Abstract
In common and durum wheats (Triticum aestivum L. and T. durum Desf.), variant waxy (Wx) alleles have been reported for three Wx proteins (Wx-A1, -B1 and -D1), responsible for amylose synthesis in flour starch. Five variant alleles, Wx-A1c, -A1e, -B1c, -B1d and -D1c, were examined to elucidate their effects on amylose content in flour starch. Common wheat lines carrying a Wx protein produced by one variant (e.g., Wx-A1c) and one control (e.g., Wx-A1a) allele were bred and their starches were compared. Results showed that Wx-A1e did not produce amylose (waxy phenotype), whereas three alleles (Wx-A1c, -B1c and -B1d) reduced amylose, and -D1c might have increased it slightly. Most data on blue value, swelling power and starch paste clarity in water and dimethyl sulphoxide also suggested the variant Wx alleles either reduced or increased amylose content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flour starch of hexaploid wheat (Triticum aestivum L.) usually consists of about 25% amylose (essentially a linear glucose polymer) and about 75% amylopectin (branched glucose polymer). The amylose percentage in starch of wheat flour affects the physicochemical properties of flour or its end-products. For example, compared to normal amylose level, low amylose starch may have higher swelling power (SP), lower set back and final viscosity in a Rapid Visco Analyzer (Sasaki and Matsuki 1998; Yamamori and Quynh 2000; Wickramasinghe et al. 2003), produce a softer gel (Ishida et al. 2003), and/or affect the eating quality of noodles (Oda et al. 1980). Thus, amylose content in wheat flour has been of interest for researchers of flour quality, food products, and breeding (Seib 2000).
Cereal starch is produced by the coordinated activities of starch synthase, starch branching enzyme, debranching enzyme, and other enzymes (Rahman et al. 2000). Waxy (Wx) protein is a (starch) granule-bound starch synthase I responsible for amylose synthesis. Because hexaploid common wheat consists of AABBDD genomes, it has three homoeologous Wx genes, i.e. Wx-A1, Wx-B1 and Wx-D1. The gene Wx-A1 belongs to the A genome, -B1 to the B genome, and -D1 to the D genome (Chao et al. 1989).
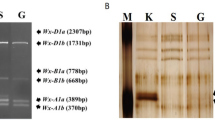
Analyses of the wheat Wx protein by two-dimensional gel electrophoresis showed protein polymorphism on isoelectric points or molecular weight; that is, the Wx protein was separated into the Wx-A1, -B1 and -D1 proteins (Nakamura et al. 1993). The Wx-A1a, -B1a and -D1a alleles are wild types present in the cultivar Chinese Spring, whereas b alleles are null types producing no Wx protein (Nakamura et al. 1993).
Gel electrophoresis using world wheat cultivars has found further variant Wx proteins or alleles; for example, Wx-A1c, -B1c and -D1c are in hexaploid common wheat (Yamamori et al. 1994), and Wx-A1d, -A1e and -B1d occur in tetraploid durum wheat (Yamamori et al. 1995). Other variant Wx alleles have been reported (Kiribuchi-Otobe et al. 1998; Rodríguez-Quijano et al. 1998; Nieto-Taladriz and Rodrígues-Quijano 2000; Marcoz-Ragot et al. 2000; Yasui 2004).
Variant Wx proteins may yield different amounts of amylose if they alter the ability to produce amylose. In fact, mutant alleles Wx-D1f(e) and g, which were generated by chemical mutagenesis, produced mutant Wx-D1 protein and lower amylose content than the wild allele Wx-D1a (Kiribuchi-Otobe et al. 1998; Yanagisawa et al. 2001; Yasui 2004). In this report, different types of wheat, with different amylose contents, were used to examine starch properties in order to determine which traits are usable for distinguishing them. In addition, the main purpose of the study was to describe the effects of the variant alleles Wx-A1c, -A1e, -B1c, -B1d, and -D1c (Yamamori et al. 1994, 1995) on amylose and starch.
