Abstract
Key message
Pm57, a novel resistant gene against powdery mildew, was transferred into common wheat from Ae. searsi and further mapped to 2S s #1L at an interval of FL0.75 to FL0.87.
Abstract
Powdery mildew, caused by the fungus Blumeria graminis f. sp. tritici, is one of the most severe foliar diseases of wheat causing reduction in grain yield and quality. Host plant resistance is the most effective and environmentally safe approach to control this disease. Tests of a set of Chinese Spring–Ae. searsii (SsSs, 2n = 2x = 14) Feldman & Kislev ex K. Hammer disomic addition lines with a mixed isolate of the powdery mildew fungus identified a novel resistance gene(s), designed as Pm57, which was located on chromosome 2Ss#1. Here, we report the development of ten wheat–Ae. searsii recombinants. The wheat chromosomes involved in five of these recombinants were identified by FISH and SSR marker analysis and three of them were resistant to powdery mildew. Pm57 was further mapped to the long arm of chromosome 2Ss#1 at a fraction length interval of FL 0.75 to FL 0.87. The recombinant stocks T2BS.2BL-2Ss#1L 89-346 (TA5108) with distal 2Ss#1L segments of 28% and 89(5)69 (TA5109) with 33% may be useful in wheat improvement. The PCR marker X2L4g9p4/HaeIII was validated to specifically identify the Ae. searsii 2Ss#1L segment harboring Pm57 in T2BS.2BL-2Ss#1L against 16 wheat varieties and advanced breeding lines, and the development of more user-friendly KASP markers is underway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common or bread wheat, Triticum aestivum L. (2n = 6x = 42, AABBDD), is an allohexaploid species and grown worldwide. Yield stability and wheat quality are globally important. However, the wheat crop is frequently affected by diseases, pests, and abiotic stresses including lodging, heat, drought, salt and alkali, leading to yield instability and reduced grain quality.
Powdery mildew of wheat, caused by the fungus Blumeria graminis f. sp. tritici, is one of the most damaging foliar diseases of wheat (Bennett 1984). The disease often occurs in fields rich in nitrogen content and high in stand density in regions of high humidity and moderate temperature. Once infection occurs, the fungus can rapidly grow on the leaves, leaf sheaths, and spikes of the wheat plant, decreasing photosynthesis, diverting nutrients, and increasing respiration and transpiration in infected plants, leading to serious losses of both yield and grain quality (Fried et al. 1981; Everts et al. 2001; Conner et al. 2003; Wang et al. 2005).
Although powdery mildew can be controlled by fungicides, breeding resistant varieties is widely considered to be the most economical and environmentally safe approach to prevent or slow the disease spread. At present, 55 powdery mildew-resistance genes have been named (http://maswheat.ucdavis.edu/CGSW/2013-2014_Supplement.pdf) (McIntosh et al. 2014; Hao et al. 2015; Ma et al. 2015; Zhang et al. 2016). Eighteen of the resistance genes were derived from wheat relatives including Aegilops tauschii Coss. (Pm19, Pm34, and Pm35), Ae. speltoides Tausch (Pm12, Pm32, and Pm53), Ae. geniculata Roth. (Pm29), Ae. longissima (Schweinf. & Muschl. in Muschl.) Eig (Pm13), Secale cereale L. (Pm7, Pm8, Pm17 and Pm20), Dasypyrum villosum (L.) Candargy (Pm21 and Pm55), Thinopyrum intermedium (Host) Barkworth & D.R. Dewey (Pm40 and Pm43), Thinopyrum elongatum (Host) Barkworth & D.R. Dewey (Pm51), and Agropryron cristatum (L.) Gaertn. (Pm2b) (http://maswheat.ucdavis.edu/CGSW/2013-2014_Supplement.pdf). However, new sources of powdery mildew resistance are constantly sought.
Aegilops searsii Feldman & Kislev ex Hammer (2n = 2x = 14, SsSs), native to sub-Mediterranean regions, is one of the S-genome diploid species belonging to the section Sitopsis (Jaub. & Spach) Zhuk. (Feldman and Kislev 1977), which also includes Ae. speltoides (SS, 2n = 2x = 14), Ae. bicornis (Forsskål) Jaub. & Spach (SbSb, 2n = 2x = 14), Ae. longissima (SlSl, 2n = 2x = 14), and Ae. sharonensis Eig (SshSsh, 2n = 2x = 14) (van Slageren 1994).
