Abstract
Key message
Two novel Wx - B1 null alleles that enlarge the genetic variability for this wheat gene were characterized, whose effects on wheat quality could be different to those of the Wx - B1b allele.
Abstract
The starch composition of wheat grain has a primary influence on flour quality. Wheat starch consists of two types of glucose polymers: amylose (22–35 % of the total) and amylopectin (68–75 % of the total). Amylose is synthesized by waxy proteins. Several studies have contributed to the catalogue of waxy alleles available for breeders, and the search for novel alleles of these and other proteins related to flour quality continues. In this report, we describe the characterization of two novel Wx-B1 alleles (Wx-B1k and Wx-B1m) in a collection of macha, Indian dwarf and club wheat. Several accessions lacking Wx-B1 protein were detected, and some were caused by the common Wx-B1b null allele. Of the other accessions, four from Indian dwarf wheat showed the insertion of 4 bp within the seventh exon, and one from club wheat had a deletion of four nucleotides in the second exon. These mutations were novel and provisionally catalogued as Wx-B1k and Wx-B1m, respectively, and could be used to enlarge the genetic variability for this gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To define the processing and end-use quality of wheat, grain hardness and gluten properties have often been considered the most important factors. They are responsible for two of the most important properties of wheat dough: its water absorption capacity and its visco-elastic properties. However, the increase in demand for novel, high-quality processed food has focussed attention on other components of the grain that also have an important effect on wheat industrial quality. One example is starch (Rahman et al. 2000), the main component of wheat grain, representing 65–75 % of its dry weight.
Starch is formed by two glucose polymers: linear amylose (22–35 % of the total) and highly branched amylopectin (68–75 % of the total) (James et al. 2003). Because the amylose/amylopectin ratio affects such processing properties of wheat as gelatinization, pasting and gelation (Zeng et al. 1997), it is reasonable to expect that this could affect end-use quality of different wheat products such as bread, pasta and noodles (Martin et al. 2008; Miura and Tanii 1994; Park and Baik 2007) as well their shelf-life (Hayakawa et al. 2004) and nutritional value (Regina et al. 2006).
In recent decades, the role of different enzymes controlling starch composition/properties have been examined (Morell et al. 2001). Different starch synthases (SSI, SSII and SSIII) have been shown to function together with the branching (SBEI and SBEII) and debranching enzymes (SDBEs) in the synthesis of amylopectin, while the granule-bound starch synthase I (GBSSI) or waxy protein is the sole enzyme responsible for amylose synthesis. In common wheat (Triticum aestivum L. ssp. aestivum; 2n = 6 × = 42, BBAADD), one waxy protein for each genome has been detected—these Wx proteins are encoded by genes Wx-A1, Wx-B1 and Wx-D1 located on chromosomes 7AS, 4AL (translocated from original 7BS) and 7DS, respectively (Yamamori et al. 1994). Although different studies on the variation of these enzymes have been carried out and several variants of starch (amylose and amylopectin) synthesis enzymes have been detected (Yamamori et al. 1994, 1995; Yamamori and Endo 1996), variability in modern wheat cultivars is not very wide according to data in the Wheat Gene Catalogue (McIntosh et al. 2013). However, the use of the null variants detected has permitted the development of wheat lines with novel starch properties (Nakamura et al. 1995, 2006; Yamamori 2009; Yamamori and Quynh 2000; Yamamori and Yamamoto 2011), which have shown remarkable differences in terms of industrial (Graybosch 1998) and nutritional quality (Yamamori et al. 2006).
