Abstract
Objective
This study aimed to estimate the cost effectiveness of non-vitamin K oral anticoagulants (NOACs) compared with warfarin for stroke prevention in patients with non-valvular atrial fibrillation (NVAF) in Thailand where suboptimal anticoagulation control is common.
Materials and Methods
A hypothetical cohort of 65-year-old patients with NVAF and their disease progression was simulated in the Markov model. The following anticoagulant agents were used: warfarin, dabigatran, rivaroxaban, and apixaban. Warfarin with high, intermediate, and low time in therapeutic ranges (TTR) was used as the three different reference treatments. Baseline clinical events were obtained from a recently published real-world study in Thailand. A lifetime horizon was utilized in this model, and all analyses were performed from societal and healthcare perspectives. The results were reported as incremental cost-effectiveness ratios (ICERs) in 2021 US dollars per quality-adjusted life-year (QALY) gained. The sensitivity analyses were performed to assess the influence of parameter uncertainty.
Results
Apixaban was a cost-effective intervention compared with warfarin with low and intermediate TTR groups. In the low TTR group, the ICERs were $779 and $816 per QALY gained from the societal and healthcare perspectives, respectively, and in the intermediate TTR group, the ICERs were $2038 and $3159 per QALY gained from the societal and healthcare perspectives, respectively. Both ICERs were below the accepted willingness-to-pay threshold ($4806) in the context of Thailand’s healthcare.
Conclusions
In a developing country where suboptimal anticoagulation control is common, apixaban was the cost-effective alternative to warfarin for patients with both low and intermediate TTR control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Previous cost-effectiveness studies using mostly input parameters from a developed health system showed that non-vitamin K oral anticoagulants (NOACs) were not cost effective compared with an old drug, i.e. warfarin. However, when using effectiveness and safety parameters that derived from a real-world study in a developing country, NOACs were found to be a cost-effective alternative to warfarin, particularly among patients with poor anticoagulation control. |
1 Introduction
Stroke is one of the most significant causes of mortality and morbidity in patients with non-valvular atrial fibrillation (NVAF) [1, 2]. The NVAF population are at a fivefold increased risk of stroke compared with the general population [3]. Vitamin K antagonists (e.g. warfarin) have long been the mainstay in stroke prevention for NVAF patients; however, maintaining optimal anticoagulation control with warfarin presents a major challenge [4]. The common quality indicator of anticoagulation control among warfarin users is the percentage of time in therapeutic range (TTR) [5]. Various guidelines suggest that maintaining TTR ≥ 65% is required to achieve optimal clinical outcomes [6, 7]. On the other hand, studies have shown that suboptimal warfarin control (TTR < 65%) was associated with increased risks of stroke, major bleeding, or even death [8,9,10,11]. Suboptimal anticoagulation control is a global problem but this issue is more pronounced and more common in resource-restraint countries [4, 12,13,14]. A recent nationwide registry in Thailand showed that 64% of warfarin users had suboptimal TTR control (TTR < 65%) [11]. Furthermore, a recent multicenter cohort study in Thailand showed that 30.7, 18.6, and 50.7% of Thai warfarin users had high TTR (TTR ≥ 65%), intermediate TTR (TTR 51–64%) and poor TTR (TTR ≤ 50%), respectively [15]. Studies from other developing countries and emerging economies showed a similar pattern [12,13,14, 16]. The currently preferred anticoagulants recommended by most international guidelines are the non-vitamin K oral anticoagulants (NOACs) due to their favorable efficacy and safety profile and ease of use compared with warfarin [1, 2, 17]; however, NOACs were found to be cost effective compared with warfarin, mostly in high-income countries [18]. Recent cost-effectiveness studies conducted in the context of Thailand’s healthcare showed that NOACs were not cost effective compared with warfarin [19,20,21]. The two main reasons for these findings were (1) efficacy and safety parameters applied in the model were derived from trials conducted in developed countries; and (2) the willingness-to-pay (WTP) threshold in Thailand (Thai Baht [THB] 160,000 or US dollars (US$) 4806, with a conversion rate of THB33.29 to US$1) is significantly less than developed countries. Recently, a multicenter, real-world study compared the effectiveness and safety of NOACs with warfarin in Thailand. Results showed that NOACs were associated with a 54% reduction in the risk of major bleeding. In addition, among warfarin users with poor TTR (TTR ≤ 50%), NOACs were associated with a 42% reduction in the risk of thromboembolism. This magnitude of benefits appeared much larger than findings reported from trials conducted mostly in developed countries [15]. With a large difference in the magnitude of benefits, the cost-effectiveness equation may subsequently be affected or altered. As a result, this study was conducted to assess the cost effectiveness of NOACs compared with different levels of warfarin control for stroke prevention in NVAF patients. The results from this study may provide useful information for developing countries where warfarin treatment is commonly suboptimal.
