Abstract
Key message
A new powdery mildew resistance gene Pm54 was identified on chromosome 6BL in soft red winter wheat.
Abstract
Powdery mildew is causing increasing damage to wheat production in the southeastern USA. To combat the disease, a continuing need exists to discover new genes for powdery mildew resistance and to incorporate those genes into breeding programs. Pioneer® variety 26R61 (shortened as 26R61) and AGS 2000 have been used as checks in the Uniform Southern Soft Red Winter Wheat Nursery for a decade, and both have provided good resistance across regions during that time. In the present study, a genetic analysis of mildew resistance was conducted on a RIL population developed from a cross of 26R61 and AGS 2000. Phenotypic evaluation was conducted in the field at Plains, GA, and Raleigh, NC, in 2012 and 2013, a total of four environments. Three quantitative trait loci (QTL) with major effect were consistently detected on wheat chromosomes 2BL, 4A and 6BL. The 2BL QTL contributed by 26R61 was different from Pm6, a widely used gene in the southeastern USA. The other two QTL were identified from AGS 2000. The 6BL QTL was subsequently characterized as a simple Mendelian factor when the population was inoculated with a single Blumeria graminis f. sp. tritici (Bgt) isolate in controlled environments. Since there is no known powdery mildew resistance gene (Pm) on this particular location of common wheat, the gene was designated Pm54. The closely linked marker Xbarc134 was highly polymorphic in a set of mildew differentials, indicating that the marker should be useful for pyramiding Pm54 with other Pm genes by marker-assisted selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Powdery mildew, caused by the obligate fungus Blumeria graminis f. sp tritici (Bgt), is one of the most damaging diseases of wheat (Triticum aestivum L.). The pathogen can attack all above-ground wheat parts including leaves, stems, and spikes. The disease commonly occurs at economically damaging levels in the southeastern USA, where the climate is maritime or semi-continental (Cowger et al. 2012). Yield losses from powdery mildew ranging from 17 to 34 % have been reported in these regions (Johnson et al. 1979; Leath and Bowen 1989). Although fungicides controlling the disease are available, deployment of resistant cultivars remains the most reliable, economical, and environmentally safe approach to control this disease (Bennett 1984).
To date, about 77 formally designated Pm genes have been cataloged at 49 loci (Pm1–Pm53, Pm18 = Pm1c, Pm22 = Pm1e, Pm23 = Pm4c, Pm31 = Pm21) with the loci Pm1, Pm3, Pm4, Pm5 and Pm24 having 5, 17, 4, 5 and 2 alleles, respectively (Hao et al. 2008; Hsam et al. 1998; McIntosh et al. 2013, 2014; Singrün et al. 2003; Xie et al. 2012a). In the soft red winter wheat (SRWW) growing region of the southeastern USA, cultivars having Pm3a, Pm8 and Pm17 are widely grown even though the effectiveness of these resistance genes is limited as virulent Bgt isolates are now common in the field (Cowger et al. 2009; Hao et al. 2012a). The gene Pm6 that was transferred to common wheat lines ‘CI 12632’ and ‘CI 12633’ from tetraploid T. timopheevii (Allard and Shands 1954; Jørgensen and Jensen 1973) has also been widely deployed in these areas. The cultivars ‘Arthur’, ‘Arthur 71’, ‘Abe’ and other Arthur-type wheat or their derivatives were all supposed to possess Pm6 or the combination Pm2 + Pm6 (Bennett 1984). The recessive gene Pm5a, originally derived from Yaroslav emmer via the hard spring wheat variety ‘Hope’ (Lebsock and Briggle 1974; McFadden 1930), was also common in the SRWW region, since Hope was used extensively as a parent in wheat breeding programs (Bennett 1984). In addition, cultivar ‘Roane’ was assumed to have inherited Pm4a from ‘IN65309C1-18-2-3-2’, a line developed by Purdue University (Griffey et al. 2001), and ‘NC-Neuse’ probably possessed Pm1a from CI 13868 (Murphy et al. 2004). The genes Pm3b, Pm3e, and Pm3f were also reported in SRWW based on amplification of Pm3 allele-specific functional markers (Tommasini et al. 2006), but in low frequencies compared with the more common Pm3a allele (Uniform Eastern and Southern Soft Red Winter Wheat Nursery Reports, http://www.ars.usda.gov/Main/docs.htm?docid=21894). Apart from the formally named genes used in these regions, quantitative trait loci (QTL) located on chromosomes 1BL, 2AL and 2BL were also reported in the SRWW cultivar ‘Massey’ and its derivative ‘USG 3209’ (Liu et al. 2001; Tucker et al. 2007).