Materials and methods
Wheat materials
For flour starch characterization, five types of wheat were used (Yamamori and Quynh 2000; Yamamori et al. 2006), viz. waxy (amylose-free) wheat (abbreviation wx) with no Wx protein, wheat with Wx-A1 protein (WxA), wheat with Wx-A1 and -D1 (WxAD) and wheat with Wx-A1, -B1 and -D1 (wild type, WxABD). High amylose wheat (HA) has Wx-A1, -B1 and -D1 but lacks another starch synthase (starch synthase IIa or SGP-1). Owing to the loss of SGP-1, starch of HA wheat appears to have a high content of amylose (Yamamori et al. 2006).
The five variant Wx alleles were Wx-A1c, -A1e, -B1c -B1d and -D1c (Yamamori et al. 1994, 1995) and seeds used were F7 or F8 generations. These were harvested in 2000 or 2001. Wx-A1c was derived from five common wheat cultivars (Pakistan Zairaishu WB 357, Pakistan Zairaishu WB 6, Pakistan Zairaishu (49P 70–27), Pakistan Zairaishu QT 105, Pakistan Zairaishu WB 27), Wx-B1c from four cultivars (Chousen 40, Junbuk 12, Cikotaba, AF 24), Wx-D1c from one cultivar (Scoutland), Wx-A1e from Triticum durum (KU 3659) and Wx-B1d was derived from T. durum (KU 4213D).
After crossing between the above wheats and a waxy wheat (which had no Wx proteins), lines with only one Wx protein produced by the variant Wx allele were selected. The presence or absence of Wx proteins was determined by one- or two- dimensional SDS-gel electrophoresis (Yamamori and Quynh 2000). Because Wx-A1e and -B1d were derived from durums (2n = 28), hexaploid (2n = 42) progenies were selected.
For control samples of variant Wx alleles, wheats with one Wx protein produced by either of Wx-A1a, Wx-B1e and Wx-D1a were used (Yamamori and Quynh 2000). The alleles Wx-A1a and Wx-B1e were derived from the cultivar Bai Huo, whereas Wx-D1a was derived from Kanto 107.
Starch isolation
Hammer-crushed grains were homogenized in a solution consisting of 2% sodium dodecyl sulfate (SDS) and 10% glycerol. Homogenates were passed through a 100 μm nylon mesh and centrifuged. A yellowish layer was removed with a spatula, and then mixed with the SDS solution. This was repeated twice, then the pellet was washed with distilled water twice, and twice with acetone. The resulting starch was used for measuring amylose, blue value (BV) and maximum absorbance (λmax).
Water-washed starches (Yamamori et al. 2006) were used for the other analyses, viz. swelling power (SP), glucoamylase digestibility, starch paste clarity, and freeze-thaw stability.
Amylose content
Amylose was measured using an amylose/amylopectin assay kit (Megazyme Int. Ireland, Ireland) by the concanavalin A (Con A) method according to the manufacturer’s protocol.
Swelling power (SP)
SP of water-washed starches was examined as per Yamamori et al. (2006). Starches (160 mg) were weighed in a 10 ml test tube, then 5 or 6.5 ml of 0.1% AgNO3 was added. Capped test tubes were incubated at 70°C in a water bath for 10 min with shaking, then incubated in boiling water for 10 min. After cooling, tubes were centrifuged at 2,100×g for 10 min and the supernatant was removed. SP was measured as sediment weight divided by dry sample weight (g/g).
SP using 40 mg starch followed Konik-Rose et al. (2001) with a slight modification: Instead of 1.0 ml of distilled water, 1.5 ml of 0.1% AgNO3 was used.
Glucoamylase digestibility
The procedure followed the methods of Wickramasinghe et al. (2003) with slight modifications. Starch (20 mg) was suspended in 500 μl of distilled water with 250 μl of 0.1 M acetate buffer (pH 5.0) and 250 μl of glucoamylase (5 units). After shaking at 40°C for 4 h, 2 ml of ethanol and 10 ml of distilled water were added and centrifuged. Sugars of the supernatant (0.5 ml) were measured; 1 ml of 3–5 dinitrosalicylic acid reagent was added, and the mixture was soaked in a boiling water bath for 5 min, then 4.5 ml of distilled water was added. Absorbance at 535 nm was then measured. The value was expressed as mg of maltose released per gram of starch.