2 Methods
2.1 Overall Description
A cost-effectiveness analysis was performed to compare NOACs versus warfarin for stroke prevention in NVAF patients in the context of Thailand’s healthcare. A decision tree and Markov model were adopted to capture total cost and health outcomes through a patient’s lifespan using societal and healthcare perspectives. Our results were presented as incremental cost-effectiveness ratios (ICERs) in 2021 US$ per quality-adjusted life-year (QALY) gained. The WTP threshold of US$4806 per QALY (THB160,000/QALY) was used to determine whether the option was cost effective. A lifetime horizon was utilized in this model, and all analyses were performed from the societal and healthcare perspectives. The cycle length was 3 months and a 3% annual discount rate was applied to all costs and outcomes.
2.2 Interventions
The following anticoagulant agents were considered: warfarin, dabigatran, rivaroxaban and apixaban. We did not perform separate analyses for each dose of NOAC because there was only a small to no difference in the pricing of full and reduced doses of NOAC in Thailand; hence we used the average price of both doses. In addition, the effectiveness and safety data that we obtained were from a real-world study where both full and reduced doses were used based on the discretion of physicians. Warfarin treatment was further subdivided into three categories based on TTR—warfarin ≥ 65% (high TTR), warfarin 51–64% (intermediate TTR), and warfarin ≤ 50% (low TTR)—and used as reference treatments.
2.3 Economic Model
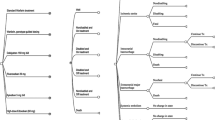
A hypothetical cohort of 65-year-old NVAF patients and their disease progression was simulated in the Markov model (Fig. 1). Patients entered this model in an NVAF ‘well’ state without any contraindications to anticoagulant therapy. From the initial ‘well’ NVAF health state, patients can experience one of the following key clinical events: ischemic stroke (IS), myocardial infarction (MI), intracranial hemorrhage (ICH), major extracranial hemorrhage (ECH), and death. According to the severity of attack, IS and ICH were subdivided into minor and major, and hemorrhagic stroke was included in ICH. IS, ICH, and MI were modeled as closed health states. Once patients experience one of these three events, they cannot transit into other health states. Patients can experience recurrent events with or without severity in a closed state, or exit the model as death. However, major ECH was modeled as a transition health state. Patients undergoing major ECH were allowed to return into the ‘well’ NVAF state.
Schematic of the Markov model. M represented a Markov model with ten health states. Health states highlighted in blue are permanent health states, while the remainder are transient health states. AF atrial fibrillation, ICH intracranial hemorrhage, IS ischemic stroke, MI myocardial infarction, TTR time in therapeutic range
2.4 Model Assumptions
Consistent with previous studies, this model included several important assumptions. First, similar adherence was observed among all treatment strategies; second, therapeutic effects occurred shortly after treatment start and remained persistent over time; and third, no switching or stopping of treatment was allowed after patients encountered any clinical events. In other words, patients stayed in the same treatment category for the duration of the model. We adapted this model from a previously published study [19], which was originally developed in another published study [20] and was validated by specialist physicians in internal medicine and neurosurgery.