Since Ae. searsii was first reported by Feldman and Kislev (1977), a Chinese Spring wheat–Ae. searsii amphiploid (2n = 8x = 56, AABBDDSsSs) (Feldman et al. 1979), 7 derived disomic chromosome addition lines, 14 ditelosomic addition lines, 21 disomic chromosome substitutions, and 31 ditelosomic substitution lines have been produced (Friebe et al. 1995).
Several Ae. searsii genes controlling high-molecular-weight glutenin subunits were identified (Sun et al. 2006; Garg et al. 2009) and Liu et al. (2011a) reported a novel gene, Sr51, conferring resistance to the stem rust race Ug99, located on the short arm of chromosome 3Ss#1. Recent screening of the complete set of wheat–Ae. searsii chromosome addition lines in China showed that addition line with chromosome 2Ss#1 confers resistance to powdery mildew. In this paper, we describe the development of compensating 2Ss#1 wheat–Ae. searsii recombinants and the mapping of this powdery mildew-resistance gene, designated Pm57, to a distal segment of the long arm of 2Ss#1.
Materials and methods
Plant materials
Lines TA3581 and TA3809 were used to develop the wheat–Ae. searsii recombinant population. TA3581 is a wheat–Ae. searsii disomic chromosome addition line where a pair of 2Ss#1 chromosomes is added to the chromosome complement of Chinese Spring wheat (DA 2Ss#1) (Friebe et al. 1995). The #1 designation is used to distinguish between the same Ae. searsii chromosome derived from different Ae. searsii accessions (Raupp et al. 1995). TA3809 is a Chinese Spring ph1b mutant stock lacking the Ph1 gene and thereby permitting homoeologous recombination. TA3581, TA3809, and Chinese Spring wheat were used to develop 2Ss#1-specific markers and evaluate response to powdery mildew following inoculation with an isolate mixture of B. graminis f. sp. tritici collected in Henan Province, China. All stocks used in this study were provided by the Wheat Genetics Resource Center at Kansas State University and are maintained at the experimental station of Henan Agricultural University.
Developing segregating populations for 2Ss#1 recombinant selection
DA 2Ss#1 (TA3581) was crossed as a male with the ph1b mutant stock (TA3809), and the F1 plants were either self-pollinated or backcrossed with TA3809 to produce F2 or BC1F1 populations. Individuals homozygous for ph1b and monosomic for 2Ss#1 (2n = 43) were selected using the ph1b-specific marker ABC302.3 (Wang et al. 2002) and 2Ss#1-specific markers developed in this study. The homozygous (ph1b/ph1b) plants with monosomic addition of 2Ss#1 (2n = 43) were self-pollinated to produce the 2Ss#1 recombinant population. Three mapped FlcDNA-based markers and STS-PCR markers located in the distal and proximal regions of each arm of chromosome 2Ss#1 were used to identify putative recombinants. Plants missing at least one of the 2Ss#1-specific markers were chosen for further genomic in situ hybridization (GISH) analysis to select 2Ss#1 recombinants.
Screening of 2Ss#1-specific markers
STS-PCR, SSR, and mapped full-length cDNA (FlcDNA)-based markers of wheat group-2 chromosomes were used to select 2Ss#1-specific PCR markers. SSR primers used in the study were selected based on the SSR physical map of Sourdille et al. (2004) and the consensus SSR map of Somers et al. (2004). PCR was performed in 15 µL reaction mixtures containing 1X PCR buffer (Bioline USA Inc., Taunton, MA, USA), 2 mM MgCl2, 0.25 mM dNTPs, a 10 pmol mixture of forward and reverse primers, 0.02 unit/µl of Taq DNA polymerase (Bioline USA Inc., Taunton, MA, USA), and 100–200 ng of genomic DNA. The procedure of PCR amplification procedure was according to Liu et al. (2011a). STS-PCR primers specific for wheat group-2 chromosomes were designed by Qi et al. (2007) based on wheat expressed sequence tags (EST) mapped to wheat homoeologous group 2. STS-PCR amplification and PCR-amplified product digestion with six different four-base recognition restriction enzymes (AluI, HaeIII, MseI, MspI, RsaI, and MboI) were according to Liu et al. (2011a). Mapped FlcDNA-based markers were selected based on the location of fluorescence in situ hybridization (FISH)-mapped FLcDNAs on the group-2 chromosome arms (Danilova et al. 2014). PCR primers were designed based on group-2-specific FlcDNA sequences flanking the introns by primer 3 (Liu et al. 2016). PCR amplification and PCR product digestion were performed following STS-PCR protocols. SSR-PCR products were resolved in 2.5% agarose gels; STS-PCR and mapped FlcDNA-based PCR products were resolved in 1.5% agarose gels following four-base recognition enzyme digestion, and visualized by ethidium bromide staining under UV light. Genomic DNA was isolated from 5- to 10-cm segments of young leaves using a BioSprint 96 workstation following the protocol described in the BioSprint DNA Plant Handbook (Cat. no. 941558, QIAGEN Inc., Valencia, CA, USA), or isolated following DNA mini-preparation protocols (Qi et al. 2007).
