Abstract
In most agro-ecosystems the organisms that feed on plant roots have an important impact on crop yield and can impose tremendous costs to farmers. Similar to aboveground pests, they rely on a broad range of chemical cues to locate their host plant. In their turn, plants have co-evolved a large arsenal of direct and indirect defense to face these attacks. For instance, insect herbivory induces the synthesis and release of specific volatile compounds in plants. These volatiles have been shown to be highly attractive to natural enemies of the herbivores, such as parasitoids, predators, or entomopathogenic nematodes. So far few of the key compounds mediating these so-called tritrophic interactions have been identified and only few genes and biochemical pathways responsible for the production of the emitted volatiles have been elucidated and described. Roots also exude chemicals that directly impact belowground herbivores by altering their behavior or development. Many of these compounds remain unknown, but the identification of, for instance, a key compound that triggers nematode egg hatching to some plant parasitic nematodes has great potential for application in crop protection. These advances in understanding the chemical emissions and their role in ecological signaling open novel ways to manipulate plant exudates in order to enhance their natural defense properties. The potential of this approach is discussed, and we identify several gaps in our knowledge and steps that need to be taken to arrive at ecologically sound strategies for belowground pest management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction—Belowground Herbivory and Plant Defense
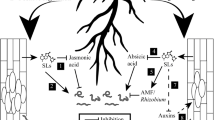
For decades, plants roots have been mainly considered as defenseless victims of soil-dwelling pests and a passive sink for leaf-produced photoassimilates. However, an increasing number of recent studies emphasize that, instead of being idle victims, roots play a major role in defending themselves and aboveground tissues, and in shaping their surrounding habitat via production and exudation of organic chemicals (Bais et al., 2006; Erb et al., 2009; van Dam, 2009). In fact, a large number of soil organisms have been shown to rely on root exudates as a carbon source (Walker et al., 2003), dramatically diverging from the formal assumption that the soil fauna is largely dependent on aboveground litter for carbon (Huhta, 2006). Beside anchoring the plant in soil and being the principal channel of nutrient transfer from the soil to the aboveground tissues of the plants and further trophic levels, roots are a prime source of carbon in soil. This makes roots preferential targets for soil-dwelling herbivores such as insects, nematodes, and other microbes. However, roots possess defense mechanisms that allow them to resist herbivore attacks (see Erb et al., 2012, this issue). Indeed, they have evolved a broad arsenal of direct defense molecules as well as indirect defenses that involve finely tuned communication and chemical interactions of the roots with the soil microfauna (Huber-Sannwald et al., 1997; Boff et al., 2001; van Tol et al., 2001; Mathesius et al., 2003; Callaway et al., 2004; Rasmann et al., 2005, 2011; Ali et al., 2010). In this review, we highlight some of these chemically mediated interactions (Fig. 1), and we argue that the chemical cues that are involved can be used to improve belowground pest control and crop production.
Schematic representation of chemically-mediated interactions between plants and soil-dwelling organisms. (1) Without root signal, the organisms are either waiting for cues or randomly move around until they detect a chemical cue. (2) General nonspecific semiochemicals emitted by roots may trigger a shift from random movement or immobility to a biased random movement. (3) More specific chemical cues may allow the organism (friend or foe) to recognize and locate a potential partner to establish an interaction with. (4) Subsequent acceptance or rejection takes place at the surface of roots due to the presence of contact chemosensory cues, being either feeding stimulants or deterrents for herbivorous organisms or cues that indicate a partner for the establishment of a mutually beneficial interaction. (5) Soil properties have an obvious impact on soil chemically mediated interactions; the clay-humic complex may favor or slow down the diffusion of the volatiles depending on the chemical interactions taking places at this interface. Moreover, soil porosity, connectivity, or particle size distribution impact the mobility and behavior of soil-dwelling organisms. Understanding each of these steps will allow us to manipulate the system in order to favor or to inhibit beneficial and detrimental interactions, respectively [modified after Johnson and Gregory (2006)]
Plant–Insect Interactions and Belowground Pest Management
Rasmann and Agrawal (2008) estimate that about 17 % of all insect families of North America contain species of root feeders (including chewers, sap suckers, and gall makers). Common insect orders such as Orthoptera, Lepidoptera, Diptera, Homoptera, Coleoptera, and Hymenoptera have immature root-feeding instars (Brown and Gange, 1990). Because of their direct impact on plant development and fitness, root-feeding insects play an important role in both agricultural and natural ecosystems (Blossey and Hunt-Joshi, 2003; Wardle et al., 2004; Rasmann and Agrawal, 2008). Indeed, as belowground herbivory induces changes in the physiology and morphology of the roots, soil-dwelling herbivores have the potential to shape the ecosystems at the plant community level (De Deyn et al., 2003), belowground fauna (Wardle, 2006), as well as aboveground insect communities (Bezemer and van Dam, 2005).
Various volatile organic compounds have been identified as arthropod attractants belowground. A comprehensive review by Wenke et al. (2010) provides an inventory of a wide range of compounds used by belowground insect herbivores to locate their food source. Johnson and Nielsen (2012, this issue) discuss in detail how insect–plant interactions are mediated by belowground volatiles. The simplest and most ubiquitous of such signals in the soil is carbon dioxide (CO2) emitted by respiring roots, but also many other biotic sources. Johnson and Gregory (2006) listed more than 20 studies in which CO2 was shown to be a major attractant for root feeding arthropods. Whereas low concentrations are known to trigger chemotaxis and attract insects, high concentrations of CO2 may actually result in disorientation (Johnson and Gregory, 2006). CO2 is such an ambiguous signal that is unlikely to be of great use by itself. We, therefore, recently argued that CO2 is a response activator rather than a key attractant per se (Turlings et al., 2012). This notion is based on the principle that where there are roots there is CO2, whereas the reverse does not hold; where there is CO2 there are not necessarily roots. The same idea holds for hemophagous insects in search of a blood meal and indeed it has been found that the presence of CO2 strongly increases their responsiveness to more specific cues (Dekker et al., 2005; Turner et al., 2011). Indeed, besides CO2, there are several compounds that have been identified as potent specific attractants to root feeders. For instance, several disulfides and trisulfides attract root-feeding larvae of the fly Delia antiqua in Allium cepa (Carson and Wong, 1961). Fatty acids in oaks (Quercus sp.) and monoterpenes in carrots (Daucus carota ssp. sativus) attract larvae of the forest cockchafer, Melolontha hippocastani (Weissteiner and Schütz, 2006). Johnson et al. (2005) showed the attraction of Sitona lepidus to formononetin, a flavonoid emitted by nodualted roots of white clover Trifolium repens. In laboratory assays, the scolyt beetle Hylastinus obscurus was shown to be attracted to volatile exuded by roots of red clover Trifolium pretense (Quiroz et al., 2005).