2.5 Input Parameters
Baseline clinical events for warfarin interventions (low, intermediate, and high TTR) were obtained from a recently published real-world study from Thailand [15]. We applied relative risks of NOACs to compare with the reference treatments by constructing a parametric survival model using Weibull regression. Consistent with previous studies, we assumed warfarin does not affect the MI outcome in AF patients. To account for the increased risk of event associated with aging, we adjusted the risk by a factor of 1.46, 1.97, and 1.30 per decade of life for stroke, bleeding, and MI, respectively. These adjustment factors were used in a previous cost effectiveness of NOAC in the Thai context [19]. The relative risk of recurrence of clinical events was assumed to be independent of interventions and was estimated to be 2.2 for IS, 2.72 for bleedings, and 2.04 for MI [20, 22, 23]. Disease-specific severity distribution and case fatality of each health state were also adopted from previous studies. Parameters used in our model are presented in electronic supplementary Table 1.
2.6 Probability Data
A life table of the general Thai population provided age-specific all-cause mortality inflated by an excess risk of 1.5 for patients with well-state NVAF [24]. Patients could die from both disease-specific events and other causes within 3 months. Higher mortality was applied to survivors of major clinical events depending on the disease severity levels, using standardized mortality ratios from the literature.
2.7 Costs and Utilities
Both direct and indirect costs were included in the total cost. All cost values were extracted from a published work and converted to 2021 US$ [19]. The baseline utility was 0.84, adopted from published literature [19]. The baseline utility was then adjusted to account for the disutility associated with aging and the occurrence of clinical events. The analysis considered no variation in utility values among interventions.
2.8 Statistical Analyses
The parametric model was applied by fitting the Weibull regression. All analyses were performed using STATA MP 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). One-way sensitivity analysis was undertaken to determine how sensitive the base-analysis result was to fluctuations in the input parameters. The results of one-way sensitivity analyses were presented in Tornado diagrams. We also performed a scenario analysis to assess the impact of a price reduction of NOACs, which may occur with the introduction of generic products. A price reduction of 20 and 50% was chosen based on findings from a previous study suggesting that such a reduction may be adequate to meet the Thai WTP threshold [19]. In addition, multivariate probabilistic sensitivity analyses (PSAs) were conducted with 1000 Monte Carlo simulations using Microsoft Excel 2022 (Microsoft Corporation Redmond, WA, USA) to explore the uncertainties of parameters. Transition probabilities were drawn from a parametric survival model based on a Weibull regression, using the method of Cholesky matrix decomposition [25]. Utilities were assigned using a beta distribution, while costs were assigned using a gamma distribution. Relative risks were assigned by log-normal distribution. The results of PSA were presented as cost-effectiveness acceptability curves (CEAC) to show the probability of NOACs being cost effective compared with warfarin.
3 Results
3.1 Base-Case Analysis
We performed base-case analyses of warfarin compared with NOACs from the societal and healthcare perspectives. We considered warfarin with varying TTR control degrees as three different populations and calculated three ICERs compared with NOACs. Apixaban was found to be cost effective compared with both the low and intermediate TTR groups (electronic supplementary Table 2). Compared with the low TTR group, the ICER was $779 and $816 per QALY gained from the societal and healthcare perspectives, respectively, and compared with the intermediate TTR group, the ICER was $2038 and $3159 per QALY gained from the societal and healthcare perspectives, respectively (Figs. 2a–c, 3a–c). All ICERs were below the accepted WTP threshold in the Thai healthcare setting.
