Abstract
Lead concentrations in the tissues of perch and its parasites were determined as mg/kg dw. Lead was found at higher concentrations in the acanthocephalans (11.56) than in different tissues (liver, gonads and muscle with skin and bone) of perch. With respect to fish tissues, the highest concentrations of lead were present in the liver (1.24), followed by the gonads (0.57) whereas the lowest concentrations were in the muscle with skin and bone (0.21). The bioconcentration factors for lead indicated that parasites accumulate metals to a higher degree than fish tissues—lead concentrations in acanthocephalans were 9.32, 19.27 and 55.05 higher than in liver, gonads and muscles of host, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Certain parasites, particularly intestinal acanthocephalans (Sures 2001, 2002, 2003, 2004, 2006; Thielen et al. 2004; Turčeková et al. 2002) and cestodes (Jankovská et al. 2009, 2010a, b; Tenora et al. 2002; Turčeková et al. 2002) can accumulate heavy metals at concentrations that are orders of magnitude higher than those in the host tissues or the environment. Pollution in aquatic biotope is, due to anthropogenic influence, still a subject of many considerations.
In this investigation, we studied Acanthocephalus lucii and its fish host under field conditions to assess the biological availability of lead in the stream “Jevanský potok”, near the city of Prague, Czech Republic. Metal accumulation in A. lucii was compared among different tissues (muscle, liver, gonads) of perch (Perca fluviatilis). Furthermore, element levels in A. lucii were compared with data published for this locality (Jevanský potok) by Kálal et al. (1990).
Materials and Methods
Fifteen specimens of perch (Perca fluviatilis) naturally infected with the adults of the intestinal acanthocephalan parasite Acanthocephalus lucii (Fig. 1; Table 1) were sampled in stream Jevanský potok (Fig. 2) about 30 km from Prague in December 2009 and were frozen immediately. After the fish were brought to the laboratory they were necropsied and acanthocephalans were removed from the gut (no other intestinal helminths were found). Tissues samples (muscle, liver, gonads) were taken with the aid of stainless steel scissors and forceps which had been previously cleaned with redistilled water. Tissue samples and parasites were frozen at −26°C in polypropylene containers until further processing.
Microphotograph of Acanthocephalus lucii from perch (Perca fluviatilis). The parasite was observed by Olympus BX51 microscope via differential interference contrast at a total magnification of 200x. This picture was taken by Olympus E-410 camera and the benchmark was created by integrated computized system (QuickPhoto Micro 2.2) (original photo by J. Vadlejch co-author)
Lead concentrations in the tissues of perch and its parasites were determined as mg/kg dry weight (dw). The content of lead was determined in digested samples of the tissues and parasites by electrothermal atomic absorption spectrometry (ETAAS). Frozen samples were dried by the lyophilisation using the LYOVAC GT 2 (LEYBOLD-HERAEUS, GmbH, Germany) and microwave digested in an acid solution using MWS-3 + (Berghof Products + Instruments, Germany). The dried samples were weighted into the Teflon digestion vessel DAP-60S (minimum 200 mg of the muscles, 100 mg of the gonads, 50 mg of the livers and mg 15 mg of the parasites). Then 2 mL of nitric acid 65%, p.a. ISO (Merck) and 3 mL H2O2 30%, TraceSelect (Fluka) were added. The mixture was shaken carefully and vessel was after 12 h waiting closed and heated in the microwave oven. The decomposition proceeded for 1 h in the temperature range 100–190°C.
The digests obtained were transferred into the 50 mL silica beaker and evaporated to wet residue, then diluted with 1.5% nitric acid prepared from HNO3 65%, p.a. ISO (Merck) and deionised water (Barnstead). The digests were transferred to probes and adjusted with 1.5% HNO3 to 10 mL.
The concentrations of lead in the digests were measured by ETAAS technique using Varian AA 280Z (Varian, Australia) with graphite tube atomizer GTA 120 and PSD 120 programmable sample dispenser. Standard solution ASTASOL (Analytika, CR) of lead was used in the preparation of a calibration curve for the measurement. The evaluation of the concentrations was done by standard addition method and ammonium dihydrogen phosphate GR (Merck) was used as matrix modifier.
The quality of analytical data was assessed by simultaneous analysis of certified reference material CRM 12-02-01 (Bovine Liver) (4% of all the samples). Analytical data obtained for Pb in this CRM 12-02-01 (Bovine liver) was found in the confidence interval given by the producer of the CRM for this element. The background of the trace element laboratory was monitored by analysis of 17.5% blanks prepared under the same conditions, but without samples, and experimental data were corrected by mean concentration of lead in blanks, and compared with detection limit (mean ± 3SD of blanks) which was 0.19 ng.mL−1.
Descriptive statistics (Table 2) were computed for lead content. According to results of Shapiro–Wilk’s normality Kruskal–Wallis test was used for comparison of lead content within parasite and different tissues of its host. For evaluation of possible sex influence on lead content Mann–Whitney U test was used. Relationships between element content within parasite and different tissues of its host was tested using Spearman correlation (Table 3). All statistical analyses were made by Statistica ver. 9 (StatSoft Inc. 2009).
Results and Discussion
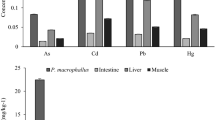
In the present study, 100% of the perch were infected with an average of 20 (2–42) worms per intestine. Lead was present at significantly higher levels (p = 0.01) in the acanthocephalans than in the tissues of its host (Table 2; Fig. 3). When comparing the different fish tissues to each other, the highest concentrations of lead were present in the liver (1.24), followed by the gonads (0.57), as well as in muscle with skin and bone (0.21).
The bioconcentration factor (BF) facilitates the comparison of the relative accumulation capacity of helminths with respect to the host tissues. The bioconcentration of lead in A. lucii compared to the host muscle, gonads and liver tissues is shown in Table 2, supplemented with basic descriptive statistics.
In the case of muscle and gonads, there is no difference of lead content dependent on host sex. The Mann–Whitney U test revealed only statistically significant differences (p = 0.01) in lead content in the liver. Male perch have on average 1.51 mg/kg dw whereas females have 0.44 mg/kg dw. Lead concentration in A. lucii is independent of host sex as well.
Spearman correlation between lead concentrations in the tissues of perch and those in its parasites revealed no significant associations (Table 3). Only weak positive correlation (ρ = 06) was found between number of parasites and lead concentration in host gonads.
Data on metal concentrations in the soft tissues of fish sampled in Jevanský potok were collected by Kálal et al. (1990). In monitored area (Jevanský potok) increased lead burden in fish from the year 1989 (Kálal et al. 1990) to the year 2009 (this study). The average lead concentrations in the soft tissues of fish are listed in Table 2.
For the evaluation and inspection of environmental conditions, many different organisms (bio-indicators) are used. The true interpretation of environmental load by heavy metals and other pollutants is based on a proper selection of a suitable organism and its examination.
Regarding the principle of bioaccumulation in the foodchain, apex predators are frequently chosen as bio-indicators because their bodies contain maximum levels of monitored substances. In the case of freshwater environments it can be various species of fish.
Based on our results and in accordance with experiments of different authors (mainly Sures and co-workers) it is evident that tissues of certain endoparasites, namely acanthocephalans or cestodes, accumulate heavy metals easily and contain significantly higher concentrations in comparison with tissues and organs of its host.
The increasing interest in the relationship between parasitism and pollution, and the potential usefulness of parasites as indicators of the biological accumulation of heavy metals has thus far been focused mainly on animals in aquatic ecosystems (Sures 2001; Tenora et al. 2002; Turčeková et al. 2002). In this investigation, we studied the acathocephalan parasite Acanthocephalus lucii and its fish host (Perca fluviatilis) under field conditions to assess the biological availability of lead in the Jevanský potok. Lead accumulation in A. lucii was compared to accumulation in different tissues (muscle, gonads and liver) of perch (P. fluviatilis).
The enormous accumulation of Pb in acanthocephalan (Pomporchynchus laevis) was for the first time described by Sures et al. (1994). The mean Pb value of P. laevis from naturally infected chub was 2,700 times higher than muscle, 770 times higher than the liver and 280 times higher than the intestine of the host. Sures (2002) worked with the perch (Perca fluviatilis) and its acanthocephalan A. lucii and he found out that the mean Pb value of A. lucii from infected perch was 26.45 times higher than the liver of perch and lead concentrations in the muscle was not detected. In this study the mean (median) Pb value of A. lucii were 9.32 (15.10), 19.27 (25.32) and 55.05 (41.32) times higher than the liver, gonad and muscle of perch, respectively (Table 2). Thus it seems that the lead accumulation by acanthocephalan A. lucii is not so high as its by acanthocephalan P. laevis. Nevertheless it is still more than concentrations in host tissues (Perca fluviatilis). When comparing aquatic and terrestrial ecosystems, the mean Pb values of sheep tapeworms (Moniezia expansa) were 4, 5 and 458 times higher than those in the kidney, liver and muscle of sheep (Jankovská et al. 2010b).
Pb concentrations in different tissues of host s and parasites dependant on host sex showed significant difference in the liver only (Fig. 4).
Furthermore, lead levels in perch tissues were also compared to data published by Kálal et al. (1990) in the same locality (Jevanský potok). The lead burden in fish increased several times (Table 4) from 1989 (Kálal et al. 1990) to 2009 (this study). The increasing Pb concentration in Jevanský potok stream is related to increasing environmental pollution near Prague (this locality serves as a residential and recreational area for many people who work in the city of Prague; this creates pollution due to increased traffic volume and so on). Therefore it is important to utilize new, more sensitive bioindicators for environmental pollution.
This study supports the idea that acanthocephalans are very useful for biomonitoring studies on metal in aquatic ecosystems. Not including parasitic helmiths (above all acanthocephalans and tapeworms) in accumulation bioindication studies with animals (as still customarily done) may lead to false results.
References
Jankovská I, Miholová D, Langrová I, Bejček V, Vadlejch J, Kolihová D, Sulc M (2009) Influence of parasitism on the use of small terrestrial rodents in environmental pollution monitoring. Environ Pollut 157:2584–2586
Jankovská I, Miholová D, Bejček V, Vadlejch J, Sulc J, Száková J, Langrová I (2010a) Influence of parasitism on trace element contents in tissues of red fox (Vulpes vulpes) and its parasites Mesocestoides spp. (Cestoda) and Toxascaris leonina (Nematoda). Arch Environ Contam Toxicol 58:469–477
Jankovská I, Vadlejch J, Száková J, Miholová D, Kunc P, Knížková I, Langrová I (2010b) Experimental studies on the lead accumulation in the cestode Moniezia expansa (Cestoda: Anoplocephalidae) and its final host (Ovis aries). Ecotoxicology 19(5):928–932
Kálal L, Pružina I, Cibulka J, Svatoš Z, Száková J (1990) The contents of risk metals in the Jevany creek watershed fish. Sborník Vysoké školy zemědělské v Praze, fakulta agronomická, B52:27-34 (in czech)
StatSoft, Inc (2009) STATISTICA (data analysis software system), version 9.0. www.statsoft.com
Sures B (2001) The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquatic Ecol 35:245–255
Sures B (2002) Competition for minerals between Acanthocephalus lucii and its definitive host perch (Perca fluviatilis). Int J Parasitol 32:1117–1122
Sures B (2003) Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology 126:S53–S60
Sures B (2004) Environmental parasitology: relevancy of parasites in monitoring environmental pollution. Trends Parasitol 20:170–177
Sures B (2006) How parasitism and pollution affect the physiological homeostasis of aquatic hosts. J Helminthol 80:151–157
Sures B, Siddall R, Taraschewski H (1999) Parasites as accumulation indicators of heavy metal pollution. Parasitol Today 15:16–21
Sures B, Taraschewski H, Jackwerth E (1994) Lead accumulation in Pomphorhynchus laevis and its host. J Parasitol 80:355–357
Tenora F, Baruš V, Prokeš M (2002) Next remarks to the knowledge of heavy metal concentrations in gravid tapeworm species parasitizing aquatic birds. Helminthologia 39:143–148
Thielen F, Zimmermann S, Baska F, Taraschewski H, Sures B (2004) Pomphorhynchus laevis (Acanthocephala) from barbel as a bioindicator for metal pollution in the Danube River near Budapest, Hungary. Environ Pollut 129:421–429
Turčeková L, Hanzelová V, Špakulová M (2002) Concentration of heavy metals in perch and its endoparasites in the polluted water reservoir in Eastern Slovakia. Helminthologia 39(1):23–28
Acknowledgments
This study was supported by project MSM 6046070901 of the Ministry of Education, Youths and Sports, Czech Republic. The authors declare that the experiments comply with the current laws of the Czech Republic, in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jankovská, I., Miholová, D., Petrtýl, M. et al. Intestinal Parasite Acanthocephalus lucii (Acanthocephala) from European Perch (Perca fluviatilis) as a Bioindicator for Lead Pollution in the Stream “Jevanský potok” Near Prague, Czech Republic. Bull Environ Contam Toxicol 86, 342–346 (2011). https://doi.org/10.1007/s00128-011-0210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0210-6