ABSTRACT
BACKGROUND
The decision as to whether to use more expensive novel oral anticoagulants (NOACs) or invest resources for quality improvement of warfarin therapy requires input from both clinical and economic analyses.
OBJECTIVE
Cost-effectiveness of NOACs compared to warfarin therapy at various levels of patient-time in therapeutic range (TTR) in patients with atrial fibrillation was examined, from the healthcare provider’s perspective.
DESIGN, SUBJECTS AND INTERVENTION
A Markov model was used to compare life-long economic and treatment outcomes of warfarin and NOACs in a hypothetical cohort of 65-year-old atrial fibrillation patients with CHADS2 scores of 2 or above. Model inputs were derived from clinical trials published in the literature.
MAIN MEASURES
The outcome measure was incremental cost per quality-adjusted life-year (QALY) gained (ICER).
KEY RESULTS
Using United States Dollar (USD) 50,000 as the threshold of willingness-to-pay per QALY, NOACs therapy was cost-effective when TTR of warfarin therapy was 60 % or below, or monthly cost of warfarin management increased by two-fold or more to achieve 70 % TTR. Warfarin therapy was cost-effective when TTR of warfarin was 70 % with up to a 1.5-fold increment in monthly cost of care, or when TTR reached 75 % with monthly cost of warfarin care increased up to three-fold. At TTR 60 %, 70 % and 75 %, NOACs was cost-effective when monthly drug cost was < USD 200, < USD 122–185 and < USD 85–145, respectively. 10,000 Monte Carlo simulations showed NOACs to be cost-effective 83.6 %, 50.7 % and 32.7 % of the time at TTR of 60 %, 70 % and 75 %, respectively.
CONCLUSIONS
The acceptance of NOACs as cost-effective was highly dependent upon drug cost, anticoagulation control for warfarin, and anticoagulation service cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Warfarin is a vitamin K antagonist, and for many decades it had been the only effective oral anticoagulant to reduce the risk of ischemic stroke in patients with atrial fibrillation.1 The anticoagulation effect of warfarin, measured by the international normalized ratio (INR), is subject to wide inter-individual and intra-individual variability that possibly leads to hemorrhagic events despite careful dosage titration.2 Recently, novel oral anticoagulants (NOACs), including direct thrombin inhibitor (dabigatran) and direct factor Xa inhibitors (rivaroxaban and apixaban) became available. All three NOACs have shorter half-life than warfarin. Dabigatran and rivaroxaban are mostly (> 60 %) excreted by renal elimination, whereas apixaban is eliminated mainly by fecal route, with 25 % renal excretion. Both apixaban and dabigatran are administered twice daily and rivaroxaban is taken once daily. It is anticipated that, because of the short half-life periods, in combination with the lack of specific requirement on coagulation monitoring, patients on NOAC therapy would need follow-up to ensure drug adherence.3
The efficacy and safety of the NOACs were compared with warfarin in randomized clinical trials for prevention of stroke in patients with atrial fibrillation.4–6 The data showed that the NOACs were either associated with lower rates of stroke and systemic embolism,4,5 or were non-inferior to warfarin for stroke prevention.6 Major bleeding rates of the NOACs and warfarin were similar. Indirect comparative studies mostly reported no profound significant difference in efficacy between these three NOACs, and apixaban was consistently found to be associated with significantly less major bleeding than dabigatran (150 mg twice daily) and rivaroxaban.7–9
Warfarin underuse is common due to the complexity of anticoagulation care. Warfarin therapy with good INR control (patient-time in therapeutic range (TTR) > 75 %) is associated with lower event rates when compared to poor INR control (TTR < 60 %).10 The majority of patients on warfarin achieve only suboptimal INR control, as indicated by the mean TTR 60 % (range 55–64 %) of 22,000 patients in warfarin arms of three NOAC clinical trials.4–6 The cost-effectiveness analyses based upon the treatment outcomes of these trials reported that NOACs were more cost-effective than warfarin when anticoagulation control was suboptimal.11–17
For anticoagulation centers with average TTR of 60 % or below, the possible options are either the use of more expensive NOACs, or providing additional resources to improve TTR. Various therapeutic strategies to improve TTR have been examined and the findings suggest that employment of a clinical factor-guided dosing algorithm,18 frequent INR monitoring,19–21 and management of anticoagulation care by specialists in the use of anticoagulants22,23 are effective interventions. Each alternative has different economic and clinical implications for patients, clinicians and decision-makers to consider. The objective of the present study was to evaluate the cost-effectiveness of NOACs compared to warfarin therapy at various levels of TTR in patients with atrial fibrillation, from the perspective of healthcare provider.