GISH and FISH analysis of recombinants
Chromosome identification was according to Gill et al. (1991). Genomic DNA isolation and GISH probe labeling were according to Liu et al. (2011a). Root tips were collected from putative recombinants missing at least one 2Ss#1-specific marker, treated in an N2O gas chamber for 2.5 h followed by a treatment with 90% acetic acid on ice for 30 min (Danilova et al. 2012). The root tips were washed three times with 75% ethanol and fixed in an ethanol:glacial acetic acid (3:1 v/v) solution for 2–7 days. Squash preparations were made after staining with 1% acetocarmine. GISH was according to Liu et al. (2011a) with minor modifications. The ratio of genomic Ae. searsii DNA and CS blocking DNA was 1:100–120. For FISH, pAs1 and (GAA)9 oligonucleotide probes were used to identify wheat and wheat–Ae. searsii recombinant chromosomes. Probe pAs1 preferentially paints tandem repeats on D-genome chromosomes, and (GAA)9 paints all A- and B-genome chromosomes except 1 A and also labels 1D, 2D, and 7D (Danilova et al. 2012, 2014). After hybridization and slide washing, a drop (25–30 µl) of Vectashield mounting medium containing 1 µg/ml PI (Vector laboratories Inc, Burlingame, CA, USA) for GISH or DAPI (Vector laboratories Inc, Burlingame, CA, USA) for FISH was added to each slide and then covered with a 24 × 30 cm glass coverslip. Fluorescent images were captured with a SPOT2.1 charge-coupled device (CCD) camera (Diagnostic Instruments, Sterling Heights, MI, USA) using an epifluorescence Zeiss Axioplan 2 microscope. Images were further processed with Adobe Photoshop CS3 (Version 10.0.1) (Adobe Systems Inc., San Jose, CA, USA).
Powdery mildew assay
Powdery mildew assays were conducted at the Institute of Plant Protection, Henan Academy of Agricultural Sciences. A mixture of Blumeria graminis f. sp. tritici isolates were collected in Henan Province, and inoculated after the first leaf of each seedling had fully unfolded. Powdery mildew infection types (IT) were scored 7–10 days post-inoculation, when the susceptible controls were heavily infected. A 0–4 infection-type scale, as described by Wang et al. (2005) and Xiao et al. (2013), was used to evaluate the resistance. Plants with infection types of 0–2 were considered resistant, whereas an infection type of 3–4 was considered susceptible.
Results
Selecting Ae. searsii 2Ss#1-specific molecular markers
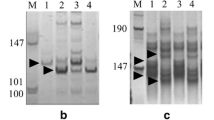
A total of 96 STS-PCR primers, 44 SSR primers, and 95 mapped FlcDNA-based markers were screened to select chromosome 2Ss#1-specific PCR markers. One STS-PCR marker (Xbe586063) and three mapped FlcDNA-based markers (X2S3-2, X2S13, and X2L4g9p4) were selected as 2Ss#1-specific markers (Fig. 1). None of the SSR markers screened in this study was specific for 2Ss#1. Markers Xbe586063 and X2L4g9p4 were located on the long arm of 2Ss#1 in deletion bins 2BL6-0.89-1.00 and 2AL1-0.85-1.00, respectively. Markers X2S3-2 and X2S13 were mapped to the short arm of group-2 chromosomes in deletion bins 2AS5-0.78-1.00 and 2DS1-0.33-0.47, respectively (Table 1). These markers were used to screen the recombinant population.
