Abstract
Some novel 4-thiazolidinone derivatives have been synthesized by the condensation of isatin/5-chloroisatin with thiosemicarbazide to yield thiosemicarbazones, which were then cyclized to form corresponding thia-3,4,9-triaza-fluoren-2-ylamines. These were reacted with substituted aldehydes to give corresponding Schiff bases, which were cyclized using thioglycolic acid in the presence of zinc chloride to obtain the 4-thiazolidinone derivatives. All the synthesized compounds were characterized by spectral (IR, MS and NMR) and elemental analysis. The compounds were screened for their antibacterial activity against Gram-positive bacteria (B. subtilis, S. aureus, B. pumilus and M. luteus), Gram-negative bacteria (P. aeruginosa, E. coli and P. fluorescens) and for antifungal activity against A. niger and P. chrysogenum by agar-diffusion method. The minimum inhibitory concentrations of these compounds were also determined by tube dilution method. The antimicrobial effectiveness of all the compounds was found to be concentration dependent. Two compounds—2-methyl-3-(1-thia-3, 4, 9-triaza-fluoren-2-yl)-thiazolidin-4-one (7aI) and 2-naphthalen-1-yl-3-(1-thia-3, 4, 9-tri aza-fluoren-2-yl)-thiazolidin-4-one (7aII)—exhibited good antibacterial activity. The antibacterial activity of all the compounds was found to be better than the antifungal activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 4-thiazolidinones are the derivatives of thiazolidine with the carbonyl group at the 4-position, belonging to an important group of heterocyclic compounds containing sulphur and nitrogen in a five membered ring. 4-Thiazolidinone template is one of the privileged structure fragments in modern medicinal chemistry considering its broad pharmacological activity (Verma and Saraf, 2008) and affinity for various biotargets as antimicrobial (Vicini et al., 2006; Bondock et al., 2007; Pooja et al., 2011a, b), anti-inflammatory (Ottana et al., 2005), anti-HIV (Rawal et al., 2005, 2007a, b, 2008a, b; Rao et al., 2003, 2004), anti-tuberculosis (Babaoglu et al., 2003), anti-convulsant (Capan et al., 1996; Gursoy et al., 2005; Ergenc et al., 1999), etc. Isatin is an endogenous compound, i.e. derivative of indigo dye isolated in 1988 (Sridhar et al., 2001). The chemistry of isatin and its derivatives is particularly interesting because of their potential application in medicinal chemistry. 2-Amino-11-hydronaphtho[2,1:5,6]pyrano[4,3-d]thiazole on treatment with isatin, chloroacetyl chloride and mercaptoacetic acid affords corresponding N[naphtha [1,2b] pyrano3,4d]thiazol-8-yl]spiro-[3H-indole-(1H,2H)3,4-(2H)-3chloroazetidine-2,2-diones and N[naphtha [1, 2b]pyrano[3,4-d]thiazol-8-yl]spirol-[3H-indole-(1H, 2H)-3,2-(4H)-thiazolidine]-2,4-dione with good antimicrobial activity (Pai et al., 2006). Jarrahpour et al. (2007) synthesized bis-Schiff bases of isatin by condensation of isatin, benzylisatin and 5-fluoroisatin with primary aromatic amines which possess significant antiviral, antibacterial and antifungal activity. Bhambi et al. (2009) synthesized 3′{4(1acetyl-5(4-flurophenyl)-2pyrazoline-3yl)phenyl}1-N-ethoxyphthalimido-4′-spiro[indole-3,2′-[1,3] thiazolidene]-2,4′-1H-dione, which was formed by reacting 3{4-(1-acetyl-5-(4-chlorophenyl)-2pyrazoline-3-yl)phenyl}-4′H-spiro[indole-3,2′-[1,3]thiazolidene]-2,4′-1H-dione, in DMF and sodium hydride, which showed good antibacterial activity. Bis-Schiff bases, N-Mannich bases, phthalimidoxy substituted and spiro-thiazolidinone derivatives of isatin possess antimicrobial activity against a variety of Gram-positive bacteria, Gram-negative bacteria and some fungi (Pal et al., 2011). Bhati et al. (2008) synthesized various derivatives of 4-thiazolidinones with Schiff and Mannich bases of isatin and screened them for their anti-inflammatory, ulcerogenic and analgesic activities. In the present study, the compounds are conjugates of two heterocyclic moieties, i.e. isatin and thiazolidinone (Scheme 1), and are being investigated for their antimicrobial activity.
