Abstract
Background
Emerging evidence indicates combination therapy with anticoagulants and antiplatelet agents for atrial fibrillation (AF) will be increasingly required. Numerous studies compare the efficacy and cost-effectiveness of anticoagulation alone in AF, i. e., non-vitamin K oral anticoagulants (NOACs) vs. warfarin. However, the addition of antiplatelet agents with their potential for decreasing thromboembolic stroke counter-balanced by an increased bleeding risk has received less attention. Thus, we evaluated the cost–utility of this combination therapy.
Method and results
We obtained event estimates from our recent meta-analysis of four randomized clinical trials designed to compare NOACs with warfarin in patients with AF. We examined patient subgroups within each trial that received antiplatelet therapy in addition to anticoagulation. Utilities were derived from the literature and cost estimates from the German health-care system. A decision tree was constructed and populated with these parameters. We used a 1-year time horizon because combination therapy is not recommended beyond this time. We calculated the incremental cost–effectiveness ratio (ICER) per quality-adjusted life-year (QALY). The derived ICER was 13,168.50 € per QALY. NOAC prices exerted considerable influence on the calculation. Nevertheless, there is potential for ICER shifts in favor of warfarin, e.g., if warfarin-mediated anticoagulation control is improved and thereby adverse events decrease. Conversely, if NOAC adherence decreases, adverse events could increase.
Conclusion
The derived ICER was 13,168.50 € per QALY, consistent with NOACs being cost-effective vs. warfarin when anticoagulation is used with antiplatelet agents.
Nevertheless, country-, practice-, and patient-related factors influence the ICER. Our cost–utility calculation should be used a starting point for decision-making.
Zusammenfassung
Hintergrund
Aktueller Evidenz zufolge ist die Kombinationstherapie aus Antikoagulanzien und Thrombozytenaggregationshemmern bei Vorhofflimmern (VHF) häufig und zunehmend. In zahlreichen Studien wird nur die Wirksamkeit und Wirtschaftlichkeit zwischen Nicht-Vitamin-K-abhängigen oralen Antikoagulanzien (NOAK) und Warfarin verglichen. Die Aufmerksamkeit richtet sich jedoch weniger auf die zusätzliche Gabe von Thrombozytenaggregationshemmern mit ihrem Potenzial eines verminderten Risikos für thromboembolisch bedingte Schlaganfälle bei gleichzeitig erhöhtem Blutungsrisiko. Daher untersuchten die Autoren das Kosten-Nutzen-Verhältnis dieser Kombinationstherapie.
Methoden und Ergebnisse
Aus der aktuellen Metaanalyse von 4 randomisierten klinischen Studien zum Vergleich von NOAK mit Warfarin bei VHF-Patienten wurden Ereignisschätzwerte der Patientensubgruppen mit zusätzlicher Thrombozytenaggregationshemmung ermittelt. Die Nutzwerte („utilities“) wurden der Literatur entnommen, die Kostenschätzwerte aus Daten des deutschen Gesundheitssystems. Ein Entscheidungsbaum wurde entwickelt und mit diesen Parametern bestückt. Die Autoren benutzten einen Ein-Jahres-Horizont, da die Kombinationstherapie darüber hinaus nicht empfohlen wird. Es wurde das inkrementelle Kosten-Effektivitäts-Verhältnis (ICER) pro qualitätskorrigiertem Lebensjahr („quality-adjusted life-year“, QALY) berechnet. Der ermittelte ICER-Wert betrug 13.168,50 € pro QALY. Die Preise für NOAKs beeinflussen den ICER beträchtlich. Trotzdem besteht ein Potenzial für Verschiebungen des ICERs zugunsten von Warfarin, z. B., wenn die Steuerung der Antikoagulation mittels Warfarin verbessert würde und somit weniger Komplikationen darunter aufträten bzw. wenn unter verminderter Compliance der NOAK-Therapie mehr Komplikationen aufträten.
