Abstract
Background
Strokes attributed to atrial fibrillation (AF) represent a major cause of adult disability and a great burden to society and healthcare systems.
Objectives
Our objective was to assess the cost effectiveness of apixaban, a direct acting oral anticoagulant (DOAC), versus warfarin or aspirin for patients with AF in the Greek healthcare setting.
Methods
We used a previously published Markov model to simulate clinical events for patients with AF treated with apixaban, the vitamin K antagonist (VKA) warfarin, or aspirin. Clinical events (ischemic and hemorrhagic stroke, intracranial hemorrhage, other major bleed, clinically relevant non-major bleed, myocardial infarction, and cardiovascular [CV] hospitalizations) were modeled using efficacy data from the ARISTOTLE and AVERROES clinical trials. The cohort’s baseline characteristics also sourced from these trials. Among VKA-suitable patients, 64.7% were men with a mean age of 70 years and average CHADS2 (cardiac failure, hypertension, age, diabetes, stroke2) score of 2.1, whereas 58.5% of VKA-unsuitable patients were men with a mean age of 70 years and a CHADS2 score of 2.0. A panel of experts (cardiologists and internists) provided information on the resource use associated with the management of AF. Cost calculations reflect the local clinical setting and a third-party payer perspective (€, discounted at 3%).
Results
Based on a simulation of 1000 VKA-suitable patients over a lifetime horizon, the use of apixaban versus warfarin resulted in 26 fewer strokes and systemic embolisms in total, 65 fewer bleeds, 41 fewer myocardial infarctions, and 29 fewer CV-related deaths, with an incremental cost-effectiveness ratio (ICER) of €14,478/quality-adjusted life-year (QALY). For VKA-unsuitable patients, apixaban versus aspirin resulted in 72 fewer strokes and systemic embolisms and 57 fewer CV-related deaths, with an ICER of €7104/QALY. Sensitivity analyses indicated that results were robust.
Conclusions
Based on the present analysis, apixaban represents a cost-effective treatment option versus warfarin and aspirin for the prevention of stroke in patients with AF from a Greek healthcare payer perspective over a lifetime horizon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The novel oral anticoagulant apixaban represents a cost-effective option versus aspirin and warfarin for the prevention of stroke and systemic embolism in patients with atrial fibrillation (AF) in the Greek healthcare setting. |
Use of apixaban versus warfarin in the treatment of AF leads to an incremental quality-adjusted life-year (QALY) gain of 0.222 and an incremental cost-effectiveness ratio (ICER) of €14,478/QALY from a Greek healthcare payer perspective. |
For VKA-unsuitable patients with AF treated in the Greek healthcare setting, apixaban compared with aspirin resulted in an incremental QALY gain of 0.284 and an estimated ICER of €7104/QALY. |
1 Introduction
Atrial fibrillation (AF) is a heart rhythm disorder that causes poor blood flow and increases the likelihood of heart failure and stroke events [1]. It is very often intermittent, and its symptoms, such as irregular pulse, shortness of breath, and palpitations, are unspecific and sporadic [2, 3]. As a result, about one-third of patients with AF remain undiagnosed and untreated, with inestimable consequences to their health and quality of life [4].
The ever-increasing aging population, with associated increases in hypertension, diabetes, and thyroid disease, which represent underlying causes of AF, inevitably increase the prevalence of AF, which is expected to double by 2050 compared with 2004, to reach 4% of the global population [5, 6]. Worldwide, there were an estimated 33.5 million patients with AF in 2010, with 5 million new cases occurring each year [7]. In Greece, the overall AF prevalence was estimated to be about 3.9% of the general population in 2002–2003, showing an increasing trend with increasing age [8, 9].
AF is associated with a fivefold risk of stroke and a threefold incidence of congestive heart failure and higher mortality [10, 11]. About 15–20% of all strokes are attributed to AF and result in severe disability and increased probability of death [12, 13]. Furthermore, cardioembolic stroke events are much more severe in patients with AF than in non-AF patients and increase the risk of remaining disabled by 50% [12, 13]. Indeed, strokes attributed to AF represent a major cause of adult disability and are more likely to be fatal, inflicting a greater burden to society and healthcare systems.