Incremental costs and effects (measured in QALYs) of NOACs relative to warfarin from a societal perspective. a Low TTR; b intermediate TTR; c high TTR. QALYs quality-adjusted life-years, NOACs non-vitamin K oral anticoagulants, TTR time in therapeutic range, USD US dollars, ICER incremental cost-effectiveness ratio
Incremental costs and effects (measured in QALYs) of NOACs relative to warfarin from a healthcare perspective. a Low TTR; b intermediate TTR; c high TTR. QALYs quality-adjusted life-years, NOACs non-vitamin K oral anticoagulants, TTR time in therapeutic range, USD US dollars, ICER incremental cost-effectiveness ratio
3.2 Sensitivity Analyses
The ten largest influential variables were presented in tornado diagrams in a decreasing hierarchy of influence (Fig. 4a–c for the low TTR group; Fig. 5a–c for the intermediate TTR group; and electronic supplementary Fig. 1 for the high TTR group). The events of IS, ICH, and major ECH, the prices of NOACs, and the discount rates were found to influence the ICERs throughout all interventions. In the PSA, apixaban’s probability of being cost effective was around 60, 57, and 35% compared with the low, intermediate, and high TTR groups, respectively, at a WTP threshold of $4806 (Fig. 6a–c and electronic supplementary Fig. 2a–c). Scenario analysis suggests that a 50% reduction in apixaban price would make it cost effective in all TTR groups (electronic supplementary Table 3)
Results of one-way sensitivity analysis (Tornado diagram) for base-case analysis comparing each NOAC with low TTR warfarin. a Dabigatran; b rivaroxaban; c apixaban. NOAC non-vitamin K oral anticoagulant, TTR time in therapeutic range, QALY quality-adjusted life-year, AF atrial fibrillation, IS ischemic stroke, USD US dollars, RR relative risk
Results of one-way sensitivity analysis (Tornado diagram) for base-case analysis comparing each NOAC with intermediate TTR warfarin. a Dabigatran; b rivaroxaban; c apixaban. NOAC non-vitamin K oral anticoagulant, TTR time in therapeutic range, QALY quality-adjusted life-year, AF atrial fibrillation, USD US dollars, RR relative risk
4 Discussion
Cost-effectiveness analysis has become a standard measurement on the value of health intervention with policy implication [26]; however, accurate analysis relies partly on the availability of data from a local context. Directly applying efficacy and safety data from randomized controlled trials conducted in advanced health systems into a cost-effectiveness analysis model of a developing country may not accurately represent the true value of a health intervention in such a healthcare context. Unfortunately, data from randomized controlled trials or real-world data from developing countries are extremely limited. As a result, most cost-effectiveness studies in developing countries have been conducted using data from developed countries, despite this limitation.
Recently, the first multicenter, real-world study in Thailand was conducted in 2055 patients, comparing the effectiveness and safety of NOACs with warfarin at varying TTR levels, with an average follow-up time of > 2 years. Results of the study showed that NOACs provided a much larger magnitude of benefits compared with warfarin, especially when TTR is less than optimal [15]. The main reason for this is because warfarin performance relies heavily on the quality of anticoagulation control [27]. With poor TTR, the incidence of thromboembolism and bleeding increase exponentially [27]. Poor anticoagulation control is a global problem that puts large numbers of patients around the world at risk for adverse outcomes [4]. While NOACs are an attractive alternative to warfarin, access to these agents remain limited in developing countries, mainly due to cost issue [28].
Previously, there were three studies that evaluated the cost effectiveness of NOACs compared with warfarin in the Thai healthcare setting, all of which showed that NOACs were not cost effective. However, these previous studies were performed with input parameters derived mostly from published literature in the developed countries, especially the efficacy and safety data. With the availability of real-world effectiveness and safety data from Thailand, we were able to re-examine this issue by conducting a new cost-effectiveness analysis using key input parameters of effectiveness and safety that represent the actual performance of NOACs in the Thai health system. We were also able to assess the value of an NOAC compared with varying degrees of TTR control among warfarin users, making our analysis unique compared with other studies. As for other key input parameters, we adopted the best available parameters at the time of conducting this work. Since the studies by Rattanachotphanit et al. [20] and Ng et al. [19] were conducted in a local Thai context, a number of input parameters from these studies were also adopted in our study. When local data were not available, input parameters were taken from literature reviews, particularly the most recent systematic review and network meta-analysis.
The results of our study showed that an NOAC, apixaban in this case, was found to be cost effective when compared with warfarin with low and intermediate TTR, which contradicts the conclusion from previous cost-effectiveness studies in Thailand. However, none of the NOACs are shown to be cost effective compared with the high TTR group. This finding may help streamline the potential use of an NOAC to patients whose TTR remains poor despite their best attempts with warfarin. Overall, this strategy seems more logical compared with a complete substitution of an NOAC for warfarin, which may present a challenge in terms of the budget impact to a resource-limited health system. In addition, a price reduction of 50% will make apixaban cost effective in all TTR groups and may represent an attractive replacement for warfarin in this indication. This is possible with either price negotiation with the branded product or the introduction of generic product with at least 50% lower cost. Although NOACs are available in Thailand’s market, these drugs are currently not reimbursed fully in the universal coverage scheme. Results of our analysis may serve as useful information for relevant healthcare agencies when a reimbursement policy is made.