Schlussfolgerung
Für die Kombinationstherapie eines Thrombozytenaggregationshemmers mit einem NOAK wurde ein ICER von 13.168,50 € pro QALY ermittelt, was als kosteneffizient angesehen werden kann, auch wenn in Deutschland keine festen Grenzwerte hierfür existieren. Dennoch beeinflussen landes-, praxis- und patientenbezogene Faktoren den ICER-Wert. Die vorliegende Kosten-Nutzwert-Berechnung sollte als Startpunkt für Entscheidungsfindungen dienen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Numerous studies employ meta-analysis and cost-effectiveness evaluation to compare non-vitamin K oral anticoagulants (NOACs) versus warfarin in patients with nonvalvular atrial fibrillation (AF; [1,2,3,4,5,6]). However, critical gaps in knowledge remain; little information is available comparing these agents when anticoagulation treatment occurs together with antiplatelet therapy. Such evaluation is necessary for three reasons. (1) This combination therapy is appropriate for patients with AF who develop coronary artery disease and require stent placement. The converse event sequence also occurs; patients on antiplatelet therapy who then develop AF and require anticoagulation. For example, combination therapy with clopidogrel or aspirin (ASA) and an oral anticoagulant is recommended with a Class IIaA indication (after an initial phase of triple therapy) for up to 12 months after acute coronary syndrome [7]. (2) Combination therapy is relatively common [8,9,10]; for example, ~40% of participants in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial of dabigatran versus warfarin used ASA at the start of the study [9]. (3) The prevalence of combination therapy will likely increase; for example, anticoagulation therapy has increased with the introduction of NOACs [11]. In addition, the incidence of AF is projected to increase [12], while risk factors for other cardiovascular diseases that may require antiplatelet therapy are also on the rise [13]. Consequently, combination therapy will be increasingly encountered and required.
Combination therapy raises the prospect of enhanced protection against thromboembolic complications, but with the potential for increased bleeding. Sufficient data are now available to provide estimates of these events. We recently conducted a meta-analysis to assess efficacy and risk in a subgroup of patients with AF on ASA enrolled in randomized clinical trials (RCTs) comparing NOACs with warfarin. Our analysis revealed NOACs plus ASA were more effective in reducing major thromboembolic complications than warfarin plus ASA and were as safe as the latter in terms of major bleeding [14]. We now aim to extend this investigation to evaluate the cost–utility of combination therapy.
Methods
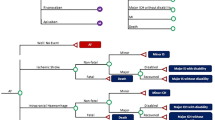
We developed a decision tree for the two patient groups with nonvalvular AF; NOAC + ASA and VKA + ASA. In our model, potential clinical states and outcomes were assigned specific probabilities based on our meta-analysis and other published data. The first branches in the decision tree contained the following clinical states: no change, myocardial infarction (MI), stroke or systemic emboli, major bleeding, and noncardiovascular death (Fig. 1). Potential sequelae were included at the subsequent branch-level for each of these states (Fig. 1). We defined “disabling myocardial infarction” as development of heart failure after MI and “disabling stroke” as a modified Rankin Score of 3–6. After major bleeding, we assumed two potential states: death or good functional status.
Recently published guidelines recommended that combination therapy should be discontinued in stabilized event-free patients 1 year after stenting [7]. Therefore, we restricted our economic analysis to a 1-year time horizon.
We used the model outputs to calculate the incremental cost-effectiveness ratio (ICER) of NOAC + ASA versus VKA + ASA. As a measure of utility, quality-adjusted life-years (QALYs) were estimated. Costs incorporated in our model reflect the German health-care payers’ perspective.
Strategy for identifying data sources
Data from our prior meta-analysis [14] of subgroups from four RCTs (RE-LY [8], Rivaroxaban Versus Warfarin in Nonvalvular Atrial Fibrillation, ROCKET-AF, [9], Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation, ARISTOTLE, [15], and Edoxaban Versus Warfarin in Nonvalvular Atrial Fibrillation, ENGAGE, [16]) were used to estimate the probabilities of first branch-level events in the model. The probabilities for subsequent outcomes were extracted from registries and published studies (Table 1). We assumed, if not otherwise indicated, subsequent outcome probabilities for disability, death, and good functional status were independent of treatment group. By contrast, clinical states at the first branch-level were dependent upon treatment.