Species in the primary wheat gene pool could be good candidates as gene sources for the search of novel Wx variants to enable diversification of the starch properties of wheat. Within the hexaploid species of this gene pool, there are some neglected or underutilized subspecies, such as club, Indian dwarf or macha wheat, which have not been screened for waxy proteins variability. Club wheat [T. aestivum L. ssp. compactum (Host) Mackey], characterized by a compact spike, is distributed throughout the Old World (Filatenko and Hammer 2014) and has commercial importance in the US Pacific Northwest area for production of flours suitable for making cookies. The distribution areas of the other two subspecies are more limited. Indian dwarf wheat [T. aestivum L. ssp. sphaerococcum (Percival) Mackey] has small stature and small round grains and originated in India and Pakistan (Hosono 1954). Macha wheat [T. aestivum L. ssp. macha (Dekapr. & A.M. Menabde) Mackey] is a hulled wheat endemic to the Caucasus area. An important advantage of these three cultivated subspecies is that they cross readily with modern wheat and have little linkage drag of unwanted traits. Until now, several studies using Indian dwarf wheat have shown different interesting traits for genetic improvement, e.g. rust resistance (Chen et al. 2012), salt tolerance (Badridze et al. 2009), concentration of bioactive compounds in grain (Giambanelli et al. 2013) and even high grain yield (Zwer et al. 1995). Consequently, these wheat subspecies could be used to widen the genetic diversity of modern wheat for waxy proteins and other agronomic traits.
The aim of the present study was to evaluate the variability for waxy proteins in a collection of club, Indian dwarf and macha wheat, together with the molecular characterization of the polymorphic waxy alleles found.
Materials and methods
Plant material
Forty-three accessions of club wheat, twenty-seven accessions of macha wheat, and twenty-three accessions of Indian dwarf wheat obtained from the National Small Grain Collections (Aberdeen, USA) were analyzed in this study (Electronic Supplementary Material, ESM-1). The bread wheat cvs. Chinese Spring and Kanto 107 were used as standards.
Starch extraction and electrophoretic analysis
Whole grain flour from a single grain was mixed with 1 ml of distilled water and incubated at 4 °C for 24 h. The homogenate was filtered through Miracloth and centrifuged at 14,000g for 1.5 min. The pellet was washed with 1 ml of buffer A [55 mM Tris–HCl pH 6.8, 2.3 % (w/v) sodium dodecyl sulphate, 2 % (w/v) dithiotreitol, 10 % (v/v) glycerol], according to Echt and Schwartz (1981). Then 1 ml of buffer A was added to the pellet and left for 30 min at room temperature. The pellet was washed three times with distilled water, once with acetone and then air-dried. The residue was mixed with 80 μl of buffer A containing 0.02 % (w/v) bromophenol blue, heated in a boiling bath for 2 min, cooled in ice and centrifuged.
Aliquots of supernatant (20 μl) were loaded in vertical SDS-PAGE slabs in a discontinuous Tris–HCl-SDS buffer system (pH: 6.8/8.8) at a polyacrylamide concentration of 12 % (w/v, C: 0.44 %). The Tris–HCl/glycine buffer system of Laemmli (1970) was used. Electrophoresis was performed at a constant current of 30 mA/gel and 18 °C, continuing for 4 h after the tracking dye migrated off the gel. Protein bands were visualised by silver staining.
For two-dimensional polyacrylamide-gel electrophoresis (2D-PAGE), starch purified as described above was incubated at room temperature in 300 μl of lysis buffer [8 M urea, 2 % Triton X-100, 2 % ampholine pH 3.5–10 (Pharmacia LKB) and 5 % 2-mercaptoethanol]. After centrifugation, the supernatant containing the solubilised proteins was subjected to 2D-PAGE using isoelectric focusing (IEF) for the first dimension and modified SDS-PAGE for the second (Nakamura et al. 1993). IEF gels contained 2.5 % (v/v) ampholines (pH 3.5–10/5–8, 1:1). Focusing was begun from the acidic end (0.01 M H3PO4) and continued at 400 V for 15 h and then 800 V for 60 min at room temperature. Proteins were revealed by silver staining according to Silver stain kit (Wako Pure Chemical Industries, Ltd., Japan).
DNA extraction and PCR amplification
For DNA extraction, approximately 100 mg of young leaf tissue was excised, immediately frozen in liquid nitrogen and stored at −80 °C. Genomic DNA was extracted by the CTAB method (Stacey and Isaac 1994).