Starch paste clarity
The paste clarity of starch in water was determined according to the method of Craig et al. (1989). Starch (50 mg, db) was suspended in 5 ml of distilled water in a capped glass tube, and the tube was soaked in a 95°C water bath for 30 min with shaking every 5 min. After cooling to room temperature, paste clarity (percent transmittance (%T) at 650 nm) was measured by spectrophotometer (U-200A, Hitachi, Japan) against a water blank. It was also measured after being stored at 4°C for 1, 3 and 7 days.
The paste clarity of starch in dimethyl sulphoxide (dehydrated DMSO containing max. 0.005% water, Wako Co., Japan) was determined according to the method of Singh et al. (2006). Starch (50 mg, db) was suspended in 10 ml of dehydrated DMSO, and the tube was shaken at 23°C. Paste clarity (%T at 640 nm) was measured against a DMSO blank after 4, 8, 16, 24, 36, 48 and 60 h.
Freeze-thaw stability of starch gel
The freeze-thaw stability of starch was examined according to the method of Zheng and Sosulski (1998) with slight modifications. Starch paste was prepared using a Rapid Visco Analyzer (RVA) (Newport Scientific, Australia); 1 g (dry basis) of water-washed starch in 12.5 ml of distilled water was subjected to the RVA (Yamamori et al. 2006). Using about 2.0 g of cooled fresh gel, the amount of water (free water) separating from the gel after centrifugation for 10 min at 4,500×g was measured. Using cooked gel in a plastic 50 ml test tube, the amounts of expelled and absorbed water were determined through three freeze-thaw cycles (freezing at −20°C for 16 h, thawing at 40°C for 2 h) over 76 h. The amount of expelled water was measured as separated water by decanting the thawed gel. After removing the expelled water, the absorbed water was measured as separated water by centrifuging about 2.0 g of gel at 4,500×g. Net syneresis (%) was calculated using the formula: expelled water + absorbed water − free water (%).
Apparent blue value (BV) and maximum absorbance (λmax)
The BV and λmax were measured as described by Yamamori et al. (2006). Absorption curves of gelatinized starch-iodine complexes were measured at 500–700 nm to determine the absorbance at 680 nm (apparent BV) and λmax of the iodine-starch complex.
Results and discussion
Starch characterization of wheats with five different amylose levels
Amylose content in starches of the five wheats (wx, WxA, WxAD, WxABD and HA) are shown in Table 1. Statistical analyses showed they produced different levels of amylose. Starch SP was distinguishable among the five wheats though SP of wx was not determined because the wx starch did not form a rigid gel (Table 1); amylose content was negatively correlated with the value of SP.
Glucoamylase treatment produced more maltose in the order of WxABD, WxAD < WxA < HA < wx (Table 1). Except for HA, amylose content tended to be negatively correlated with maltose release. These results corroborated those of previous studies (Sasaki and Matsuki 1998; Yamamori and Quynh 2000; Wickramasinghe et al. 2003). In the following analyses of wheats having variant Wx alleles, amylose and SP were examined.
Paste clarity in water varied among starches of the five wheats; wheat with higher amylose content showed a lower %T at 650 nm (Fig. 1). After boiling, the %T of WxA, WxAD, WxABD and HA decreased as days passed while that of wx was comparatively stable and high. The %T of five wheat starches differed from each other within 1 day. On the other hand, paste clarity in DMSO increased over time (Fig. 1). After 36–60 h, WxABD, WxAD, WxA and wx were distinguishable while the HA starch showed a unique trend. In the following analyses of the variant Wx alleles, paste clarities in both water and DMSO were examined.