We were unable to find utility values for oral anticoagulation therapy with concomitant antiplatelet use. However, the utility for VKA/NOAC alone was 0.987/0.998 and for ASA alone, 0.998 [17]. Therefore, we assumed the additional use of an antiplatelet would not change these utilities in a material way and we summed the values. Consequently, we extracted values for the final health states (Table 2) from publications describing utility values in patients with AF on NOACs or VKA alone [17,18,19]. VKA-related utilities were derived from patients for whom time trade-off and standard gamble methods were used to estimate quality of life; contributing factors included international normalized ratio (INR) measurements, diet, and lifestyle changes [17]. A utility value of “1” represents full health and a value of “0” denotes death. We assumed patients who made a complete recovery after MI, stroke/systemic embolism, and major bleeding had the same utility as patients with stable health status.
Costs were expressed in euro (Table 3). Average prices of antiplatelet, NOACs, and VKAs reflect German costs and were derived from the Red List [20]. For VKA treatment, we also included costs associated with required monitoring of the INR. Routine care costs for both NOACs and VKA patients associated with general practitioner visits were included [21]. The one-time costs or outcomes were taken from the Institute for Payment Regulations in German Hospitals (Institut für Entgeldsystem im Krankenhaus, InEK), according to German diagnosis related groups (G-DRGs; [22]). Patients who survived MI/stroke/systemic embolism were assumed to participate in inpatient rehabilitation programs for as long as 4 weeks. These costs were based on expert opinion. After stroke/systemic embolism, or MI with subsequent disability, additional long-term health-maintenance costs were attributed on the basis of educated estimates; we assumed one hospitalization per year together with four visits to outpatient specialists [21, 22].
Sensitivity analysis
Deterministic sensitivity analyses were conducted to evaluate the effects of uncertainty in key input parameters and to test assumptions made in the calculation of the final cost-effectiveness.
We performed a one-way sensitivity analysis on the cost of NOACs. Their relatively high, but likely decreasing, costs would be expected to exert considerable influence. Conversely, the cost of ASA is sufficiently low (~0.03 €/day) to be neglected and thus was omitted from sensitivity analyses.
Additional one-way sensitivity analyses were conducted to examine potential variation in treatment costs for stroke/systemic embolism, MI, and major bleeding. We assumed, in the initial calculation, an average price for acute therapy independent of event severity. However, the DRG system permits differential billing based on severity; therefore, we examined a range of costs.
The type and severity class of AF influence utility values for AF [4, 23,24,25,26]. Therefore, we also ran a two-way sensitivity analysis to explore the effect of such changes; absolute utility values for AF ranged from 0.59 to 0.85, which were corrected for antiplatelet and anticoagulant use. Corresponding differences in values between NOAC and VKA utilities ranged from 0.007 to 0.013 [4, 23, 24] and were also incorporated into the sensitivity analysis.
Results
The expected 1‑year costs were ~50% lower for VKA + ASA versus NOAC + ASA (969.12 € versus 1914.58 €). NOAC + ASA treatment was estimated to provide an incremental 0.0718 QALY increase within our 1‑year time horizon. Hence, the resulting ICER was 13,168.50 € per QALY gained (Table 4).
Medication costs exerted a considerable effect on ICER estimation (Table 5). By contrast, the cost of (one-time) acute stroke therapy had a less pronounced effect (Table 6). Our range was from mild cases (cost = 6000 €) to severe cases that required complex intensive care therapy (22,000 €). Similarly, we used a range of costs for MI to reflect event severity; from early discharge after uncomplicated MI (6000 €) to prolonged hospitalization associated with the development of cardiogenic shock (18,000 €). This range also had a limited effect on ICER; ICER ranged between 13,112.79 €/QALY and 13,279.93 €/QALY. Likewise, a fourfold increase in costs for major bleeding (2000–8000 €) produced a limited change in ICER; 11,921.05 €/QALY to 14,415.95 €/QALY.
Two-way sensitivity analysis with different utilities for the baseline state in AF patients revealed remarkable differences in the ICER (Table 7). Patients treated with NOACs do not require INR measurements and have fewer dietary restrictions than VKA-treated patients. It therefore seems likely NOAC-associated utility values will always be higher than those associated with VKA treatment.
Table 8 shows a two-way sensitivity analysis designed to examine the influence of the probability of stroke incidence on ICER (€/QALY).