The genomic sequence of the Wx gene contains twelve exons and eleven introns, with a coding region around 2800 bp. Primers designed by Guzmán and Alvarez (2012) were used to amplify the coding region of Wx genes in three regions or fragments: the first from second to fourth exon (Wx1Fw/1Rv); the second from fourth to the seventh exon (Wx2Fw/2Rv); and the last fragment from the seventh to the twelfth exon (Wx3Fw/3Rv).
All amplifications were performed in 20 μl of final reaction volume containing 50 ng of DNA genomic, 1.25 mM MgCl2, 0.2 mM dNTPs, 4 μl 10× PCR buffer and 0.75 U Taq polymerase (Promega, Madison, WI, USA). The primer concentrations were 0.4, 0.3 and 0.2 μM of each primer for the first, second and third fragment, respectively. Furthermore, the primers BDFL and BRD designed by Nakamura et al. (2002) were used to detect Wx-B1b allele following the author’s instructions. Also, a new reverse primer (Wx1.3Rv) was designed to amplify the beginning region of these genes. PCR conditions as well as primers sequence are available in Table 1.
Analysis of PCR products, cloning and sequencing analysis
Amplification products were fractionated in vertical PAGE gels at 8 % (w/v C: 1.28 %), and the bands were visualized by ethidium bromide staining. PCR products were purified using Sureclean Plus (Bioline) and cloned into pGEM T-easy vector (Promega) for sequencing. Wx-B1 inserts were selected from the mix of Wx-1 inserts (Wx-A1, Wx-B1 and Wx-D1) based on the size and digestion pattern with specific endonucleases of each insert. Inserts were sequenced from at least three different clones using an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Carlsban, CA, USA). The sequences were analyzed and compared to the sequences of cv. Chinese Spring available in the databases (Wx-A1a: AB019622, Wx-B1a: AB019623, and Wx-D1a: AB019624) using Geneious Pro ver. 5.0.4 software (Biomatters Ltd.).
Results
Waxy protein polymorphism and PCR analysis
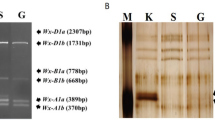
The SDS-PAGE electrophoresis of waxy proteins did not detect polymorphism for Wx-A1 or Wx-D1 proteins in any of the three species. All samples showed two bands with the same mobility as those of cv. Chinese Spring (Fig. 1a). However, five accessions of club wheat and 13 of Indian dwarf wheat lacked the Wx-B1 protein (ESM-1). In contrast, all accessions of macha wheat showed a band similar to Wx-B1 protein of Chinese Spring. These results were confirmed by 2D-PAGE (Fig. 1b).
Waxy protein polymorphism by SDS-PAGE and 2D electrophoresis means. All the samples had Wx-A1 and Wx-D1 proteins. Lanes are as follows: 1 PI 355512, 2 PI 422411, 3 PI 357307 (lack of Wx-B1), 4 PI 442911 (lack of Wx-B1), 5 PI 565431 (lack of Wx-B1), 6 cv. Chinese Spring, 7 PI 272580 (lack of Wx-B1), 8 PI 272581 (lack of Wx-B1), 9 PI 278650, and 10 CItr 4528
To identify the cause of the lack of Wx-B1 protein, PCR markers described by Nakamura et al. (2002) that permit identification of the Wx-B1b allele (the most common Wx-B1 null allele) were used to screen these accessions. In general, accessions that produced the three waxy proteins had the three PCR product bands corresponding to each Wx-1 gene (Fig. 2, lane 4). Compositions of the 18 accessions that did not show the wild composition are presented in Table 2. Thirteen of the accessions lacking Wx-B1 protein did not show the corresponding Wx-B1 band as did cv. Kanto 107 (Fig. 2, lane 8), a positive control for the Wx-B1b allele (Table 2). However, five of the accessions showed a PCR product corresponding to Wx-B1. The accession PI 442911 showed a band of the same apparent size as for Chinese Spring (Fig. 2, lane 3), and accessions CItr 4923, PI 272580, PI 330556 and PI 352499 showed a Wx-B1 band with less electrophoretic mobility (Fig. 2, lane 5).