Our study has several limitations. First, since the data input into our model were derived from a retrospective real-world study, the confounding factors of residual effects and unmeasured confounders, which are a non-randomized measure of treatment effect from not using a randomized treatment allocation, along with missing events and lack of adjudication, may remain despite statistical adjustment.
Additionally, the data were from tertiary-level hospitals, which may not limit the generalizability. Second, our model did not incorporate the issue of anticoagulant interruption after major bleeding or switching to aspirin, which can occur in real clinical practice. Third, our model assumed that clinical events were mutually exclusive, which may not reflect the actual clinical scenario where patients may simultaneously encounter more than one event. Fourth, our findings may be affected if there are changes in several factors, mainly the event rates of adverse clinical outcomes and the pricing of NOACs. Since the patents of some NOACs have expired, a drastic change in pricing may occur with the introduction of generic products. Such a change will mostly change the cost-effectiveness equation seen in our study, as in our scenario analysis. Fifth, although different disutility values, including the acute and maintenance phases, should be considered to distinguish and better reflect different health states, we applied the same disutility values for the acute and maintenance phases similar to a previous study [20], due to the unavailability of separate disutility data from a local context. Lastly, we were unable to include edoxaban in our analysis, despite it being shown to have a favorable safety and efficacy profile, especially in the elderly [29, 30], because we did not have real-world effectiveness and safety data of edoxaban in the Thai population. At the time when the real-world study by Mitsuntisuk et al was conducted, edoxaban was still unavailable in most study sites [15].
5 Conclusions
In a developing country where anticoagulation control with warfarin is suboptimal, apixaban was found to be a cost-effective alternative to warfarin, particularly among patients with poor (TTR ≤ 50%) to intermediate (51–64%) TTR levels. This finding can be useful to inform policy makers and health authorities to consider the inclusion of NOACs in a reimbursement scheme to provide access to this life-saving drug for the population, particularly for those whose TTR remains suboptimal despite best attempts with warfarin. From a larger perspective, since poor anticoagulation control is a global issue, our finding may be applied or utilized in other developing countries with a similar healthcare context. Moreover, the results of our study highlight the importance and value of using real-world data from a local healthcare context as essential parameters to ensure that the true value of a health intervention can be accurately assessed. As a result, attempts to create and utilize real-world data in health policy decision making is urgently needed among all developing countries around the world.
References
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–32.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42(5):373–498.
Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–57.
Haas S, Ten Cate H, Accetta G, Angchaisuksiri P, Bassand JP, Camm AJ, et al. Quality of vitamin K antagonist control and 1-year outcomes in patients with atrial fibrillation: a global perspective from the GARFIELD-AF Registry. PLoS ONE. 2016;11(10): e0164076.
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9.
Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–429.
Chiang CE, Okumura K, Zhang S, Chao TF, Siu CW, Wei Lim T, et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm. 2017;33(4):345–67.
Razouki Z, Ozonoff A, Zhao S, Jasuja GK, Rose AJ. Improving quality measurement for anticoagulation: adding international normalized ratio variability to percent time in therapeutic range. Circ Cardiovasc Quality Outcomes. 2014;7(5):664–9.
Chan EW, Lau WC, Siu CW, Lip GY, Leung WK, Anand S, et al. Effect of suboptimal anticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: a population-wide cohort study. Heart Rhythm. 2016;13(8):1581–8.
Hirano T, Kaneko H, Mishina S, Wang F, Morita S. Suboptimal anticoagulant management in Japanese patients with nonvalvular atrial fibrillation receiving warfarin for stroke prevention. J Stroke Cerebrovasc Dis. 2017;26(10):2102–10.
Krittayaphong R, Chantrarat T, Rojjarekampai R, Jittham P, Sairat P, Lip GYH. Poor time in therapeutic range control is associated with adverse clinical outcomes in patients with non-valvular atrial fibrillation: a report from the Nationwide COOL-AF Registry. J Clin Med. 2020;9(6):1698. https://doi.org/10.3390/jcm9061698.