PCR patterns of Chinese Spring (lane 1), Chinese Spring–Ae. searsii disomic addition line 2Ss#1 (TA3587) (lane 2) and Ae. searsii (TA1840) (lane 3). a Xbe586063/RsaI (2BL6-0.89-1.00); b X2L4g9p4/HaeIII (2AL1-0.85-1.00); c X2S13/MspI (2DS1-0.33-0.43); d X2S3-2/MseI (2AS5-0.78-1.00). Polymorphic fragments of 2Ss#1 are marked by arrows
Developing wheat–Ae. searsii recombinants
A total of 398 plants derived from F2 or BC1F1 plants homozygous for ph1b and monosomic for 2Ss#1were screened with four 2Ss#1-specific markers. Nineteen plants lacking the 2Ss#1 short-arm markers and 21 plants missing the long-arm markers were selected as putative recombinants. GISH analysis initially identified one Robertsonian translocation (RobT) (89(5)185) and 10 recombinants (89-152, 89-160, 89-346, 89-358, 89(2)362, 89(2)378, 89(5)12, 89(5)69, 89(5)102, and 89(6)88). Line 89-160, 89-358, 89(5)12, and 89(5)102 did not survive and the other six surviving recombinants (89-152, 89-346, 89(2)362, 89(2)378, 89(5)69 and 89(6)88) and the RobT 89(5)185 were further characterized (Fig. 2). Of the remaining plants with dissociated 2Ss#1 specific markers, 5 had telosomes, 19 had complete 2S#1 chromosomes, and 5 plants were not identified.
GISH/GAA-FISH patterns of wheat–Ae. searsii recombinants. Ae. searsii chromatin is visualized in red in 89(5)69, 89-346, 89(5)185 telosome on the right, 89(6)88, 89(2)378, and 89(2)362 chromosomes on the right, in yellow-green in 89(5)185 RobT on the left and 89-152, and in green in 89(2)362 chromosome on the left; and GAA FISH sites are visualized in green. Powdery mildew reactions are indicated by R (resistant) and S (susceptible); arrowheads point to the translocation breakpoints. (Color figure online)
Based on the analyses of 2Ss#1-specific markers and GISH patterns, line 89(5)69 and 89-346 had translocated chromosomes with the terminal segments of 2Ss#1 long arm replacing the distal regions of wheat chromosome long arms. Further measurements of ten recombinant chromosomes indicated that the fragment length (FL) of the translocation breakpoints (FL equals distance from the centromere to the translocation breakpoint divided by the complete lengths of this chromosome arm) averaged 0.67 in line 89(5)69 and 0.72 in line 89-346, with distal 2Ss#1 segments of 33 and 28% of the long arms, respectively. Line 89(2)378 and 89(2)362 had recombinant chromosomes of Ae. searsii 2Ss#1 where the distal 36 and 25% of the arms were replaced by wheat segments. Recombinant line 89(6)88 had translocated chromosomes where both distal segments of 2Ss#1 were replaced by wheat segments, with one breakpoint at FL 0.87 at long arm and another at FL 0.70 at the short arm. Line 89-152 had interstial translocations where proximal portions of the short arm were replaced by 2Ss#1 derived segments between FL 0.82 and FL 0.35 (Fig. 2).
Powdery mildew responses and mapping of resistance gene Pm57
In powdery mildew tests of self-pollinated offspring of recombinant lines 89(5)69, 89-346 and 89(6)88 and the RobT 89(5)185 were resistant with infection types 0–1, and recombinants 89(2)362, 89(2)378, and 89-152 and Chinese Spring were susceptible with infection types of 3–4 (Fig. 3). Heterozygous recombinant 89(5)69, 89-346, and 89(6)88 plants were also resistant, indicating that the gene conferring powdery mildew resistance is dominant and was designated as Pm57. The three resistant recombinants 89(5)69, 89-346, and 89(6)88, and the RobT 89(5)185, all share a distal segment of the 2Ss#1L, limiting the Pm57 gene to this segment.