The number of hospitalized patients with AF has increased by 60% in the past 20 years, and this, in combination with the severity of the stroke events experienced by patients with AF, inevitably leads to a substantial increase of the associated healthcare costs [14]. The mean cost of a severe stroke was estimated to be about three times higher than that of a mild stroke, mainly because severe stroke extends the hospitalization length by 20% (4–8 days in European countries) and requires intensive hospital care. More specifically, in Greece, the mean cost per patient and per day in an acute stroke unit was calculated at €2864 and €244, respectively [15]. One study estimated that the mean inpatient cost of a patient with AF could reach €6445 with the total cost of stroke in Europe being estimated at about €38 billion and the total annual cost of AF in Greece was €272 in 2007, with 1-year follow-up assessed at €1507 per patient and mean inpatient admission at €1363 [16, 17]. Therefore, to reduce the overall burden of stroke, the main target should be AF management.
Management of AF consists of relieving symptoms and controlling the risk of stroke [11, 18]. Pharmaceutical treatment includes administering heart rate and/or antiarrhythmic agents, and international guidelines recommend antithrombotic therapy in both cases depending on the risk of stroke [19]. The most recent European guidelines recommend that patients with AF who have more than one stroke risk factor should receive effective stroke-prevention therapy, either with oral anticoagulants (OACs), such as vitamin K antagonists (VKAs) with well-controlled VKA therapy, or one of the direct acting oral anticoagulants (DOACs) [11]. Regarding antiplatelet drugs, acetylsalicylic acid (aspirin) is widely used in patients with AF and a low risk of stroke or in those who are unable to receive warfarin [20]. However, both therapies present several limitations that restrict their administration.
Warfarin has been shown to be very effective in preventing stroke. Several systematic reviews have revealed that warfarin can reduce the risk of stroke by 62–68% and the risk of death by 26–33% [21]. Nevertheless, warfarin is characterized by a very narrow therapeutic window, and regular blood pressure monitoring is required to ensure patients remain within optimal ranges, i.e., between 2.0 and 3.0 as defined by the international normalized ratio (INR) [19]. Thus, patients in whom the INR is too high are at increased risk of bleeding, whereas patients in whom the INR is too low are inadequately protected from stroke. Moreover, warfarin has been found to strongly interact with food and other drugs, further complicating its maintenance within the therapeutic ranges, and justifying its underuse [22, 23]. On the other hand, aspirin reduces the risk of all strokes by approximately 22% compared with placebo but is associated with an increased risk of bleeding and is less effective than warfarin [24, 25]. Hence, these limitations highlight the need for more effective and safer OACs.
Apixaban is a direct factor Xa inhibitor approved by the European Medicines Agency and is used to prevent stroke as well as blood clots in other organs in patients with AF who present one or more risk factors (previous stroke, hypertension, diabetes, heart failure) or are aged >75 years [26]. Two primary clinical studies, ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) and AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment), have highlighted the efficacy of apixaban. ARISTOTLE compared apixaban with warfarin, whereas AVERROES compared apixaban with aspirin in patients unable to tolerate warfarin. In both trials, apixaban proved superior in preventing stroke or systemic embolism [27, 28].
Healthcare systems must prioritize effective and high-quality management of AF to alleviate the economic and societal burden it engenders [29] and are therefore obliged to restrain their expenses as well as provide patients with the most efficacious treatments. In countries under financial constraints, such as Greece, adequate resource allocation is key factor in achieving this, especially for diseases associated with high clinical and economic burden such as AF. In that context, economic evaluations of new treatments provide evidence of their value from clinical and economic viewpoints.
The present study aimed to perform a cost-effectiveness analysis of apixaban versus treatment strategies using warfarin for VKA-suitable or aspirin for VKA-unsuitable patients in a setting under fiscal restraints, as in the case of Greece.
2 Methods
2.1 Model Structure
We adapted a previous Markov decision tree model [30] for the Greek healthcare setting to analyze the cost effectiveness of apixaban versus warfarin or aspirin for VKA-suitable or VKA-unsuitable patients, respectively. The model included the following mutually exclusive health states: non-valvular AF (NVAF), ischemic stroke (mild, moderate, severe), hemorrhagic stroke (mild, moderate, severe), myocardial infarction (MI), systemic embolism (SE), NVAF without anticoagulation, and death, which was considered as an absorbing health state in which patients remained for the rest of the simulation. All patients started at the NVAF state and could remain in the NVAF state until one stroke, bleed (intracranial hemorrhage [ICH], other major bleed, or clinically relevant non-major [CRNM] bleed), systemic embolism, MI, cardiovascular hospitalization unrelated to the events modelled, or death. We applied half-cycle correction to avoid overestimation or underestimation of the expected values.