The absorbed water of wx wheat starch was not measured because the starch gel fell to the bottom of the tube after centrifugation. Thus, net syneresis of wx was not estimated. Net syneresis of starches of the other four wheats rose as their amylose content increased (Fig. 2). After two or three freeze-thaw cycles, the differences among the starches became more distinct.
Free water and net syneresis in freeze-thaw stability of starch gels. For each wheat, four lines were used and values were derived from four measurements (one measurement per sample). Columns with the same subscript letter in the cycle are not significantly different (P < 0.05, Tukey’s studentized range test)
The values of SP, starch paste clarity in water and net syneresis of the five wheats seemed to be either negatively or positively related to amylose content. However, regarding glucoamylase digestibility and paste clarity in DMSO, the values for HA starch were closer to those of the wx starch than to those for WxABD. This uniqueness of the HA starch might be attributed to its altered structure of amylopectin which was caused not by the Wx protein but the starch synthase IIa (Yamamori et al. 2000; Hanashiro et al. 2004).
Wx-A1c and -A1e (variant alleles of Wx-A1)
On a two-dimensional electrophoretic gel, the variant Wx-A1 protein coded by Wx-A1c showed slightly lower molecular weight (higher mobility in SDS-gel electrophoresis) and a slightly more basic isoelectric point than that coded by the wild allele Wx-A1a (Yamamori et al. 1994). Amylose percentages in starch produced by Wx-A1c which was derived from five Pakistani cultivars ranged from 12.3 to 14.8% in 2000 and 9.5 to 12.4% in 2001 whereas Wx-A1a was 23.2% in 2000 and 17.6% in 2001 (Table 2). The observation that amylose in 2001 was lower than in 2000 might be attributable to experimental error. However, the results suggest that Wx-A1c yielded about 60% of the amylose level generated by Wx-A1a.
Both the BV (0.214–0.228) and λmax (555–557 nm) of the starch-iodine complex from Wx-A1c were significantly lower than those of Wx-A1a (0.286 and 569 nm), suggesting there was less amylose in starch from the Wx-A1c genotype compared with the Wx-A1a genotype. The SP of 160 mg starch produced by Wx-A1c ranged from 30.3 to 32.6 g/g, statistically greater than that of Wx-A1a starch (28.0 g/g). The SP of 40 mg starch produced by Wx-A1c (20.4–22.1 g/g) was also higher than that of the Wx-A1a starch (18.2 g/g). Because wheat starches with lower amylose tend to increase SP (Sasaki and Matsuki 1998; Yamamori and Quynh 2000), these results mean that Wx-A1c generates less amylose in starch than the wild Wx-A1a.
Paste clarities of the Wx-A1c starch in water were significantly higher than those of Wx-A1a at 0, 1, 3 and 7 days after treatment (Table 3). Furthermore, paste clarities of the Wx-A1c starch in DMSO at 36, 48 and 60 h after treatment were also significantly higher than those of Wx-A1a starch. These results provide further evidence that less amylose is produced by Wx-A1c (40%) than by Wx-A1a.
The Wx-A1 protein from the variant Wx-A1e of T. durum showed a more basic isoelectric point on the electrophoretic gel (Yamamori et al. 1995). Starch from Wx-A1e showed 0.5% amylose which was the same as waxy starch from the null allele Wx-A1b, and the BV of the Wx-A1e starch (0.084) was almost the same as that of Wx-A1b (0.086) (Table 2). Although λmax of Wx-A1e starch (534 nm) was greater than that of Wx-A1b (529 nm), it was lower than Wx-A1a starch (569 nm). The flour starches and pollen grains of Wx-A1e stained by a KI-I2 solution turned red-brown like those of waxy wheat, showing a waxy (amylose-free) phenotype. These results mean that Wx-A1e produces the Wx-A1 protein but it does not produce amylose, presumably due to the inability to synthesize amylose.
This kind of alteration was reported for the allele Wx-D1f(e); in the mutant Tanikei A6599-4, Wx-D1f produced the Wx-D1 protein, but showed much less amylose or waxy phenotype (1.6% amylose of Wx-D1f was slightly more than typical waxy (0.4%) (Kiribuchi-Otobe et al. 1998). The point mutation in Wx-D1f generated an alanine-to-threonine change and decreased enzymatic activity in the Wx-D1 protein (Yanagisawa et al. 2001, 2003).