METHODS
Decision Model
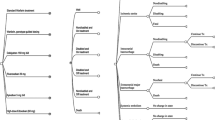
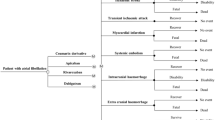
The life-long outcomes of two types of anticoagulants in a hypothetical cohort of 65-year-old patients with newly diagnosed atrial fibrillation were simulated by a Markov model (Fig. 1): (1) Adjusted-dose vitamin K antagonist (warfarin) and (2) NOACs. Markov modeling is a form of decision analysis in which hypothetical patients proceed through different Markov health states over time, based on probability inputs of the model. The Markov states of this model included well, post-myocardial infarction, post-extra-cranial bleeding, major, mild or no neurologic deficit as a result of ischemic stroke or intra-cranial bleeding, and dead. Patients of all treatment arms entered the model at the “well” Markov state. In each cycle, they might or might not experience an event (myocardial infarction, ischemic stroke or major bleeding) and transit to other Markov health states. Two tiers of outcomes were simulated for each study arm: Total direct medical cost and quality-adjusted life-years (QALYs) gained were calculated over a maximum period of 25 years with monthly cycles. Comparisons of cost and QALYs between NOACs and warfarin therapy were performed at different TTR levels.
The patient selection criteria were adopted from those of the NOACs clinical trials.4–6 Patients with atrial fibrillation aged 65 years or above with high risk for stroke (CHADS2 score of 2 or above) were included. Exclusion criteria included presence of severe heart-valve disorders or severe stroke within 6 months, and creatinine clearance of < 30 ml per minute. The warfarin dose was adjusted to an INR of 2–3. The INR control might be in, below or above the target range, and patients might consequently experience bleeding or ischemic events. In the NOACs arm, patients were initiated on a NOAC (dabigatran 150 mg twice daily, rivaroxaban 20 mg daily, or apixaban 5 mg twice daily). Patients who survived ischemic stroke resumed the initial anticoagulation therapy. Those who survived an intra-cranial bleeding event stopped the current anticoagulation therapy and started on aspirin alone. Patients who experienced extra-cranial bleeding might resume the initial anticoagulation therapy or switch to aspirin.24
Clinical Inputs
The clinical inputs of the model were retrieved from the literature. A Literature search on MEDLINE over the period from 1990 to 2013 was performed using keywords “atrial fibrillation”, “warfarin”, “dabigatran”, “rivaroxaban”, “apixaban”, “bleeding”, “thromboembolism”, “QALY” and “INR”. The selection criteria of clinical trials on anticoagulation treatment and related events were: (1) reports in English; (2) patients were at least 18 years of age; and (3) control of INR and/or the incidence of major events (bleeding or ischemic event) were reported. All articles retrieved by this process were screened for relevance to the present model. Case reports were excluded. A publication was included if it had data relevant to the model inputs. The preferred types of studies were meta-analyses and randomized controlled trials. When there were multiple sources available for a model input, the base-case value of this variable would be estimated using the pooled average weighted against the number of patients in each study. When both randomized and observational trials provided data for a model input, the base-case value of this variable was derived from randomized trials. Data from both randomized and observational trials provided the range of this variable for sensitivity analysis.
Clinical inputs are shown in Table 1. Outcome analysis reported that TTR > 75 % was associated with lower event rates, whereas TTR < 60 % had higher event rates.10 In the three clinical trials of NOACs versus warfarin for patients with atrial fibrillation, the weighted mean TTR in warfarin groups of was estimated to be 60 %.4–6 Comparisons of cost and QALYs between NOACs and warfarin therapy were therefore performed at TTR levels of 60 %, 70 % and 75 %.