Discussion
We found NOAC + ASA to be a cost-effective alternative to treatment with VKA + ASA. The calculated ICER was 13,168.50 € per gained QALY for a time horizon of 1 year. These benefits coincide with a lower incidence of stroke and systemic embolism and a generally better safety profile [14].
Sensitivity analysis indicated that NOAC costs exerted the greatest effect on ICER, reducing the cost per QALY gained. This is important because NOAC cost might be anticipated to decrease when patent protection expires; dabigatran in 2018, apixaban in 2019, rivaroxaban in 2021, and edoxaban in 2031. However, although it appears intuitively reasonable to assume prices decrease with patent expiration, the reality is more complex. Studies in Canada, the United States, and Europe demonstrated that after patent expiration, prices for original drugs remained constant or even increased [27]. Government policies, the use of other “clinically substitutable” in-patent agents, the influence of prescribers and pharmacists, and even consumer brand loyalty could all play roles in maintaining or increasing prices [28,29,30]—the so-called generics paradox [31]. However, generics, if they become available, are cheaper and thereby reduce the total cost burden from the health-care payer perspective. In Europe, countries where the overall market share for generic medicines is high appear to have larger post-patent price decreases than countries with low generic market share [32]. Consequently, Germany, which has long provided an environment conducive to promotion of generic medicines, may see larger price declines than countries with more recent adoption of such policies [33].
In contrast to NOAC costs, because probabilities for stroke and MI were small, the cost range used in the model for these outcomes had relatively little influence on ICER. However, as anticipated, we found ICER decreased slightly with higher stroke treatment costs. Similarly, for MI, ICER decreased slightly as treatment costs increased. Conversely, for major bleeding, ICER increased with rising treatment costs.
Other model parameters with potential for significant variation are the probabilities used for stroke or systemic emboli and major bleeding. The risk for such events primarily depends, for VKA therapy, on the degree of anticoagulation control and, for NOAC therapy, on medication adherence. Because both could differ from the values used in the model, they merit consideration.
One index used to assess anticoagulation control with VKA therapy is the time in therapeutic range (TTR; i. e., what proportion of time is spent with an INR within the specified target range). Adverse events occur most often when INR is outside this range. If INR is below target, the risk of thrombosis increases, and if above target, the risk of bleeding increases. Consequently, high TTR values are associated with reduced likelihood of stroke and bleeding [34, 35]. Anticoagulation control varies widely and appears to depend, at least in part, upon practice setting. A meta-analysis, conducted on studies from the United States, revealed higher TTR values in anticoagulation clinics (63%) than those achieved by community-based management (51%; [36]). Country-related differences were found in a study that compared INR control in France, Germany, Italy, and the United Kingdom [37]. The proportion of patients with good control, defined as TTR >70%, ranged between 44% in Germany and 65% in the United Kingdom, while mean TTR ranged from 65 to 73% in the same countries. However, these differences were attributed to different approaches to INR monitoring—the use of specialized anticoagulation clinics in the United Kingdom versus general practitioner management in the other countries. The mean or median TTR in all four of the RCTs we used to construct the model was below 70% [16, 38,39,40]; i. e., below what would be considered an indicator of “good control”. It should be emphasized that TTRs ≥70% can be achieved. Thus, there may be circumstances in which our model overestimates the probability of VKA-associated adverse events because INR control in the RCTs was “suboptimal.” Similarly, there may be circumstances in which our model underestimates the probability of NOAC-associated adverse events because of potentially reduced medication adherence in real-world therapy [41,42,43]. In fact, a cost-effectiveness study of NOACs versus warfarin (as sole therapies) for stroke prevention in patients with AF using Slovenian cost data found that if TTR was above 70%, then warfarin was more cost-effective [44]. It remains to be determined whether the same conclusion is reached when combination therapy is used or if the results apply to other countries.
Comparison with other studies
As far as we are aware, this is the first cost–utility study to examine combination therapy and thus direct comparison with other studies cannot be made. Also, because combination therapy has yet to be prospectively examined in patients with AF, there is insufficient information available to derive event estimates beyond the follow-up periods conducted in the RCTs: ~2–3 years. Furthermore, for most cases, antiplatelet therapy will be prescribed for a finite duration [45]. Therefore, we decided to limit our time horizon to 1 year (we calculated events per year averaged over the follow-up) and omit Markov-chain analysis.