Bgt isolates in the southeastern USA usually have the highest levels of genetic diversity in the country, including isolates present in the states of North Carolina (NC) and Georgia (GA) (Parks et al. 2008). Some Pm genes start to lose effectiveness even before commercial deployment. For example, little or no virulence to Pm12 was detected in this region in the early 1990s (Niewoehner and Leath 1998), but in 2005 a low frequency of Pm12 virulence was observed among NC isolates, an intermediate level among Virginia isolates and a high level among GA isolates (Parks et al. 2008). To the best of our knowledge, Pm12, derived from Aegilops speltoides (Jia et al. 1996), has never been used in commercial wheat cultivars. To meet the challenges of pathogen diversity and increasingly complex virulent Bgt isolates, there is an urgent need to discover new genes or QTL for Pm resistance and promptly incorporate them into breeding materials.
The SRWW cultivars, ‘AGS 2000’ and ‘Pioneer® variety 26R61’ (shortened as 26R61), were both released in 2000 and have been used as checks in the Uniform Southern Soft Red Winter Wheat Nursery (USSRWWN) for more than a decade. Both cultivars have generally exhibited good resistance to Bgt in the field across locations and years. In seedling tests conducted in 2001–2002, AGS 2000 was resistant or moderately resistant to 27 of 30 Bgt isolates, and 26R61 was resistant or moderately resistant to 28 of the same isolates (USSRWWN Reports, http://www.ars.usda.gov/Main/docs.htm?docid=21894). However, information on the genetic basis of powdery mildew resistance in the two cultivars is limited. Hao et al. (2012a) reported that both 26R61 and AGS 2000 possessed the 1BL.1RS translocation and therefore Pm8; AGS 2000 also has Pm3a. As Pm3a has lost its effectiveness in southeastern USA and Pm8 is becoming less effective in the states of GA and NC (Parks et al. 2008), other Pm genes must be conferring resistance in these cultivars. The primary objective of the present study was to determine the unknown factors for mildew resistance in the two cultivars using a RIL population with good genome coverage of molecular markers.
Materials and methods
Plant materials
Two SRWW cultivars, 26R61 (PI 612153) and AGS 2000 (PI 612956), were crossed and 178 F7:8 recombinant inbred lines (RILs) were developed by single-seed descent (Hao et al. 2011). Cultivar 26R61 (Omega 78/S76/Arthur 71/3/Stadler//Redcoat/Wisconsin 1/5/Coker 747/6/PIO2555 sib) was developed by Pioneer Hi-Bred, and AGS 2000 (PIO2555/PF84301//Florida 302) was developed and released jointly by the University of Georgia and University of Florida (Johnson et al. 2002). The population name was abbreviated as PR61/A2000. Three lines (42, 149 and 172) were omitted from mapping and QTL analysis due to high percentages of missing molecular data.
The cultivars Arthur, Arthur 71, Coker 747 and Coker 68-15 were included in the study. Coker 747 (Arthur/Coker 68-15) is a Pm6 differential line used in the USDA Powdery Mildew Differential Collection and Resistance Nursery at Raleigh, NC. Pm6 was presumably inherited from Arthur. Coker 68-15 was used as a negative check, and was assumed to have no Pm gene (Shi et al. 1998). Arthur 71 (thought to have Pm6) was a five-time backcross derivative of Arthur and a parent of the cultivar 26R61.
A set of Pm differentials including 39 formally named Pm genes, 5 temporarily named genes, and 2 susceptible checks (Chancellor and Coker 68-15) was also included to test the polymorphism of markers closely linked with the newly identified gene. All the germplasm are maintained by the USDA-ARS Plant Science Research Unit, North Carolina State University, Raleigh, NC, USA.
Evaluation of powdery mildew response in the field
The PR61/A2000 RILs and parents were evaluated for mildew response under natural infection in the field at Plains, GA, and Raleigh, NC, in 2011–2012 and 2012–2013, hereafter referred to as 2012 and 2013. Plants were grown in randomized complete blocks with two replications in Plains and three replications in Raleigh. Both parents were randomly interspersed with the population five to ten times each depending upon environment.
Powdery mildew response was assessed at the adult-plant stage when the most susceptible checks (Saluda or Chancellor) approached maximum disease severities. A numeric 0–9 scale was adopted based on disease severity (DS), where 0 indicated immunity, DS = 0; 1, 0 < DS ≤ 10 %; 2, 10 % < DS ≤ 20 %; 3, 20 % < DS ≤ 30 %; 4, 30 % < DS ≤ 40 %; 5, intermediate type, 40 % < DS < 60 %; 6, 60 % ≤ DS < 70 %; 7, 70 % ≤ DS < 80 %; 8, 80 % ≤ DS < 90 %; 9, full susceptibility, DS ≥ 90 %. This method provides a fast and repeatable way of scoring powdery mildew response (Bennett and Westcott 1982), and has been routinely used in regional screening nurseries. For each environment, mean response values over the replications were used for QTL analysis.