PCR analysis using primers BDFL/BRD from Nakamura et al. (2002). Lanes are as follows: 1 PI 422411, 2 PI 357307 (lack of Wx-B1), 3 PI 442911 (lack of Wx-B1), 4 cv. Chinese Spring, 5 PI 272580 (lack of Wx-B1), 6 PI 272581 (lack of Wx-B1), 7 PI 278650, and 8 Kanto 107
Wx-B1 sequences analysis
The Wx-B1 genes of accessions that showed a PCR product different from the Wx-B1b allele were cloned and sequenced to identify the reason for the absence of the Wx-B1 protein. The sequences obtained were compared to the Wx-B1a allele of Chinese Spring. The four accessions of Indian dwarf wheat showed the same mutation, carrying the insertion of four nucleotides (GGTA) in the seventh exon at position 1437 from the start codon (Fig. 3). This insertion caused a frameshift mutation that generated a stop codon (TGA) within the ninth exon, which would make a truncated protein. Comparing this sequence with others available in NCBI GenBank by BLAST revealed that this mutation was novel and, therefore, the allele was provisionally named Wx-B1k (NCBI ID: KP726909), according to international nomenclature (McIntosh et al. 2013).
In the case of accession PI 442911 (club wheat), its Wx-B1 sequence (NCBI ID: KP726910) showed a deletion of four nucleotides (AACA) in the second exon (position 157 from the start codon) compared to the Wx-B1a allele (Fig. 4). This deletion caused a frame shift in the open reading frame (ORF) that translated in a stop codon 69 bp downstream. This mutation was also novel and provisionally catalogued as Wx-B1m.
Development of a molecular marker to detect Wx-B1m allele
Because the variation of the novel Wx-B1m allele was detected in the beginning of the gene, the sequence between positions −31 and 466 was amplified by the use of the Wx-1Fw primer together with a new reverse primer (Wx1.3Rv, Table 1). Three products of 492 bp (Wx-B1a), 480 bp (Wx-D1a) and 472 bp (Wx-A1a) were expected to be obtained in Chinese Spring by PCR with this primer pair. The PCR product was run in a polyacrylamide gel (Fig. 5, lanes 1-3). Chinese Spring (lane 1) showed only two bands, indicating that probably two of the Wx-1 products (Wx-A1 and Wx-D1) were co-migrating. This is in consonance with the profile of Kanto 107 (lane 3), which lacked the whole of Wx-B1 gene, and its Wx-A1b gene is 23 nucleotides smaller than the Wx-A1a allele of Chinese Spring (Vrinten et al. 1999). In any case, the co-migration of both bands was confirmed by digesting the PCR product with the DdeI endonuclease, which had target sequences only in the Wx-A1 product (lanes 4–6). The PCR digestion confirmed that the lower band of Chinese Spring (lane 1) and PI 442911 (lane 2) was composed by Wx-A1 and Wx-D1 products. Therefore, the upper band corresponded to Wx-B1, which presented two variants in addition to those of Kanto 107. In genotype PI 442911 (Wx-B1m), the Wx-B1 band showed higher mobility than those of Chinese Spring (Wx-B1a), due to the deletion of four nucleotides as described above. Consequently, the use of this PCR assay permitted discrimination among Wx-B1a, -B1b and -B1m alleles.
Molecular marker to detect Wx-B1m allele. In lanes 1–3 PCR products resulted from the amplification with primers Wx1-Fw and Wx1.3Rv. In lanes 3–6 the amplification products are digested with endonuclease DdeI, which has target sequences in Wx-A1 amplicon. Lanes are as follows: 1 and 4 cv. Chinese Spring (Wx-B1a), 2 and 5 PI 442911 (Wx-B1m), and 3 and 6 cv. Kanto 107 (Wx-B1b)
The use of the primers designed by Nakamura et al. (2002) permits a rapid identification of the wheat lines that carry the Wx-B1k allele. The lines carrying this allele showed a Wx-B1 band with reduced mobility (Fig. 2) compared to Wx-B1a due to the insertion of four nucleotides as described previously.