We used a lifetime horizon and discounted costs and health outcomes after the first year at 3%. The perspective was that of the Greek healthcare system.
2.2 Model Population
The base-case model simulated a cohort of 1000 patients with NVAF and different stroke risks per treatment arm. Patients were assigned to receive apixaban if they were considered ‘VKA suitable’ and aspirin if ‘VKA unsuitable’. The model also allowed a mixed analysis of the two subpopulations.
Baseline characteristics for VKA-suitable and VKA-unsuitable patients were sourced from AVERROES and ARISTOTLE, respectively [26, 27]. The inputs required were mean age, sex, and CHADS2 (cardiac failure, hypertension, age, diabetes, stroke2) score distribution information. Mean age and sex reflect predicted life expectancy, whereas CHADS2 is an index that assesses the risk of patients experiencing a stroke event and is therefore used to guide anticoagulation therapy. In the analysis, 77% of patients were VKA suitable: males 64.7%, mean age 70 years, mean CHADS2 score 2.1. The rest were VKA unsuitable: males 58.5%, mean age 70 years, average CHADS2 score 2.0.
2.3 Risk of Clinical Events
Data concerning clinical event rates also derived from AVERROES (apixaban 5 mg twice daily vs. aspirin 81–324 mg/day) and ARISTOTLE (apixaban 5 mg twice daily vs. warfarin, dose-adjusted to maintain an INR of 2.0–3.0) [27, 28]. Table 1 depicts the clinical event rates for apixaban per 100 patients and the hazard ratios (HRs) of the comparators versus apixaban obtained from these studies.
The model allows stroke, bleeding, and MI risks to be adjusted, taking into account their increased risk with ageing. These risks were increased by 1.40 for stroke, 1.97 for bleeding, and 1.30 for MI per decade, as reported in the literature. Severity distributions for stroke and hemorrhagic stroke according to the modified Rankin scale (mRS) score were also obtained from AVERROES and ARISTOTLE (Table 2).
2.4 Resource Use and Cost
As Greek-specific data were lacking, we obtained information on resource use from an expert panel of healthcare practitioners (cardiologists and internists) to reflect local clinical practice (additional detail is provided in the electronic supplementary material). The costs associated with patient monitoring and resource use are reported in €, year 2015 values, and only direct medical costs are considered. The analysis was undertaken from a third-party payer perspective, with a lifetime horizon, and costs and outcomes were discounted at a rate of 3% per annum.
Treatment prices were sourced from the most recent official price list available at the time of model adaptation (December 2015, Price Bulletin, Greek Ministry of Health [31]). A cost was attributed to the occurrence of each acute clinical event and to the recommended monthly maintenance treatment. Costs for acute care episodes were derived from the Greek diagnosis-related group (DRG) price list and a study by Gioldasis et al. [35]. Maintenance costs were calculated based on the proportion of acute to total per year costs, as previously reported [36], and the allocation of (the remaining) maintenance costs were calculated on a monthly basis (Table 3).
2.5 Utilities
Utility inputs were sourced from a UK-based utility catalog [37] and assigned to patients according to their health states. Utility estimations were calculated by applying a baseline AF-specific utility score and subtracting the disutility decrements associated with each event, modeled for a particular duration of time [37, 38] (Table 4).
2.6 Model Outcomes
For each treatment strategy, model outcomes included cardiovascular events as well as the number of life-years and quality-adjusted life-years (QALYs) gained. Cost outcomes per treatment alternative comprised costs attributed to therapy, routine care, monitoring, cardiovascular events, and management.
Treatment alternatives were compared by estimating the incremental cost-effectiveness ratios (ICERs), expressed as the cost per QALY gained, comparing apixaban with warfarin for VKA-suitable patients or with aspirin for the VKA-unsuitable population.
2.7 Sensitivity Analysis
We conducted extensive univariate and probabilistic sensitivity analyses (PSA) to test the robustness of the model and the corresponding results.
Univariate sensitivity analysis was used to examine the effect on results of changes in key model parameters. Each parameter was varied according to 95% confidence intervals and standard deviations when applicable, with keeping other parameters constant. If confidence intervals and standard deviations were unavailable, the standard deviation was assumed to be 25% of the mean.