There are, however, numerous publications that examined the cost–utility of NOACs versus warfarin without addition of antiplatelet agents. Although comparisons should be made with caution because of different cost models and different methodologies, it is interesting to note that, for example, recent cost–utility evaluations of apixaban versus warfarin reported estimated ICERs/QALY of ~10,500–14,500 € in The Netherlands, France, and Greece [23, 46, 47]. That these ICER values are similar to ours could indicate the net effect of adding ASA therapy has limited effect on cost–utility.
Limitations
Our study examined aggregated estimates obtained from four different trials that compared NOACs with VKA. Therefore, we were unable to compare the different NOACs with each other or with VKA. Second, we only analyzed data from subgroups of the original RCTs; this could have introduced selection bias. Third, we focused only on ASA. Comparative studies comparing combination therapy of NOAC versus warfarin with a single P2Y12 inhibitor and comparable dosages of NOAC as we did in our meta-analysis have yet to be published. Studies such as PIONEER AF-PCI [48] and RE-DUAL PCI [49] could not be included because they enrolled a different patient population. Specifically, they included only patients undergoing percutaneous coronary intervention with the urgent need for a P2Y12 inhibitor, while we focused on ASA therapy. Fourth, country-specific cost estimates were used, which limits generalizability. Moreover, these costs may not reflect real costs because the German DRG-based health-care system allows services to be subsidized if necessary. However, the costs do reflect those paid by the German health insurance system. We did not include indirect costs, such as productivity loss, because our analysis was based on a 65-year-old patient with AF (i. e., retired).
The health-state probabilities used were extracted from the four NOAC RCTs, observational studies, and international registries. We further assumed that all extracted estimates are applicable to Germany. The choice of time horizon can exert considerable influence on results and thereby on the interpretation [50]. Short time horizons can potentially mislead if there are high initial costs for therapies that provide benefit over extended periods. In our analysis, this was not the case. In addition, although temporal changes in benefit-to-risk ratios likely occur (cumulative adverse event risk increases and drug costs recur), the relative change over prolonged periods remains unexamined. Hence, for these and the clinical reasons described earlier, we limited our analysis to a 1-year time horizon.
Implications for practice and conclusion
In conclusion, NOACs used in combination with ASA therapy are cost-effective from a German public health-care insurance perspective with an ICER of 13,168.50 €/QALY. Even though the German health-care system imposes no thresholds on ICER, the ICER we calculated would be considered an acceptable level for those countries that do impose thresholds. The cost–utility analysis allows for a comparison across different health programs and policies because it uses a common unit of measure (cost/QALYs gained).
The cost, morbidity, and mortality of adverse events associated with AF and its treatment mean that selecting effective and safe therapies is of paramount importance. In the RE-LY, ROCKET-AF, ARISTOTLE, and ENGAGE trials, NOACs were shown to be a valuable alternative to VKAs in terms of efficacy and safety. However, “real-life,” long-term benefits of NOACs when used together with antiplatelet agents await confirmation.
Our findings should not be regarded as a universal endorsement of superior cost–utility for combination NOAC + ASA versus VKA + ASA. There may be circumstances when ICER values shift in favor of VKA + ASA; for example, when this therapy’s probability of stroke decreases or as the probability of stroke with NOAC + ASA increases. These two constructs can occur with improved TTR for the former and poor medication adherence for the latter. In addition, there are country-specific considerations that should be taken into account. Instead, our findings should be considered as a starting point for decision-making and calculation.