Single conidium isolation
In the spring of 2012, a severe powdery mildew epidemic occurred in the field at Plains, GA. Cultivar AGS 2000 was nearly immune to the disease and 26R61 was moderately resistant. Leaves with fresh conidiospores were collected from 26R61 on April 13, 2012, and cultured in a growth chamber maintained at 17 °C, relative humidity at least 70 %, and a 12:12 h (light:dark) photoperiod. Conidiospores were isolated three times from a single conidium according to the protocol described by Namuco et al. (1987) with minor modifications. Briefly, one pot of 26R61 covered with a plastic bag was placed in a Bgt-free growth chamber. The plants were inoculated at the three-leaf stage with the inoculum collected in the field. After about 10 days, when new conidiospores were visible, a small leaf segment with a single-colony conidium was cut out, and the conidiospores were shaken onto 26R61 seedlings in another pot and covered with a plastic bag. The isolation was repeated twice to increase the probability of avoiding an isolate mixture. The resulting single-conidial isolate, designated PL-12 (Plains in 2012), was avirulent to AGS 2000 and virulent to 26R61. It was increased and maintained on 26R61 seedlings and used to inoculate the entire PR61/A2000 population.
Evaluation of powdery mildew response in growth chamber
Inoculation of isolate PL-12 onto the RIL population was conducted in two separate growth chambers, with two replicates of the population in each chamber. About ten seeds of each line were planted in a 15 cm pot. The pots in each replicate were randomly distributed in the growth chamber. AGS 2000 and 26R61 were included at 20 pot intervals as resistant and susceptible controls, respectively. The growth chamber was programmed the same as the chamber used in the single conidium isolation. Plants were inoculated at the three-leaf stage by shaking conidiospores from susceptible 26R61 plants onto the test seedlings.
Reactions were scored 15–20 days after inoculation when the susceptible parent 26R61 was heavily infected, and then repeated once a week later. Five major classes of infection types (ITs) were categorized: 0 (resistant), no visible symptoms or a few flecks; 1 (moderately resistant), necrosis with low to medium sporulation; 2 (segregating), both resistant and susceptible plants observed; 3 (moderately susceptible), no necrosis with medium to high sporulation; and 4 (susceptible), no necrosis with full sporulation. In later mapping of the resistance gene, IT 0 and 1 classes were pooled as resistant; those with 3 and 4 as susceptible; and those with 2 as segregating.
Data analysis and QTL mapping
The SAS 9.1 statistical package was used for statistical analysis and output of histograms (SAS Institute, Cary, NC, USA). The genetic linkage maps used for QTL analysis were described by Hao et al. (2012b) with updates of QTL target regions in the present study. Two SNP markers from a 9 k iSelect Beadchip Assay were also added near the major QTL region on 6BL (Cavanagh et al. 2013). The maps included 972 loci on 24 linkage groups, with gaps for chromosomes 2A, 4D, and 7D, and spanned 2,757 cM, with 1,125, 916, and 716 cM in the A, B, and D genomes, respectively.
QTL detection was conducted in Windows QTL Cartographer 2.5 as follows: the composite interval mapping (CIM) method was used; walk speed was set as 1.0 cM and the control parameters were default; and the LOD (logarithm of odds) threshold was set as 3.0. To declare significance levels, LOD scores were calculated from 1,000 permutations for each trait at P = 0.05, 0.01 and/or 0.001, respectively (Wang et al. 2012). QTL designation referred to the guidelines for nomenclature of QTL in wheat (McIntosh et al. 2013). The function ‘effectplot’ in program R/qtl was used to create effect plots of phenotypes against genotypes at selected loci (Broman et al. 2003).
Molecular marker analysis
As a major QTL had been detected at a location near Pm6 on chromosome 2BL, to clarify the relationship of the two resistance sources, genotyping was conducted on 26R61 and AGS 2000 with the Pm6 diagnostic marker NAU/STS BCD135-2 (Ji et al. 2008).
In addition, the simple sequence repeat (SSR) marker Xbarc134, which was closely linked with another major QTL on chromosome 6BL, was used to genotype a set of differential lines. PCR was performed using a touchdown program described by Hao et al. (2008), and amplified products were separated in a 6 % (w/v) polyacrylamide gel using a Mega-Gel High Throughput Vertical Unit, following the procedure reported by Wang et al. (2003).
Results
Phenotypic analysis
The parent AGS 2000 was highly resistant in the field in Plains in 2012 with an average score of 0.05 on the 0–9 scale. The mean score of parent 26R61 was 1.90. The difference between the two values was highly significant (P < 0.001), but there was no significant difference in the other three environments (Raleigh 2012, Plains 2013 and Raleigh 2013) (Fig. 1). For RILs at the adult-plant stage, the rating data were continuous and the distribution deviated significantly from a normal distribution (P < 0.01) in all environments except in Raleigh 2012 (Fig. 1), suggesting that both major and minor mildew resistance QTL might be involved. Transgressive segregation (Fig. 1) implied that both parents possessed favorable additive allele(s).