We also conducted a PSA to test the robustness of the findings. A series of ICERs were calculated by running a PSA for 2000 simulations during which event rates, costs, and utilities were varied simultaneously by drawing random values based on a set of pre-specified types of distributions. The time horizon, population characteristics, and model settings were kept constant. We used Beta distributions for probabilities and utilities of clinical events, Gamma distributions for cost inputs and, finally, Dirichlet distributions for patient distribution among a number of different occurrences, such as stroke severity.
3 Results
3.1 Base-Case Analysis
Based on a simulation of a cohort of 1000 VKA-suitable patients over a lifetime, the use of apixaban versus warfarin was predicted to reduce the number of cardiovascular events, increase the number of life-years and QALYs gained, and yield an ICER less than an implicit threshold of €30,000/QALY. Currently, there is no official willingness-to-pay threshold in Greece. The World Health Organization recommends a threshold corresponding to three times per capita gross domestic product (GDP), i.e., €72,918/QALY in Greece [39, 40]. However, we adopted a more conservative implicit threshold of €30,000/QALY, reflecting the context of economic crisis. The drug-acquisition cost is higher for apixaban, but this is offset because the clinical event costs are lower than for warfarin and aspirin (Table 5).
More specifically, when compared with warfarin, apixaban resulted in 26 fewer strokes (ischemic and hemorrhagic, including recurrent strokes) and systemic embolisms in total, 65 fewer bleeds (including other ICH, major bleeds, and CRNM bleeds), 41 fewer MIs, and 29 fewer cardiovascular-related deaths, but 28 more cardiovascular-related hospitalizations (Table 5). The incremental gain in QALYs with apixaban versus warfarin was estimated to be 0.222 at an incremental cost of €3210.11, resulting in an ICER of €14,477.55/QALY
For the cohort of VKA-unsuitable patients, apixaban resulted in 72 fewer strokes (ischemic and hemorrhagic, including recurrent strokes) and systemic embolisms and 57 fewer cardiovascular-related deaths than did aspirin. However, it also engendered 107 more bleeds (including other ICHs, major bleeds, and CRNM bleeds) and 37 more cardiovascular-related hospitalizations. The incremental gain in QALYs for that population was 0.284 at an incremental cost of €2019.24, and the respective ICER was therefore estimated at €7104.31/QALY (Table 5).
3.2 Univariate Sensitivity Analysis
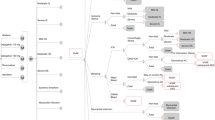
According to the results of the univariate sensitivity analysis, the daily cost of apixaban and that of the comparator, and the utility values for AF, were the key parameters that influenced the ICER for the VKA-suitable cohort. Apixaban always remained a cost-effective strategy, with ICERs ranging from €7172.54/QALY to €20,281/QALY (Fig. 1).
Tornado charts illustrating results from the univariate sensitivity analysis for the incremental cost-effectiveness ratio for (a) vitamin K antagonist-suitable and (b) VKA-unsuitable cohorts. AF atrial fibrillation, CFR case-fatality rate, CRNMB clinically relevant non-major bleed, HR hazard ratio, ICER incremental cost-effectiveness ratio, ICH intracranial hemorrhage, MI myocardial infarction, OMB other major bleed, SE systemic embolism, SEFU systemic embolism follow-up, VKA vitamin K antagonist
For VKA-unsuitable patients, the stroke risk for apixaban was the key driver of the model, while the daily cost of apixaban and the age of patients also significantly influenced the ICERs. As previously, the outcomes of the analysis were favorable for apixaban, with the relevant ICERs ranging from €4554.53/QALY to €13,551.80/QALY, well below a willingness-to-pay threshold of €30,000/QALY.
3.3 Probabilistic Sensitivity Analysis
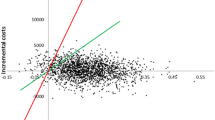
The results of the PSA suggest that the findings of the cost-effectiveness analysis were robust. Apixaban was found to be more effective and more costly than warfarin and aspirin, as suggested by the cost-effectiveness planes in which the PSA iterations were concentrated in the north-east quadrant. Given a willingness-to-pay threshold of €30,000/QALY, apixaban was cost effective in 79 and 76% of cases compared with warfarin and aspirin, respectively (Fig. 2).