Wx-B1c and -B1d (variant alleles of Wx-B1)
The Wx-B1 protein from the variant Wx-B1c showed a slightly more basic isoelectric point than the alleles Wx-B1a of Chinese Spring and Wx-B1e of Bai Huo (Yamamori et al. 1994; Yamamori and Quynh 2000). The amylose content of Wx-B1c from four cultivars was less than that of Wx-B1e in both years, except for AF 24 in 2000 (Table 2). On average, Wx-B1c produced about 85% of the amylose level of Wx-B1e.
The BV and λmax of Chousen 40 and Junbuku 12 were lower than those of Wx-B1e, but those of Cikotaba and AF 24 were not significantly different from Wx-B1e. In SP of 160 mg starch, Wx-B1c from AF 24 was not significantly different from Wx-B1e, whereas other starches from Wx-B1c genotypes were different. In 40 mg starch SP, Junbuku 12, Cikotaba, AF 24 and their mean were higher than Wx-B1e. These results suggest the amylose level in starch produced by Wx-B1c genotypes was somewhat less (15%) than those with Wx-B1e.
Though the starch paste clarity of Wx-B1c in water was greater than that of Wx-B1e, it was statistically different from Wx-B1e only at 1 day after treatment (Table 3). On the other hand, the starch paste clarity of Wx-B1c in DMSO was lower than that of Wx-B1e. Paste clarity in water seemed to provide supporting evidence that Wx-B1c produces less amylose than Wx-B1e, but paste clarity in DMSO did not.
The Wx-B1 protein from the variant Wx-B1d of T. durum showed a slightly higher basic isoelectric point and a lower molecular weight compared to wild Wx-B1a (Yamamori et al. 1995). Wx-B1d produced 21.0% amylose which was less than Wx-B1e (24.3%) (Table 2). Although the BV and λmax of Wx-B1d genotypes did not differ from those with Wx-B1e, the SP of 160 mg starch was significantly higher than that of Wx-B1e. The starch paste clarity of Wx-B1d in water was statistically higher than that of Wx-B1e on the 4 days examined, but its paste clarity in DMSO did not differ from that of Wx-B1e (Table 3). These results suggest that, like Wx-B1c, Wx-B1d also produced somewhat less amylose than Wx-B1e.
In this study, the allele Wx-B1e was used as a control for the variants Wx-B1c and -B1d, because the only available wheat possessing only the Wx-B1 protein was bred using Bai Huo carrying Wx-B1e (not -B1a) as a parent (Yamamori and Quynh 2000). The Wx protein from Wx-B1e showed a slightly higher molecular weight on an electrophoretic gel than that from Wx-B1a and the allele was reported to occur in 12–16% of common wheat cultivars (Marcoz-Ragot et al. 2000; Yamamori and Quynh 2000).
In durum wheat, amylose from the genotype Wx-A1a Wx-B1e(c′) appeared to be higher (by ~2.9%) than that from Wx-A1a Wx-B1a (Nieto-Taladriz and Rodrígues-Quijano 2000; Rodríguez-Quijano et al. 2003), suggesting that Wx-B1e produces slightly more amylose than Wx-B1a. However, Wx-B1e in Bai Huo has not been compared to Wx-B1a in a common wheat.
Wx-D1c (variant allele of Wx-D1)
The Wx-D1 protein from Wx-D1c showed a slightly more basic isoelectric point than that from Wx-D1a (Yamamori et al. 1994). The average amylose values of Wx-D1c from Scoutland in the two harvest years were a little higher than those of Wx-D1a, but statistical differences were not detected (Table 2). Compared to Wx-D1a, the BV of Wx-D1c starch showed a higher value, but it was not statistically significant. The λmax of Wx-D1c was greater than Wx-D1a, and the SP’s of 160 and 40 mg starches were smaller than Wx-D1a. The starch paste clarity of Wx-D1c in water was significantly lower than that of Wx-D1a at 3 and 7 days after treatment, but it was not lower on the other 2 days (days zero and one) (Table 3). The starch paste clarity of Wx-D1c in DMSO was lower than that of Wx-D1a at 36, 48 and 60 h after treatment. These results suggest that Wx-D1c may produce a little more amylose (by 1–2%) than Wx-D1a.