Materials and methods
Chemistry
All the chemicals and solvents used in the study were procured as LR grade from S. D. Fine Chem. Ltd., Mumbai and Sigma-Aldrich Chemie, Germany. Thin layer chromatography (TLC) was used for monitoring the progress of the reactions and product formation. The TLC of the synthesized compounds was carried out on 0.25 mm precoated plates of silica gel 60F254, E. Merck, Darmstadt, Germany with different solvent systems. Spots were detected under UV lamp (short and long wavelengths) and in an iodine chamber. The melting points were determined by open capillary method and are uncorrected. Infrared spectra (νmax in cm−1) of the synthesized compounds were recorded on Shimadzu FTIR-8400S and Perkin Elmer 881in the range of 400–4000 cm−1 in potassium bromide. Mass spectra were recorded on a JEOL SX 102/DA-600 instrument using direct analysis in real time (DART) method and fast atomic bombardment (FAB) method. 1HNMR spectra (ppm, δ) were recorded on a Brucker ADVANCE DRX 300 MHz/200 MHz spectrometer, with TMS as the internal standard. Microanalyses for C, H, and N were performed on an Elementar Vario EL III at SAIF, Central Drugs Research Institute, Lucknow, India. Turbidity measurements were made on a Shimadzu 1700 UV-Visible spectrophotometer.
General procedure for the synthesis of thiosemicarbazone derivatives (3a–b)
Equimolar quantities (0.004 mol) of isatin/5-substituted isatin (1a–b) and thiosemicarbazide (2) were dissolved in 90 % ethanol and refluxed for 1 h in the presence of a few drops of glacial acetic acid. The completion of the reaction was checked by TLC using solvent system chloroform: methanol (95:5). Excess ethanol was distilled off and the residue was poured into ice water. The solid product was filtered, washed with water, dried and recrystallized using ethanol.
3-Thiosemicarbazido indole-2-one (3a)
Yield 80.10 %, melting range 180–183 °C; IR (KBr) 1132, 1593 & 1466, 1674, 1593, and 3172 cm−1; ms: m/z 221 [M + 1]. Anal. Calcd. for C9H8N4OS: C, 49.08; H, 3.66; N, 25.44; O, 7.26; S, 14.56.
5-Chloro-3-thiosemicarbazido-indole-2,3,-dione (3b)
Yield 68.35 %, melting range 155–160 °C; IR (KBr) 1049, 1134, 1612 & 1470, 1687, 1470, 3163 and 3478 cm−1; ms: m/z 255 [M + 1]. Anal. Calcd. for C9H7ClN4OS: C, 42.44; H, 2.77; Cl, 13.92; N, 22.00; O, 6.28; S, 12.59.
General procedure for the synthesis of thia-3,4,9-triaza-fluoren-2-ylamine derivatives (4a–b)
Equimolar quantities of 3a–b and 4–5 drops of cold conc. H2SO4 were dissolved in ethanol and refluxed for about 8 h. The completion of the reaction was checked by TLC using chloroform:methanol (98:2) as the solvent system. The reaction mixture was cooled and neutralized with liquor ammonia. The neutralized mixture was then poured into ice water, filtered, dried and recrystallized using rectified spirit.
1-Thia-3,4,9-triaza-fluoren-2-ylamine (4a)
Yield 45.40 %, melting range 230–235 °C; IR (KBr) 3421, 3336, 1623, 1701, 3176 and 3421 cm−1; ms: m/z 255 [M + 2]. Anal. Calcd. for C9H6N4S: C, 53.45; H, 2.99; N, 27.70; S, 15.86.
6-Chloro-1-thia-3,4,9-triaza-fluoren-2-ylamine (4b)
Yield 59.35 %, melting range 170–175 °C; IR (KBr) 3421, 3342, 1611, 1689, 3414 and 3161 cm−1; ms: m/z 237 [M+2]. Anal. Calcd. for C9H5ClN4S: C, 45.67; H, 2.13; Cl, 14.98; N, 23.67; S, 13.55.
General procedure for the synthesis of Schiff base derivatives (6aI–6aIII and 6bI–6bIII)
Equimolar quantities of 4a–b and appropriate aldehydes were dissolved in 20 mL of absolute ethanol, in the presence of 5–6 drops of glacial acetic acid, and the reaction mixture was refluxed till the completion of the reaction. The completion of the reaction (8–9 h) was checked by TLC using chloroform: methanol (95:5) as the solvent system. The hot mixture was then poured onto crushed ice. The crude product so obtained was purified by recrystallization from ethanol.