Discussion
The role of the null Wx alleles in the amylose content of wheat flour has been widely investigated in different studies; genotypes with the null allele for Wx-B1 showed a greater decrease in amylose content compared to those with Wx-A1 or Wx-D1 nulls (Araki et al. 2000; Miura and Sugawara 1996; Yamamori and Quynh 2000). The detection of all null genotypes for the three Wx genes in modern wheat has been unsuccessful, with most detected genotypes null for Wx-A1 or Wx-B1 and in some rare occasions null for Wx-D1 and both Wx-A1 and Wx-B1. The available waxy wheat lines are products of crosses between partial-waxy genotypes in modern breeding. For bread wheat (Nakamura et al. 1995), the first successful cross was between two partial-waxy cultivars, Kanto 107 (Wx-A1b, Wx-B1b and Wx-D1a) and Bai Huo (Wx-A1a, Wx-B1a and Wx-D1b).
Other studies have since shown the existence of null Wx alleles derived from other genetic events (Guzmán et al. 2015; Saito and Nakamura 2005; Vanzetti et al. 2010). In the current study, two novel null alleles for Wx-B1 were detected in a collection of two hexaploid wheat species, which enlarges the availability of null Wx alleles for wheat breeders. Paradoxically, some of these genetic materials were analyzed by Li et al. (2013) showed differences to the variation detected in the current study.
Based exclusively on the use of PCR molecular markers, Li et al. (2013) reported the presence of numerous genotypes with null Wx alleles in macha wheat due to the absence of amplified PCR products. However, our data, based on the combined use of protein and DNA analysis, showed conflicting results with that of Li et al. (2013). These authors found in macha wheat two accessions (PI 361862 and PI 572911) with null alleles for the three waxy loci (Wx-A1, -B1 and -D1), one accession (PI 572913) with null alleles for Wx-B1 and -D1 and another one (PI 572910) with null alleles for Wx-A1 and -D1. Additionally, two more macha accessions (PI 572906 and PI 290507) were described in their study as null for Wx-D1 or for Wx-B1, respectively. It is important to mention that before the study of Li et al. (2013), no wheat accession with null alleles for all three Wx genes had been reported. This kind of wheat (waxy wheat, 0 % amylose) has only been generated in breeding programs, as mentioned above (Nakamura et al. 1995; Yasui et al. 1997; Zhao et al. 1998). Besides, the null allele for Wx-D1 is extremely rare. Yamamori et al. (1994) found one line lacking Wx-D1 protein in a collection of 1960 cultivars of different geographical origins (frequency of 0.05 %). Guzmán et al. (2010) also identified one accession lacking Wx-D1 protein in a collection of 420 spelt lines (0.23 %). However, Li et al. (2013) described five macha accessions having the Wx-D1 null allele in a total of 23 accessions analyzed (21.73 %). In the current study, all the macha wheat accessions described by Li et al. (2013) were analyzed by SDS-PAGE. In contrast to their results, no polymorphism was found and all accessions showed three waxy proteins. The screening of the same collection with BDFL and BRD primers also confirmed the absence of null alleles (data not shown). The reason to explain why Li et al. (2013) obtained different results from ours is not known, but probably is due to false negatives that occurred in their PCR analysis, which led to misclassification.
A contrasting discrepancy was observed in the analyzed accessions of Indian dwarf wheat. In this study, some accessions (PI 282451, PI 282452, PI 324492 and PI 352498) were found to carry the Wx-B1b null allele according to protein electrophoresis as well as PCR using the BDFL and BRD primers. These results were in agreement with those of Li et al. (2013); however, they described PI 272580, PI 330556 and PI 352499 as having the wild allele for Wx-B1. We demonstrated here that these accessions had a novel Wx-B1k null allele by molecular characterization of the Wx-B1 gene. The molecular characterization was carried out because the protein electrophoresis analysis (SDS-PAGE and 2D) revealed that these accessions lacked the Wx-B1 protein and this was not in agreement with the result obtained with the BDFL and BRD primers. This fact strengthens the idea that for appropriate evaluation of waxy protein variability, both protein and DNA analysis (PCR and/or sequencing) should be combined for waxy protein/gene alleles to avoid misclassification due to failures or inconclusive results with only one method (Ortega et al. 2015).
