Abstract
Toxicity of cadmium, copper and zinc was tested on four green algal species (Ankistrodesmus fusiformis, Chlorella ellipsoidea, Monoraphidium contortum and Scenedesmus acuminatus) isolated from a highly polluted river (Matanza–Riachuelo River, Buenos Aires, Argentina). The relative abundance of these species in river waters showed that C. ellipsoidea was the most abundant species (mean 4,540 ind mL−1), whereas the less abundant species was S. acuminatus (mean 220 ind mL−1). The most sensitive species was A. fusiformis, which EC50 were Cd = 141 μg L−1, Cu = 72 μg L−1, and Zn = 199 μg L−1, whereas C. ellipsoidea was the most resistant species to copper (EC50 = 489 μg L−1) and cadmium (EC50 = 429 μg L−1), and M. contortum and S. acuminatus were the most resistant species to zinc (EC50 = 381 and 394 μg L−1, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The individual effects of copper, cadmium, and zinc on microalgae have been well documented. In trace amounts, copper and zinc are essential micronutrients that play an important role in many enzyme systems. However, at concentrations above those required for optimal growth, these metals have been shown to cause adverse effects on growth, photosynthesis and chlorophyll concentration (Omar 2002; Küpper et al. 2002). Similar effects have been observed in algal cells growing at low concentrations of cadmium (Das et al. 1997).
Algae comprise an essential component of aquatic ecosystems. In particular, green unicellular freshwater algae with high metabolism rates play the basic role in the primary production (Sabater and Carrasco 2001). Important factors such as the effects of possible adaptation/acclimation to natural conditions could have considerable implications on the metal toxicity data (Janssen and Heijerick 2003). Recently, Piotrowska-Niczyporuk et al. (2012) suggested that phytohormones and polyamine play an important role in the algal adaptation ability to metal contamination of the aquatic environment by inhibiting heavy metal biosorption and stimulating antioxidant enzyme activities. On the other hand, aquatic plants have evolved enzymatic (superoxide dismutase, catalase, ascorbate peroxidase) and non-enzymatic (ascorbate, glutathione) antioxidant mechanisms to prevent the oxidative stress caused by metals (Kalinowska and Pawlik-Skowronska 2010; Takami et al. 2012).
Heavy metal pollution in aquatic ecosystems constitutes a serious problem in Argentina due to the few environmental controls and political regulation of industrial discharges. This regional situation leads to deterioration of the water quality of many rivers in Argentina, and modifies greatly the aquatic ecosystems. Particularly, the Matanza–Riachuelo River is considered one of the most polluted rivers of Latin America. Previous monitoring studies indicated eutrophic conditions as well as higher metal concentrations than the limits established for the protection of aquatic life (Magdaleno et al. 2001). This paper describes the effects of cadmium, copper and zinc on the growth of pure cultures of four species of phytoplankton, representatives of the Matanza–Riachuelo River. We isolated and cultured the green algae (Chlorococcales) species based on their abundance and their easy maintenance and culture in the laboratory conditions. We analyzed the algal growth inhibition and chlorophyll a content at 96 h of culture, and estimated the respective effective concentrations of each metal that caused 50 % inhibition (EC50).
Materials and Methods
Four sampling sites in the Matanza–Riachuelo river, Buenos Aires, Argentina (GPS: 34°37′54.8″S 58°20′37.4″W) were chosen for the study (Fig. 1), according to the characteristics of the area: S1 (rural area), S2 (low industrialized urban area); S3 (forest area); S4 (high industrialized urban area). Monthly samples were taken for 10 months were carried out in order to investigate the natural abundance and distribution of the isolated species.
Surface water samples for the algae population abundance analysis were collected in triplicate into 150 mL polypropylene flasks, and immediately fixed with 4 % Lugol solution. Counts were performed according to the Utermöhl (1958) method, considering an estimated error lower than 10 % for the most frequent species. For qualitative analysis, approximately 40 L of water were filtered with a 25-μm mesh net and stored in a flask with 3 % formalin. One filtered sample without fixation was collected in December (the month of the greatest abundance of species) to select and isolate the living organisms. The green algae (Chlorococcales) were isolated using a micropipette under an inverted microscope. Individual organisms were transferred to tubes containing liquid Bold’s Basal Medium (BBM), according with Bischoff and Bold (1963). These cultures were maintained at 24 ± 1°C and continuous cool-white fluorescent light illumination (80 μM photons m−2 s−1). The unialgal cultures were purified axenically and the axenic conditions were checked by incubation into Tryptein Soy Agar (for bacteria detection) and Sabuoraud Agar (for fungus detection). These strains were kept in BBM agar medium as “stock cultures” in the Microalgal Culture Collection of the Phycology Laboratory of the Biodiversity and Experimental Biology Department (Faculty of Exacts and Natural Sciences, University of Buenos Aires, Argentine).