Out-of-range INR was defined as < 1.8 or > 3.2. The rates of major bleeding (including intracranial and extracranial hemorrhage) and ischemic stroke in therapeutic range of INR (INR ≤ 3 and INR ≥ 2) and the risks for stroke in under-coagulated patients (INR < 2) and major bleeding in over-coagulated patients (INR > 3) were estimated in a meta-analysis of outcomes of warfarin anticoagulation for patients with atrial fibrillation.25 The risk of major bleeding in under-coagulated patients and major thromboembolic events in over-coagulated patients were both assumed to be the same as patients with in-range INRs.
The relative risks of major bleeding, ischemic event and myocardial infarction of the NOAC groups, compared to warfarin, were obtained from the results of the meta-analysis.26 The rate of ischemic stroke and percentage of ischemic strokes with major, minor or no deficit on aspirin were derived from prospective trials.4,27–29 The rate of major bleeding on aspirin was estimated from relative risk of bleeding on aspirin versus warfarin and the bleeding rate.30,31 The mortality rates of intracranial hemorrhage, extracranial hemorrhage, ischemic stroke and acute myocardial infarction within 30 days of an event were estimated from observational studies.29,32,33
Utility and Cost Inputs
The QALYs gained in each study arm were estimated from the time spent in different states (on warfarin, NOAC or aspirin, myocardial infarction, major neurologic deficit, mild neurologic deficit, no neurologic deficit, major hemorrhage and dead) and the utility score of each health state (Table 1).34–37 The QALYs were discounted with a rate of 3 % annually.38 The one-time treatment cost and monthly cost of major events (extracranial hemorrhage, intracranial hemorrhage, stroke and myocardial infarction) were estimated from the perspective of healthcare payers.39–42 The monthly cost of anticoagulation care management, including staff time, laboratory tests and administrative cost, was estimated from economic analyses on anticoagulation care.43 The potential increment of anticoagulation service cost to improve anticoagulation control was examined from a range of no increment to three-fold of the anticoagulation service cost, for extra costs due to increased INR testing frequency and hiring more experienced clinicians to the service. The monthly warfarin drug cost was estimated from retail pricing of generic warfarin.44 The costs of apixaban, dabigatran and rivaroxaban were retrieved from retail pricing and were found to be comparable (United States Dollar [USD] 150 per month). The drug cost of the NOACs was examined over a wide range (USD 109-240) to identify the threshold value.44 The monthly monitoring cost for NOACs (including drug therapy compliance and safety) was estimated from the cost for medication therapy management.45 All costs were discounted to 2013 costs with an annual rate of 3 %.
Cost-Effectiveness Analysis and Sensitivity Analysis
The incremental cost per QALY gained (ICER) of a more effective option compared to the less effective arm was calculated using the following equation: Δcost/ΔQALYs. Using the threshold of USD 50,000 as the willingness-to-pay per QALY, the most effective strategy with an ICER of USD 50,000 or less was considered as cost-effective.46
Sensitivity analysis was performed by TreeAge Pro 2009 (TreeAge Software, Inc., Williamstown, MA, USA) and Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA), to examine the robustness of the model results, with variation of all parameters. Threshold values of influential factors were identified by one-way sensitivity analysis over the high/low values. To evaluate the impact of the uncertainty in all variables simultaneously, a probabilistic sensitivity analysis was performed using Monte Carlo simulation. The cost and QALYs of each study arm were recalculated 10,000 times by randomly drawing each of the model input from a triangular probability distribution.
RESULTS
The expected life-long cost and QALYs in the NOACs arm were USD 98,524 and 9.970, respectively. Both cost and QALYs of the NOACs arm were consistently higher than those of warfarin at various levels of TTR and at different increments of monthly cost of anticoagulation care (Table 2). Using the threshold of USD 50,000 as the willingness-to-pay per QALY, the NOACs group was more cost-effective than warfarin arm when the TTR of warfarin therapy was 60 %, or the monthly cost of warfarin care was increased by two-fold or above to achieve 70 % TTR. Warfarin therapy was more cost-effective than the NOACs when TTR of warfarin was 70 % with up to 1.5-fold increment in monthly cost of care, or when TTR reached 75 % with monthly cost of warfarin care increased up to three-fold.