Johnson and Gregory (2006) proposed a conceptual model for chemically mediated plant host location and acceptance by belowground insect pests that can readily be adapted to general belowground chemical signaling pathways (Fig. 1). Interfering with any of these steps would disrupt the insect’s ability to find or accept its host, and thus offers a way to control pest insects. Following this approach, Bernklau et al. (2004) managed to interfere with the host-finding behavior of the larvae of the western corn rootworm Diabrotica virgifera virgifera. The larvae of this chrysomelid beetle are an important pest of maize, and rely, among other volatile cues, on CO2 to locate the root system of its host (Bernklau and Bjostad, 1998a, b). In a laboratory assay, significantly fewer D. v. virgifera larvae were recovered from maize roots in soil with CO2-producing granules than from maize roots in control soil, suggesting that the increase in CO2 prevented the insect larvae from locating the roots of their host plant (Bernklau et al., 2004). By testing the same strategy in the field, they found that CO2 application resulted in a significant decrease in damage done by D. v. virgifera to the maize root (Bernklau et al., 2004).
In an earlier study, Bjostad and Hibbard (1992) identified a more specific cue, the 6-methoxy-2-benzoxazolinone (MBOA), as an attractant for D. v. virgifera larvae. MBOA is one of several benzoxazinoids that maize seedlings produce and release as toxic and anti-feedents against insects in soil (Bjostad and Hibbard, 1992). D. v. virgifera have evolved resistance to benzoxazinoids (Abou Fakhr et al., 1994; Robert et al., 2012a) and even rely on this cue to locate the host plant (Bjostad and Hibbard, 1992) and to identify the most nutritious maize roots (Robert et al., 2012a). Knowing the importance of MBOA as a foraging cue for the pest, Hibbard et al. (1995) employed it to reduce larval damage on maize roots in the field. They baited a soil insecticide with MBOA to lure D. v. virgifera larvae to their death (Hibbard et al., 1995). Similarly, Bernklau and Bjostad (2005) could reduce the effective dose of the insecticide thiamethoxan by 50 % when they mixed it with feeding stimulants. Recently, attempts to lure foraging D. v. virgifera larvae by using alginate capsules as dispensers of attractants and feeding stimulants have been undertaken. In the laboratory, larvae of the chrysomelid pest were found to be as much attracted towards the capsules as towards the roots of a maize seedling. However, in the field, the attractive coating of the capsules did not help to further reduce D. v. virgifera damage on the maize roots (Hiltpold et al., 2012). Hence, this approach needs to be improved, but it has interesting potential in pest management, especially because the capsules can be used to deliver biocontrol agents such as entomopathogenic nematodes (Hiltpold et al., 2012) into pest-infested fields. By luring the pests towards the capsules, their efficacy can be further enhanced.
Even though examples are scarce, it is evident that the manipulation of chemically mediated host recognition and/or food acceptance has great potential in controlling insect pests (Fig. 1). However, basic knowledge on chemical attraction of pests towards their host and the chemical cues that they use as host acceptance signals is largely missing. Having such information would help breeders to select varieties with the right chemical profile, or it might even be possible to genetically engineer plants to make them emit less attractive volatiles or even repel the pests. Thus, affecting the acceptance of food sources by an insect herbivore could provide ecologically sound solutions to pest problems.
Belowground Tritrophic Interactions as an Inspiration for Insect Pest Control Strategies
Plants cannot run away to escape herbivory, but they have evolved many other defense traits (Howe and Jander, 2008). One strategy that appears to provide protection against herbivory is the release of herbivore induced volatile organic compounds (Fig. 1), which increases the plant’s attractiveness to the natural enemies of herbivores (e.g., Dicke and Vet, 1999; Dicke et al., 2003; Turlings and Wäckers, 2004; Kessler and Morrell, 2010). Such interactions also take place belowground. For instance, females of the predatory mites Neoseiulus cucumeris respond to belowground volatiles signals of tulip bulbs infested by the rust mite Aceria tulipae, but not to volatiles of untreated or mechanically wounded bulbs (Aratchige et al., 2004). Single root-emitted chemicals can have a dual beneficial effect for the plant. For instance, dimethyl disulfide is emitted from cabbage roots damaged by the cabbage root fly Delia radicum (Ferry et al., 2007; Danner et al., 2012, this issue). This volatile both attracts the main predators of D. radicum (i.e., two staphylinids, Aleochara bilineata and Aleochara bipustulata, and carabid beetles of the genus Bembidion) and it inhibits oviposition by cabbage root fly females (Ferry et al., 2009). In a field experiment, the authors placed dispensers to continuously release dimethyl disulfide in broccoli plots. The number of predators increased in the plots that received the dispensers (Ferry et al., 2009). In this particular experiment, the increase in predators did not improve the quality of the harvested plants at the end of the season, but such approaches should help pest management at higher pest densities.
Boff et al. (2001) and van Tol et al. (2001) found that the emission of odorous volatiles by insect damaged roots results in the attraction of entomopahtogenic nematodes. These insect-killing microscopic worms are frequently used in insect–pest management (Grewal et al., 2005), but rarely in large-scale agriculture. Exploiting their ability to detect damaged roots might be extremely interesting in the context of pest control improvement. To date, only few additional tritrophic interaction that rely on belowground herbivore-induced volatiles have been described in agricultural ecosystems (Rasmann et al., 2005; Rasmann and Turlings, 2008; Ali et al., 2010) or in natural ecosystems (Rasmann et al., 2011). Ali et al. (2010) recently showed that the entomopathogenic nematode Steinernema diaprepesi is significantly more attracted by citrus roots damaged by the larvae of the curculionid pest Diaprepes abbreviates than by mechanically damaged roots. However, this agronomically interesting trait also is abused by pests, as insect-induced roots of citrus tree also attract the plant parasitic nematode Tylenchulus semipenetrans (Ali et al., 2011). Consequently, this may interfere with the possible exploitation of citrus induced volatiles in biological control strategies that target Diaprepes abbreviates, specifically in cases where rootstocks are not naturally resistant to this nematode pest.