Identifying wheat chromosome segments in the recombinant chromosomes
FISH, using Ae. searsii genomic DNA together with pAs1 and (GAA)9, was used to identify six homozygous 2Ss#1L recombinants and to the wheat chromosomes involved in these recombinants. In recombinants 89(5)69 and 89-346, a distal segment of 2Ss#1L was translocated to the long arm of wheat chromosome 2B resulting in a T2BS.2BL-2Ss#1L recombinant chromosome (Fig. 2). The recombinant chromosome in 89(2)378 consisted of 2Ss#1S, part of 2Ss#1L, and a distal segment derived from the long arm of wheat chromosome 2A (T2Ss#1S.2Ss#1L-2AL) (Fig. 2). The recombinant 89(6)88 had a pair of chromosomes where the distal parts of the short and long arms of 2Ss#1 were replaced by homoeologous segments derived from wheat chromosome 2A resulting in Ti2AS-2Ss#1S.2Ss#1L-2AL (Fig. 2). GISH analysis of the self-pollinated offspring of line 89(2)362 identified a new recombinant with a shortened Ae. searsii segment. The original recombinant present in line 89(2)362 consisted of 2Ss#1S, part of 2Ss#1L, and a distal segment derived from an unidentified wheat chromosome, whereas in the progeny of this plant a new Ti2DS.2DL-2Ss#1L-2DL recombinant was identified; (Fig. 2). The recovery of a new recombinant in the progeny of 89(2)362 suggests that this plant originally may have had a dicentric wheat–Ae. searsii chromosome that underwent undetected breakage fusion-bridge cycles. Similarly, modified recombinants derived from cycling dicentric chromosomes were reported previously during the production of wheat–Th. intermedium recombinants (Liu et al. 2011b, 2013).
In the progeny of line 89(5)185, which originally had a RobT where the short arm of an unidentified wheat chromosome was translocated to 2Ss#1L, we recovered plants with a 2Ss#1L telosome (Fig. 2) that was most likely produced by centric misdivision of the univalent RobT. Homozygous recombinant stocks of the remaining lines were obtained by combining GISH and SSR marker analysis of self-pollinated progenies of the recombinant plants.
In addition to FISH identification, wheat group 2-specific SSR markers were also used for identifying the wheat segments involved in the recombinants, and SSR marker analysis supported the FISH data. Figure 4 shows recombinant 89(6)88 (Ti2AS-2SsS.2Ss#1L-2AL), retaining both distal wheat 2A SSR markers Xbarc124 and Xgwm265, but losing the 2A-specific proximal markers Xgwm425 and Xbarc15. The markers Xbarc124 and Xgwm265 are located in the deletion bins 2AS5-0.78-1.00 and 2AL1-0.85-1.00, respectively, and the markers Xgwm425 and Xbarc15 were mapped in the deletion bins C-2AS5-0.78 and C-2AL1-0.85. Thus, the recombinant stock 89(6)88 was confirmed to have two independent recombination events in both arms of 2Ss#1, as suggested by FISH analysis (Fig. 2).
SSR marker analysis of wheat–Ae. searsii recombinant line 89(6)88. From left to right, Lane 1 100 bp DNA marker; lane 2 Chinese Spring; lane 3 Chinese Spring–Ae. searsii disoimc addition line of 2Ss#1 (DA2Ss); lane 4 N2AT2D; lanes 5–7 homozygous plant 88-7, 88-20, 88-23 derived from heterozygous recombinant 89(6)88; lane 8 plant 88-12 without translocated chromosomes derived from heterozygous recombinant 89(6)88 (the negative control line). All the homozygous recombinants have retained the wheat 2A distal markers of the short (Xbarc124) and long arms (Xgwm265) but have lost the wheat 2A proximal markers Xgwm425 (short arm specific) and Xbarc15 (long arm specific). SSR marker analysis showed recombinant 89(6)88 contained translocated chromosomes where both distal segments of 2Ss#1 were replaced by homoeologous segments of wheat chromosome 2A, confirming that line 89(6)88 can be designed as Ti2AS-2SsS.2Ss#1L-2AL
Further comparison of the 2Ss#1 segments showed that all resistant recombinants share an Ae. searsii segment ranging between FL 0.72 and FL 0.87, whereas in the susceptible recombinant 89(2)362, the 2Ss#1L segment is located between FL 0.51 and FL 0.75 and between FL 0.00 and FL 0.64 in the susceptible recombinant 89(2)378. These data map Pm57 onto chromosome arm 2Ss#1L and between FL 0.75 and FL 0.87, corresponding to 12% the long arm length of the recombinant chromosomes (Fig. 5).