References
Almutairi AR, Zhou L, Gellad WF, Lee JK, Slack MK, Martin JR, Lo-Ciganic WH (2017) Effectiveness and safety of non-vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta-analyses. Clin Ther 39(7):1456–1478 e1436. https://doi.org/10.1016/j.clinthera.2017.05.358
Capodanno D, Capranzano P, Giacchi G, Calvi V, Tamburino C (2013) Novel oral anticoagulants versus warfarin in non-valvular atrial fibrillation: a meta-analysis of 50,578 patients. Int J Cardiol 167(4):1237–1241. https://doi.org/10.1016/j.ijcard.2012.03.148
Coyle D, Coyle K, Cameron C, Lee K, Kelly S, Steiner S, Wells GA (2013) Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health 16(4):498–506. https://doi.org/10.1016/j.jval.2013.01.009
Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, Wang PJ, Turakhia MP (2011) Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 154(1):1–11. https://doi.org/10.7326/0003-4819-154-1-201101040-00289
Hicks T, Stewart F, Eisinga A (2016) NOACs versus warfarin for stroke prevention in patients with AF: a systematic review and meta-analysis. Open Heart 3(1):e279. https://doi.org/10.1136/openhrt-2015-000279
You JH (2014) Novel oral anticoagulants versus warfarin therapy at various levels of anticoagulation control in atrial fibrillation—a cost-effectiveness analysis. J Gen Intern Med 29(3):438–446. https://doi.org/10.1007/s11606-013-2639-2
Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN (2018) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J 39(3):213–260. https://doi.org/10.1093/eurheartj/ehx419 (The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS))
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, Committee R‑LS, Investigators (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151. https://doi.org/10.1056/NEJMoa0905561
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, Investigators RA (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891. https://doi.org/10.1056/NEJMoa1009638
Secemsky EA, Butala NM, Kartoun U, Mahmood S, Wasfy JH, Kennedy KF, Shaw SY, Yeh RW (2016) Use of chronic oral anticoagulation and associated outcomes among patients undergoing percutaneous coronary intervention. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.004310
Barnes GD, Lucas E, Alexander GC, Goldberger ZD (2015) National trends in ambulatory oral anticoagulant use. Am J Med 128(12):1300–1305 e1302. https://doi.org/10.1016/j.amjmed.2015.05.044
Go AS, Hylek EM, Phillips KA, Chang YC, Henault LE, Selby JV, Singer DE (2001) Prevalence of diagnosed atrial fibrillation in adults—National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 285(18):2370–2375. https://doi.org/10.1001/jama.285.18.2370
Kapadia S (2017) Trends in cardiovascular risk profiles. Cleve Clin J Med 84(12 Suppl 4):e6–e9. https://doi.org/10.3949/ccjm.84.s4.02
Bennaghmouch N, de Veer A, Bode K, Mahmoodi BK, Dewilde WJM, Lip GYH, Brueckmann M, Kleine E, Ten Berg JM (2017) The efficacy and safety of the use of non-vitamin-K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation and concomitant aspirin therapy: a meta-analysis of randomized trials. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.117.028513
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, Committees A, Investigators (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992. https://doi.org/10.1056/NEJMoa1107039
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, Investigators EA-T (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369(22):2093–2104. https://doi.org/10.1056/NEJMoa1310907
Gage BF, Cardinalli AB, Owens DK (1996) The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 156(16):1829–1836
Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V (2011) Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 31(6):800–804. https://doi.org/10.1177/0272989x11401031
Krejczy M, Harenberg J, Wehling M, Obermann K, Lip GYH (2015) Cost-effectiveness of anticoagulation in patients with nonvalvular atrial fibrillation with Edoxaban compared to Warfarin in Germany. Biomed Res Int. https://doi.org/10.1155/2015/876923
online RL (2017) Arzneimittelinformation für Deutschland. http://online.rote-liste.de/. Accessed 1 Sept 2017
Bundesvereinigung K (2017) EBM Einheitlicher Bewertungsmaßstab. http://www.kbv.de/html/online-ebm.php. Accessed 1 Sept 2017