For Wx-D1, seven alleles (Wx-D1a to -D1 g) have been reported (Yasui 2006); Wx-D1a is the wild standard, -D1b, d, and e are null alleles whereas f and g produce less amylose than a.
The present analyses suggest that the amylose synthesizing ability of Wx alleles is Wx-A1b(null) = Wx-A1e < Wx-A1c < Wx-A1a < Wx-B1c ≑ Wx-B1d ≤ Wx-D1a < Wx-D1c ≦ Wx-B1e. In our previous study (Yamamori and Quynh 2000), amylose content in flour starch was related to the amount of Wx protein; the amounts of Wx protein and amylose increased in the order of Wx-A1a < -D1a < -B1e. The present study indicates that Wx proteins with altered pIs or molecular weights also influence amylose content. The findings of Wx allelic variation for amylose production will contribute to the genetic and precision control of amylose percentage in flour. In addition, this study has shown that wheat lines carrying one of three Wx proteins are useful for examining the effect of the Wx protein because the influences of the other two Wx proteins are excluded.
References
Chao S, Sharp PJ, Worland AJ, Warham EJ, Koebner RMD, Gale MD (1989) RFLP-based genetic maps of wheat homoeologous group 7 chromosomes. Theor Appl Genet 78:495–504. doi:10.1007/BF00290833
Craig SAS, Maningat CC, Seib PA, Hosney RC (1989) Starch paste clarity. Cereal Chem 66:173–182
Hanashiro I, Ikeda I, Yamamori M, Takeda Y (2004) Increased amylose content and altered molecular structures of wheat starch by SGP-1 null mutation. J Appl Glycosci 51:217–221
Ishida N, Miura H, Noda T, Yamauchi H (2003) Mechanical properties of white salted noodles from near-isogenic wheat lines with different wx protein-deficiency. Starch/Stärke 55:390–396
Kiribuchi-Otobe C, Yanagisawa T, Yamaguchi I, Yoshida H (1998) Wheat mutant with waxy starch showing stable hot paste viscosity. Cereal Chem 75:671–672. doi:10.1094/CCHEM.1998.75.5.671
Konik-Rose CM, Moss R, Rahman S, Appels R, Stoddard F, McMaster G (2001) Evaluation of the 40 mg swelling test for measuring starch functionality. Starch/Stärke 53:14–20
Marcoz-Ragot C, Gateau I, Koenig J, Delaire V, Branlard G (2000) Allelic variants of granule-bound starch synthase proteins in European bread wheat varieties. Plant Breed 119:305–309. doi:10.1046/j.1439-0523.2000.00510.x
Nakamura T, Yamamori M, Hirano H, Hidaka S (1993) Identification of three Wx proteins in wheat (Triticum aestivum L.). Biochem Genet 31:75–86. doi:10.1007/BF02399821
Nieto-Taladriz MT, Rodrígues-Quijano M (2000) Polymorphism of waxy proteins in Spanish durum wheats. Plant Breed 119:277–279. doi:10.1046/j.1439-0523.2000.00448.x
Oda M, Yasuda Y, Okazaki S, Yamauchi Y, Yokoyama Y (1980) A method of flour quality assessment for Japanese noodles. Cereal Chem 57:253–254
Rahman S, Li Z, Batey I, Cochrane MP, Appels R, Morell M (2000) Genetic alteration of starch functionality in wheat. J Cereal Sci 31:91–110. doi:10.1006/jcrs.1999.0291
Rodríguez-Quijano M, Mieto-Tlaldrizo MT, Carrillo JM (1998) Polymorphism of waxy proteins in Iberian hexaploid wheats. Plant Breed 117:341–344. doi:10.1111/j.1439-0523.1998.tb01951.x
Rodríguez-Quijano M, Lucas R, Carrillo JM (2003) Waxy proteins and amylose content in tetraploid wheats Triticum dicoccum Schulb., Triticum durum L. and Triticum polonicum L. Euphytica 134:97–101. doi:10.1023/A:1026157713582
