Crop Sci 5:277–282
Myslek P, Kalisinska E (2006) Contents of selected heavy metals in the liver, kidneys and abdominal muscle of the brown hare (Lepus europaeus Pallas) in Central Pomerania, Poland. Pol J Vet Sci 9:31–41
Onosaka S, Tanaka K, Cherian MG (1984) Effects of cadmium and zinc in tissue levels of metallothionein. Environ Health Perspect 54:67–92
Pedersen S, Lierhagen S (2006) Heavy metal accumulation in arctic hares (Lepus arcticus) in Nunavut, Canada. Sci Tot Environ 368:951–955
Venalainen ER, Niemi A, Hirvi T (1996) Heavy metals of hares in Finland. 1980–82 and 1992–93. Bull Environ Contam Toxicol 56:251–258
Wren DC (1986) Mammals as biological monitors of environmental metal levels. Environ Monit Assess 6:127–144
Acknowledgments
The authors would like to thank the colleagues from the Department of Residue Analysis of Institute of Meat Hygiene and Technology and the Hunting Association of Serbia for their selfless help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrović, Z., Teodorović, V., Dimitrijević, M. et al. Environmental Cd and Zn Concentrations in Liver and Kidney of European Hare from Different Serbian Regions: Age and Tissue Differences. Bull Environ Contam Toxicol 90, 203–207 (2013). https://doi.org/10.1007/s00128-012-0901-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0901-7