One of the best studied belowground tritrophic interactions involves maize roots (Rasmann et al., 2005). Upon attack by the voracious larvae of D. v. virgifera, the roots of many maize varieties emit the sesquiterpene (E)-β-caryophyllene (Rasmann et al., 2005; Köllner et al., 2008), which is highly attractive to the entomopathogenic nematode Heterorhabidtis megidis in the laboratory as well as in the field (Rasmann et al., 2005; Köllner et al., 2008; Hiltpold et al., 2010c). However, most of the American maize varieties have lost the ability to produce (E)-β-caryophyllene (Rasmann et al., 2005; Köllner et al., 2008), probably because the herbivore induced cue also recruits D. v. virgifera larvae (Robert et al., 2012b), which may have changed breeders to unintentionally select against this trait. Nevertheless, plants that do not emit this signal may suffer from more rootworm damage than plants that are able to recruit the entomopahtogenic nematodes (Rasmann et al., 2005; Hiltpold et al., 2010c, 2011). To restore the ability of maize to indirectly protect its roots with the emission of (E)-β-caryophyllene, the terpene synthase gene Ovtps6 from Oreganum vulgare (Crocoll et al., 2010) was introduced to a maize variety that normally is unable to produce the sesquiterpene (Degenhardt et al., 2009). The transformation resulted in maize lines that constitutively emitted (E)-β-caryophyllene (Degenhardt et al., 2009). When these transformed lines were compared to untransformed isogenic lines, significantly more nematodes H. megidis were attracted toward the genetically engineered plants than toward the controls both in the laboratory and in the field, resulting in a better protection of the emitting roots (Degenhardt et al., 2009). This first field demonstration that genetic engineering can be used to enhance indirect defenses against insect pests illustrates the potential of exploiting plant mediated signaling for crop protection. However, such approach is feasible only in combination with the right species of nematode (Hiltpold et al., 2010c). In fact, Heterorhabditis bacteriophora, which is highly virulent against D. v. virgifera (Kurzt et al., 2009), does not respond well to (E)-β-caryophyllene (Hiltpold et al., 2010c). To overcome this drawback, a strain of H. bacteriophora was selected in the laboratory for enhanced responsiveness to (E)-β-caryophyllene (Hiltpold et al., 2010a). The selection resulted in a strain that responded 6-fold better than the original strain and with equivalent virulence and persistence (Hiltpold et al., 2010a, b). The application of this strain in the field significantly increased the mortality of D. v. virgifera larvae feeding on the roots of plants emitting (E)-β-caryophyllene (Hiltpold et al., 2010a). A recent study on chemotaxis of H. bacteriophora and Steinernema feltia has revealed several new compounds that induce movement in the tested entomopahtogenic nematodes (Hallem et al., 2011). Further research is needed to determine the full potential of using these belowground signals for insect pest control.
Only few inducible and constitutively emitted volatiles involved in belowground tritrophic interactions are known, but an increasing effort is invested in this field of research. Little is known also about the impact of abiotic factors in the soil on the diffusion of these volatiles (Hiltpold and Turlings, 2008) or about the foraging behavior of the beneficials such as the nematodes (Kruitbos et al., 2010; Wilson et al., 2012). Understanding more about the complex interactions at each trophic level will not only reveal the intricacies of these fascinating interactions in the rhizosphere, but may also lead to ecologically sound alternatives in pest management in agricultural systems.
Management of Plant Parasitic Nematodes Using Root-Produced Exudates
After insects, the second most important group of root feeders encompasses the plant parasitic nematodes. All species are obligate parasites, feeding exclusively on the cytoplasm of living plant cells. The most economically important groups of nematodes are the sedentary endoparasites including the genera Heterodera and Globodera (cyst nematodes) and Meloidogyne (root-knot nematodes). Cyst and root-knot nematodes differ in their parasitic life-cycle strategies, but they both rely on volatile cues to locate the host plant. With the exception of ambiguous CO2 emissions, it is largely unknown what triggers the attraction of plant parasitic nematode towards host plants (discussed by Rasmann et al., 2012, this issue). Carbon dioxide has been shown to attract several nematode species (Klingler, 1963; Dusenbery, 1980, 1987; Pline and Dusenbery, 1987), but aggregation and attraction of plant parasitic nematodes also have been demonstrated in response to plant root exudates (Prot, 1980; Rolfe et al., 2000; Curtis et al., 2009; Reynolds et al., 2011). Only recently, has it been found that plant parasitic nematodes follow gradients of herbivore-induced terpene volatile organic compounds (Ali et al., 2011). In their study, a series of terpenoids were identified as possible attractants for the nematode Tylenchulus semipenetrans, including α- and, β-pinene, limonene, geijerene, and pregeijerene (Ali et al., 2011). The identification of such specific volatiles offers the possibility of employing a confusion strategy to disrupt nematodes’ host location and acceptance efforts, analogous to the pheromone confusion technique used in insect pest control (e.g., Joshi et al., 2011; Levi-Zada et al., 2011; Vacas et al., 2011, 2012; Schmera and Guerin, 2012). Further research will be needed to understand fully the mechanisms behind nematode attraction in order to develop lures that can compete with the plant-produced attractants.
On the one hand, root exudates may attract plant parasitic nematodes, but they are also involved in plant defense against these pests. For instance, root tip exudates can trigger a loss of motility, inducing quiescence and thus reducing the ability of the nematodes to successfully infect the plant (Zhao et al., 2000). Such temporal alteration of plant parasitic nematode motility in contact with root exudates has been observed for several plant species (Hubbard et al., 2005), suggesting that this defense strategy is widespread. Attempts to identify the active compounds have so far failed (Hubbard et al., 2005). Once identified, synthetic versions of the active compound(s) might be employed by spraying them to immobilize plant parasitic nematodes in the field. It is evident that further fundamental research into possible other ecological roles of such compounds is essential in order to establish whether or not they could be ecologically sound alternatives in plant parasitic nematode control.