For the powdery mildew resistance in the seedling stage, AGS 2000 was uniformly resistant (IT = 0) to the Bgt isolate PL-12, and 26R61 was fully susceptible (IT = 4). Most of the RILs had ITs of 0 or 4; a few lines were 1 or 3; some segregating lines (IT = 2) were also observed and presumed to be heterozygous (Fig. 2).
QTL detection for powdery mildew resistance in the field
Three QTL of major effect located on chromosomes 2BL, 4A and 6BL were detected in all environments on the basis of whole-genome scanning and the CIM analysis (Supplementary Fig. S1). Together, these QTL explained 37–46 % of the phenotypic variation across locations (Table 1).
The 2BL QTL, with resistance contributed by 26R61, was designated QPm.uga-2BL; it was closely linked to marker wPt-0694 (Fig. 3; Table 1). This QTL explained approximately 6 % of phenotypic variation in Plains, and 12–18 % in Raleigh; the peak LOD values were highly significant (P < 0.001) in Raleigh in both years, but only suggestive in Plains (Table 1).
The 4A QTL (QPm.uga-4A) explained 8–18 % of phenotypic variation and the 6BL QTL (QPm.uga-6BL) explained 7–30 %. Peak LOD values for both the 4A and 6BL QTL were significant in all environments (Table 1). QPm.uga-4A was closely linked with marker wPt-3515 (Fig. 4a) and QPm.uga-6BL was closely linked with marker Xbarc134 (Fig. 4b). The mean mildew severity for the lines with the AA genotype (26R61) was lower than those lines with the BB genotype (AGS 2000) at the wPt-0694 locus on 2BL, but higher at the loci wPt-3515 and Xbarc134 on chromosomes 4A and 6BL, respectively, indicating that both the 4A and 6BL QTL were contributed by AGS 2000 (Fig. 5). In addition, two smaller QTL (QPm.uga-4B and QPm.uga-5A) were detected in Raleigh 2012 (Table 1).
Effect plots of three markers closely linked to QTL for powdery mildew resistance in wheat; wPt-0694 is on chromosome 2BL, wPt-3515 on chromosome 4A and Xbarc134 on chromosome 6BL. AA represents the allele from 26R61, BB represents the allele from AGS 2000. Mildew severity ratings are on a 0–9 scale; 0 = immunity, 9 = maximum susceptibility. Error bars are ±1 SE
Identification of a major gene for resistance to isolate PL-12 in AGS 2000
When seedlings of the RIL population were inoculated with Bgt isolate PL-12 in growth chambers, only one major QTL was identified based on whole-genome scanning (Supplementary Fig. S2). This QTL on 6BL was very closely linked with the marker Xbarc134, and was presumed to be the same as QPm.uga-6BL detected in field environments. The QTL explained up to 78 % of the total phenotypic variation in the controlled environment, with very high LOD values at the peak position (Supplementary Fig. S3, Table S1) suggesting the action of a single major gene. Following pooling of the IT 0 and 1 classes as resistant (AGS 2000 genotype), the IT 3 and 4 groups as susceptible (26R61 genotype), and those classified IT 2 as segregating, the resistance gene in AGS 2000 was definitively placed on the genetic map of 6BL (Fig. 6). This gene was temporarily designated PmA2K.
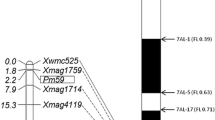
Genetic map and physical location of powdery mildew resistance QTL PmA2K on the long arm of wheat chromosome 6B; deletion bins are indicated as intervals on the physical map (e.g. 0.40–1.00). Pm20 from Secale cereale L. is on the distal third of the long arm of chromosome 6R in a T6BS.6RL translocation
Relationship between QPm.uga-2BL and Pm6
As this study had identified a major QTL on 2BL close to Pm6, and Pm6 is widespread in southeastern USA wheat germplasm, a need exists to clarify the relationship of the two resistance sources. The Pm6 diagnostic marker NAU/STS BCD135-2 produced the typical amplification pattern shown in Fig. 7; Pm6-specific bands were amplified in the Pm6 differential line Coker 747, but not in the susceptible check Coker 68-15. Cultivars Arthur and Arthur 71 yielded the same amplification as Coker 747, indicating that both possessed Pm6. The parents 26R61 and AGS 2000 were both negative for the marker, indicating that Pm6 was not present in these cultivars, and that QPm.uga-2BL is different from Pm6 (Fig. 7).