4 Discussion
This analysis was performed to assess whether apixaban can be considered a first-line cost-effective alternative to warfarin and aspirin for the prevention of stroke in patients with AF, particularly in a setting under financial constraints where a plethora of health cost-containment policies have been implemented over the last few years. Indeed, in a time where health resources are becoming even scarcer, healthcare systems are obliged to operate in an unfavorable economic environment. Implementation of adequate health policies and processes to ensure access to efficacious treatments is crucial, particularly when considering highly complex disorders such as AF that are characterized by an ever-increasing prevalence. In that context, cost-effectiveness analyses represent an indispensable tool for evaluating new pharmaceutical technologies.
Our findings suggest that apixaban is the optimal option for stroke prevention in patients with AF compared with standard of care. The ICERs of the analysis were estimated well below the willingness-to-pay threshold of €30,000/QALY, at €14,477.55/QALY and €7104.31/QALY versus warfarin and aspirin, respectively. In most cases, apixaban was associated with fewer cardiovascular events and better health outcomes.
In our study, apixaban was related to 107 more bleeds in total, in VKA-unsuitable patients, as well as with 37 cardiovascular-related hospitalizations. The first finding was mainly driven by non-major bleeding events (see Table 5), which is in line with the findings of AVERROES. More specifically, in this study, there was a non-significant difference with respect to major bleeding events between apixaban-treated and aspirin-treated VKA-unsuitable patients with AF. Moreover, non-major bleeds do not need hospitalization and thus do not encumber the national health system. With respect to the 37 more cardiovascular-related hospitalizations, one could hypothesize that these were of minor importance, since the number of strokes was significantly lower and the number of MIs was equal in apixaban-treated versus aspirin-treated patients. Last but not least, apixaban led to 57 fewer cardiovascular-related deaths than did aspirin.
The results of our analysis seem to generally be in line with other published studies. However, differences in methodology mean results may not always be directly comparable [30, 44–49]. Stevanović et al. [41] compared apixaban with warfarin from the perspective of the Netherlands healthcare system. The analysis resulted in an estimated ICER of €10,576/QALY, highlighting a lower number of events with apixaban [41], whereas the respective ratio from a French perspective was €12,227/QALY [42]. Kongnakorn et al. [43] compared apixaban with aspirin and found apixaban to be a cost-effective alternative to aspirin from a Belgian viewpoint, with an ICER of €7423/QALY, which is remarkably close to our findings. Several other studies have evaluated the comparative cost effectiveness of apixaban from European, US, and Canadian perspectives. As mentioned, differences in methodology and currencies do not allow direct comparison with our results [30, 44–49]. However, a systematic review of the economic models for newer anticoagulants highlighted that apixaban was dominant compared with aspirin from an economic viewpoint and cost effective when compared with warfarin, with ICERs ranging from $US11,400 to $US25,059 (year 2010–2011 values) [50].
Like all cost-effectiveness studies, our analysis may involve some limitations. First, because Greek-specific references are lacking, data for utilities and clinical inputs were sourced from ARISTOTLE and AVERROES or other references. These trials were multinational, so all the estimates derived from several countries. As such, some of the limitations of the present study derive from the limitations of ARISTOTLE and AVERROES. The main limitation of AVERROES was its early termination, which could eventually have inflated the benefit estimations. However, as stated by the investigators, the robustness of the findings was ensured as the statistical threshold for stopping the trial was very high [28]. The main limitations of ARISTOTLE could be considered to be that the observation period was 1.8 years and that it did not provide long-term information on the effects of apixaban. Nevertheless, these remained consistent over the study period. Moreover, about 31% of patients were also treated with aspirin [27]. Furthermore, although clinical trials are the gold standard for evaluating the outcomes of a therapy, they do not reflect real-world clinical practice. Patients are highly selected according to strict criteria and are monitored in a very tightly controlled environment, thus data do not represent real-world outcomes. Another limitation is the use of CHADS2 rather than CHA2DS2-VASC (congestive heart failure, hypertension, age >75 years2, diabetes, stroke, or transient ischemic attack2, vascular disease, age 65–74, sex) for stroke risk stratification. CHA2DS2-VASC overcomes the limitations of CHADS2, which is more likely to under-evaluate patient risk, by integrating more common risk factors. Finally, as stated, the analysis was undertaken from a third-party payer perspective and therefore does not include societal costs such as productivity losses and informal care costs, which can account for up to 21–25% of total costs for patients who have experienced cardiovascular events [51, 52]. Therefore, the benefits of apixaban may have been underestimated, as the inclusion of such costs in the analysis usually favors the treatment that averts the most clinical events compared with treatment alternatives.
5 Conclusions
Apixaban can be regarded as an optimal first-line treatment option for stroke prevention in the Greek healthcare setting. Reduced morbidity and mortality are the main advantages that can enhance adequate management of this condition. In that context, healthcare systems should be sufficiently prepared for the arrival of innovative drugs, albeit associated with high treatment costs. Indeed, health systems in settings with scarce health resources, an ageing population, and increased multiple comorbidities face new challenges. Therefore, the evidence for each treatment option should be considered meticulously with a view to wise resource allocation, not only for the sustainability of healthcare but also to ensure patient access to such therapies.
References
Steinberg JS. Atrial fibrillation: an emerging epidemic? Heart. 2004;90:239–40.
National Institute for Health and Clinical Excellence. Guideline 180-Atrial fibrillation: the management of atrial fibrillation. https://www.nice.org.uk/guidance/cg180/resources/guidance-atrial-fibrillation-the-management-of-atrial-fibrillation-pdf. Accessed Sept 2015.
Kaba RA, Camm AJ, Williams TM, Sharma R. Managing atrial fibrillation in the global community: the European perspective. Glob Cardiol Sci Pract. 2013;2013:173–184
Turakhia MP, Shafrin J, Bognar K, Goldman DP, Mendys PM, Abdulsattar Y, et al. Economic burden of undiagnosed nonvalvular atrial fibrillation in the United States. Am J Cardiol. 2015;116:733–9.
Moukabary T, Gonzalez MD. Management of atrial fibrillation. Med Clin North Am. 2015;99:781–94.
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25.
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47.
Korantzopoulos P, Andrikopoulos G, Vemmos K, Goudevenos JA, Vardas PE. Atrial fibrillation and thromboembolic risk in Greece. Hellenic J Cardiol. 2012;53:48–54.
Ntaios G, Manios E, Synetou M, Savvari P, Vemmou A, et al. Prevalence of atrial fibrillation in Greece: the arcadia rural study on atrial fibrillation. Acta Cardiol. 2012;67:65–9.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8.
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–413.
Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36:1115–9.
Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke. 2001;32:392–8.
Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–72.
Kritikou P, Spengos K, Zakopoulos N, Tountas Y, Yfantopoulos J, Vemmos K. Resource utilization and costs for treatment of stroke patients in an acute stroke unit in Greece. Clin Neurol Neurosurg. 2016;142:8–14.
Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996;27:1765–9.
Ringborg A, Nieuwlaat R, Lindgren P, Jönsson B, Fidan D, Maggioni AP, et al. Costs of atrial fibrillation in five European countries: results from the Euro Heart Survey on atrial fibrillation. Europace. 2008;10:403–11.
European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360–420.
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–98.
European Stroke Organisation. (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507.
Kirschhof P, Adamou A, Knight E, Lip GYH, Norrving B, de Pouvourville G, et al. Working Group Report: Stroke Prevention in Patients with Atrial Fibrillation. 2009. http://ec.europa.eu/health-eu/doc/strokecrisis.pdf. Accessed Sept 2015.
Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31:326–43.
Frykman V, Beerman B, Rydén L, Rosenqvist M, Medical Products Agency; Swedish Society of Cardiology. Management of atrial fibrillation: discrepancy between guideline recommendations and actual practice exposes patients to risk for complications. Eur Heart J. 2001;22:1954–9.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67.
Casado-Arroyo R, Lanas A, Brugada P. A balanced view of efficacy and safety of aspirin in cardiovascular diseases. Curr Pharm Des. 2015;21(35):5101–7.
European Medicines Agency. Summary of opinion (post authorisation)—Eliquis (apixaban). EMA. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/002148/WC500132869.pdf. Accessed Sept 2015.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom-Lundqvist C, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace. 2016;18:37–50.
Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35:1897–906.
Greek Ministry of Health. Official pricelists, Athens. http://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn. Accessed 15 Jan 2016.
Lopes RD, Al-Khatib SM, Wallentin L, Yang H, Ansell J, Bahit MC, et al. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet. 2012;380:1749–58.
Lip GY, Eikelboom J, Yusuf S, Shestakovska O, Hart RG, Connolly S, et al. Modification of outcomes with aspirin or apixaban in relation to female and male sex in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Stroke. 2014;45:2127–30.