In our study, the analysis of a set of club wheat showed five accessions lacking Wx-B1 protein, four of which had the Wx-B1b null allele as detected with BDFL and BRD primers. The other accession had a novel allele (Wx-B1m), characterized by the deletion of four nucleotides in the second exon. As for Wx-B1k, this produced a frameshift in the ORF (i.e. premature appearance of a stop codon) which would result in absence of the protein. This kind of frameshift mutation leading to lack of the protein has been described several times in Wx-A1 (Saito et al. 2004; Saito and Nakamura 2005; Vanzetti et al. 2010), but only once in Wx-B1 (Guzmán et al. 2015). This is important because the common null mutation for Wx-B1 (Wx-B1b) implies the deletion of the entire gene and the surrounding region (67 kb), in which other genes related to quality could be included (Saito et al. 2009). Additionally, Wx-B1 protein has been shown to have a greater impact on amylose synthesis compared to Wx-A1 and Wx-D1 (Miura and Sugawara 1996; Yamamori and Quynh 2000), so the detection of variability is important to provide more sources of variation for starch modification.
Currently, several null alleles have been described that differ from the first ones (b alleles) described by Vrinten et al. (1999). Most molecular markers developed to screen for Wx-1 gene variability (Li et al. 2013; McLauchlan et al. 2001; Nakamura et al. 2002) were designed to detect those first described b null alleles. The sole use of these markers could generate misclassification. In the case of the novel Wx-B1k allele, the BDFL and BRD primers could be used to detect it in breeding programs, due to the slightly reduced mobility of its amplicon compared with the wild allele Wx-B1a. We have designed a molecular marker for the rapid detection of the novel Wx-B1m allele in breeding programs.
In conclusion, this study demonstrated the importance of combining both protein and molecular characterization analysis for appropriate analysis of variability of waxy proteins. Several accessions lacking Wx-B1 protein were detected, with some caused by the common Wx-B1b null allele. Two novel Wx-B1 null alleles were identified in Indian dwarf and club wheat, which could be used to enlarge the genetic variability for this gene. The differential effects of these novel null alleles in wheat quality compared to Wx-B1b need to be studied in further research.
Author contribution statement
JBA and CG conceived and designed the study. MA performed the experiments. MY performed the 2-D PAGE analysis. All authors analyzed the data and wrote the paper. All them have read and approved the final manuscript.
References
Araki E, Miura H, Sawada S (2000) Differential effects of the null alleles at the three Wx loci on the starch-pasting properties of wheat. Theor Appl Genet 100:1113–1120
Badridze G, Lidner A, Asch F, Börner A (2009) Variation in salt tolerance within a Georgian wheat germplasm collection. Genet Resour Crop Evol 56:1125–1130
Chen SS, Chen GY, Chen H, Li YM, Liu YX, Liu DC, Lan XJ, Zheng YL (2012) Mapping stripe rust resistance gene YrSph derived from Triticum sphaerococcum Perc. with SSR, SRAP, and TRAP markers. Euphytica 185:19–26
Echt CS, Schwartz D (1981) Evidence for the inclusion of controlling elements within the structural gene at the waxy locus in maize. Genetics 99:275–284
Filatenko AA, Hammer K (2014) Wheat landraces from Oman: a botanical analysis. Emir J Food Agric 26:119–136
Giambanelli E, Ferioli F, Koçaoglu B, Jorjadze M, Alexieva I, Darbinyan N, D’Antuono LF (2013) A comparative study of bioactive compounds in primitive wheat populations from Italy, Turkey, Georgia, Bulgaria and Armenia. J Sci Food Agric 93:3490–3501