Using TTR of 60 % and no increment in monthly cost of anticoagulation care as the base-case model input, one-way sensitivity analysis was performed, and the results showed that the cost-effectiveness of NOACs group (base-case ICER = USD 35,804) was sensitive to: (1) TTR of the warfarin anticoagulation control, (2) monthly drug cost of NOACs, (3) relative risk of ischemic stroke with NOACs versus warfarin, and (4) utility value of NOACs. Figure 2 shows the variation of ICER of the NOACs over the ranges of these four variables. The ICER of NOACs would become > USD 50,000 (and warfarin would be more cost-effective) when TTR of warfarin anticoagulation control was > 67 %, monthly drug cost of NOACs was > USD 200, relative risk of ischemic stroke with NOACs was > 0.831, or the utility value of NOACs was < 0.981. The variation of these four model inputs would change the ICER of NOACs from USD 22,386 to as high as > USD 79,000.
Three-way sensitivity analysis was further conducted to examine the effect of NOAC monthly cost and increment of monthly cost of anticoagulation care on the cost-effectiveness of the two study arms at three levels of TTR (60 %, 70 % and 75 %) (Fig. 3a–c). The results indicated that the NOACs would be more cost-effective than warfarin therapy managed at TTR of 60 % if the monthly cost of NOACs was < USD 200. At higher TTR levels (70 % and 75 %), lower monthly drug cost was needed (< USD 122–185 and < USD 85–145, respectively) for the NOACs to be more cost-effective than warfarin therapy.
The probabilistic sensitivity analysis was performed by 10,000 Monte Carlo simulations at TTR = 60 %, 70 % and 75 %. The probabilities of each strategy to be cost-effective at different TTR levels were examined in the acceptability curve over a wide range of willingness-to-pay per QALY, from USD 0 to USD 150,000 (Fig. 4a–c). Using USD 50,000 as the threshold willingness-to-pay, the NOACs were cost-effective 83.6 %, 50.7 % and 32.7 % of the time at TTR of 60 %, 70 % and 75 %, respectively. If the willingness-to-pay threshold further extended to USD 100,000 per QALY, the probabilities of NOACs to be cost-effective at TTR of 60 %, 70 % and 75 % would become 98.6 %, 87.7 % and 74.0 % of time, correspondingly.
DISCUSSION
In the present study, the potential life-long cost and effectiveness of NOACs versus warfarin therapy controlled at different levels of TTR for patients with atrial fibrillation were examined. The ICER of NOACs (USD 35,804) was well below the threshold of cost-effectiveness (USD 50,000 per QALY) at low TTR (60 %). These results were compatible with a recent cost-effectiveness analysis of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation, in which the ICERs of each NOAC versus warfarin ranged between USD 3,190 and USD 15,026 per QALY.17 In this study, Harrington et al. compared each NOAC with warfarin using the event rates from three NOAC clinical trials on patients with atrial fibrillation.4–6 The mean TTRs of warfarin arms in these trials were 55–64 %, similar to the base-case anticoagulation control in the warfarin arm of present study. Harrington’s data therefore supported the base-case analysis results of NOACs versus warfarin (at TTR level of 60 %).
If warfarin therapy was controlled at high TTR (75 %), the additional cost versus the marginal gain in QALYs of NOACs exceeded the threshold of USD 50,000 per QALY, even when the increment in monthly cost for anticoagulation service was as high as three-fold. The 10,000 Monte Carlo simulations verified the cost-effectiveness results at different TTR levels; the probability of NOACs to be cost-effective was high (83.6 % of the time) at low TTR (60 %), and it decreased to 32.7 % of the time at high TTR levels (75 %).