Ethylidene-(1-thia-3,4,9-triaza-fluoren-2-yl)-amine (6aI)
Yield 77.45 %, melting range 260–265 °C; IR (KBr) 1595, 1689 and 3247 cm−1; ms: m/z 229 [M+1]. Anal. Calcd. for C11H8N4S: C, 57.88; H, 3.53; N, 24.54; S, 14.05.
Naphthalen-1-ylmethylene-(1-thia-3,4,9-triaza-fluoren-2-yl)-amine (6aII)
Yield 61.23 %, melting range 190–195 °C; IR (KBr) 1595, 1689 and 3247 cm−1; ms: m/z 341 [M+1]. Anal. Calcd. for C20H12N4S: C, 70.57; H, 3.55; N, 16.46; S, 9.42.
Pyridin-2-ylmethylene-(1-thia-3,4,9-triaza-fluoren-2-yl)-amine (6aIII)
Yield 55.65 %, melting range 176–180 °C; IR (KBr) 1620, 1720, 3174 and 3421 cm−1; ms: m/z 292 [M+1]. Anal. Calcd. for C15H9N5S: C, 61.84; H, 3.11; N, 24.04; S, 11.01.
6-Chloro-1-thia-3,4,9-triaza-fluoren-2-yl-ethylidene-amine (6bI)
Yield 77.85 %, melting range 210–215 °C; IR (KBr) 1624, 1701, 3173 and 3422 cm−1; ms: m/z 263 [M+1]. Anal. Calcd. for C11H7ClN4S: C, 50.29; H, 2.69; Cl, 13.49; N, 21.33; S, 12.21.
6-Chloro-1-thia-3,4,9-triaza-fluoren-2-yl-naphthalen-1-ylmethylene-amine (6bII)
Yield 88.35 %, melting range 225–230 °C; IR (KBr) 1632, 1710, 3175 and 3428 cm−1; ms: m/z 375 [M+1]. Anal. Calcd. for C20H11ClN4S: C, 64.08; H, 2.96; Cl, 9.46; N, 14.95; S, 8.55.
6-Chloro-1-thia-3,4,9-triaza-fluoren-2-yl-pyridin-2-ylmethylene-amine (6bIII)
Yield 69.33 %, melting range 200–205 °C; IR (KBr) 1636, 1723, 3110 and 3437 cm−1; ms: m/z 326 [M+1]. Anal. Calcd. for C15H8ClN5S: C, 55.30; H, 2.48; Cl, 10.88; N, 21.50; S, 9.84.
General procedure for the synthesis of thiazolidin-4-one derivatives (7aI–7aIII and 7bI–7bIII)
Equimolar quantities of 6aI–6aIII and 6bI–6bIII were dissolved in 50 mL of methanol. An equimolar quantity of thioglycolic acid was also added dropwise, in the presence of anhydrous zinc chloride and the mixture was refluxed till the completion of the reaction. The completion of the reaction was checked by TLC using different solvent systems. Excess of ethanol was distilled off and the residue was poured into ice water. The solid product was filtered, washed with water, dried and recrystallized using ethanol.
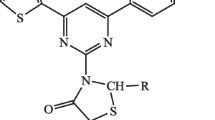
2-Methyl-3-(1-thia-3,4,9-triaza-fluoren-2-yl)-thiazolidin-4-one (7aI)
Crystalline solid, yield 40.12 %, melting range 230–232 °C; IR (KBr) 1485, 1620, 1672 and 3193 cm−1; 1H-NMR (CDCl3, δ, ppm): 1.25 (s, 3H), 2.96–2.97 (s, 2H), 3.61–3.75 (s, 1H), 6.0–7.787 (m, 4H); ms: m/z 301 Anal. Calcd. for C13H10N4OS2: C, 51.64; H, 3.33; N, 18.53. Found: C, 51.88; H, 3.97; N,18.86.
2-Naphthalen-1-yl-3-(1-thia-3,4,9-tri aza-fluoren-2-yl)-thiazolidin-4-one (7aII)
Amorphous powder, yield 45.27 %, melting range 250–255 °C; IR (KBr) 1483, 1618, 1678 and 3193 cm−1; 1H-NMR (CDCl3, δ, ppm): 3.23 (s, 2H), 5.76 (s, 1H), 7.10–7.77 (m, 11H); ms: m/z 413 Anal. Calcd. for C22H14N4OS2: C, 63.75; H, 3.40; N, 13.52. Found: C, 62.93; H, 3.23; N, 13.12.