Genotyping differentials with marker Xbarc134
Marker Xbarc134 tightly linked to the PmA2K was highly polymorphic between AGS 2000 (the source of PmA2K) and a set of Pm differentials (Supplementary Fig. S4, Table 2). No polymorphism was identified between AGS 2000 and the cultivars or lines 81–7241 (Pm4c), Coker 747 (Pm6), Amigo (Pm17), TAM W-104 (Pm?), L501 (Pm32), CH5025 (Pm43), and NC09BGTUM15 (MlUM15), or the difference was too minor to detect using 6 % (w/v) polyacrylamide gels. In addition, Chancellor produced the same amplification pattern as CI 14114 (Pm1a), CI 14118 (Pm2), CI 14120 (Pm3a), CI 14121 (Pm3b), CI 14122 (Pm3c), CI 15888 (Pm3f), CI 14124 (Pm4a) and CI 14125 (Pm5a). This was expected due to the near-isogenic relationships of these differential lines and the differing locations of the respective Pm genes from 6BL. Similarly, because of the common recurrent parent Saluda, the same amplification pattern was found in differentials NC96BGTA5 (Pm25), NC97BGTD7 (Pm34), NC96BGTD3 (Pm35), NC99BGTAG11 (Pm37), NC96BGTA4 (Pm-NCA4) and NC96BGTA6 (Pm-NCA6), as well as Coker 68-15 (none), a parent of Saluda. However, NC06BGTAG12 (MlAG12), NC06BGTAG13 (MlAG13) and NC09BGTUM15 (MlUM15) had different patterns, indicating that during backcrossing, Saluda chromatin at the Xbarc134 locus was replaced by that of the respective Pm donor parents (Supplementary Fig. S4, Table 2).
Discussion
Since their release, the soft red winter wheat cultivars AGS 2000 and 26R61 have exhibited moderate to high levels of resistance to powdery mildew at locations in the southeastern USA. In the present study, the factors in 26R61 and AGS 2000 contributing to powdery mildew resistance in the field were revealed. Three QTL, one from 26R61 on chromosome 2BL and the other two from AGS 2000 on chromosomes 4A and 6BL, were stably detected across environments. The fact that both parents contributed resistance QTL probably accounted for the transgressive segregation in the RIL population. Based on the LOD values and percentages of phenotypic variation explained, QPm.uga-2BL played a key role in mildew resistance at Raleigh; QPm.uga-6BL contributed significantly at Plains, particularly in 2012; and QPm.uga-4A was a major determinant of resistance at both locations.
The chromosome 2BL QTL was closely linked with the marker wPt-0694, and flanked by markers wPt-3755 and wPt-0510, both located in the distal 11 % of wheat chromosome 2BL in physical map (Wilkinson et al. 2012). QPm.uga-2BL was further assigned to deletion bin 2BL6-0.89–1.00. Within that deletion bin or nearby, three formally named Pm genes (Pm6, Pm33 and Pm51, the last is unpublished) and three temporarily named powdery mildew resistance genes (MlZec1, MlAB10 and PmJM22) have been identified, as well as QTL detected in Massey or its derivative USG 3209, ‘RE9001’, ‘Fukuho-Komugi’, ‘Lumai 21’ and ‘Naxos’ (Cowger et al. 2012; Lu et al. 2012; McIntosh et al. 2013, 2014). Erayman et al. (2004) suggested that the distal part of 2BL is a gene-rich region of the wheat genome which may be true in particular for Pm genes. According to the marker data (Fig. 7), QPm.uga-2BL should be different from Pm6, a commonly used gene in the southeastern USA, but its relationship to other resistance genes remains uncertain.
Similarly, several QTL have been reported on chromosome 4A, and most of them are of minor effect and only detected in certain environments, except QPm.tut-4A, the race non-specific resistance QTL from a T. militinae translocation (Jakobson et al. 2006, 2012). QPm.tut-4A and a minor QTL identified in US hard winter wheat cultivar ‘2174’ are both closely linked to the marker Xgwm160 and assigned to the most distal part of 4AL (Chen et al. 2009; Jakobson et al. 2012). They are presumed to be different from the QTL detected in the present study on chromosome 4AS or near the centromere (Fig. 4a). The present 4A QTL is about 68 and 180 cM, respectively, from two QTL detected in Swiss wheat cultivar ‘Forno’ on 4AL based on the common marker Xgwm397 (Keller et al. 1999), indicating that they are also different. However, the present 4A QTL is very near a minor QTL detected in French wheat cultivar ‘Courtot’ and also based on the marker Xgwm397 (Bougot et al. 2006). Another minor QTL was also reported on 4A in French wheat line ‘RE714’, but it is difficult to compare their locations because the genetic map of 4A for RE714 only included RFLP (restriction fragment length polymorphism) markers and the chromosomal assignments of the markers were also uncertain (Chantret et al. 2001; Mingeot et al. 2002). In the present research, QPm.uga-4A was stably detected in all field environments and contributed similar major effects on phenotypic variation in both GA and NC. Since Bgt race frequencies in the two states differ (Parks et al. 2008), it is likely that the 4A QTL from AGS 2000 represents a race non-specific adult-plant resistance or horizontal resistance locus (Nelson 1978). To the authors’ knowledge, this is the first QTL detected on chromosome 4A in southeastern USA wheat germplasm, and it should be valuable for breeding mildew resistant wheat cultivars in the SRWW growing regions.