Because plant parasitic nematodes rely on plants as food sources, they not only use plant chemicals to locate roots, but they also synchronize egg hatching with the phenology of their host plants. It has been amply demonstrated that plant parasitic nematode egg hatching is stimulated by root exudates (e.g., Perry and Clarke, 1981; Perry and Gaur, 1996; Dennijs and Lock, 1992; Gaur et al., 2000; Devine and Jones, 2001; Wesemael et al., 2006; Pudasaini et al., 2008; Khokon et al., 2009; Oka and Mizukubo, 2009). For instance, a key hatch-stimulating substance for soybean cyst nematode was successfully isolated from soybean roots (Masamune et al., 1982). Sometime later, solanoeclepin A, a hatching stimulus for the potato cyst nematodes Globodera rostochiensis and G. pallida Stone, was isolated by Mulder et al. (1992) and its structure was resolved by Schenk et al. (1999). It is easy to imagine various applications of such compounds in crop protection; these compounds can be applied to the field before the plants have been sown or germinated. This should result in nematode hatching in the absence of their actual host plants, and the free-roaming nematode can be expected to die of starvation or chemical pesticides before damages occur. However, the challenge of this idealistic plan of attack is the availability of enough material to treat large crop fields. Until recently, the hatch-stimulating chemicals have been isolated only in minute quantities from natural sources, but Tanino et al. (2011) have developed a potent laboratory synthesis methodology of solanoeclepin, thus opening the way to a new management strategy of plant pathogenic nematodes.
Specific root secondary metabolites or breakdown products also have a direct impact on plant parasitic nematode survival. For instance, Brassicaceae plants contain various glucosinolates (McCully et al., 2008) that are released upon pest damage and degraded into toxic breakdown products such as (iso)thiocyanates (Halkier and Gershenzon, 2006). Belowground, glucosinolates and their breakdown products can efficiently reduce the populations of plant parasitic nematodes (e.g., Potter et al., 1998, 2000; Lazzeri et al., 2004; Oliveira et al., 2011). The release of these toxic compounds into the soil does not alter communities of beneficial organisms such as earthworms or collembola (Kabouw et al., 2010), and this approach has been widely used for the protection of subsequent crops (Matthiessen and Kirkegaard, 2006; Lazzeri et al., 2010). Moreover, breeding for increased concentrations of glucosynolates in roots of Brassica plants has resulted in a better control of nematode pests (Potter et al., 2000), and can be an effective way to manipulate belowground chemical ecology to control plant parasitic nematodes.
Enhancing Plant Production by Exploiting Chemically Mediated Interactions Between Roots and Microbes
Plants have to face several foes in soil, but they also can interact with beneficial microbes to increase their biomasses or, in agriculture, yield. Indeed, there are myriads of microorganisms that interact with plants with different levels of intimacy, ranging from symbionts to co-inhabitants of the same niche without particular interaction, and each interaction might be of interest in the context of plant protection and production.
Plant Interactions with Free-Living Nematodes
There are numerous free-living nematodes in soil that do not need an insect or a host plant to complete their life cycle. These nematodes are usually bacterivorous, carnivorous, or fungivorous (Neher, 2010). Nonetheless, they can interact with plant roots in various negative or positive ways. On the negative side, they transmit viruses or plant pathogenic bacteria (Raaijmakers et al., 2009). On the positive side, they also can carry beneficial microorganisms and enhance root growth. For instance, Caenorhabditis elegans mediates positive interactions between plant roots and rizhobia, thus resulting in a increased number of bacterial colonies (Horiuchi et al., 2005) and potential increases in nodulation. Caenorhabditis elegans are attracted by dimethyl sulfide toward Medicago truncatula, and thereby transport the beneficial rizho-bacterium Sinorhizobium meliloti close to the root systems (Horiuchi et al., 2005). Nematodes also are able to carry fungal spores that adhere to their cuticular mucilage (Bonkowski et al., 2009), and thus they serve as potential vectors for beneficial plant symbiotic fungi. Beside these transporter activities in the rhizosphere, free-living nematodes also enhance plant nutrient availability by grazing on microbial communities and increasing their turnover and metabolic activity (Bonkowski et al., 2009). Obviously, nematodes are not the only animal feeding on bacteria in the rhizosphere. Other organisms such as amoeba also positively impact nutrient turnover around roots (Rosenberg et al., 2009). A better understanding of such interactions and knowledge of the chemicals that are involved in their establishment could lead to novel strategies to enhance nutrient availability and uptake in the rhizosphere. The favoring of natural nutrient cycles in crop production also will reduce the need for fertilizer input and can contribute to a more sustainable agriculture and food production.
Root Volatile Involved in Communication with Symbiotic Fungi
Simple root volatile organic compounds such as carbon dioxide play an important generic role in belowground interactions with other organisms (Johnson and Gregory, 2006). However, CO2 also has been shown to mediate highly specific interactions. Indeed, carbon dioxide is crucial in the growth of the vesicular-arbuscular fungus Gigaspors margarita, an obligate biotrophic symbiont (Bécard and Piché, 1989). A synergistic effect of CO2 and root exudate factors in the hyphal growth was measured; carbon dioxide and root exudates taken alone had little or no effect, but when mixed together, they significantly stimulated hyphal growth (Bécard and Piché, 1989). Further experimentation has suggested that, in this particular interaction, carbon dioxide serves as an essential source of carbon for fungal growth (Bécard and Piché, 1989). Since then, numerous plant exudates, mainly belonging to the sesquiterpene lactone family, have been shown to mediate plant–microbe interactions. For example, the strigolactone 5-desoxy-strigol, isolated from Lotus japonicus, triggers hyphal branching in G. margarita (Akiyama et al., 2005). Very recently, the first component involved in strigolactone root exudation has been described (Kretzschmar et al., 2012). The identification of the ABC transporter in Petunia ssp. opens new opportunities to manipulate strigolactone dependent processes (Badri et al., 2009; Kretzschmar et al., 2012).
Conclusion
This review summarizes our current knowledge of direct and indirect interactions between soil fauna, rhizosphere microorganisms, and plant roots, and highlights the importance of such knowledge for the development of methods to fight soil pests. Research into belowground chemically mediated interactions is drastically increasing, and no longer is restricted to interactions between roots and microbial symbionts, but involves many other soil-dwelling organisms. It is increasingly recognized that, similar to aboveground interactions, a coevolution between plants and herbivores has taken place belowground that has led to sophisticated reciprocal adaptations. Microbial communities (see Effmert et al. 2012, this issue) and bacterivorous fauna jointly have strong effects on root growth and architecture, even though plants might only be passive benefiters and not directly shape these interactions. Conversely, an increasing number of examples suggest that roots are active players in the rhizosphere and that they are able to influence and shape their environment, thus ensuring their protection and optimizing their performance. They have been shown to chemically influence soil microorganisms and fauna for their own benefit: entomopahtogenic nematodes are recruited by insect herbivore damaged roots; plant pathogenic nematodes are immobilized by root tip exudates; and root diffusates can attract free-living nematodes that carry potentially beneficial bacteria and initiate symbiosis between plants and beneficial fungi.