Mapping of powdery mildew-resistance gene Pm57. Chromosome 2Ss#1L chromatin is shown in black and wheat chromatin in grey, arrows point to the translocation breakpoints, and numbers on the right indicate the fragment lengths (FL) of the breakpoints. All resistant recombinants share a 2Ss#1L segment between FL 0.75 and FL 0.87, limiting Pm57 to this interval
Validation of Pm57 linked markers
X2L4g9p4/HaeIII, an FLcDNA-based PCR marker is specific for the 2Ss#1 long arm and was used to identify putative 2Ss#1 recombinants tagging the Ae. searsii segments in 89(5)69 and 89-346 (T2BS.2BL-2Ss#1L) (Fig. 1). The corresponding full-length cDNA (AK331687) of X2L4g9p4 was mapped at FL0.86, 0.91 and 0.89 2A, 2B and 2D long arms by single gene FISH (Danilova et al. 2014). The usefulness of marker X2L4g9p4/HaeIII in tagging the 2Ss#1L segments carrying Pm57 was validated with 12 wheat varieties and advanced breeding lines. The result showed that X2L4g9p4 PCR primers generated polymorphic DNA bands of 660 bp in the CS-Ae. searsii 2Ss#1 addition line, 89(5)185 (2Ss#1L telosome), recombinants 89(5)69 and 89-346 (T2BS.2BL-2Ss#1L), compared to both CS and 11 different wheat cultivars and advanced breeding lines from USA and China (Fig. 6). Thus marker X2L4g9p4/HaeIII can be used to track the transfer of Pm57 in breeding programs and the development of more user friendly KASP markers is underway.
PCR amplification of marker X2L4g9p4/HaeIII. Lane M 100 bp DNA ladder, lanes 1–4 TA3581 (CS-Ae. searsii 2Ss#1 disomic addition line); TA5108 (89(5)69; T2BS.2BL-2Ss#1L); TA5109 (89-346 T2BS.2BL-2Ss#1L); and 89(5)185 (2Ss#1L telosome); lanes 5–16 wheat varieties Chinese Spring, Lakin, Postrock, Pingan 8, Jingmai 66, Zhengzhou 366, Xinong 979, Pingan 0518, Pingan 602, Yanzhan 4110, Zhoumai 18, and advanced breeding line 1113-411-5-243. The arrow points to the polymorphic X2L4g9p4/HaeIII band of 660 bp amplified from the distal Ae. searsii 2Ss#1L segments carrying Pm57
Discussion
Developing wheat–alien recombinants is a very efficient approach for utilizing the gene pool of distantly related relatives of wheat in crop improvement. Although wheat–alien translocations occur spontaneously, their frequency is very low. Several strategies have been used to transfer alien genes to wheat. The transfer of alien chromosome arms can be achieved using the centric breakage–fusion mechanism of univalents at meiotic metaphase I (Sears 1952). Radiation treatment (Sears 1956) and induced homoeologous recombination (Riley et al. 1968a, b) can be used for transferring alien chromosome segments smaller than complete chromosome arms. In this study, we identified ten wheat–Ae. searsii recombinants from 398 individuals derived from F2 or BC1F1 plants homozygous for ph1b and monosomic for chromosome 2Ss#1. The frequency of recovered recombinants in this study was 2.76%, which is slightly higher than that previously reported for ph1b-induced wheat–Ae. searsii 3Ss#1 recombinants (1.94%) (Liu et al. 2011a). Of the five 2Ss#1 recombinants identified by FISH, all involved homoeologous group-2 chromosomes, indicating that they are genetically compensating and agronomically useful.
A single Ae. searsii-derived gene Sr51, conferring stem rust resistance was identified and transferred to wheat in the form of T3AL.3Ss#1S, T3BL.3Ss#1S, and T3DL.3Ss#1S RobTs (Liu et al. 2011a). In this study, we report the transfer and mapping of a novel powdery mildew-resistance gene, Pm57, to the wheat–Ae. searsii recombinant chromosome T2BS.2BL-2Ss#1L. Ten of the catalogued powdery mildew-resistance genes are located on group-2 chromosomes, however, none are derived from Ae. searsii, indicating that Pm57 is most probably new source of resistance.