2-Pyridin-2-yl-3-(1-thia-3,4,9-triaza-fluoren-2-yl)-thiazolidin-4-one (7aIII)
Crystalline solid, yield 36.87 %, melting range 210–211 °C; IR (KBr) 1453, 1593, 1620 and 1693 cm−1; 1H-NMR (CDCl3, δ, ppm): 3.29–3.48 (s, 2H), 5.70–5.88 (s, 1H), 7.01–7.87(m, 8H); ms: m/z 365 Anal. Calcd. for C17H11N5OS2. C, 55.87; H, 3.03; N, 19.16. Found: C, 55.15; H, 3.94; N, 19.78.
3-(6-Chloro-1-thia-3,4,9-triaza-fluoren-2-yl)-2-methyl-thiazolidin-4-one (7bI)
Crystalline solid, yield 56.55 %, melting range 295–297 °C; IR (KBr): 767, 1378, 1443, 1474, 1611 and 1688 cm−1; 1H-NMR (CDCl3, δ, ppm): 1.25 (s, 3H); 3.58 (s, 1H); 5.92 (s, 1H); 7.26–7.68 (m, 4H); ms: m/z 335. Anal. Calcd. for C13H9ClN4OS2. C, 46.36; H, 2.69; N, 16.63. Found: C, 46.23; H, 2.25; N, 16.38.
3-(6-Chloro-1-thia-3,4,9-triaza-fluoren-2-yl)-2-naphthalen-1-yl-thiazolidin-4-one (7bII)
Crystalline solid, yield 50.23 %, melting range 265–268 °C; IR (KBr) 767, 1365, 1440, 1473, 1611 and 1688 cm−1; 1H-NMR (CDCl3, δ, ppm): 3.83 (s, 1H); 5.58 (s, 1H); 7.26–7.89 (m, 11H); ms: m/z 447. Anal. Calcd. for C22H13ClN4OS2. C, 58.86; H, 2.92; N, 12.48. Found: C, 58.21; H, 2.79; N, 12.98.
3-(6-Chloro-1-thia-3, 4, 9-triaza-fluoren-2-yl)-2-pyridin-2-yl-thiazolidin-4-one (7bIII)
Crystalline solid, yield 30.23 %, melting range 280–284 °C; IR (KBr) 761, 1465 and 1634 cm−1. 1H-NMR (CDCl3, δ, ppm): 3.19 (s, 1H); 5.57 (s, 1H); 6.89–7.68 (m, 8H); ms: m/z 401. Anal. Calcd. for C17H10ClN5OS2. C, 51.06; H, 2.52; N, 17.51. Found: C, 51.69; H, 2.34; N, 17.98.
Microbiological activities
Test microorganisms
The standard strains were procured from the microbial type culture collection (MTCC), Institute of Microbial Technology, Chandigarh, India. The antibacterial activities of the synthesized compounds were screened against the bacterial strains: Bacillus pumilus (MTCC 1456), Pseudomonas fluorescens (MTCC 2421), Micrococcus luteus (MTCC 1538), Pseudomonas aeruginosa (MTCC 424), Escherichia coli (MTCC 1573), Bacillus subtilis (MTCC 441) and Staphylococcus aureus (MTCC 1430). For antifungal screening Penicillium chrysogenum (MTCC 161), Aspergillus niger (MTCC 2546) were selected.
Antimicrobial screening
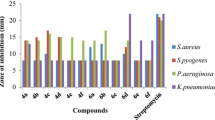
Compounds 7aI–7aIII and 7bI–7bIII were dissolved in 10 % DMSO at the concentrations of 100, 250, 500, 750, 1,000, 1,250 μg/mL. Norfloxacin and fluconazole were used as the standard drugs for antibacterial and antifungal studies, respectively. Nutrient broth suspension of test microorganism (10 mL) was added to 100 mL of sterile molten nutrient agar growth media (cooled to 45 °C), mixed well and poured into sterile petri plates. The agar was allowed to solidify and was then punched to make six wells/cups using a 6 mm sterile cork borer (separate borer for each organism) to ensure proper distribution of wells in periphery and one in centre. Agar plugs were removed and 50 μl solutions of test samples (each compound in six concentrations) was poured into the corresponding marked well by micropipettes. Triplicate plates of each organism were prepared. The plates were left at room temperature for 2 h to allow diffusion of samples and then incubated face upward, at corresponding temperature of each organism for 48 h (Gautam et al., 2010). The diameters of zone of inhibition were measured to the nearest millimeters (the cup size also included) and are presented in Table 1.
Minimum inhibitory concentration (MIC)
A series of glass tubes containing different concentrations of the synthesized compounds (in dimethyl sulphoxide) with Mueller–Hinton broth was inoculated with the required quantity of the inoculum to obtain a suspension of microorganism which contains 105 colony forming units per millilitre. One growth control tube was prepared with the addition of the compound and one blank tube was prepared without the addition of the microorganism. The tubes were incubated at 37 °C for 24 h. The turbidity produced in each tube was recorded by using a UV-visible spectrometer (Agrawal et al., 2011; Pandey et al., 2011). The observed MICs (μg/mL) are presented in Table 2.