In our efforts to exploit root signals in crop protection, it should be realized that herbivorous insects also may use root signaling to locate their food source, and that root chemicals can trigger egg hatching in plant parasitic nematodes. Only a multidisciplinary approach to disentangle all aspects of root ecology will allow us to use chemically mediated belowground interactions to our benefit. Special effort should be invested in understanding the role of fungi in belowground interactions, indeed, as “root extensions”, hyphae must play a central role in local as well as in long distance belowground signaling. Co-evolutionary perspectives are lacking in rhizosphere ecology and belowground food webs. A good understanding of these processes would help in approaches that conserve well-established beneficial interactions during domestication and breeding of cultivars. In general, analytic methodologies that are employed for the description of aboveground interactions are in part transferable to belowground chemical ecology. It is important to note that, before the techniques that are discussed here can be applied, it is essential to evaluate the overall consequences of the manipulations. Hundreds of species of microorganisms can be found in a handful of soil and changing one parameter might have unexpected consequences on the established ecosystem services and threaten soil sustainability. Because soils are complex and heterogeneous ecosystems, the application of various strategies cannot be generalized, and will have to be carefully assessed in case by case studies. Hence, with the increasing interest in what might be called a new frontier in biological sciences, a cooperative and holistic approach appears crucial to tackle the complexity of the rhizosphere. This should allow us to benefit optimally from generated knowledge for sustainable agricultural practices.
References
Abou Fakhr, E. M., Hibbard, B. E., and Bjostad, L. B. 1994. Tolerance of western corn rootworm larvae (Coleoptera: Chrysomelidae) to 6-methoxy-2-benzoxazolinone, a corn semiochemical for larval host location. J. Econ. Entomol. 87:647–652.
Akiyama, K., Matsuzaki, K., and Hayashi, H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827.
Ali, J. G., Alborn, H. T., and Stelinski, L. L. 2010. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J. Chem. Ecol. 36:361–368.
Ali, J. G., Alborn, H. T., and Stelinski, L. L. 2011. Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J. Ecol. 99:26–35.
Aratchige, N. S., Lesna, I., and Sabelis, M. W. 2004. Below-ground plant parts emit herbivore-induced volatiles: olfactory responses of a predatory mite to tulip bulbs infested by rust mites. Exp. Appl. Acarol. 33:21.
Badri, D. V., Quintana, N., el Kassis, E. G., Kim, H. K., Choi, Y. H., Sugiyama, A., Verpoorte, R., Martinoia, E., Manter, D. K., and Vivanco, J. M. 2009. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol. 151:2006–2017.
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. 2006. The role of root exudates in rhizosphere interations with plants and other organisms. Annu. Rev. Plant Biol. 57:233–266.
Bécard, G. and Piché, Y. 1989. Fungal growth-stimulation by CO2 and root exudates in vesicular-arbusular mycorrhizal symbiosis. Appl. Environ. Microbiol. 55:2320–2325.
Bernklau, E. J. and Bjostad, L. B. 1998a. Behavioral responses of first-instar western corn rootworm (Coleoptera: Chrysomelidae) to carbon dioxide in a glass bead bioassay. J. Econ. Entomol. 91:444–456.
Bernklau, E. J. and Bjostad, L. B. 1998b. Reinvestigation of host location by western corn rootworm larvae (Coleoptera: Chrysomelidae): CO2 is the only volatile attractant. J. Econ. Entomol. 91:1331–1340.
Bernklau, E. J. and Bjostad, L. B. 2005. Insecticide enhancement with feeding stimulants in corn for western corn rootworm larvae (Coleoptera: Chrysomelidae). J. Econ. Entomol. 98:1150–1156.
Bernklau, E. J., Fromm, E. A., and Bjostad, L. B. 2004. Disruption of host location of western corn rootworm larvae (Coleoptera: Chrysomelidae) with carbon dioxide. J. Econ. Entomol. 97:330–339.
Bezemer, T. M. and van Dam, N. M. 2005. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 20:617–624.
Bjostad, L. B. and Hibbard, B. E. 1992. 6-Methoxy-2-benzoxalinone—a semiochemical for host location by western corn-rootworm larvae. J. Chem. Ecol. 18:931–944.
Blossey, B. and Hunt-Joshi, T. R. 2003. Belowground herbivory by insects: Influence on plants and aboveground herbivores. Annu. Rev. Entomol. 48:521–547.
Boff, M. I. C., Zoon, F. C., and Smits, P. H. 2001. Orientation of Heterorhabditis megidis to insect hosts and plant roots in a Y-tube sand olfactometer. Entomol. Exp. Appl. 98:329–337.
Bonkowski, M., Villenave, C., and Griffiths, B. 2009. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321:213–233.
Brown, V. K. and Gange, A. C. 1990. Insect herbivory below ground. Adv. Ecol. Res. 20:1–58.
Callaway, R. M., Thelen, G. C., Rodriguez, A., and Holben, W. E. 2004. Soil biota and exotic plant invasion. Nature 427:731–733.
Carson, J. F. and Wong, F. F. 1961. Onion flavor and odor—Volatile flavor components of onions. J. Agric. Food Chem. 9:140–143.
Crocoll, C., Asbach, J., Novak, J., Gershenzon, J., and Degenhardt, J. 2010. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol. Biol. 73:587–603.
Curtis, R. H. C., Robinson, A. F., and Perry, R. N. 2009. Hatch and host location, pp. 139–162, in R. Perry, M. Moens, and J. Starr (eds.), Root-knot Nematodes. CABI, Wallingford.
DANNER H., SAMUDRALA D., CRISTESCU S. M., and VAN DAM N. 2012. Tracing hidden herbivores: Time-resolve non-invasive analysis of belowground volatiles by proton-transfer-reaction mass spectrometry (PTR-MS). J. Chem. Ecol., this issue.
de Deyn, G. B., Raaijmakers, C. E., Zoomer, H. R., Berg, M. P., de Ruiter, P. C., Verhoef, H. A., Bezmer, T. M., and van der Putten, W. H. 2003. Soil invertebrate fauna enhances grassland succession and diversity. Nature 422:711–713.
Degenhardt, J., Hiltpold, I., Köllner, T. G., Frey, M., Gierl, A., Gershenzon, J., Hibbard, B. E., Ellersieck, M. R., and Turlings, T. C. J. 2009. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 106:13213–13218.