Based on the positions of the closely linked marker Xbarc134 and proximal markers Xgdm147 and wPt-1541, PmA2K was physically mapped to deletion bin 6BL5-0.40–1.00 (Fig. 6). At least three formally named genes (Pm12, Pm20 and Pm27) and one temporarily named gene (PmG3M) have been identified on chromosome 6B. Pm12 was introgressed into line #31 from A. speltoides, and was located on the short arm of translocation chromosome 6BS-6SS.6SL (Jia et al. 1996). Pm27 was introduced from T. timopheevii in line 146–155-T of common wheat, and located on a translocation segment near the centromeric region of 6B (Järve et al. 2000). Thus, Pm12 and Pm27 were located in different deletion bins from PmA2K (Fig. 6). However, two other genes, PmG3M and Pm20, were assigned to the same deletion bin as PmA2K (Fig. 6). PmG3M was 23.3 cM distal to the marker Xbarc134 (Xie et al. 2012b), whereas PmA2K was proximal to the marker at 1.2 cM (Fig. 6); thus, the genetic distance between the two genes is about 24.5 cM. PmG3M was derived from wild emmer wheat (T. dicoccoides) whereas PmA2K came from common wheat (T. aestivum). It is thus concluded that PmA2K is probably different from PmG3M. Further, there are four lines of evidence that PmA2K is different from Pm20: (1) Pm20, derived from Secale cereale L. cv. ‘Prolific’, is located on the distal third of the 6RL segment in a T6BS.6RL translocation (Friebe et al. 1994), whereas PmA2K is not from rye; (2) normal crossing-over was observed between markers in the PmA2K region at the distal end of 6BL, supporting a common wheat origin for PmA2K; (3) Pm20 has never been used in wheat production in the USA or elsewhere because of unfavorable linkage drag (B. Friebe, personal communication); and (4) ‘KS93WGRC28’, the germplasm containing Pm20, was developed in 1993 and released in 1995 (Friebe et al. 1995), later than the cross made in 1989 to develop AGS 2000 (experimental name GA89482E7) (Johnson et al. 2002), and AGS 2000 should not have KS93WGRC28 or its progenitors in its pedigree. In addition to the genes already discussed, one QTL was detected on chromosome 6BL in CIMMYT breedling line SHA3/CBRD (Lu et al. 2012), but its location was near the centromere and about 55 cM proximal to PmA2K, indicating they are also different. Thus, it is concluded that PmA2K is different from any named gene or QTL reported on this particular chromosome arm. The gene is formally designated Pm54.
For the 2BL and 4A QTL, the closely linked molecular markers were all diversity arrays technology (DArT) markers. Since there are limitations on their direct utilization in breeding programs, it would be advantageous to convert them to more easily used PCR-based markers according to their sequences (http://www.diversityarrays.com/). For the gene Pm54 on 6BL, the most closely linked marker is an easily used SSR marker; genotyping the differentials with the marker Xbarc134 demonstrates that the marker is suitable for marker-assisted selection in pyramiding Pm54 with most of the differential genes (Supplementary Fig. S4, Table 2). However, knowing the polymorphism in a panel of SRWW cultivars would be more useful to aid the introgression of Pm54 with molecular markers. Cautions should also be given for the appearing virulent Bgt isolates for this gene in SRWW growing regions. In conclusion, the QTL reported here and their corresponding closely linked molecular markers will help diversify the genetic sources of Pm resistance in SRWW and will facilitate the breeding process.
Author Contribution Statement
YH and JJ designed research; YH, RP, CC, ZC, YW, DB, JPM, MG, GBG and JJ performed research; YH and YW analyzed data; YH wrote the paper.