Lip GY, Connolly S, Yusuf S, Shestakovska O, Flaker G, Hart R, et al. Modification of outcomes with aspirin or apixaban in relation to CHADS(2) and CHA(2)DS(2)-VASc scores in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Circ Arrhythm Electrophysiol. 2013;6:31–8.
Gioldasis G, Talelli P, Chroni E, Daouli J, Papapetropoulos T, Ellul J. In-hospital direct cost of acute ischemic and hemorrhagic stroke in Greece. Acta Neurol Scand. 2008;118:268–74.
Geitona M, Papadimitriou A, Kyriopoulos J. Economic Evaluation of prevention of cerebral strokes via the administration of dipyridamol and aspirin twice daily. Neurologia. 1999;8:250–7.
Sullivan P, Slejko J, Sculpher M, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31:800–4.
Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11.
Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28.
World Health Organization. Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health. In: WHO Commission on Macroeconomics and Health: Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health. Geneva: WHO; 2001.
Stevanović J, Pompen M, Le HH, Rozenbaum MH, Tieleman RG, Postma MJ. Economic evaluation of apixaban for the prevention of stroke in non-valvular atrial fibrillation in the Netherlands. PLoS One. 2014;9:e103974.
Lanitis T, Cotté FE, Gaudin AF, Kachaner I, Kongnakorn T, Durand-Zaleski I. Stroke prevention in patients with atrial fibrillation in France: comparative cost-effectiveness of new oral anticoagulants (apixaban, dabigatran, and rivaroxaban), warfarin, and aspirin. J Med Econ. 2014;17:587–98.
Kongnakorn T, Lanitis T, Lieven A, Thijs V, Marbaix S. Cost effectiveness of apixaban versus aspirin for stroke prevention in patients with non-valvular atrial fibrillation in Belgium. Clin Drug Investig. 2014;34:709–21.
Ademi Z, Pasupathi K, Liew D. Cost-effectiveness of apixaban compared to warfarin in the management of atrial fibrillation in Australia. Eur J Prev Cardiol. 2015;22:344–53.
Lee S, Anglade MW, Meng J, Hagstrom K, Kluger J, Coleman CI. Cost-effectiveness of apixaban compared with aspirin for stroke prevention in atrial fibrillation among patients unsuitable for warfarin. Circ Cardiovasc Qual Outcomes. 2012;5:472–9.
Lanitis T, Kongnakorn T, Jacobson L, De Geer A. Cost-effectiveness of apixaban versus warfarin and aspirin in Sweden for stroke prevention in patients with atrial fibrillation. Thromb Res. 2014;134:278–87.
Canestaro WJ, Patrick AR, Avorn J, Ito K, Matlin OS, Brennan TA, et al. Cost-effectiveness of oral anticoagulants for treatment of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2013;6:724–31.
Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–81.
Lee S, Mullin R, Blazawski J, Coleman CI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PLoS One. 2012;7:e47473.
Limone BL, Baker WL, Kluger J, Coleman CI. Novel anticoagulants for stroke prevention in atrial fibrillation: a systematic review of cost-effectiveness models. PLoS One. 2013;8:e62183.
Luengo-Fernandez R, Leal J, Gray A, Petersen S, Rayner M. Cost of cardiovascular diseases in the United Kingdom. Heart. 2006;92:1384–9.
Liu JL, Maniadakis N, Gray A, Rayner M. The economic burden of coronary heart disease in the UK. Heart. 2002;88:597–603.
European Medicine Agency. Eliquis summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf. Accessed 10 Dec 2015.
Beswick AD, Rees K, Griebsch I, et al. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups. Health Technol Assess. 2004;8(41):iii–iv, ix–x, 1–152.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was financially supported by Pfizer Hellas.
Conflicts of interest
AB was an employee of Pfizer Hellas at the time of manuscript submission. PS is an employee of Pfizer Hellas. KA, NB, EK, FT, and JK have no conflicts of interest that might be relevant to the contents of this manuscript.
Disclaimer
Nothing contained in this paper is intended to guarantee the appropriateness of any medical treatment or to be used for therapeutic purposes or as a substitute for a health professional’s advice. All authors read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Athanasakis, K., Boubouchairopoulou, N., Karampli, E. et al. Cost Effectiveness of Apixaban versus Warfarin or Aspirin for Stroke Prevention in Patients with Atrial Fibrillation: A Greek Perspective. Am J Cardiovasc Drugs 17, 123–133 (2017). https://doi.org/10.1007/s40256-016-0204-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-016-0204-1