Powdery mildew of wheat can cause severe yield loss, especially under high nitrogen conditions and stand densities. In a recent study of 908 wheat varieties and advanced lines from 2009 to 2013 in Henan, 63.9% of entries had the T1BL.1RS translocation with Pm8, and only 18 entries (1.98%) had Pm2, Pm4a, Pm21, or more than one resistance gene (Cao et al. 2015). To broaden the genetic variability, employing additional powdery mildew-resistance genes in cultivar improvement is highly recommended. The resistant powdery mildew recombinant lines 89(5)69 and 89-346 identified in this study as T2BS.2BL-2Ss#1L and Pm57 linked maker X2L4g9pe/HaeIII will be useful in breeding powdery mildew-resistant wheat cultivars. The distal 2Ss#1L segments in those lines differ in size, being 28% in 89-346 (designated as TA5108) and 33% in 89(5)69 (designated as TA5109). We are currently transferring the T2BS.2BL-2Ss#1L recombinant chromosomes to locally adapted Chinese cultivars, which will then be evaluated for various agronomic traits under field conditions. Small quantities of TA5108 and TA5109 are available upon request.
Author contribution statement
WL, D-HK, QY, and FB performed the molecular marker and GISH analyses; YS and CL evaluated the powdery mildew resistance; and WL, D-HK, BSG, and BF participated in writing the manuscript.
References
Bennett FGA (1984) Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol 33:279–300
Cao T, Chen Y, Li D, Zhang Y, Wang X, Zhao H, Liu Z (2015) Identification and molecular detection of powdery mildew resistance of new bred wheat varieties (lines) in Henan province, China. Acta Agron Sinica 41:1172–1182
Conner RL, Kuzyk AD, Su H (2003) Impact of powdery mildew on the yield of soft white spring wheat cultivars. Can J Plant Sci 83:725–728
Danilova TV, Friebe B, Gill BS (2012) Single-copy gene fluorescence in situ hybridization and genome analysis: Acc-2 loci mark evolutionary chromosomal rearrangements in wheat. Chromosoma 121:597–611
Danilova TV, Friebe B, Gill BS (2014) Development of a wheat single gene FISH map for analyzing homoeologous relationship and chromosomal rearrangements within the Triticeae. Theor Appl Genet 127:715–730
Everts KL, Leath S, Finney PL (2001) Impact of powdery mildew on milling and baking quality of soft red winter wheat. Plant Dis 85:423–429
Feldman M, Kislev M (1977) Aegilops searsii, a new species of section Sitopsis (Platystachys). Isr J Bot 26:190–201
Feldman M, Strauss I, Vardi A (1979) Chromosome pairing and fertility of F1 hybrids of Aegilops longissima and Ae. searsii. Can J Genet Cytol 21:261–272
Friebe B, Tuleen NA, Gill BS (1995) Standard karyotype of Triticum searsii and its relationship with other S-genome species and common wheat. Theor Appl Genet 91:248–254
Fried PM, Mackenzie DR, Nelson RR (1981) Yield loss caused by Erysiphe graminis f. sp. tritici on single culms of “Chancellor” wheat and four multilines. Z Pflanzenkrankh Pflanzenschutz 88:256–264
Garg M, Tanaka H, Ishikawa N, Takata K, Yanaka M, Tsujimoto H (2009) A novel pair of HMW glutenin subunits from Aegilops searsii improves quality of hexaploid wheat. Cereal Chem 86:26–32
Gill BS, Friebe B, Endo TR (1991) Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 34:830–839
Hao Y, Parks R, Cowger C, Chen Z, Wang Y, Bland D, Murphy J, Guedira M, Brown-Guedira G, Johnson J (2015) Molecular characterization of a new powdery mildew resistance gene Pm54 in soft red winter wheat. Theor Appl Genet 128:465–476
Liu W, Jin Y, Rouse M, Friebe B, Gill BS, Pumphrey MO (2011a) Development and characterization of wheat-Ae. searsii Robertsonian translocations and a recombinant chromosome conferring resistance to stem rust. Theor Appl Genet 122:1537–1545
Liu W, Seifers DL, Qi LL, Friebe B, Gill BS (2011b) A compensating wheat-Thinopyrum intermedium Robertsonian translocation conferring resistance to wheat streak mosaic virus and Triticum mosaic virus. Crop Sci 15:2382–2390