In order to adjust the five metal concentrations of each algal test, preliminary bioassays were carried on using a wide range of concentrations (10–1,000 μg L−1). Different concentrations were prepared from the stock solutions (1 g L−1) of cadmium (Cd-CdCl2), copper (Cu-CuSO4.5H2O), and zinc (Zn-ZnSO4.7H2O). Measurements by flame atomic absorption spectrometry showed more than 98 % similitude between nominal and real concentrations. The Algal Assay Medium (AAM) proposed by USEPA (1989), but prepared with doubled concentrations of nitrogen and phosphorus, and without ethylene diaminetetracetic acid (EDTA) was used as the controls and test dilutions. Prior to experiments, cultures maintained in BBM agar were transferred to liquid 10 % BBM, and then to liquid AAM in order to acclimatize the algae at the medium test conditions. After 10 days, the exponential growing phase culture was used as inoculum. Bioassays were conducted with initial cell densities of 5 ± 0.2 × 104 cell mL−1. Controls, together with the metal concentrations were prepared in triplicate using 125-mL flasks containing 25 mL of medium AAM. Temperature and light conditions for the toxicity tests were identical to those used for culture maintenance. Cultures were continuously shaken at 100 rpm, and the algal growth was estimated after 96 h incubation. Cell densities (cells mL−1) were obtained by absorbance readings (750 nm) and counting chamber (Neubauer). The chlorophyll a (Chl a) content was determined following the Marker et al. (1980) methodology. Each toxicity test was conducted three times. Growth rate was determined using the equation: μ = ln(x 2 /x 1 )/(t 2 − t 1 ), where x 1 and x 2 denote absorbances at time intervals t 1 and t 2 , respectively, and μ denotes the specific growth rate at each metal concentration. Statistical tests were carried out using the software package STATISTICA ver. 5.0. ANOVA and Student’s t test were employed to determine if treatments were significantly different from each other, and Pearson correlation coefficients were used to analyze differences between algal growth and concentration of Chl a. ANOVA was also employed to determine significant differences between algal densities in the four sites. The mean EC50 values of the three tests and of each metal was calculated using Probit analysis.
Results and Discussion
According to Magdaleno et al. (2001), the green algae represents the sub-dominant group in the total phytoplankton of the Matanza–Riachuelo River (mean 25,000 ind mL−1). The isolated and purified algal species were identified as Ankistrodesmus fusiformis, Chlorella ellipsoidea, Monoraphidium contortum, and Scenedesmus acuminatus. Table 1 shows the mean, maximum and minimum algal densities in the four sites. The most abundant species was C. ellipsoidea (mean 4,540 ind mL−1), followed by M. contortum (mean 580 ind mL−1), A. fusiformis (mean 440 ind mL−1), and S. acuminatus (mean 220 ind mL−1). No significant differences were observed between algal densities among the sites (p < 0.05), showing similar biotic and abiotic conditions of algal growing.
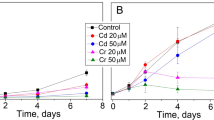
The four strains showed different sensitivities when they were exposed to cadmium, copper and zinc in laboratory conditions during 96 h. Cell densities reached by the controls at 96 h growth were as follows (Fig. 2): A. fusiformis (2.5 × 106 cells mL−1), C. ellipsoidea (10.5 × 106 cells mL−1), M. contortum (8.3 × 106 cells mL−1), and S. acuminatus (4.0 × 106 cells mL−1). The algal growth inhibition with respect to the control at Cd 200 μg L−1 were 99 % in A. fusiformis, 55 % in M. contortum, 2 % in S. acuminatus, and 26 % in C. ellipsoidea at 250 μg L−1. On the other hand, 200 μg L−1 of copper produced 94 %, 11 %, 54 %, and 53 % of growth inhibition in A. fusiformis, C. ellipsoidea, M. contortum and S. acuminatus, respectively. M. contortum and S. acuminatus were more resistant to zinc than the other species, showing 17 % and 16 % of growth inhibition at 200 μg L−1, whereas A. fusiformis and C. ellipsoidea showed 65 % and 61 % inhibition at the same concentration (Fig. 2). The four species showed high correlation coefficients (Pearson correlation coefficients) between cell densities and concentration of Chl a (up to 0.92) at all treatments (Fig. 3).