The one-way sensitivity analysis also found a threshold value for utility of NOACs (< 0.981) that would weaken the cost-effectiveness of NOACs. It is believed that all NOACs requiring less periodic blood testing and follow-ups would have better quality of life (thus higher utility value) than warfarin therapy. The base-case utility value for all NOACs (0.994) was therefore higher than that of warfarin (0.987). The current finding is consistent with previously reported cost-effectiveness analyses of dabigatran versus warfarin, in which the ICER of dabigatran would increase as its utility score decreased.13,47
The three-way sensitivity analysis explored the interaction of two cost-driving factors (monthly cost of NOACs and increment in monthly cost of anticoagulation service), as well as different levels of INR control on the cost-effectiveness of warfarin and NOACs. The results showed that at no increment in monthly service cost, warfarin therapy would be the cost-effective option at TTR 60 %, 70 % and 75 % if the monthly drug cost of NOACs was >USD 200, >USD 122 and >USD 85, respectively. When the cost of service increased, better anticoagulation control of warfarin therapy with higher TTR would be required for it to be cost-effective.
The present results were supported by the findings reported by Shah and Gage, that dabigatran would be more cost-effective than warfarin therapy when the TTR was < 57.1 %, and warfarin therapy would be more cost-effective than dabigatran when the average TTR was > 72.6 %.16 It is understood that additional resources would be required to improve anticoagulation control, yet the impact of increment in cost of service for higher TTR was not factored in previous cost-effectiveness studies of warfarin versus NOAC. The cost of service to achieve better anticoagulation control could have important influence on the cost-effectiveness comparison between NOACs and warfarin. In the present study, the effect of various levels of service cost and anticoagulation control were examined thoroughly in both base-case analysis and sensitivity analyses, and these findings provided crucial information for the cost-effectiveness consideration between improving anticoagulation control of warfarin and the use of NOACs.
This study is an example of decision analysis to compare the potential changes in economic and clinical outcomes of using NOACs versus investing resources on anticoagulation service to improve INR control of warfarin therapy. The results demonstrated a number of influential factors (relative risk reduction of stroke by NOACs, drug cost of NOACs, TTR of an anticoagulation service and the corresponding cost) that indicated the target values for warfarin management and the NOACs to be cost-effective, and therefore assisted clinicians and administrators to be better informed on the decision of resource allocation for anticoagulation therapy.
The present model was limited by projecting life-long events using key model inputs from clinical trials of 2-year follow-up. Projecting life-long outcomes using short-term clinical trial data may weaken the robustness of the model findings. Continuing monitoring the post-market surveillance data of NOACs is warranted to update the decision model. The cost items were limited to the resources of anticoagulation therapy and related complications. The current model only simulated benefits of investing resources in anticoagulation service for patients with atrial fibrillation, and outcomes of other warfarin users (such as patients with mechanical heart valve replacement) were not considered. The full benefits of investing resources on anticoagulation service might therefore be underestimated. All the model inputs were examined in sensitivity analysis and probabilistic sensitivity analysis over a wide range to test for robustness of the results.
In conclusion, the NOACs appeared to gain higher life-long QALYs and to cost more than warfarin therapy. The acceptance of the NOACs as a more cost-effective option than warfarin therapy is highly dependent upon the level of anticoagulation control for warfarin, cost for anticoagulation service, and drug cost of the NOACs.
REFERENCES
Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–37.
You JHS, Chan FWH, Wong RSM, Cheng G. Is INR, between 2.0 and 3.0 the optimal level for Chinese patients on warfarin therapy for moderate-intensity anticoagulation? Br J Clin Pharmacol. 2005;59:582–7.
Mantha S, Cabral K, Ansell J. New avenues for anticoagulation in atrial fibrillation. Clin Pharmacol Ther. 2013;93:68–77.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients atrial fibrillation. N Engl J Med. 2011;365:981–92.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Lip GYH, Larsen TB, Skjoth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2010;60:738–46.
Rasmussen LH, Larsen TB, Graungaard T, Skjoth F, Lip GYH. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012;345:e7097. doi:10.1136/bmj.e7097.
Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:480–6.
White HD, Cruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control. Arch Intern Med. 2007;167:239–45.
Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman GI. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845–51.
Lee S, Mullin R, Blasawski J, Coleman GI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PLoS ONE. 2012;7:e47473. doi:10.1371/journal.pone.0047473.
Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fribrillation. Ann Intern Med. 2011;154:1–11.
Soresens SV, Kansal AR, Connolly S, Reng S, Linnehan J, Bradley-Kennedy C, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillatioin: A Canadian payer perspective. Thromb Haemost. 2011;105:908–19.
Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economics analyses. BMJ. 2011;343:d6333. doi:10.1136/bmj.d6333.
Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123:2562–70.
Harrington AR, Armstrong EP, Nolan PE, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–81.
Kim YK, Nieuwlaat R, Connolly SJ, et al. Effect of a simple two-step warfarin dosing algorithm on anticoagulant control as measured by time in therapeutic range: a pilot study. J Thromb Haemost. 2010;8:101–6.
Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulations: A literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000;9:283–92.
Bloomfield HE, Krause A, Greer N, et al. Meta-analysis: effect of patient self-testing and self-management of long-term anticoagulation on major clinical outcomes. Ann Intern Med. 2011;154:472–82.
Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet. 2006;367:404–11.
Chan FWH, Wong RSM, Lau WH, Chan TYK, Cheng G, You JHS. Management of Chinese patients on warfarin therapy in two models of anticoagulation service — A prospective randomized trial. Br J Clin Pharmaco l. 2006;62:601–9.
Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158:1641–7.
Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484–91.
Reynolds MW, Fahrbach K, Hauch O, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation. A systematic review and metaanalysis. Chest. 2004;126:1938–45.
Dentali F, Riva N, Crowther M, Turpie AGG, Lip GYH, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation. A systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–91.
Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–8.
Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–92.
Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–26.
van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–8.
Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503.
Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–5.
Hanger HC, Fletcher VJ, Wilkinson TJ, Brown AJ, Frampton CM, Sainsbury R. Effect of aspirin and warfarin on early survival after intracerebral haemorrhage. J Neurol. 2008;255:347–52.
Gage BF, Cardinalli AB, Owen DK. The effect of stroke and stroke prophylaxis with aspirin and warfarin on quality of life. Arch Intern Med. 1996;156:1829–36.
Thomson R, Parkin D, Ecclles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet. 2000;355:956–62.
Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102.
Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–20.
Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2005:189–90.
Centers for Medicare & Medicaid Services website (assessed on Nov 6, 2012): http://www.cms.gov/MedicareFeeforSvcPartsAB/03_MEDPAR.asp
Agency for Healthcare Research and Policy. HCUPnet. http://hcupnet.ahrq.gov/. Accessed Nov 6, 2012.
Mark DB, Knight JD, Cowper PA, Davidson-Ray L, Anstrom KJ. Long-term economic outcomes associated with intensive versus moderate lipid-lowering therapy in coronary artery disease: results from the Treating to New Targets (TNT) Trial. Am Heart J. 2008;156:698–705.
Tsevat J, Kuntz KM, Orav EJ, Weinstein MC, Sacks FM, Goldman L. Cost-effectiveness of pravastatin therapy for survivors of myocardial infarction with average cholesterol levels. Am Heart J. 2001;141:727–34.
Menzin J, Boulanger L, Hauch O, et al. Quality of anticoagulation control and costs of monitoring warfarin therapy among patients with atrial fibrillation in clinic settings: A multi-site managed-care study. Ann Pharmacother. 2005;39:446–51.
www.pharmacychecker.com. Accessed on 1 February 2013.
Barnett MJ, Frank J, Wehring H, et al. Analysis of pharmacist-provided medication therapy management (MTM) services in community pharmacies over 7 years. J Manag Care Pharm. 2009;15:18–31.
Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russel LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1253–8.
You JHS, Tsui KKN, Wong RSM, Cheng G. Cost-effectiveness of dabigatran versus genotype-guided management of warfarin therapy for stroke prevention in patients with atrial fibrillation. PLoS One. 2012;7:e39640. doi:10.1371/journal.pone.0039640.
Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L, Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875–6.
ACTIVE Writing Group of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel TrialWith Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12.
Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17.
Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459–67.
O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293:699–706.
Acknowledgements
This work was funded by General Research Fund from the Research Grants Council of the Hong Kong (Project no. CUHK477612).
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, J.H.S. Novel Oral Anticoagulants Versus Warfarin Therapy at Various Levels of Anticoagulation Control in Atrial Fibrillation—A Cost-Effectiveness Analysis. J GEN INTERN MED 29, 438–446 (2014). https://doi.org/10.1007/s11606-013-2639-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-013-2639-2