References
Allard RW, Shands RG (1954) Inheritance of resistance to stem rust and powdery mildew in cytologically stable spring wheats derived from Triticum timopheevi. Phytopathology 44:266–274
Bennett FGA (1984) Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol 33:279–300
Bennett FG, Westcott B (1982) Field assessment of resistance to powdery mildew in mature wheat plants. Plant Pathol 31:261–268
Bougot Y, Lemoine J, Pavoine M, Guyomar’ch H, Gautier V, Muranty H, Barloy D (2006) A major QTL effect controlling resistance to powdery mildew in winter wheat at the adult plant stage. Plant Breed 125:550–556
Broman KW, Wu H, Sen Ś, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Reynolds M, da Silva ML, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci 110:8057–8062
Chantret N, Mingeot D, Sourdille P, Bernard M, Jacquemin JM, Doussinault G (2001) A major QTL for powdery mildew resistance is stable over time and at two development stages in winter wheat. Theor Appl Genet 103:962–971
Chen Y, Hunger RM, Carver BF, Zhang H, Yan L (2009) Genetic characterization of powdery mildew resistance in US hard winter wheat. Mol Breed 24:141–152
Cowger C, Parks R, Marshall D (2009) Appearance of powdery mildew of wheat caused by Blumeria graminis f. sp. tritici on Pm17-bearing cultivars in North Carolina. Plant Dis 93:1219
Cowger C, Miranda L, Griffey C, Hall M, Murphy JP, Maxwell J (2012) Wheat powdery mildew. In: Sharma I (ed) Disease resistance in wheat. CAB International, Oxfordshire, pp 84–119
Erayman M, Sandhu D, Sidhu D, Dilbirligi M, Baenziger P, Gill KS (2004) Demarcating the gene-rich regions of the wheat genome. Nucleic Acids Res 32:3546–3565
Friebe B, Heun M, Tuleen N, Zeller FJ, Gill BS (1994) Cytogenetically monitored transfer of powdery mildew resistance from rye into wheat. Crop Sci 34:621–625
Friebe B, Gill BS, Tuleen NA, Cox TS (1995) Registration of KS93WGRC28 powdery mildew resistant T6BS-6RL wheat germplasm. Crop Sci 35:1237
Griffey C, Price T, Sisson A, Das W, Pridgen M, Vaughn T, Rohrer M, Brann W (2001) Registration of ‘Roane’ wheat. Crop Sci 41:1359
Hao Y, Liu A, Wang Y, Feng D, Gao J, Li X, Liu S, Wang H (2008) Pm23: a new allele of Pm4 located on chromosome 2AL in wheat. Theor Appl Genet 117:1205–1212
Hao Y, Chen Z, Wang Y, Bland D, Buck J, Brown-Guedira G, Johnson J (2011) Characterization of a major QTL for adult plant resistance to stripe rust in US soft red winter wheat. Theor Appl Genet 123:1401–1411
Hao Y, Chen Z, Wang Y, Bland D, Parks R, Cowger C, Johnson J (2012a) Identification of Pm8 suppressor at the Pm3 locus in soft red winter wheat. Crop Sci 52:2438–2445
Hao Y, Wang Y, Chen Z, Bland D, Li S, Brown-Guedira G, Johnson J (2012b) A conserved locus conditioning Soil-borne wheat mosaic virus resistance on the long arm of chromosome 5D in common wheat. Mol Breed 30:1453–1464
Hsam S, Huang X, Ernst F, Hartl L, Zeller F (1998) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.) 5. Alleles at the Pm1 locus. Theor Appl Genet 96:1129–1134
Jakobson I, Peusha H, Timofejeva L, Järve K (2006) Adult plant and seedling resistance to powdery mildew in a Triticum aestivum × Triticum militinae hybrid line. Theor Appl Genet 112:760–769
Jakobson I, Reis D, Tiidema A, Peusha H, Timofejeva L, Valárik M, Kladivová M, Šimková H, Doležel J, Järve K (2012) Fine mapping, phenotypic characterization and validation of non-race-specific resistance to powdery mildew in a wheat–Triticum militinae introgression line. Theor Appl Genet 125:609–623
Järve K, Peusha H, Tsymbalova J, Tamm S, Devos K, Enno T (2000) Chromosomal location of a Triticum timopheevii-derived powdery mildew resistance gene transferred to common wheat. Genome 43:377–381
Ji J, Qin B, Wang H, Cao A, Wang S, Chen P, Zhuang L, Du Y, Liu D, Wang X (2008) STS markers for powdery mildew resistance gene Pm6 in wheat. Euphytica 163:159–165
Jia J, Devos K, Chao S, Miller T, Reader S, Gale M (1996) RFLP-based maps of the homoeologous group-6 chromosomes of wheat and their application in the tagging of Pm12, a powdery mildew resistance gene transferred from Aegilops speltoides to wheat. Theor Appl Genet 92:559–565
Johnson JW, Baenziger PS, Yamazaki WT, Smith RT (1979) Effects of powdery mildew on yield and quality of isogenic lines of ‘Chancellor’ wheat. Crop Sci 19:349–352
Johnson JW, Barnett RD, Cunfer BM, Buntin GD, Bland DE (2002) Registration of ‘AGS 2000’ wheat. Crop Sci 42:661
Jørgensen JH, Jensen CJ (1973) Gene Pm6 for resistance to powdery mildew in wheat. Euphytica 22:423
Keller M, Keller B, Schachermayr G, Winzeler M, Schmid J, Stamp P, Messmer M (1999) Quantitative trait loci for resistance against powdery mildew in a segregating wheat × spelt population. Theor Appl Genet 98:903–912