GmbH I (2017) Fallpauschalen-Katalog 2017. http://www.g-drg.de/cms/G-DRG-System_2016/Fallpauschalen-Katalog. Accessed 1 Sept 2016
Stevanovic J, Pompen M, Le HH, Rozenbaum MH, Tieleman RG, Postma MJ (2014) Economic evaluation of apixaban for the prevention of stroke in non-valvular atrial fibrillation in the Netherlands. PLoS ONE 9(8):e103974. https://doi.org/10.1371/journal.pone.0103974
Sullivan PW, Arant TW, Ellis SL, Ulrich H (2006) The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics 24(10):1021–1033
Berg J, Lindgren P, Nieuwlaat R, Bouin O, Crijns H (2010) Factors determining utility measured with the EQ-5D in patients with atrial fibrillation. Qual Life Res 19(3):381–390. https://doi.org/10.1007/s11136-010-9591-y
Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof P, Gupta D (2014) The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace 16(7):965–972. https://doi.org/10.1093/europace/eut395
Vandoros S, Kanavos P (2013) The generics paradox revisited: empirical evidence from regulated markets. Appl Econ 45:3230–3239
Costa-Fonta J, Rudisilla C, Ta S (2014) Brand loyalty, patients and limited generic medicines uptake. Health Policy (New York) 116:224–233. https://doi.org/10.1016/j.healthpol.2014.01.015
Gonzalez J, Sismeiro C, Dutta S, Stern P (2008) Can branded drugs benefit from generic entry? The role of detailing and price in switching to non-bioequivalent molecules. Int J Res Mark 25:247–260. https://doi.org/10.1016/j.ijresmar.2008.08.002
Vandoros S (2014) Therapeutic substitution post-patent expiry: the cases of ACE-inhibitor and proton pump inhibitors. Health Econ 23:621–630. https://doi.org/10.1002/hec.2935
Kanavos P, Vandoros S (2011) Determinants of branded prescription medicine prices in OECD countries. Health Econ Policy Law 6:337–367. https://doi.org/10.1017/S1744133111000090
Dylst P, Simoens S (2011) Does the market share of generic medicines influence the price level? A European analysis. Pharmacoeconomics 10:875–882
Dylst P, Simoens S (2010) Generic medicine pricing policies in Europe: current status and impact. Pharmaceuticals (Basel) 3:471–481. https://doi.org/10.3390/ph3030471
Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM (2009) Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 124(1):37–41. https://doi.org/10.1016/j.thromres.2008.09.016
Nieuwlaat R, Connolly BJ, Hubers LM, Cuddy SM, Eikelboom JW, Yusuf S, Connolly SJ, Investigators A (2012) Quality of individual INR control and the risk of stroke and bleeding events in atrial fibrillation patients: a nested case control analysis of the ACTIVE W study. Thromb Res 129(6):715–719. https://doi.org/10.1016/j.thromres.2011.08.024
Baker WL, Cios DA, Sander SD, Coleman CI (2009) Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm 15(3):244–252. https://doi.org/10.18553/jmcp.2009.15.3.244
Cotte FE, Benhaddi H, Duprat-Lomon I, Doble A, Marchant N, Letierce A, Huguet M (2014) Vitamin K antagonist treatment in patients with atrial fibrillation and time in therapeutic range in four European countries. Clin Ther 36(9):1160–1168. https://doi.org/10.1016/j.clinthera.2014.07.016
Piccini JP, Hellkamp AS, Lokhnygina Y, Patel MR, Harrell FE, Singer DE, Becker RC, Breithardt G, Halperin JL, Hankey GJ, Berkowitz SD, Nessel CC, Mahaffey KW, Fox KA, Califf RM, Investigators RA (2014) Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial. J Am Heart Assoc 3(2):e521. https://doi.org/10.1161/JAHA.113.000521
Wallentin L, Lopes RD, Hanna M, Thomas L, Hellkamp A, Nepal S, Hylek EM, Al-Khatib SM, Alexander JH, Alings M, Amerena J, Ansell J, Aylward P, Bartunek J, Commerford P, De Caterina R, Erol C, Harjola VP, Held C, Horowitz JD, Huber K, Husted S, Keltai M, Lanas F, Lisheng L, McMurray JJ, Oh BH, Rosenqvist M, Ruzyllo W, Steg PG, Vinereanu D, Xavier D, Granger CB, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Investigators (2013) Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 127(22):2166–2176. https://doi.org/10.1161/CIRCULATIONAHA.112.142158
Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ, Investigators R‑L (2010) Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 376(9745):975–983. https://doi.org/10.1016/S0140-6736(10)61194-4
Brown JD, Shewale AR, Talbert JC (2016) Adherence to Rivaroxaban, Dabigatran, and Apixaban for stroke prevention in incident, treatment-naive nonvalvular atrial fibrillation. J Manag Care Spec Pharm 22(11):1319–1329. https://doi.org/10.18553/jmcp.2016.22.11.1319