Bahit MC, Granger CB, Alexander JH, Mulder H, Wojdyla DM, Hanna M, et al. Regional variation in clinical characteristics and outcomes in patients with atrial fibrillation: findings from the ARISTOTLE trial. Int J Cardiol. 2020;302:53–8.
Semakula JR, Mouton JP, Jorgensen A, Hutchinson C, Allie S, Semakula L, et al. A cross-sectional evaluation of five warfarin anticoagulation services in Uganda and South Africa. PLoS ONE. 2020;15(1): e0227458.
Chebrolu P, Patil S, Laux TS, Al-Hammadi N, Jain Y, Gage B. Quality of anticoagulation with warfarin in rural Chhattisgarh, India. Indian J Med Res. 2020;152(3):303–7.
Mitsuntisuk P, Nathisuwan S, Junpanichjaroen A, Wongcharoen W, Phrommintikul A, Wattanaruengchai P, et al. Real-world comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in a developing country. Clin Pharmacol Ther. 2021;109(5):1282–92.
Liang HF, Du X, Zhou YC, Yang XY, Xia SJ, Dong JZ, et al. Control of anticoagulation therapy in patients with atrial fibrillation treated with warfarin: a study from the Chinese Atrial Fibrillation Registry. Med Sci Monit. 2019;25:4691–8.
Lip GY, Banerjee A, Boriani G, en Chiang C, Fargo R, Freedman B, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121–201.
Noviyani R, Youngkong S, Nathisuwan S, Bagepally BS, Chaikledkaew U, Chaiyakunapruk N, et al. Economic evaluation of direct oral anticoagulants (DOACs) versus vitamin K antagonists (VKAs) for stroke prevention in patients with atrial fibrillation: a systematic review and meta-analysis. BMJ Evid Based Med. 2022;27(4):215–23.
Ng SS, Nathisuwan S, Phrommintikul A, Chaiyakunapruk N. Cost-effectiveness of warfarin care bundles and novel oral anticoagulants for stroke prevention in patients with atrial fibrillation in Thailand. Thromb Res. 2020;185:63–71.
Rattanachotphanit T, Limwattananon C, Waleekhachonloet O, Limwattananon P, Sawanyawisuth K. Cost-effectiveness analysis of direct-acting oral anticoagulants for stroke prevention in Thai patients with non-valvular atrial fibrillation and a high risk of bleeding. Pharmacoeconomics. 2019;37(2):279–89.
Jarungsuccess S, Taerakun S. Cost-utility analysis of oral anticoagulants for nonvalvular atrial fibrillation patients at the police general hospital, Bangkok, Thailand. Clin Ther. 2014;36(10):1389-94.e4.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–42.
Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014;36(2):192-210. e20.
World Health Organization. Global health observatory data repository: life tables by country, Thailand. Available at: https://apps.who.int/gho/data/?theme=main&vid=61640.2020. (July 1).
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York: Oxford University Press; 2006.
Culyer AJ, Chalkidou K. Economic evaluation for health investments en route to Universal Health Coverage: cost-benefit analysis or cost-effectiveness analysis? Value Health. 2019;22(1):99–103.
Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res. 2009;124(1):37–41.
Banerjee A. Access to medicines-an old problem needing new solutions. BMJ. 2022;376: o824.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Okumura K, Akao M, Yoshida T, Kawata M, Okazaki O, Akashi S, et al. Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med. 2020;383(18):1735–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
M. Sakil Syeed, Teerawat Nonthasawadsri, Richard E. Nelson, Nathorn Chaiyakunapruk and Surakit Nathisuwan declare they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Data availability statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Consent for publication
Not applicable.
Author contributions
All authors have made substantial contributions to this study. All authors were involved in the conception and design of the study. SS, TN, and SN were responsible for acquisition of the data. All authors were responsible for data analysis and data interpretation, drafting of the manuscript, and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Syeed, M.S., Nonthasawadsri, T., Nelson, R.E. et al. Integrating Real-World Evidence in Economic Evaluation of Oral Anticoagulants for Stroke Prevention in Non-valvular Atrial Fibrillation in a Developing Country. Am J Cardiovasc Drugs 23, 173–183 (2023). https://doi.org/10.1007/s40256-023-00570-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00570-z