Sasaki T, Matsuki J (1998) Effect of wheat starch structure on swelling power. Cereal Chem 75:525–529. doi:10.1094/CCHEM.1998.75.4.525
Seib P (2000) Reduced-amylose wheats and Asian noodles. Cereal Foods World 45:504–512
Singh J, McCarthy OJ, Singh H (2006) Physico-chemical and morphological characteristics of New Zealand Taewa (Maori potato) starches. Carbohydr Polym 64:569–581. doi:10.1016/j.carbpol.2005.11.013
Wickramasinghe HAM, Miura H, Yamauchi H, Noda T (2003) Properties of starches from near-isogenic wheat lines with different Wx protein deficiencies. Cereal Chem 80:662–666. doi:10.1094/CCHEM.2003.80.6.662
Yamamori M, Quynh NT (2000) Differential effects of Wx-A1, -B1 and -D1 protein deficiencies on apparent amylose content and starch pasting properties in common wheat. Theor Appl Genet 100:32–38. doi:10.1007/s001220050005
Yamamori M, Nakamura T, Endo TR, Nagamine T (1994) Waxy protein deficiency and chromosomal location of coding genes in common wheat. Theor Appl Genet 89:179–184
Yamamori M, Nakamura T, Nagamine T (1995) Polymorphism of two waxy proteins in the emmer group of tetraploid wheat, Triticum dicoccoides, T. dicoccum, and T. durum. Plant Breed 114:215–218. doi:10.1111/j.1439-0523.1995.tb00796.x
Yamamori M, Fujita S, Hayakawa K, Matsuki J, Yasui T (2000) Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor Appl Genet 101:21–29. doi:10.1007/s001220051444
Yamamori M, Kato M, Yui M, Kawasaki M (2006) Resistant starch and starch pasting properties of a starch synthase IIa-deficient wheat with apparent high amylose. Aust J Agric Res 57:531–535. doi:10.1071/AR05176
Yanagisawa T, Kiribuchi-Otobe C, Yoshida H (2001) An alanine to threonine change in the Wx-D1 protein reduces GBSS I activity in waxy mutant wheat. Euphytica 121:209–214. doi:10.1023/A:1012051705517
Yanagisawa T, Kiribuchi-Otobe C, Hirano H, Suzuki Y, Fujita M (2003) Detection of single nucleotide polymorphism (SNP) controlling the waxy character in wheat by using a derived cleaved amplified polymorphic sequence (dCAPS) marker. Theor Appl Genet 107:84–88
Yasui T (2004) A new allele on the Wx-D1 locus causes an altered flour-pasting profile of the low-amylose bread wheat (Triticum asetivum L.) mutant, K107Afpp4. Breed Sci 54:281–286. doi:10.1270/jsbbs.54.281
Yasui T (2006) Waxy and low-amylose mutants of bread wheat (Triticum aestivum L.) and their starch, flour and grain properties. Jpn Agric Res Q 40:327–331
Zheng GH, Sosulski FW (1998) Determination of water separation from cooked starch and flour pastes after refrigeration and freeze-thaw. J Food Sci 63:134–139. doi:10.1111/j.1365-2621.1998.tb15693.x
Acknowledgements
The author thanks the Gene Bank of the National Institute of Agrobiological Sciences (Tsukuba) and the Plant Germplasm Institute of Kyoto University for providing wheat seeds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamori, M. Amylose content and starch properties generated by five variant Wx alleles for granule-bound starch synthase in common wheat (Triticum aestivum L.). Euphytica 165, 607–614 (2009). https://doi.org/10.1007/s10681-008-9793-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9793-3