Graybosch RA (1998) Waxy wheats: origin, properties, and prospects. Trends Food Sci Technol 9:135–142
Guzmán C, Alvarez JB (2012) Molecular characterization of a novel waxy allele (Wx-A u 1a) from Triticum urartu Thum. ex Gandil. Genet Resour Crop Evol 59:971–979
Guzmán C, Caballero L, Moral A, Alvarez JB (2010) Genetic variation for waxy proteins and amylose content in Spanish spelt wheat (Triticum spelta L.). Genet Resour Crop Evol 57:721–725
Guzmán C, Ortega R, Yamamori M, Peña RJ, Alvarez JB (2015) Molecular characterization of two novel null waxy alleles in Mexican bread wheat landraces. J Cereal Sci 62:8–14
Hayakawa K, Tanaka K, Nakamura T, Endo S, Hoshino T (2004) End use quality of waxy wheat flour in various grain-based foods. Cereal Chem 81:666–672
Hosono S (1954) The classification and the distribution of wheat. In: Kihara H (ed) Study of wheat. Yokendo, Tokyo, pp 5–132
James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6:215–222
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li W, Liu A-J, Sheng Y-Z, Cheng G-Y, Pu Z-E, Liu Y-X, Kong L (2013) Genetic diversity of null alleles of waxy gene in Triticum L. J Plant Sci 8:15–23
Martin JM, Sherman JD, Lanning SP, Talbert LE, Giroux MJ (2008) Effect of variation in amylose content and puroindoline composition on bread quality in a hard spring wheat population. Cereal Chem 85:266–269
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC (2013) Catalogue of gene symbols for wheat. http://www.shigen.nig.ac.jp/wheat/komugi/genes/macgene/2013/GeneSymbol.pdf. Accessed 13 Apr 2015
McLauchlan A, Ogbonnaya FC, Hollingsworth B, Carter M, Gale KR, Henry RJ, Holton TA, Morell MK, Rampling LR, Sharp PJ, Shariflou MR, Jones MGK, Appels R (2001) Development of robust PCR-based DNA markers for each homoeo-allele of granule-bound starch synthase and their application in wheat breeding programs. Aust J Agric Res 52:1409–1416
Miura H, Sugawara A (1996) Dosage effects of the three Wx genes on amylose synthesis in wheat endosperm. Theor Appl Genet 93:1066–1070
Miura H, Tanii S (1994) Endosperm starch properties in several wheat cultivars preferred for Japanese noodles. Euphytica 72:171–175
Morell MK, Rahman S, Regina A, Appels R, Li Z (2001) Wheat starch biosynthesis. Euphytica 119:55–58
Nakamura T, Yamamori M, Hirano H, Hidaka S (1993) Identification of three Wx proteins in wheat (Triticum aestivum L.). Biochem Genet 31:75–86
Nakamura T, Yamamori M, Hirano H, Hidaka S, Nagamine T (1995) Production of waxy (amylose-free) wheats. Mol Gen Genet 248:253–259
Nakamura T, Vrinten P, Saito M, Konda M (2002) Rapid classification of partial waxy wheats using PCR-based markers. Genome 45:1150–1156
Nakamura T, Shimbata T, Vrinten P, Saito M, Yonemaru J, Seto Y, Yasuda H, Takahama M (2006) Sweet wheat. Genes Genet Syst 81:361–365
Ortega R, Guzmán C, Alvarez JB (2015) Molecular characterization of several Wx alleles in durum wheat. Biol Plant 59:220–226
Park CS, Baik BK (2007) Characteristics of French bread baked from wheat flours of reduced starch amylose content. Cereal Chem 84:437–442
Rahman S, Li Z, Batey I, Cochrane MP, Appels R, Morell M (2000) Genetic alteration of starch functionality in wheat. J Cereal Sci 31:91–110
Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T, Kosar-Hashemi B, Li Z, Sadequr R, Morell M (2006) High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci USA 103:3546–3551
Saito M, Nakamura T (2005) Two point mutations identified in emmer wheat generate null Wx-A1 alleles. Theor Appl Genet 110:276–282