According to the EC50 values (Table 2), A. fusiformis was the most sensitive strain to cadmium (EC50 = 141 μg L−1), copper (EC50 = 72 μg L−1) and zinc (EC50 = 199 μg L−1). On the other hand, C. ellipsoidea was the most resistant species to cadmium (EC50 = 429 μg L−1) and copper (EC50 = 489 μg L−1), whereas M. contortum and S. acuminatus were the most resistant species to zinc, showing very similar EC50 (381 and 394 μg L−1, respectively).
Table 3 shows the growth rate reached by algal species at each experimental metal concentration. The lowest effect level was determined as the minimum metal concentration that showed a significant difference with respect to the growth rate in the control. Growth rate was significant reduced in: A. fusiformis at Cd 25 μg L−1, Cu 25 μg L−1, and Zn 50 μg L−1; C. ellipsoidea at Cd 50 μg L−1, Cu 100 μg L−1, and Zn 50 μg L−1; M. contortum in Cd 50 μg L−1, Cu 50 μg L−1, and Zn 100 μg L−1; and S. acuminatus in Cd 300 μg L−1, Cu 100 μg L−1, and Zn 200 μg L−1. According to the maximum values of dissolved metal concentrations measured in the Matanza–Riachuelo river waters by Magdaleno et al. (2001), Cd concentration (85 μg L−1) were higher than the lowest toxic effect level for A. fusiformis, C. ellipsoidea and M. contortum, whereas Cu and Zn concentrations (330 and 270 μg L−1, respectively) were higher than the lowest effect level for the four species (Table 3). However, the four algal species were always present during the sampling in the four sites (Table 1), showing that the results obtained in laboratory test overestimated metal toxicities.
Taking into account the respective EC50 values obtained in laboratory conditions (Table 2), the four isolated strains were less sensitive to the three metals than the standard algal test Pseudokirchneriella subcapitata (Guéguen et al. 2004). The reported EC50 for cadmium, copper and zinc were 74, 19 and 79 μg L−1, respectively. Besides, the four native species can growth at higher metal concentrations than that reported by Magdaleno et al. (2001) in river waters. These differences could be due to several factors acting on metal toxicity upon algae in both natural and laboratory conditions. Abiotic factors, such as nutrient concentrations, pH, redox potential, salinity and natural organic chelators, can significantly modify metal toxicity on these organisms. Many nutrient concentrations in the algal medium (calcium, magnesium, sodium and phosphorus) were ten times lower than those found in river waters, whereas nitrate concentration was ten times higher in the algal medium with respect to the river waters. Besides, the Matanza–Riachuelo River is characterized by high levels of organic matter, and hence the presence of natural organic ligands capable to form metal complexes could reduce metal toxicity.
Biotic factors, such as growth rate, cell wall, and intracellular metabolism, could play an important role on the ability of algae to avoid metal toxicity. It was reported that the thick cell wall of C. ellipsoidea may reduce copper and cadmium toxicity (Zhou et al. 2008), and the presence of intracellular sulfur-rich peptides (phytochelatins) in C. vulgaris act as a detoxification mechanism of metal ions (Gekeler et al. 1988). Recently, Kalinowska and Pawlik-Skowronska (2010) showed that the predominant thiol peptide in living cells (glutathione) can directly chelate some heavy metals such as copper. Glutathione is also a precursor of phytochelatins with high metal binding affinity. Besides, it was demonstrated that S. acuminatus can release extracellular copper ligands (exopolysaccharides) that reduce the activities of these metal ions in the environment and culture conditions (Lombardi et al. 2005). Besides, algae can acclimate or (genetically) adapt to metal concentrations in their environment. These aquatic organisms must be able to discriminate between essential and non-essential heavy metal ions. Cells can select those metal ions essential for growth and exclude those that are not, and keep essential ions at optimal intracellular concentrations (Perales-Vela et al. 2006).