Leath S, Bowen K (1989) Effects of powdery mildew, triadimenol seed treatment, and triadimefon foliar sprays on yield of winter wheat in North Carolina. Phytopathology 79:152–155
Lebsock KL, Briggle LW (1974) Gene Pm5 for resistance to Erysiphe graminis f. sp. tritici in Hope wheat. Crop Sci 14:561–563
Liu S, Griffey C, Maroof MAS (2001) Identification of molecular markers associated with adult plant resistance to powdery mildew in common wheat cultivar Massey. Crop Sci 41:1268–1275
Lu Q, Bjørnstad Å, Ren Y, Asad M, Xia X, Chen X, Ji F, Shi J, Lillemo M (2012) Partial resistance to powdery mildew in German spring wheat ‘Naxos’ is based on multiple genes with stable effects in diverse environments. Theor Appl Genet 125:297–309
McFadden ES (1930) A successful transfer of emmer characters to vulgare wheat. J Am Soc Agron 22:1020–1034
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC (2013) Catalogue of gene symbols for wheat. In: Ogihara Y (ed) Proceeding of the 12th international wheat genetics symposium, Yokohama, Japan, 8–13 Sept 2013
McIntosh RA, Dubcovsky J, Rogers WJ, Morris CF, Appels R, Xia XC (2014) Catalogue of gene symbols for wheat: 2013–2014 supplement. Annu Wheat Newsl 60:153–175
Mingeot D, Chantret N, Baret P, Dekeyser A, Boukhatem N, Sourdille P, Doussinault G, Jacquemin J (2002) Mapping QTL involved in adult plant resistance to powdery mildew in the winter wheat line RE714 in two susceptible genetic backgrounds. Plant Breed 121:133–140
Murphy J, Navarro R, Leath S, Bowman D, Weisz P, Ambrose L, Pate M, Fountain M (2004) Registration of ‘NC-Neuse’ wheat. Crop Sci 44:1479–1480
Namuco L, Coffman W, Bergstrom G, Sorrels M (1987) Virulence spectrum of the Erysiphe graminis f. sp. tritici population in New York. Plant Dis 71:539–541
Nelson RR (1978) Genetics of horizontal resistance to plant diseases. Annu Rev Phytopathol 16:359–378
Niewoehner A, Leath S (1998) Virulence of Blumeria graminis f. sp. tritici on winter wheat in the eastern United States. Plant Dis 82:64–68
Parks R, Carbone I, Murphy JP, Marshall D, Cowger C (2008) Virulence structure of the eastern US wheat powdery mildew population. Plant Dis 92:1074–1082
Shi A, Leath S, Murphy J (1998) A major gene for powdery mildew resistance transferred to common wheat from wild einkorn wheat. Phytopathology 88:144–147
Singrün C, Hsam S, Hartl L, Zeller F, Mohler V (2003) Powdery mildew resistance gene Pm22 in cultivar Virest is a member of the complex Pm1 locus in common wheat (Triticum aestivum L. em Thell.). Theor Appl Genet 106:1420–1424
Tommasini L, Yahiaoui N, Srichumpa P, Keller B (2006) Development of functional markers specific for seven Pm3 resistance alleles and their validation in the bread wheat gene pool. Theor Appl Genet 114:165–175
Tucker D, Griffey C, Liu S, Brown-Guedira G, Marshall D, Maroof MAS (2007) Confirmation of three quantitative trait loci conferring adult plant resistance to powdery mildew in two winter wheat populations. Euphytica 155:1–13
Wang D, Shi J, Carlson S, Cregan P, Ward R, Diers B (2003) A low-cost, high-throughput polyacrylamide gel electrophoresis system for genotyping with microsatellite DNA markers. Crop Sci 43:1828–1832
Wang S, Basten CJ, Zeng ZB (2012) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm. Accessed 17 Sept 2014
Wilkinson PA, Winfield MO, Barker GL, Allen AM, Burridge A, Coghill JA, Edwards KJ (2012) CerealsDB 2.0: an integrated resource for plant breeders and scientists. BMC Bioinform 13:219
Xie W, Ben-David R, Zeng B, Dinoor A, Xie C, Sun Q, Röder MS, Fahoum A, Fahima T (2012a) Suppressed recombination rate in 6VS/6AL translocation region carrying the Pm21 locus introgressed from Haynaldia villosa into hexaploid wheat. Mol Breed 29:399–412
Xie W, Ben-David R, Zeng B, Distelfeld A, Röder MS, Dinoor A, Fahima T (2012b) Identification and characterization of a novel powdery mildew resistance gene PmG3M derived from wild emmer wheat, Triticum dicoccoides. Theor Appl Genet 124:911–922
Acknowledgments
The authors acknowledge financial support by the National Research Initiative of USDA’s Cooperative State Research, Education and Extension Service, CAP (Grant No. 2006-55606-16629). Critical review of the manuscript from Dr. R.A. McIntosh is highly appreciated.
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical standard
The experiments reported in the manuscript are in accordance with the ethical standards in the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Frank Ordon.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hao, Y., Parks, R., Cowger, C. et al. Molecular characterization of a new powdery mildew resistance gene Pm54 in soft red winter wheat. Theor Appl Genet 128, 465–476 (2015). https://doi.org/10.1007/s00122-014-2445-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2445-1