Dekker, T., Geier, M., and Carde, R. T. 2005. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 208:2963–2972.
Dennijs, L. and Lock, C. A. M. 1992. Differential hatching of the potato cyst nematodes Gobodera rostochiensis and Globodera pallida in root diffusates and water of differing ionic composition. Neth. J. Plant Pathol. 98:117–128.
Devine, K. J. and Jones, P. W. 2001. Effects of hatching factors on potato cyst nematode hatch and in-egg mortality in soil and in vitro. Nematology 3:65–74.
Dicke, M. and Vet, L. E. M. 1999. Plant–carnivore interactions: evolutionary and ecological consequences for plat, herbivore and carnivore, pp. 483–520, in H. Olff, V. K. Brown, and R. H. Drent (eds.), Herbivores: Between Plants and Predators. Blackwell Science, Oxford.
Dicke, M., van Poecke, R. M. P., and de Boer, J. G. 2003. Inducible indirect defence of plants: From mechanisms to ecological functions. Basic Appl. Ecol. 4:27–42.
Dusenbery, D. B. 1980. Responses of the nematode Caenorhabditis elegans to controlled chemical stimulation. J. Comp. Physiol. 136:327–331.
Dusenbery, D. B. 1987. Behavioral responses of Meloidogyne incognita to temperature and carbon dioxide. J. Nematol. 19:519–519.
EFFMERT U., KALDERAS J., WARNKE R., and PIECHULLA B. 2012. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol., this issue.
Erb, M., Lenk, C., Degenhardt, J., and Turlings, T. C. J. 2009. The underestimated role of roots in defense against leaf attackers. Trends Plant Sci. 14:653–659.
ERB M., GLAUSER G., and ROBERT C. A. M. 2012. Induced immunity against below ground insect herbivores—activation of defenses in the absence of a jasmonate burst. J. Chem. Ecol., this issue.
Ferry, A., Dugravot, S., Delattre, T., Christides, J. P., Auger, J., Bagneres, A. G., Poinsot, D., and Cortesero, A. M. 2007. Identification of a widespread monomolecular odor differentially attractive to several Delia radicum ground-dwelling predators in the field. J. Chem. Ecol. 33:2064–2077.
Ferry, A., le Tron, S., Dugravot, S., and Cortesero, A. M. 2009. Field evaluation of the combined deterrent and attractive effects of dimethyl disulfide on Delia radicum and its natural enemies. Biol. Control. 49:219–226.
Gaur, H. S., Beane, J., and Perry, R. N. 2000. The influence of root diffusate, host age and water regimes on hatching of the root-knot nematode, Meloidogyne triticoryzae. Nematology 2:191–199.
Grewal, P. S., Ehlers, R. U., and Shapiro, D. I. 2005. in P. S. Grewal, R. U. Ehlers, and D. I. Shapiro (eds.), Nematodes as Biocontrol Agents. CABI Publishing, Wallingford.
Halkier, B. A. and Gershenzon, J. 2006. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57:303–333.
Hallem, E. A., Dillman, A. R., Hong, A. V., Zhang, Y. J., Yano, J. M., Demarco, S. F., and Sternberg, P. W. 2011. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 21:377–383.
Hibbard, B. E., Peairs, F. B., Pilcher, S. D., Schroeder, M. E., Jewett, D. K., and Bjostad, L. B. 1995. Germinating corn extracts and 6-methoxy-2-benzoxazolinone—western corn-rootworm (Coleoptera: Chrysomelidae) larval attractants evaluated with soil insecticides. J. Econ. Entomol. 88:716–724.
Hiltpold, I. and Turlings, T. C. J. 2008. Belowground chemical signalling in maize: When simplicity rhymes with efficiency. J. Chem. Ecol. 34:628–635.
Hiltpold, I., Baroni, M., Toepfer, S., Kuhlmann, U., and Turlings, T. C. J. 2010a. Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J. Exp. Biol. 213:2417–2423.
Hiltpold, I., Baroni, M., Toepfer, S., Kuhlmann, U., and Turlings, T. C. J. 2010b. Selective breeding of entomopathogenic nematodes for enhanced attraction to a root signal did not reduce their establishment or persistence after field release. Plant Signal. Behav. 5:1450–1452.
Hiltpold, I., Toepfer, S., Kuhlmann, U., and Turlings, T. C. J. 2010c. How maize root volatiles influence the efficacy of entomopathogenic nematodes against the western corn rootworm? Chemoecology 20:155–162.
Hiltpold, I., Erb, M., Robert, C. A. M., and Turlings, T. C. J. 2011. Systemic root signalling in a belowground, volatile-mediated tritrophic interaction. Plant Cell Environ. 34:1267–1275.
HILTPOLD I., HIBBARD B. E., FRECKMAN D. W., and TURLINGS T. C. J. 2012. Capsules containing entomopathogenic nematodes as a Trojan horse approach to control the western corn rootworm. Plant Soil. doi:10.1007/s11104-012-1253-0.
Horiuchi, J., Prithiviraj, B., Bais, H. P., Kimball, B. A., and Vivanco, J. M. 2005. Soil nematodes mediate positive interactions between legume plants and rhizobium bacteria. Planta 222:848–857.
Howe, G. A. and Jander, G. 2008. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59:41–66.
Hubbard, J. E., Flores-Lara, Y., Schmitt, M., McClure, M. A., Stock, S. P., and Hawes, M. C. 2005. Increased penetration of host roots by nematodes after recovery from quiescence induced by root cap exudate. Nematology 7:321–331.
Huber-Sannwald, E., Pyke, D. A., and Caldwell, M. M. 1997. Perception of neighbouring plants by rhizomes and roots: morphological manifestations of a clonal plant. Can. J. Bot.-Revue Canadienne de Botanique 75:2146–2157.
Huhta, V. 2006. The role of soil fauna in ecosystems: A historical review. Pedobiologia 50:489–495.
Johnson, S. N. and Gregory, P. J. 2006. Chemically-mediated host-plant location and selection by root-feeding insects. Physiol. Entomol. 31:1–13.
Johnson, S. N., Gregory, P. J., Greenham, J. R., Zhang, X. X., and Murray, P. J. 2005. Attractive properties of an isoflavonoid found in white clover root nodules on the clover root weevil. J. Chem. Ecol. 31:2223–2229.
JOHNSON S. N., and NIELSEN U. N. 2012. Foraging in the dark—Chemically mediated host plant location by belowground insect herbivores. J. Chem. Ecol. doi:10.1007/510886-012-0106-x.