In this work we analyzed the population abundance of four algae isolated from a highly polluted river and their metal sensitivities in laboratory conditions. Among the isolated, C. ellipsoidea was the most abundant species in the Matanza–Riachuelo River, and also showed the highest growth rate in laboratory conditions. This species was also the most resistant species to cadmium and copper. However, S. acuminatus was the less abundant species in river waters but showed high resistance to cadmium and zinc. On the other hand, A. fusiformis was one of the less abundant species in river waters and also showed the lowest growth rate and metal resistance in laboratory conditions.
References
Bischoff HW, Bold HC (1963) Phycological studies. IV. Some soil algae from Enchanted Rock and related algal species. Texas: Univ Texas Public N°6318
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98(1):29–36. doi:10.1016/S0269-7491(97)00110-3
Gekeler W, Grill E, Winnacker EL, Zenk MH (1988) Algae as sequester heavy metals via phytochelatin complexes. Arch Microbiol 150:197–202
Guéguen C, Gilbin R, Pardos M, Dominik J (2004) Water toxicity and metal contamination assessment of a polluted river: the Upper Vistula River (Poland). Appl Geochem 19:153–162. doi:10.1016/S0883-2927(03)00110-0
Janssen CR, Heijerick DG (2003) Algal toxicity tests for environmental risk assessments of metals. Rev Environ Contam Toxicol 178:23–52
Kalinowska R, Pawlik-Skowronska B (2010) Response of two terrestrial green microalgae (Chlorophyta, Trebouxiophyceae) isolated from Cu-rich and unpolluted soils to copper stress. Environ Pollut 158:2778–2785. doi:10.1016/j.envpol.2010.03.003
Küpper H, Setlik I, Spiller M, Küpper FS, Prášil O (2002) Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J Phycol 38(3):429–441. doi:10.1046/j.1529-8817.2002.01148
Lombardi AT, Hidalgo TMR, Vieira AAH (2005) Copper complexing properties of dissolved organic materials exuded by the freshwater microalgae Scenedesmus acuminatus (Chlorophyceae). Chemosphere 60:453–459. doi:10.1016/j.chemosphere.2004.12.071
Magdaleno A, Puig A, de Cabo L, Salinas C, Arreghini S, Korol S, Bevilacqua S, López L, Moretton J (2001) Water pollution in an urban Argentine river. Bull Environ Contam Toxicol 67:408–415
Marker AFH, Nusch EA, Rai H, Riemann B (1980) The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Arch Hydrobiol Beih Ergebn Limnol 14:91–106
Omar HH (2002) Bioremoval of zinc ions by Scenedesmus obliquus and Scenedesmus quadricauda and its effects on growth and metabolism. Int Biodeter Biodegr 50:95–100. doi:10.1016/S0964-8305(02)00048-3
Perales-Vela HV, Peña-Castro JM, Cañizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Review. Chemosphere 64(1):1–10. doi:10.1016/j.chemosphere.2005.11.024
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Zylkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65. doi:10.1016/j.plaphy.2011.11.009
Sabater C, Carrasco JM (2001) Effects of pyridaphenthion on growth of five freshwater species of phytoplankton. A laboratory study. Chemosphere 44:1775–1781. doi:10.1016/S0045-6535(00)00575-0
Takami R, Almeida JV, Vardaris CV, Colepicolo P, Barrosa MP (2012) The interplay between thiol-compounds against chromium (VI) in the freshwater green alga Monoraphidium convolutum: toxicology, photosynthesis, and oxidative stress at a glance. Aquat Toxicol 118–119:80–87. doi:10.1016/j.aquatox.2012.03.018
US Environmental Protection Agency (1989) Algal (Selenastrum capricornutum) growth test. Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. Environmental Monitoring System Laboratory. Environmental Protection Agency
Utermöhl H (1958) Zur vervokummung der quantitativen phytoplankton methodik. Mitt Int Ver Limnol 9:1–38
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Review. Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150. doi:10.1016/j.aca.2007.11.018
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magdaleno, A., Vélez, C.G., Wenzel, M.T. et al. Effects of Cadmium, Copper and Zinc on Growth of Four Isolated Algae from a Highly Polluted Argentina River. Bull Environ Contam Toxicol 92, 202–207 (2014). https://doi.org/10.1007/s00128-013-1171-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-013-1171-8