Saito M, Konda M, Vrinten P, Nakamura K, Nakamura T (2004) Molecular comparison of waxy null alleles in common wheat and identification of a unique null allele. Theor Appl Genet 108:1205–1211
Saito M, Vrinten P, Ishikawa G, Graybosch R, Nakamura T (2009) A novel codominant marker for selection of the null Wx-B1 allele in wheat breeding programs. Mol Breed 23:209–217
Stacey J, Isaac P (1994) Isolation of DNA from plants. In: Isaac PG (ed) Methods in molecular biology: protocols for nucleic acid analysis by non-radioactive probes. Humana Press, Totawa, pp 9–15
Vanzetti LS, Pfluger L, Bainotti CT, Jensen C, Helguera M (2010) Identification of a null allele at the Wx-A1 locus in durum wheat (Triticum turgidum L. ssp durum Desf.). Plant Breed 129:718–720
Vrinten P, Nakamura T, Yamamori M (1999) Molecular characterization of waxy mutations in wheat. Mol Gen Genet 261:463–471
Yamamori M (2009) Amylose content and starch properties generated by five variant Wx alleles for granule-bound starch synthase in common wheat (Triticum aestivum L.). Euphytica 165:607–614
Yamamori M, Endo T (1996) Variation of starch granule proteins and chromosome mapping of their coding genes in common wheat. Theor Appl Genet 93:275–281
Yamamori M, Quynh NT (2000) Differential effects of Wx-A1,-B1 and-D1 protein deficiencies on apparent amylose content and starch pasting properties in common wheat. Theor Appl Genet 100:32–38
Yamamori M, Yamamoto K (2011) Effects of two novel Wx-A1 alleles of common wheat (Triticum aestivum L.) on amylose and starch properties. J Cereal Sci 54:229–235
Yamamori M, Nakamura T, Endo TR, Nagamine T (1994) Waxy protein deficiency and chromosomal location of coding genes in common wheat. Theor Appl Genet 89–89:179–184
Yamamori M, Nakamura T, Nagamine T (1995) Polymorphism of two waxy proteins in the emmer group of tetraploid wheat, Triticum dicoccoides, T. dicoccum, and T. durum. Plant Breed 114:215–218
Yamamori M, Kato M, Yui M, Kawasaki M (2006) Resistant starch and starch pasting properties of a starch synthase IIa-deficient wheat with apparent high amylose. Aust J Agric Res 57:531–535
Yasui T, Sasaki T, Matsuki J, Yamamori M (1997) Waxy endosperm mutants of bread wheat (Triticum aestivum L) and their starch properties. Breed Sci 47:161–163
Zeng M, Morris CF, Batey IL, Wrigley CW (1997) Sources of variation for starch gelatinization, pasting, and gelation properties in wheat. Cereal Chem 74:63–71
Zhao XC, Batey IL, Sharp PJ, Crosbie G, Barclay I, Wilson R, Morell MK, Appels R (1998) A single genetic locus associated with starch granule properties and noodle quality in wheat. J Cereal Sci 27:7–13
Zwer PK, Sombrero A, Rickman RW, Klepper B (1995) Club and common wheat yield component and spike development in the Pacific Northwest. Crop Sci 35:1590–1597
Acknowledgments
This research was supported by grants AGL2010-19643-C02-01 and AGL2014-52445-R from the Spanish Ministry of Economy and Competitiveness, co-financed with European Regional Development Fund (FEDER) from the European Union. We thank the National Small Grain Collection (Aberdeen, USA) for supplying the analysed material. M. Ayala thanks for the pre-doctoral scholarship program ITAIPU Binacional-Paraguay (Paraguay Government).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by E. Lagudah.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayala, M., Alvarez, J.B., Yamamori, M. et al. Molecular characterization of waxy alleles in three subspecies of hexaploid wheat and identification of two novel Wx-B1 alleles. Theor Appl Genet 128, 2427–2435 (2015). https://doi.org/10.1007/s00122-015-2597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2597-7