Joshi, N. K., Hull, L. A., Rajotte, E. G., Krawczyk, G., and Bohnenblust, E. 2011. Evaluating sex-pheromone- and kairomone-based lures for attracting codling moth adults in mating disruption versus conventionally managed apple orchards in Pennsylvania. Pest Manag. Sci. 67:1332–1337.
Kabouw, P., van der Putten, W. H., van Dam, N. M., and Biere, A. 2010. Effects of intraspecific variation in white cabbage (Brassica oleracea var. capitata) on soil organisms. Plant Soil 336:509–518.
Kessler, A. and Morrell, K. 2010. Plant volatile signalling: Multitrophic interactions in the headspace, pp. 95–122, in A. Herrmann (ed.), The Chemistry and Biology of Volatiles. Wiley, Chichester.
Khokon, M. A. R., Okuma, E., Rahman, T., Wesemael, W. M. L., Murata, Y., and Moens, M. 2009. Quantitative analysis of the effects of diffusates from plant roots on the hatching of Meloidogyne chitwoodi from young and senescing host plants. Biosci. Biotechnol. Biochem. 73:2345–2347.
Klingler, J. 1963. Die Orientierung von Ditylenchus dipsaci in Gemessenen Kunstlichen und Biologischen CO2-Gradienten. Nematologica 9:185–199.
Köllner, T., Held, M., Lenk, C., Hiltpold, I., Turlings, T. C. J., Gershenzon, J., and Degenhardt, J. 2008. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20:482–494.
Kretzschmar, T., Kohlen, W., Sasse, J., Borghi, L., Schlegel, M., Bachelier, J. B., Reinhardt, D., Bours, R., Bouwmeester, H. J., and Martinoia, E. 2012. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483:341–344.
Kruitbos, L. M., Heritage, S., Hapca, S., and Wilson, M. J. 2010. The influence of habitat quality on the foraging strategies of the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis megidis. Parasitology 137:303–309.
Kurzt, B., Hiltpold, I., Turlings, T. C. J., Kuhlmann, U., and Toepfer, S. 2009. Comparative susceptibility of larval instars and pupae of the western corn rootworm to infection by three entomopathogenic nematodes. Biocontrol 54:255–262.
Lazzeri, L., Curto, G., Leoni, O., and Dallavalle, E. 2004. Effects of glucosinolates and their enzymatic hydrolysis products via myrosinase on the root-knot nematode Meloidogyne incognita (Kofoid et White). J. Agric. Food Chem. 52:6703–6707.
LAZZERI L., D’AVINO L., and GIES D. 2010. Additional benefits of the efficacy in containing soilborne pest and pathogens with biofumigant plants and materials. Acta Hortic. 883:323–339.
Levi-Zada, A., Ben-Yehuda, S., Dunkelblum, E., Gindin, G., Fefer, D., Protasov, A., Kuznetsowa, T., Manulis-Sasson, S., and Mendel, Z. 2011. Identification and field bioassays of the sex pheromone of the yellow-legged clearwing Synanthedon vespiformis (Lepidoptera: Sesiidae). Chemoecology 21:227–233.
Masamune, T., Anetai, M., Takasugi, M., and Katsui, N. 1982. Isolation of a natural hatching stimulus, glycinoeclepin-A, for the soybean cyst nematode. Nature 297:495–496.
Mathesius, U., Mulders, S., Gao, M. S., Teplitski, M., Caetano-Anolles, G., Rolfe, B. G., and Bauer, W. D. 2003. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA 100:1444–1449.
Matthiessen, J. and Kirkegaard, J. 2006. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 25:235–265.
McCully, M. E., Miller, C., Sprague, S. J., Huang, C. X., and Kirkegaard, J. A. 2008. Distribution of glucosinolates and sulphur-rich cells in roots of field-grown canola (Brassica napus). New Phytol. 180:193–205.
MULDER J. G., DIEPENHORST P., PLIEGER P., and BRUGGEMANN-ROTGANS I. E. M.; B.V. Chemische Pharmaceutische Industrie “Luxan”, PA Elst, Netherlands, assignee. 1992. Hatching Agent for the Potato Cyst Nematode. The Netherlands.
Neher, D. A. 2010. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 48:371–394.
Oka, Y. and Mizukubo, T. 2009. Tomato culture filtrate stimulates hatching and activity of Meloidogyne incognita juveniles. Nematology 11:51–61.
Oliveira, R. D. L., Dhingra, O. D., Lima, A. O., Jham, G. N., Berhow, M. A., Holloway, R. K., and Vaughn, S. F. 2011. Glucosinolate content and nematicidal activity of Brazilian wild mustard tissues against Meloidogyne incognita in tomato. Plant Soil 341:155–164.
Perry, R. N. and Clarke, A. J. 1981. Hatching mechanisms of nematodes. Parasitology 83:435–449.
Perry, R. N. and Gaur, H. S. 1996. Host plant influences on the hatching of cyst nematodes. Fundam. Appl. Nematol. 19:505–510.
Pline, M. and Dusenbery, D. B. 1987. Responses of plant-parasitic nematode Meloidogyne incognita to carbon dioxide determined by video camera-computer tracking. J. Chem. Ecol. 13:873–888.
Potter, M. J., Davies, K., and Rathjen, A. J. 1998. Suppressive impact of glucosinolates in Brassica vegetative tissues on root lesion nematode Pratylenchus neglectus. J. Chem. Ecol. 24:67–80.
Potter, M. J., Vanstone, V. A., Davies, K. A., and Rathjen, A. J. 2000. Breeding to increase the concentration of 2-phenylethyl glucosinolate in the roots of Brassica napus. J. Chem. Ecol. 26:1811–1820.
Prot, J.-C. 1980. Migration of plant-parasitic nematodes towards plant roots. Revue de Nématologie 3:305–318.
Pudasaini, M. P., Viaene, N., and Moens, M. 2008. Hatching of the root-lesion nematode, Pratylenchus penetrans, under the influence of temperature and host. Nematology 10:47–54.
Quiroz, A., Ortega, F., Ramirez, C. C., Wadhams, L. J., and Pinilla, K. 2005. Response of the beetle Hylastinus obscurus Marsham (Coleoptera: Scolytidae) to red clover (Trifolium pratense L.) volatiles in a laboratory olfactometer. Environ. Entomol. 34:690–695.