Results and discussion
Six novel 4-thiazolidinones of isatin were synthesized by the fusion of two heterocyclic moieties. These compounds were characterized using IR, 1H-NMR, mass-spectroscopy and elemental analysis. The IR spectrum of the synthesized compounds revealed the presence of C–S–C functional group at 761–856, C–N at 1,440–1,485, C=C at 1,611–1,634, C=O at 1,672–1,694 and C–H at 3,060–3,247 cm−1. In 1H-NMR spectra, δ values of the synthesized compounds were found in the range of 1.25–3.83 for alkyl protons and 6.89–7.89 for aromatic protons. M+ and M+1 peak were observed in mass spectra of the synthesized compounds. Percentage of the carbon, hydrogen, and nitrogen in all the compounds was determined by microanalysis. The compounds were screened for antimicrobial activity against four Gram-positive bacteria, three Gram-negative bacteria and two fungal strains. The MICs of all the active compounds were also determined by tube dilution method. All the compounds (7aI–7aIII and 7bI–7bIII) were found more effective against Gram-negative strains than Gram-positive strains. The cell wall of Gram-negative bacteria is high in lipid content and low in peptidoglycan. On the other hand, the cell wall of the Gram-positive bacteria is low in lipid content and high in peptidoglycan. Compounds which were more lipophilic may have more penetration into the Gram-negative bacteria than the Gram-positive bacteria. Therefore, the compounds show better activity against Gram-negative strains than Gram-positive strains. Compound 7aI exhibited good antibacterial activity, having MIC 30 μg/mL, against Bacillus subtilis, Pseudomonas aeruginosa, Pseudomonas fluorescens and Bacillus pumilus. Compound 7aII was found to be the most effective against B. pumilus having lowest MIC (20 μg/mL) and good activity against Bacillus subtilis, Pseudomonas aeruginosa, Pseudomonas fluorescens and Escherichia coli having MIC 40–50 μg/mL. Two bacterial strains (B. pumilus and Pseudomonas aeruginosa) were found to be most sensitive against all the compounds at 20–50 μg/mL. Staphylococcus aureus was found to be the least sensitive strain against all the synthesized compounds. The antibacterial activity of all the compounds was found to be better than antifungal activity. The antibacterial activity of the compounds was in the order of 7aI > 7aII > 7bII > 7aIII > 7bI > 7bIII. The antifungal activity was in the order of 7aIII = 7bII > 7bIII > 7aII > 7aI = 7bI.
Conclusion
The present research encompasses the syntheses of some thiazolidinone analogs of 1-thia-3,4,9-triaza fluorene of thiazolidinones and their antimicrobial potential. The compounds were screened for antimicrobial activity by cup-plate and tube dilution methods. All the compounds exhibited more pronounced antibacterial activity than antifungal activity. The methyl and naphthyl substitutions at position-2 of the thiazolidinone ring resulted in better activity. Also, substitution with chlorine on position-6 of the 1-thia-3,4,9-triaza fluorene ring resulted in lowering of the activity. These findings lead to the conclusion that the more active analogs were the more lipophilic ones, thereby suggesting that better permeation through the microbial cell-wall could be the reason for this. Thus, lipophilic conjugates of thiazolidinone and 1-thia-3,4,9-triaza fluorene could be the potential antimicrobial agents of the future.
References
Agrawal M, Sonar PK, Saraf SK (2011) Synthesis of 1, 3, 5-trisubstituted pyrazoline nucleus containing compounds and screening for antimicrobial activity. Med Chem Res. doi:10.1007/s00044-011-9871-2
Babaoglu K, Page MA, Jones VC, Mcneil MR, Dong C, Naismith JH, Lee RE (2003) Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg Med Chem Lett 13:3227–3230
Bhambi D, Sharma C, Sharma S, Salvi VK, Talesara GL (2009) Synthesis and pharmacological studies of some phthalimidoxy substituted spiro-thiazolidinone derivatives of isatin. Indian J Chem 48B:1006–1012
Bhati SK, Kumar A (2008) Synthesis of new substituted azetidinoyl and thiazolidinoyl-1, 3, 4-thiadiazino (6, 5-b) indoles as promising anti-inflammatory agents. Eur J Med Chem 43:2323–2330
Bondock S, Khalifa W, Fadda AA (2007) Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem 42:948–954
Capan G, