Abstract
Key message
Using the ph1b mutant, the recombination frequency between the homoeologous region of 2B and 2G was significantly increased. By this, we narrowed Pm6 to a 0.9 Mb physical region.
Abstract
The powdery mildew (Pm) resistance gene Pm6 from Triticum timopheevii (2n = 48, AAGG) was mapped to the long arm of chromosome 2G and introduced into common wheat in the form of 2B–2G introgressions. The introgression line IGV1-465 has the shortest 2G segment, which is estimated 37 Mb in size when referring to 2BL genome reference of Chinese Spring (CS). The further fine mapping of Pm6 was impeded by the inhibition of allogeneic chromosome recombination between 2B and 2G in the Pm6 region. In the present study, to overcome 2B/2G recombination suppression, a ph1b-based strategy was employed to produce introgressions with reduced 2G fragments for the fine mapping of Pm6. IGV1-465 was crossed and backcrossed to the CSph1b mutant to produce plants with increased 2B/2G chromosome pairing frequency at the Pm6 region. A total of 182 allogeneic recombinants were obtained through two-round screening, i.e., first round of screening of 820 BC1F2:3 progenies using the flanking markers CIT02g-14/CIT02g-19 and second round of screening of 642 BC1F2:4 progenies using the flanking markers CIT02g-13/CIT02g-18, respectively. Through marker analysis using 30 chromosome 2G-specific markers located in the Pm6 region, the identified recombinants were divided into 14 haplotypes. Pm resistance evaluation of these haplotypes enabled us to narrow Pm6 to a 0.9 Mb physical region of 2BL, flanked by markers CIT02g-20 and CIT02g-18. Six wheat varieties containing Pm6 were identified from a natural population, and they showed increased Pm resistance. This implied Pm6 is still effective, especially when used in combination with other Pm resistance genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the evolution of common wheat, the presence and balance of the Ph (Pairing homoeologous) system have ensured homologous chromosome pairing and recombination, and the disruption of this balance leads to increased homoeologous chromosome recombination (Riley and Chapman 1958). Among the Ph genes, Ph1b from hexaploid wheat (Sears 1977) and Ph1c from tetraploid wheat (Giorgi 1978) on chromosome arm 5BL exhibit the most dominant effects. The recombination frequency between homoeologous chromosomes can be improved by mutation or deletion of the Ph1b gene. By introducing the Chinese Spring ph1b mutant (CS ph1b) into specific background containing a single alien chromosome or whole-arm alien translocation, compensating wheat–alien translocations or introgressions have been developed by inducing homoeologous recombination between wheat and alien chromosomes (Qi et al. 2007). Employing CS ph1b, Zhao et al. (2013) generated translocations of Haynaldia villosa 4VS with reduced fragment sizes and mapped the wheat yellow mosaic virus resistance locus to the terminal region. Through a similar approach, translocations or recombinants between wheat and Thinopyrum, Lophopyrum elongatum, Leymus and barley were developed by Xin et al. (2001), Qi et al. (2008), Mullan et al. (2009) and Rey et al.(2015), respectively.
Wheat powdery mildew, caused by Blumeria graminis f. sp. tritici (Bgt), is a worldwide disease that occurs in major wheat-producing countries including China (Li et al. 2011). The disease is favored by intensive cultivation methods associated with modern agriculture such as the growing of semi-dwarf and high-yielding cultivars under conditions with high levels of nitrogen fertilization and irrigation (Bennett 1984). This biotrophic fungus appears on susceptible varieties from seedling to mature stages and causes a significant reduction in grain yield as well as kernel quality in severe epidemic regions. Compared with the application of fungicides, breeding for resistant varieties is widely accepted as the most economical and environmentally safe approach for disease control. To date, 82 formally designated powdery mildew (Pm) resistance genes associated with 59 loci have been reported (Li et al. 2019; McIntosh et al. 2013; Sun et al. 2018; Tan et al. 2018a, b; Zhang et al. 2018, 2019; Zou et al. 2018), several of which have been extensively used in breeding, such as Pm8 (Purnhauser et al. 2011).
The cloning of Pm genes will be helpful for improving Pm resistance via a transgenic approach as well as the understanding of resistance mechanisms at the molecular level. To date, eight Pm genes have been cloned, among which six were derived from common wheat or its donor species, such as Pm2 from Aegilops squarrosa (Sanchez-Martin et al. 2016), Pm60 from Triticum urartu (Zou et al. 2018) and four genes (Pm3, Pm38, Pm39 and Pm46) from hexaploid wheat (Herrera-Foessel et al. 2014; Lagudah et al. 2009; Lillemo et al. 2008; Yahiaoui et al. 2004), and the other two are from wild relatives of wheat, i.e., Pm8 from rye (Secale cereal) (Hurni et al. 2013) and Pm21 from H. villosa (Xing et al. 2018). Pm3, Pm38, Pm39, Pm46 and Pm60 were cloned via conventional map-based cloning. Pm8 was isolated by homology-based cloning (Hurni et al. 2013). Pm2 and Pm21 were cloned through a mutant chromosome sequencing approach (Sanchez-Martin et al. 2016; Xing et al. 2018).
The tetraploid T. timopheevii (2n = 4x = 28, genome AAGG) is included in the second gene pool of wheat. Several Pm resistance genes have been transferred from T. timopheevii to hexaploid wheat, including Pm6 (Jorgensen and Jensen 1973), Pm27 (Peusha et al. 2000), Pm37 (Perugini et al. 2008) and MlAG12 (Maxwell et al. 2009). Pm6 on the long arm of chromosome 2G has been widely used in wheat breeding for Pm resistance via the development of wheat–T. timopheevii 2B/2G introgression lines. Compared with other Pm resistance genes, the resistance conferred by Pm6 exhibits a distinctly developmental stage-dependent manner, with moderate effectiveness at the seedling stage and high resistance from the fourth leaf stage onward (Bennett 1984). Although virulent isolates overcoming Pm6 resistance have occurred, Pm6 is still effective, especially when used in combination with Pm2 (Costamilan and Trigo 2005; Purnhauser et al. 2011; Švec and Miklovičová 1998; Vechet 2006). The fine mapping and further cloning of Pm6 will facilitate not only the better utilization of Pm6 in wheat breeding, but also a better understanding of the molecular mechanism of developmental stage-dependent disease resistance in plants.
In a previous study, using RFLP and/or STS, along with SSR molecular markers, Pm6 was mapped within a 0.8 cM genetic distance (Ji et al. 2006; Tao et al. 2000). With the help of comparative genomics analysis, Qin et al. (2011) allocated Pm6 to a 614 Mb–753 Mb physical region of wheat chromosome 2B. Two TaRLKs (TaRLK1 and TaRLK2) located in this region were cloned and proved to positively regulate wheat Pm resistance (Chen et al. 2016). However, we later excluded TaRLKs as candidates for Pm6 because when we further narrowed the Pm6 region, we found that the TaRLKs (~ 629 Mb region) were not included in the introgressed 2G fragment in the IGV1-465 line, which exhibits the shortest 2G fragment introgression. It is suspected that recombination between the introgressed 2G fragment and its corresponding wheat chromosome 2B may be repressed. Thus, increasing the 2B/2G recombination frequency is critical for the fine mapping and final cloning of Pm6.
To approach the Pm6 region for target gene cloning, we constructed a population with an increased homoeologous chromosome recombination frequency via the introduction of the ph1b mutant gene. We succeed in narrowing the Pm6 region from 37 to 0.9 Mb, which will greatly facilitate the discovery of the candidate gene for Pm6.
Materials and methods
Plant materials
Prins (AABBDD) is a Swedish Spring wheat variety that is susceptible to wheat powdery mildew. T. timopheevii (AAGG) is the tetraploid donor of Pm6. IGVI-438, IGVI-458, IGVI-463, IGVI-464, IGVI-465 and IGVI-466 are six T. aestivum (c.v. Prins)–T. timopheevii introgression lines with different 2G introgressed segments, all of which carry the Pm6 gene. The above materials were kindly provided by Dr. J. MacKey, Swedish Agricultural University, Uppsala, Sweden.
Common wheat variety Chinese Spring nulli-tetrasomic lines N2AT2D, N2BT2D and N2DT2B were used for the validation of genome-specific markers. The Chinese Spring ph1b mutant (CS ph1b) is a deletion line of 5BL, in which Ph1b is absent and can be used to induce meiotic recombination between the 2G introgression fragment and its counterpart 2B in common wheat. The above germplasms were kindly provided by the Wheat Genetics Resource Centre at Kansas State University, Manhattan, USA.
A natural population consisting of a panel of 387 wheat varieties was kindly provided by Professor Jizeng Jia, Chinese Academy of Agricultural Sciences, Beijing, China.
Mapping population and recombinant screening
To fine map Pm6, a strategy is proposed to improve the 2B/2G allogeneic chromosome recombination frequency in the Pm6 region by employing the Ph1 pairing control system. The workflow is delineated in Fig. 1. The introgression line IGV1-465, containing the shortest introgressed 2G segments, was crossed with CS ph1b, followed by backcrossing the F1 individuals to CS ph1b. The derived BC1F1 progenies were screened using the 2G-specific codominant marker CINAU141 and the Ph1b-specific marker ABC302.3. The BC1F1 plants that were heterozygous for Pm6 and homozygous for ph1b were self-crossed to produce BC1F2. Through analysis using the Pm6 flanking markers, the BC1F2 plants were divided into two groups: recombinant and nonrecombinant. The identified recombinants were phenotyped for Pm resistance and for the fine mapping of Pm6. Those nonrecombinants that were heterozygous for Pm6 and homozygous for ph1b (residual heterozygous lines, RHL) were further self-pollinated to generate BC1F2:3 progenies (equivalent to BC1F2) for the second round of screening of new 2B/2G recombinants, and so forth for the following generations. The identified new recombinants were phenotyped for Pm resistance, and to further narrow the genetic region of Pm6. Recombinant frequency (%) = (no. of recombinants)/(no. of plants identified) × 100.
Workflow for the identification of individuals heterozygous for Pm6/pm6 and lacking Ph1b for the subsequent screening of recombinants between 2B and 2G chromatin in the Pm6 region in their offspring. The black boxes in the chromosomes represent the Pm6 region, and the gray boxes in the chromosomes represent the pm6 region. The green boxes represent the Ph1b region, and the dotted boxes represent the Ph1b deletion. The red boxes represent the centromere. RHL: residual heterozygous lines (color figure online)
Evaluation of powdery mildew resistance
All the tested parents and recombinants were grown under controlled greenhouse conditions, and their Pm resistance was evaluated in vitro at the fifth leaf stage thereafter. The detached leaf segments of the samples were maintained on culture medium (0.5% agar and 20 mg/l 6-BA) in a Petri dish, inoculated with Bgt isolate E26 for 5–6 days in a pathogen-free environment. At 7 days postinoculation, when the susceptible variety CS ph1b became severely infected, photographs were taken. The plants were rated as resistant (R) or susceptible (S), with the former showing either no visible symptoms or necrotic flecks, while the latter exhibited no necrosis and showed high to full sporulation.
For the natural population, we determined Pm resistance using Bgt isolate E26 and the Bgt mixture. We evaluated Pm resistance in vivo. The inocula were increased on susceptible plants under spore-proof greenhouse conditions prior to setting up the disease evaluation experiment. Inoculation was accomplished by gently shaking conidia from the leaves of infected plants onto the foliage.
Bgt isolate E26 was kindly provided by Dr. Yilin Zhou (Institute of Plant Protection, Chinese Academy of Agricultural Sciences). The Bgt mixture was obtained from a native population of Bgt spores collected from Nanjing, Jiangsu Province, China. The evaluation of powdery mildew resistance was performed three times.
Marker development and marker analysis
Intron targeting (IT) markers were developed following the procedure of Wang et al. (2017) with some modifications. We extracted the coding sequences of genes located within the Pm6 region of Ta_chr2B and performed a BLASTn search against the genomic reference of Chinese Spring and the survey sequence of flow-sorted 2G (data not shown). All genes matching the 2AL, 2BL, 2DL and 2G assemblies and possessing at least one predicted exon–exon junction were selected. The intron sizes of the corresponding genes were then calculated and compared against each other. Those homologous genes whose intron sizes were at least 10% different in 2G from those in 2AL, 2BL and 2DL were chosen to design primers for IT markers. The primers were designed in the exons flanking the targeted introns using the online software Primer 3 (version 4.1.0, http://frodo.wi.mit.edu/primer3/) according to the 2G sequences, assuming that exon regions and exon–intron structures of orthologous genes are highly conserved among the four genomes. The designed primers were expected to anneal the genomic DNA of 2AL, 2BL, 2DL and 2GL sequences but to amplify different fragment sizes from different genomes.
To increase the marker density, simple sequence repeat (SSR) and single-nucleotide polymorphism (SNP) markers were developed. The corresponding genomic sequence of the Pm6 region of Ta_2B was extracted and SSR primer pairs were designed using the online software WebSat (http://wsmartins.net/websat/). For SNP marker development, IGV1-465 and CSph1b were genotyped with a Wheat55K array (Affymetrix® Axiom® Wheat55). SNPs located in the Pm6 region were employed for SNP primer design using the online software BatchPrimer3 v1.0 (https://wheat.pw.usda.gov/demos/BatchPrimer3/). The criteria for primer design were as follows: melting temperature of 55 °C and primer length of 18–25 bp (optimum: 20). All primers were synthesized by the TSINGTE Company (Nanjing, China).
Genome sequences
The reference genome sequences of Chinese Spring, T. dicoccoides, T. urartu, Ae. tauschii and Hordeum vulgare were downloaded from the EnsemblPlants Web site (http://plants.ensembl.org/index.html) and used for marker development and microcolinearity analysis.
Results
Enrichment of the marker density targeting the Pm6 region
In a previous study, Ji et al. (2006) and Qin et al. (2011) mapped a total of 29 molecular markers to the Pm6 locus. Referring to the reference genome of Chinese Spring (IWGSC 2018), these markers were assigned to wheat chromosome 2B. Seven of them were located in the introgressed 2G fragment in IGV1-465 (2G_465), flanking a physical distance of 28 Mb from 695 to 723 Mb (Fig. S1). Microcolinearity analysis indicated that the Pm6 region in 2G was highly conserved with the corresponding region of other Triticum species, including 2A from CS, T. dicoccoides and T. urartu, 2B from CS and T. dicoccoides, 2D from CS and Ae. tauschii and 2H from H. vulgare (Fig. S2).
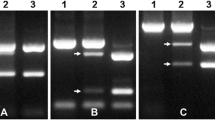
To enrich marker density in the Pm6 region, three types of markers were developed, including IT, SNP and SSR markers. For primer design, we enlarged the physical distance of the Pm6 region from 28 to 48 Mb including an additional 10 Mb of neighbor sequences in both directions. In total, 50 IT, 100 SSR and four SNP primer pairs were designed. Specific markers for the Pm6 region of 2G were screened by amplification using Prins (AABBDD), T. timopheevii (AAGG) and six T. aestivum–T. timopheevii introgression lines. We found that 30 markers generated distinct PCR products only in T. timopheevii and all the six T. aestivum–T. timopheevii introgression lines. They were referred to as 2G_465-specific markers, including 22 IT, two SNP and six SSR markers (Table 1). Amplification in Chinese Spring nulli-tetrasomic lines confirmed that one of the specific IT markers, CIT02g-14-900bp, could be used to differentiate four sub-genomes (Fig. 2).
Validation of the specificity of the IT marker CIT02g-14 for the introgressed 2G fragment. Prins: the receptor of Pm6; IGV1-465 to IGV1-466: the introgression lines with different 2G chromosome segment sizes containing Pm6. T. timopheevii: the donor of Pm6. N2AT2D, N2BT2D and N2DT2B are three nulli-tetrasomic lines of Chinese Spring. The arrow indicates the specific bands for each subgenome
Thirty-seven 2G_465-specific markers (30 developed in this research and seven reported by Qin et al. 2011) were assigned to the Ta_2BL reference sequence of Chinese Spring. It was found that CIT02g-14-900bp and CIT02g-19-750bp were the most proximal and distal to the centromere, covering a 36.9 Mb physical distance (Fig. 3). The physical distance was similar among the sub-genomes of the same origin and their progenitor but varied in different genomes/sub-genomes, with the maximum distance being observed in Td_2BL (38 Mb) of wheat and the minimum in Hv_2HL (15 Mb) of barley (Fig. 3). The genome region bordering Pm6 was determined by the two nearest neighbor markers outside 2G_465 segment, CINAU134 for CIT02g-14-900bp and CINAU140 for CIT02g-19-750bp. Among the six introgression lines with the exception of IGV1-464 and IGV1-465, CINAU134 amplified the specific band for T. timopheevii and CINAU140 amplified the specific band for T. timopheevii in only three lines, i.e., IGV1-463, IGV1-468 and IGV1-466. Therefore, we presumed that Pm6 was most likely located in Bin2, not in Bin1 and Bin 3 (Fig. 3), and CIT02g-14-900bp and CIT02g-19-750bp were used for the screening of recombinants from the segregation populations for the Pm6 locus.
Microcolinearity analysis of the Pm6 region using the developed 2G-specific markers. Left: The schema graph of chromosome 2BL of Prins, T. timopheevii and six T. aestivum–T. timopheevii introgression lines; white boxes indicate chromatin from the 2B Prins; black boxes indicate 2G introgression fragments from T. timopheevii; the red box indicates the centromere. Right: Physical location of markers used for the fine mapping of Pm6; the markers indicated with asterisks were reported by Ji et al. (2006) and Qin et al. (2011); “–” represents an absence of positional information in the reference genome
Fine mapping of Pm6 by generating allogeneic chromosome recombination induced by the Ph1 pairing control system
A total of 34 BC1F1 individuals that were heterozygous for Pm6 and homozygous for phlb were derived from the IGV1-465/CS ph1b//CS ph1b crosses. They were self-pollinated to generate BC1F2 or BC1F2 equivalents in the subsequent generations for recombinant screening.
Recombinant screening was performed in two rounds. Two flanking markers, CIT02g-14-900bp and CIT02g-19-750bp, were used for the first round of screening. A total of 164 recombinants were identified from 820 BC1F2:3 individuals derived from 214 RHLs in BC1F2. The recombinants could be classified into ten haplotypes by genotyping using another 35 specific markers (28 developed in this study and seven from previous study) (Fig. 4 II). The recombinants were evaluated for Pm resistance by inoculating the single Bgt isolate E26 at the fifth leaf stage. The results showed that only haplotypes J and K were resistant and the remaining eight haplotypes were susceptible (Fig. 4 III, IV, A–G, N). Hence, Pm6 was narrowed to a physical region flanked by two markers, CIT02g-13-500bp and CIT02g-18-300bp. The corresponding physical distances were 4 Mb in Ta_2AL, 10 Mb in Ta_2BL and 7 Mb in Ta_2DL. Due to the limited number of recombinants, ten markers within the genome region flanked by CIT02g-13-500bp and CIT02g-18-300bp co-segregated with Pm resistance (Fig. 4 II). This region covers a physical distance of 9 Mb.
Fine mapping of Pm6 using the identified recombinants. I The 2B/2G introgression chromosome in IGV1-465. The red box indicates the centromere of chromosome 2B; the black box indicates the introgressed 2G segment from T. timopheevii. II The distribution of chromosome 2G-specific markers in the 2G introgression segment. Markers above # and ## are those markers that were used for the first and second rounds of recombinant screening, respectively. III Recombinant haplotypes classified through marker analysis. White boxes indicate chromatin from wheat 2B; black boxes indicate chromatin from T. timopheevii 2G; A to N represent 14 recombinant haplotypes classified through marker analysis; S and R represent susceptible and resistant recombinants, respectively; black dotted lines indicate the positions of the markers. The region between the two dotted red lines represents the physical region of Pm6. IVPm resistance of different haplotypes. A to N: 14 recombinant haplotypes; PR: IGV1-465 (resistant parent); PS: CS ph1b (susceptible parent) (color figure online)
The second round of recombinant screening was performed using the flanking markers, CIT02g-13-500bp and CIT02g-18-300bp. Eighteen recombinants were identified from 642 BC1F2:4 individuals derived from 134 RHLs in BC1F2:3. The recombinants could be classified into four haplotypes by genotyping using the other ten specific markers. These recombinants were evaluated for their resistance to isolate E26 at the fifth leaf stage. Two haplotypes were resistant (Fig. 4 III, IV, L and M), and the remaining two were susceptible (Fig. 4 III, IV, H and I). Pm6 was further mapped to the genome region flanked by CIT02g-20-490bp and CIT02g-18-300bp, and the physical distance was narrowed to 0.9 Mb on chromosome 2B (Fig. 4 III).
The homoeologous recombination frequency and recombination frequency per million base pairs (RPM) were compared. The homoeologous recombination frequency and RPM were 20.000% and 0.541%, respectively, for the first round of screening using the BC1F2:3 population and 2.803% and 0.280% for the second round of screening using the BC1F2:4 population (Table 2). In the presence of Ph1b, recombination between 2B and 2G was extremely low (Qin et al. 2011). Our results indicated that the introduction of ph1b significantly increased the 2B/2G recombination frequency in the Pm6 region. The narrowing of this region laid a solid foundation for Pm6 candidate gene prediction and the final cloning of Pm6.
Identification of Pm6 in a natural population and validation of its contribution to Pm resistance
The Pm6 flanking markers CISSR02g-100-350bp and CIT02g-18-300bp were used to genotype a natural population consisting of 387 varieties. The specific products for both markers could be amplified from six of the varieties (Mianmai 40, Mianmai 48, Xingyi 4, Zhongyou 206, Jimai 44 and Jimai 53) (Fig. S3), and we presumed that Pm6 was present in these varieties. Through genotyping using specific markers for six other known Pm resistance genes (Pm2, Pm3b, Pm4, Pm8, Pm21 and Pm38), we found that 92 of 387 varieties harbored none of the above seven Pm resistance genes (data not shown). When the single Bgt isolate E26 was inoculated at the adult stage, the above six Pm6-containing varieties were all found to be highly resistant, while 70 out of 92 non-Pm varieties were Pm susceptible. When the Bgt mixture was inoculated at the adult stage, Mianmai 40, Mianmai 48 and Xingyi 4 were found to be highly resistant, Zhongyou 206 and Jimai 44 were moderately resistant, and Jimai 53 was susceptible. Eighty-eight out of the 92 varieties were moderately or highly susceptible to Pm. Considering the average Pm resistance level against either E26 or the Bgt mixture, Pm6-containing varieties were more resistant to Pm than 92 non-Pm varieties (Fig. S4). This implied that Pm6 was still effective against the tested Bgt mixture or E26 isolate and that the use of the Pm6 flanking markers in molecular-assisted selection was effective for Pm resistance improvement.
Discussion
The transfer of favorable genes from wild relatives is an effective way to broaden the genetic diversity of cultivated wheat for the improvement of wheat cultivars. The cloning of target genes from alien species through a map-based cloning approach is extremely difficult for two main reasons: the relatively low diversity of the target traits among the collections of specific wild species (Cao et al. 2011) and recombination suppression between alien and corresponding wheat chromosomes which makes it difficult to narrow the target region (Jayatilake et al. 2013; Wang et al. 2014; Xie et al. 2012). The suppression of recombination between chromosomes from cultivated wheat and wild wheat species has largely limited the efforts to clone alien genes by using a map-based strategy. The powdery mildew resistance genes Pm12 and Pm27 introgressed into wheat chromosome 6B of Ae. speltoides and T. timopheevii, respectively, show no recombination between the alien segments (6S or 6G) and wheat chromosome 6B (Järve et al. 2000; Jia et al. 1996). In the wheat–H. villosa translocation line T6VS/6AL, the recombination of chromosome arm 6VS with its wheat counterpart is completely suppressed (Xie et al. 2012). An introgression from an unidentified wild relative species carrying the multiple disease resistance locus Lr20/Sr15/Pm1 also showed no recombination between the alien and wheat chromatin (Neu et al. 2002).
The deletion of the Ph1 locus allows homoeologous pairing to occur relatively more easily, implying that the ph1b mutant could be exploited to overcome recombination suppression between chromosomes from common wheat and its relatives. The ph1b mutant has been applied to improve the wheat–alien recombination frequency. The effects of the ph1b mutant vary for different alien chromosomes (arms or fragments). The recombination frequencies in the homozygous ph1b background for wheat–rye translocation chromosomes involving 2RS and 2RL are 0.62% and 16.3%, respectively (Lukaszewski et al. 2004); the recombination frequencies of 4VS and 6VL in wheat–H. villosa translocation with wheat counterparts are 2.19% (Zhao et al. 2013) and 6.81% (data unpublished), respectively; the recombination frequencies of 4Hch, 5Hch and 7Hch in wheat–barley substitution lines with wheat chromosomes range from 3.3 to 7.5% (Rey et al. 2015).
In previous work, Qin et al. (2011) tried to screen recombinants in the Pm6 region from two segregating populations from a cross between IGV1-465/Prins and IGV1-466/Prins. IGV1-466 contains a larger introgressed 2G segment (2G_466) than that in IGV1-465. Initially, two flanking markers CINAU117 and CINAU139 were used, and both markers were present within 2G_466 rather than 2G_465. Among the 1816 F2 individuals from the IGV1-465/Prins cross, 36 were deemed recombinants. However, no recombination site occurred within 2G_465 (Fig. S1), indicating that there was no crossover between 2G and 2B chromatin in this population. For the IGV1-466/Prins population, six of 891 F2 individuals were identified as recombinants and the recombination sites were located within 2G_466, indicating an extremely low recombination frequency (0.005% RPM) between allogeneic 2G and 2B (Table 2). In this study, we proved that the introduction of ph1b could greatly increase the allogeneic recombination frequency, with recombination frequencies of 0.54% and 0.28% RPM being observed in the first and second rounds of recombinant screening, respectively. Through this approach, Pm6 was fine mapped within less than 1 Mb of the genome region. This again demonstrated the effectiveness of the Ph control pairing system in both reducing linkage drag and the fine mapping of target genes from an alien species in a wheat background.
A high-density map lays a foundation for the map-based cloning of target genes (Xue et al. 2008). The DNA markers used for the detection of alien chromatin introgressed into wheat germplasm show the distinct characteristic of being cross species specific. The ability to detect polymorphic loci across species is most likely attributed to the primers being positioned within regions of the genome that exhibit consistent sequence conservation. Common wheat presents a very high repetitive DNA content, accounting for 80–90% of the genome, and these repetitive DNA sequences are typically species specific (Mayer et al. 2014). Therefore, repetitive element-derived molecular markers, such as SSR, are likely not applicable to the detection of alien chromatin. A low interspecific transferability of cereal SSRs has been reported in rye species (Li et al. 2018). In this study, a total of 100 SSR primers were designed according to the sequence of wheat chromosome 2B in accordance with the homologous Pm6 region of 2G. Only six SSR markers could be used for the analysis of introgression lines, implying a low efficiency of genomic SSRs across plant species. We checked the primers for these six applicable SSR markers and found that they could be uniquely aligned within the intergenic region of the survey sequence of flow-sorted 2G (data not shown), which explains their transferability. Although the transferability of genomic SSRs across plant species is unexpected, we still found collinearity in the non-gene-coding region, implying a close relationship between the G genome of T. timopheevii and the wheat B genome (Kilian et al. 2007).
EST-SSR and IT markers are two priority options for efficient polymorphic marker development (Ishikawa et al. 2007; Yu et al. 2004; Zhang et al. 2017). A high degree of synteny exists between wheat and its wild relatives within the same homoeologous groups, and ESTs or functional genes constitute high-synteny region. The gene- or EST-based markers in conserved regions are transferable when used for the detection of alien chromatin from related species (Wang et al. 2017; Xiao et al. 2017). In addition, when these markers are genetically associated with a trait of interest, it will be helpful to predict the candidate genes by referring to the target genome region where the marker sequences are allocated (Thiel et al. 2003). We found that 22 IT markers could amplify specific amplicons in the Pm6 region, suggesting that they have become essential tools for the advanced fine mapping and map-based cloning of Pm6.
References
Bennett F (1984) Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol 33:279–300. https://doi.org/10.1111/j.1365-3059.1984.tb01324.x
Cao A, Xing L, Wang X, Yang X, Wang X, Sun Y et al (2011) Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc Natl Acad Sci 108:7727–7732. https://doi.org/10.1073/pnas.1016981108
Chen T, Xiao J, Xu J, Wan W, Qin B, Cao A et al (2016) Two members of TaRLK family confer powdery mildew resistance in common wheat. BMC Plant Biol 16:27. https://doi.org/10.1186/s12870-016-0713-8
Costamilan L, Trigo E (2005) Variability of the wheat powdery mildew pathogen Blumeria graminis f. sp. tritici in the 2003 crop season. Fitopatol Bras 30:420–422. https://doi.org/10.1590/s0100-41582005000400015
Giorgi B (1978) A homoeologous pairing mutant isolated in Triticum durum cv. Cappelli. Mutat Breed Newsl 11:4–5
Herrera-Foessel S, Singh R, Lillemo M, Huerta-Espino J, Bhavani S, Singh S et al (2014) Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor Appl Genet 127:781–789. https://doi.org/10.1007/s00122-013-2256-9
Hurni S, Brunne S, Buchmann G, Herren G, Jordan T, Krukowski P et al (2013) Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J 76:957–969. https://doi.org/10.1111/tpj.12345
Ishikawa G, Yonemaru J, Saito M, Nakamura T (2007) PCR-based landmark unique gene (PLUG) markers effectively assign homoeologous wheat genes to A, B and D genomes. BMC Genom 8:135. https://doi.org/10.1186/1471-2164-8-135
Järve K, Peusha H, Tsymbalova J, Tamm S, Devos K, Enno T (2000) Chromosomal location of a Triticum timopheevii-derived powdery mildew resistance gene transferred to common wheat. Genome 43:377–381. https://doi.org/10.1139/g99-141
Jayatilake D, Tucker E, Bariana H, Kuchel H, Edwards J, McKay A et al (2013) Genetic mapping and marker development for resistance of wheat against the root lesion nematode Pratylenchus neglectus. BMC Plant Biol 13:230. https://doi.org/10.1186/1471-2229-13-230
Ji J, Wang H, Cao A, Wang S, Zhuang L, Chen P et al (2006) STS markers for powdery mildew resistance gene Pm6 in wheat. Euphytica 2008 163(2):159–165. https://doi.org/10.1007/s10681-007-9578-0
Jia J, Devos K, Chao S, Miller T, Reader S, Gale M (1996) RFLP-based maps of the homoeologous group-6 chromosomes of wheat and their application in the tagging of Pm12, a powdery mildew resistance gene transferred from Aegilops speltoides to wheat. Theor Appl Genet 92:559–565. https://doi.org/10.1007/bf00224558
Jorgensen J, Jensen C (1973) Gene Pm6 for resistance to powdery mildew in wheat. Euphytica. https://doi.org/10.1007/BF00022656
Kilian B, Ozkan H, Deusch O, Effgen S, Brandolini A, Kohl J et al (2007) Independent wheat B and G genome origins in outcrossing Aegilops progenitor haplotypes. Mol Biol Evol 24:217–227. https://doi.org/10.1093/molbev/msl151
Lagudah E, Krattinger S, Herrera-Foessel S, Singh R, Huerta-Espino J, Spielmeyer W et al (2009) Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor Appl Genet 119:889–898. https://doi.org/10.1007/s00122-009-1097-z
Li H, Wang X, Song F, Wu C, Wu X, Zhang N et al (2011) Response to powdery mildew and detection of resistance genes in wheat cultivars from China. Acta Agron Sin 37:943–954. https://doi.org/10.1016/s1875-2780(11)60026-6
Li J, Zhou R, Endo T, Stein N (2018) High-throughput development of SSR marker candidates and their chromosomal assignment in rye (Secale cereale L.). Plant Breed 137:561–572. https://doi.org/10.1111/pbr.12619
Li G, Cowger C, Wang X, Carver B, Xu X (2019) Characterization of Pm65, a new powdery mildew resistance gene on chromosome 2AL of a facultative wheat cultivar. Theor Appl Genet 132(9):2625–2632. https://doi.org/10.1007/s00122-019-03377-2
Lillemo M, Asalf B, Singh R, Huerta-Espino J, Chen X, He Z et al (2008) The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor Appl Genet 116:1155–1166. https://doi.org/10.1007/s00122-008-0743-1
Lukaszewski A, Rybka K, Korzun V, Malyshev S, Lapinski B, Whitkus R (2004) Genetic and physical mapping of homoeologous recombination points involving wheat chromosome 2B and rye chromosome 2R. Genome 47:36–45. https://doi.org/10.1139/g03-089
Maxwell J, Lyerly J, Cowger C, Marshall D, Brown-Guedira G, Murphy J (2009) MlAG12: a Triticum timopheevii-derived powdery mildew resistance gene in common wheat on chromosome 7AL. Theor Appl Genet 119:1489–1495. https://doi.org/10.1007/s00122-009-1150-y
Mayer K, Rogers J, Dolezel J, Pozniak C, Eversole K, Feuillet C et al (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:6194. https://doi.org/10.1126/science.1251788
McIntosh R, Dubcovsky J, Rogers W, Morris C, Appels R, Xia X (2013) Catalogue of gene symbols for wheat. In: KOMUGI-integrated wheat science database at http://www.shigen.nig.ac.jp/wheat/komugi/genes/download.jsp
Mullan D, Mirzaghaderi G, Walker E, Colmer TD, Francki M (2009) Development of wheat–Lophopyrum elongatum recombinant lines for enhanced sodium ‘exclusion’ during salinity stress. Theor Appl Genet 119:1313–1323. https://doi.org/10.1007/s00122-009-1136-9
Neu C, Stein N, Keller B (2002) Genetic mapping of the Lr20-Pm1 resistance locus reveals suppressed recombination on chromosome arm 7AL in hexaploid wheat. Genome 45:737–744. https://doi.org/10.1139/g02-040
Perugini L, Murphy J, Marshall D, Brown-Guedira G (2008) Pm37, a new broadly effective powdery mildew resistance gene from Triticum timopheevii. Theor Appl Genet 116:417–425. https://doi.org/10.1007/s00122-007-0679-x
Peusha H, Enno T, Priilinn O (2000) Chromosomal location of powdery mildew resistance genes and cytogenetic analysis of meiosis in common wheat cultivar Meri. Hereditas 132:29–34. https://doi.org/10.1111/j.1601-5223.2000.00029.x
Purnhauser L, Bóna L, Láng L (2011) Occurrence of 1BL. 1RS wheat-rye chromosome translocation and of Sr36/Pm6 resistance gene cluster in wheat cultivars registered in Hungary. Euphytica 179:287–295. https://doi.org/10.1007/s10681-010-0312-y
Qi L, Friebe B, Zhang P, Gill B (2007) Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res 15:3–19. https://doi.org/10.1007/s10577-006-1108-8
Qi L, Pumphrey M, Friebe B, Chen P, Gill B (2008) Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor Appl Genet 117:1155–1166. https://doi.org/10.1007/s00122-008-0853-9
Qin B, Cao A, Wang H, You F, Liu Y et al (2011) Collinearity-based marker mining for the fine mapping of Pm6, a powdery mildew resistance gene in wheat. Theor Appl Genet 123:207–218. https://doi.org/10.1007/s00122-011-1577-9
Rey M, Calderón M, Prieto P (2015) The use of the ph1b mutant to induce recombination between the chromosomes of wheat and barley. Front Plant Sci 6:160. https://doi.org/10.3389/fpls.2015.00160
Riley R, Chapman V (1958) Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature 182:713. https://doi.org/10.1038/182713a0
Sanchez-Martin J, Steuernagel B, Ghosh S, Herren G, Hurni S, Adamski N et al (2016) Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol 17:221. https://doi.org/10.1186/s13059-016-1082-1
Sears E (1977) Genetics society of Canada award of excellence lecture an induced mutant with homoeologous pairing in common wheat. Can J Genet Cytol 19:585–593. https://doi.org/10.1139/g77-063
Sun H, Hu J, Song W, Qiu D, Cui L, Wu P et al (2018) Pm61: a recessive gene for resistance to powdery mildew in wheat landrace Xuxusanyuehuang identified by comparative genomics analysis. Theor Appl Genet 131:2085–2097. https://doi.org/10.1007/s00122-018-3135-1
Švec M, Miklovičová M (1998) Structure of populations of wheat powdery mildew (Erysiphe graminis DC f. sp. tritici Marchal) in Central Europe in 1993–1996: I. Dynamics of virulence. Eur J Plant Pathol 104:537–544. https://doi.org/10.1023/a:1008642816326
Tan C, Li G, Cowger C, Carver B, Xu X (2018a) Characterization of Pm59, a novel powdery mildew resistance gene in Afghanistan wheat landrace PI 181356. Theor Appl Genet 131:1145–1152. https://doi.org/10.1007/s00122-018-3067-9
Tan C, Li G, Cowger C, Carver B, Xu X (2018b) Characterization of Pm63, a powdery mildew resistance gene in Iranian landrace PI 628024. Theor Appl Genet 132:1137. https://doi.org/10.1007/s00122-018-3265-5
Tao W, Liu D, Liu J, Feng Y, Chen P (2000) Genetic mapping of the powdery mildew resistance gene Pm6 in wheat by RFLP analysis. Theor Appl Genet 100:564–568. https://doi.org/10.1007/s001220050074
The International Wheat Genome Sequencing Consortium (IWGSC) (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. https://doi.org/10.1126/science.aar7191
Thiel T, Michalek W, Varshney R, Graner A (2003) Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor Appl Genet 106:411–422. https://doi.org/10.1007/s00122-002-1031-0
Vechet L (2006) Reaction of winter wheat cultivars and breeding lines to Blumeria graminis f. sp. tritici. Plant Protect Sci 42:15–20. https://doi.org/10.17221/2691-PPS
Wang S, Wong D, Forrest K, Allen A, Chao S, Huang B et al (2014) Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol J 12:787–796. https://doi.org/10.1111/pbi.12183
Wang H, Dai K, Xiao J, Yuan C, Zhao R, Dolezal J et al (2017) Development of intron targeting (IT) markers specific for chromosome arm 4VS of Haynaldia villosa by chromosome sorting and next-generation sequencing. BMC Genom 18:167. https://doi.org/10.1186/s12864-017-3567-z
Xiao J, Dai K, Fu L, Vrana J, Kubalakova M, Wan W et al (2017) Sequencing flow-sorted short arm of Haynaldia villosa chromosome 4V provides insights into its molecular structure and virtual gene order. BMC Genom 18:791. https://doi.org/10.1186/s12864-017-4211-7
Xie W, Ben-David R, Zeng B, Dinoor A, Xie C, Sun Q et al (2012) Suppressed recombination rate in 6VS/6AL translocation region carrying the Pm21 locus introgressed from Haynaldia villosa into hexaploid wheat. Mol Breed 29:399–412. https://doi.org/10.1007/s11032-011-9557-y
Xin Z, Zhang Z, Chen X, Lin Z, Ma Y, Xu H et al (2001) Development and characterization of common wheat-Thinopyrum intermedium translocation lines with resistance to barley yellow dwarf virus. Wheat Glob Environ Euphytica 2001 119(1–2):163–167. https://doi.org/10.1023/A:1017508915932
Xing L, Hu P, Liu J, Witek K, Zhou S, Xu J et al (2018) Pm21 from Haynaldia villosa encodes a CC-NBS-LRR protein conferring powdery mildew resistance in wheat. Mol Plant. https://doi.org/10.1016/j.molp.2018.02.013
Xue S, Zhang Z, Lin F, Kong Z, Cao Y, Li C et al (2008) A high-density intervarietal map of the wheat genome enriched with markers derived from expressed sequence tags. Theor Appl Genet 117:181–189. https://doi.org/10.1007/s00122-008-0764-9
Yahiaoui N, Srichumpa P, Dudler R, Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37:528–538. https://doi.org/10.1046/j.1365-313X.2003.01977.x
Yu J, Dake T, Singh S, Benscher D, Li W, Gill B et al (2004) Development and mapping of EST-derived simple sequence repeat markers for hexaploid wheat. Genome 47:805–818. https://doi.org/10.1139/g04-057
Zhang X, Wei X, Xiao J, Yuan C, Wu Y, Cao A et al (2017) Whole genome development of intron targeting (IT) markers specific for Dasypyrum villosum chromosomes based on next-generation sequencing technology. Mol Breed 37:115. https://doi.org/10.1007/s11032-017-0710-0
Zhang R, Fan Y, Kong L, Wang Z, Wu J, Xing L et al (2018) Pm62, an adult-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor Appl Genet 131:2613. https://doi.org/10.1007/s00122-018-3176-5
Zhang D, Zhu K, Dong L, Liang Y, Li G, Fang T et al (2019) Wheat powdery mildew resistance gene Pm64 derived from wild emmer (Triticum turgidum var. dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5. Crop J. https://doi.org/10.1016/j.cj.2019.03.003
Zhao R, Wang H, Xiao J, Bie T, Cheng S, Jia Q et al (2013) Induction of 4VS chromosome recombinants using the CS ph1b mutant and mapping of the wheat yellow mosaic virus resistance gene from Haynaldia villosa. Theor Appl Genet 126:2921–2930. https://doi.org/10.1007/s00122-013-2181-y
Zou S, Wang H, Li Y, Kong Z, Tang D (2018) The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol 218:298–309. https://doi.org/10.1111/nph.14964
Acknowledgements
This research was supported by the Grants from the National Key Research and Development Program (2016YFD0101004, 2016YFD0102001-004), the National Natural Science Foundation of China (Nos. 31571653, 31771782, 31201204), the International Cooperation and Exchange of the National Natural Science Foundation of China (No. 31661143005), the Special Fund of Jiangsu Province for the Transformation of Scientific and Technological Achievements (BA2017138), the Creation of Major New Agricultural Varieties in Jiangsu Province (PZCZ201706), the SAAS Program for Excellent Research Team, the Science and Technology Service Programs of the Chinese Academy of Sciences (KFJ-STS-ZDTP-002), the Jiangsu Agricultural Technology System (JATS) (No. 2019429) and the Key Research and Development Major Project of Ningxia Hui Autonomous Region (No. 2019BBF02022-04).
Author information
Authors and Affiliations
Contributions
WXE and WHY designed the experimental plan. WWT, LML, TX, CAK and WMX performed the experiments. WWT, LML, TX, LYB and WMX managed the materials in the field. WWT, XJ and WXE wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Communicated by Albrecht E. Melchinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
1 The distribution of previously reported markers forPm6by Qin et al. (2011) along the wheat 2B chromosome. The black boxes indicate introgression fragments from 2G of T. timopheevii; the red arrows point to the recombination site in IGV1-466/Prins and IGV1-465/Prins F2 population. Supplementary Fig. 2: The microcolinearity analysis ofPm6region in Tritium crop. Ta, Td, At, Tu and Hv represent common wheat, Triticum dicoccoides, Aegilops tauschii, Triticum urartu and Hordeum vulgare, respectively. The lines link the homologous genes between chromosomes. Supplementary Fig. 3: The flanking marker ofPm6amplified in a natural group contains 387 materials. Recombinant represents a recombinant derived from IGV1-465/CSph1b which obtains the gene of Pm6. Lanes 1 to 6 are six wheat varieties harboring Pm6; lanes 7 to 21 are representatives without Pm6. Supplementary Fig. 4: Identification ofPm6in a natural population and validation of its contribution toPmresistance. A: Comparison of Pm resistance between wheat varieties with and without Pm6, tested with Blumeria graminis f. sp. tritici (Bgt) isolate E26 and Bgt mixture, respectively; B: the geographical distribution of Pm6-containing wheat varieties in China. (PDF 520 kb)
Rights and permissions
About this article
Cite this article
Wan, W., Xiao, J., Li, M. et al. Fine mapping of wheat powdery mildew resistance gene Pm6 using 2B/2G homoeologous recombinants induced by the ph1b mutant. Theor Appl Genet 133, 1265–1275 (2020). https://doi.org/10.1007/s00122-020-03546-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03546-8