Raaijmakers, J. M., Paulitz, T. C., Steinberg, C., Alabouvette, C., and Moenne-Loccoz, Y. 2009. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361.
Rasmann, S. and Agrawal, A. A. 2008. In defense of roots: A research agenda for studying plant resistance to belowground herbivory. Plant Physiol. 146:875–880.
Rasmann, S. and Turlings, T. C. J. 2008. First insights into specificity of below ground tritrophic interactions. Oikos 117:362–369.
Rasmann, S., Köllner, T. G., Degenhardt, J., Hiltpold, I., Toepfer, S., Kuhlmann, U., Gershenzon, J., and Turlings, T. C. J. 2005. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737.
Rasmann, S., Erwin, A. C., Halitschke, R., and Agrawal, A. A. 2011. Direct and indirect root defences of milkweed (Asclepias syriaca): trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J. Ecol. 99:16–25.
RASMANN S., ALI J.G., HELDER J., and VAN DER PUTTEN W. H. 2012. Ecology and evolution of soil nematode chemotaxis. J. Chem. Ecol., this issue.
Reynolds, A. M., Dutta, T. K., Curtis, R. H. C., Powers, S. J., Gaur, H. S., and Kerry, B. R. 2011. Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes. J. R. Soc. Interface 8:568–577.
Robert, C. A. M., Veyrat, N., Glauser, G., Marti, G., Doyen, G. R., Villard, N., Gaillard, M. D. P., Köllner, T. G., Giron, D., and Body, M. 2012. A specialist root herbivore takes advantage of defensive metabolites to locate nutritious tissues. Ecol. Lett. 15:55–64.
ROBERT C. A. M., ERB M., DUPLOYER M., ZWAHLEN C., DOYEN G. R., and TURLINGS T. C. 2012b. Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol. 194:1061–1069
Rolfe, R. N., Barrett, J., and Perry, R. N. 2000. Analysis of chemosensory responses of second stage juveniles of Globodera rostochiensis using electrophysiological techniques. Nematology 2:523–533.
Rosenberg, K., Bertaux, J., Krome, K., Hartmann, A., Scheu, S., and Bonkowski, M. 2009. Soil amoebae rapidly change bacterial community composition in the rhizosphere of Arabidopsis thaliana. ISME J. 3:675–684.
Schenk, H., Driessen, R. A. J., de Gelder, R., Goubitz, K., Nieboer, H., Bruggemann-Rotgans, I. E. M., and Diepenhorst, P. 1999. Elucidation of the structure of Solanoeclepin A, a natural hatching factor of potato and tomato cyst nematodes, by single-crystal x-ray diffraction. Croat. Chem. Acta 72:593–606.
Schmera, D. and Guerin, P. M. 2012. Plant volatile compounds shorten reaction time and enhance attraction of the codling moth (Cydia pomonella) to codlemone. Pest Manag.. Sci. 68:454–461.
Tanino, K., Takahashi, M., Tomata, Y., Tokura, H., Uehara, T., Narabu, T., and Miyashita, M. 2011. Total synthesis of solanoeclepin A. Nat. Chem. 3:484–488.
Turlings, T. C. J. and Wäckers, F. 2004. Recruitment of predators and parasitoids by herbivore injured-plants, pp. 21–75, in R. T. Cardé and J. G. Millar (eds.), Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge.
TURLINGS T. C. J., HILTPOLD I., and RASMANN S. 2012. The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil.
Turner, S. L., Li, N., Guda, T., Githure, J., Cardé, R. T., and Ray, A. 2011. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474:87–91.
Vacas, S., Alfaro, C., Primo, J., and Navarro-Llopis, V. 2011. Studies on the development of a mating disruption system to control the tomato leafminer, Tuta absoluta Povolny (Lepidoptera: Gelechiidae). Pest Manag. Sci. 67:1473–1480.
Vacas, S., Vanaclocha, P., Alfaro, C., Primo, J., Verd, M. J., Urbaneja, A., and Navarro-Llopis, V. 2012. Mating disruption for the control of Aonidiella aurantii Maskell (Hemiptera: Diaspididae) may contribute to increased effectiveness of natural enemies. Pest Manag. Sci. 68:142–148.
van Dam, N. M. 2009. Belowground herbivory and plant defenses. Annu. Rev. Ecol. Evol. Syst. 40:373–391.
van Tol, R. W. H. M., van der Sommen, A. T. C., Boff, M. I. C., van Bezooijen, J., Sabelis, M. W., and Smits, P. H. 2001. Plants protect their roots by alerting the enemies of grubs. Ecol. Lett. 4:292–294.
Walker, T. S., Bais, H. P., Grotewold, E., and Vivanco, J. M. 2003. Root exudation and rhizosphere biology. Plant Physiol. 132:44–51.
Wardle, D. A. 2006. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 9:870–886.
Wardle, D. A., Bardgett, R. D., Klironomos, J. N., Setala, H., van der Putten, W. H., and Wall, D. H. 2004. Ecological linkages between aboveground and belowground biota. Science 304:1629–1633.
Weissteiner, S. and Schütz, S. 2006. Are different volatile pattern infuencing host plant choice of belowground living insects. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 15:51–55.
Wenke, K., Kai, M., and Piechulla, B. 2010. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506.
Wesemael, W. M. L., Perry, R. N., and Moens, M. 2006. The influence of root diffusate and host age on hatching of the root-knot nematodes, Meloidogyne chitwoodi and Ma fallax. Nematology 8:895–902.
Wilson, M. J., Ehlers, R. U., and Glazer, I. 2012. Entomopathogenic nematode foraging strategies—is Steinernema carpocapsae really an ambush forager? Nematology in press.
Zhao, X. W., Schmitt, M., and Hawes, M. C. 2000. Species-dependent effects of border cell and root tip exudates on nematode behavior. Phytopathology 90:1239–1245.
Acknowledgments
We thank the journal editors for giving us the opportunity to address the aspect of rhizosphere pest control in this special issue. Our work in this field is supported by a Swiss economic stimulus grant awarded to the National Center of Competence in Research (NCCR) Plant Survival, as well as by the postdoctoral fellowship PBNEP3-13485 from the Swiss National Science Foundation awarded to IH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiltpold, I., Turlings, T.C.J. Manipulation of Chemically Mediated Interactions in Agricultural Soils to Enhance the Control of Crop Pests and to Improve Crop Yield. J Chem Ecol 38, 641–650 (2012). https://doi.org/10.1007/s10886-012-0131-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0131-9