Liu W, Danilova TV, Rouse MN, Bowden RL, Friebe B, Gill BS, Pumphrey MO (2013) Development and characterization of a compensating wheat-Thinopyrum intermedium translocation with Sr44 resistance to stem rust (Ug99). Theor Appl Genet 126:1167–1177
Liu W, Koo D, Bernd Friebe B, Gill BS (2016) A set of Triticum aestivum-Aegilops speltoides Robertsonian translocation lines. Theor Appl Genet. doi:10.1007/s00122-016-2774-3
Ma P, Xu H, Xu Y, Li L, Qie Y, Luo Q, Zhang X, Li X, Zhou Y, An D (2015) Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor Appl Genet 128:613–622
McIntosh R, Dubcovsky J, Rogers W, Morris C, Appels R, Xia X (2014) Catalogue of gene symbols for wheat: 2013–2014 supplement. Ann Wheat Newslett 60:153–175 http://www.shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp
Qi LL, Friebe B, Zhang P, Gill BS (2007) Homoelogous recombination, chromosome engineering and crop improvement. Chromosome Res 15:3–19
Raupp WJ, Friebe B, Gill BS (1995) Suggested guidelines for the nomenclature and abbreviation of the genetic stocks of wheat, Triticum aestivum L. em Thell. and its relatives. Wheat Inf Serv 81:51–55
Riley R, Chapman V, Johnson R (1968a) The incorporation of alien disease resistance in wheat by genetic interference with the regulation of meiotic chromosome synapsis. Genet Res Camb 12:198–219
Riley R, Chapman V, Johnson R (1968b) Introduction of yellow rust resistance of Aegilops comosa into wheat by genetically induced homoeologous recombination. Nature 217:383–384
Sears ER (1952) Misdivision of univalents in common wheat. Chromosoma 4:535–550
Sears ER (1956) The transfer of leaf rust resistance rom Aegilops umbellulata to wheat. Brookhaven Symp Biol 9:1–22
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Sun X, Hu S, Liu X, Qian W, Hao S, Zhang A, Wang D (2006) Characterization of the HMW glutenin subunits from Aegilops searsii and identification of a novel variant HMW glutenin subunit. Theor Appl Genet 113:631–641
van Slageren MW (1994) Wild wheats: a monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae). Agriculture University, Wageningen, p 513
Wang X, Lai J, Liu G, Chen F (2002) Development of a scar marker for the Ph1 locus in common wheat and its application. Crop Sci 42:1365–1368
Wang ZL, Li LH, He ZH, Duan XY, Zhou YL, Chen XM, Lillemo M, Singh RP, Wanh H, Xia XC (2005) Seedling and adult plant resistance to powdery mildew in Chinese bread wheat cultivars and lines. Plant Dis 89:457–463
Xiao MG, Song FJ, Jiao JF, Wang XM, Xu HX, Li HJ (2013) Identification of the gene Pm47 on chromosome 7BS conferring resistance to powdery mildew in the Chinese wheat landrace Hongyanglazi. Theor Appl Genet 126:1397–1403
Zhang R, Sun B, Chen J, Cao A, Xing L, Feng Y, Lan C, Chen P (2016) Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum, into common wheat. Theor Appl Genet. DOI:10.1007/s00122-016-2753-8
Acknowledgements
We thank Robert McIntosh and John Raupp for valuable suggestions and Duane L. Wilson for excellent technical assistance. This research was supported by the National Natural Science Foundation of China (31571658), the State Key Laboratory of Wheat and Maize Crop Science at Henan Agricultural University, Zhengzhou, China (39990022), the Kansas Wheat Commission, WGRC I/UCRC NSF contract 1338897 and the Kansas Crop Improvement Association. This paper is contribution number 17-077-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, KS 66506-5502.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Aimin Zhang.
Rights and permissions
About this article
Cite this article
Liu, W., Koo, DH., Xia, Q. et al. Homoeologous recombination-based transfer and molecular cytogenetic mapping of powdery mildew-resistant gene Pm57 from Aegilops searsii into wheat. Theor Appl Genet 130, 841–848 (2017). https://doi.org/10.1007/s00122-017-2855-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-017-2855-y