Lader E, Martin N, Cohen G, Meyer M, Reiter P, Dimova A, Parikh D (2012) Warfarin therapeutic monitoring: is 70 % time in the therapeutic range the best we can do? J Clin Pharm Ther 37(4):375–377. https://doi.org/10.1111/j.1365-2710.2011.01324.x
Martinez C, Katholing A, Wallenhorst C, Freedman SB (2016) Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost 115(1):31–39. https://doi.org/10.1160/TH15-04-0350
Janzic A, Kos M (2015) Cost effectiveness of novel oral anticoagulants for stroke prevention in atrial fibrillation depending on the quality of Warfarin anticoagulation control. Pharmacoeconomics 33(4):395–408. https://doi.org/10.1007/s40273-014-0246-7
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‑C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K (2016) 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962. https://doi.org/10.1093/eurheartj/ehw210
Athanasakis K, Boubouchairopoulou N, Karampli E, Tarantilis F, Savvari P, Bilitou A, Kyriopoulos J (2017) Cost effectiveness of Apixaban versus Warfarin or Aspirin for stroke prevention in patients with atrial fibrillation: a Greek perspective. Am J Cardiovasc Drugs 17(2):123–133. https://doi.org/10.1007/s40256-016-0204-1
Lanitis T, Cotte FE, Gaudin AF, Kachaner I, Kongnakorn T, Durand-Zaleski I (2014) Stroke prevention in patients with atrial fibrillation in France: comparative cost-effectiveness of new oral anticoagulants (apixaban, dabigatran, and rivaroxaban), warfarin, and aspirin. J Med Econ 17(8):587–598. https://doi.org/10.3111/13696998.2014.923891
Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA (2016) Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 375(25):2423–2434. https://doi.org/10.1056/NEJMoa1611594
Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH, Committee R‑DPS, Investigators (2017) Dual antithrombotic therapy with Dabigatran after PCI in atrial fibrillation. N Engl J Med 377(16):1513–1524. https://doi.org/10.1056/NEJMoa1708454
Cohen DJ, Reynolds MR (2008) Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol 52(25):2119–2126. https://doi.org/10.1016/j.jacc.2008.09.018
Vaartjes I, van Dis I, Grobbee DE, Bots ML (2010) The dynamics of mortality in follow-up time after an acute myocardial infarction, lower extremity arterial disease and ischemic stroke. BMC Cardiovasc Disord 10:57. https://doi.org/10.1186/1471-2261-10-57
Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D (2006) Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C‑reactive protein. J Am Coll Cardiol 47(5):962–968. https://doi.org/10.1016/j.jacc.2005.10.055
Park TH, Saposnik G, Bae HJ, Lee SJ, Lee KB, Lee J, Park JM, Choi JC, Kim DE, Cho YJ, Kim JT, Cha JK, Lee J, Yu KH, Lee BC, Yoon BW (2013) The iScore predicts functional outcome in Korean patients with ischemic stroke. Stroke 44(5):1440–1442. https://doi.org/10.1161/STROKEAHA.111.000748
Shah R, Hellkamp A, Lokhnygina Y, Becker R, Berkowitz SD, Breithardt G, Hacke W, Halperin JL, Hankey GJ, Fox KAA, Nessel CC, Mahaffey KW, Piccini JP, Singer DE, Patel MR, Investigators obotRASC (2016) Use of concomitan aspirin in patients with atrial fibrillation: findings from the ROCKET AF trial. Am Heart J 179:77–86
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Bode and P. Whittaker declare that they have no competing interests. J.M. ten Berg has received speaker’s fees from Astra Zeneca, BMS, Pfizer, the Medicines Company, Boehringer Ingelheim, and Lilly and has received a grant from Astra Zeneca and ZonMw. G. Hindricks reported grants for the Heart Center Leipzig from Abbott and Boston Scientific but has not received personal payments for his services.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
J.M. ten Berg and P. Whittaker shared senior authorship.
Rights and permissions
About this article
Cite this article
Bode, K., Hindricks, G., ten Berg, J.M. et al. Anticoagulant plus antiplatelet therapy for atrial fibrillation. Herz 45, 564–571 (2020). https://doi.org/10.1007/s00059-018-4747-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-018-4747-6
Keywords
- Anticoagulation agents
- Platelet aggregation inhibitors
- Coronary artery disease
- Cost–utility
- Non-vitamin K antagonist
- Warfarin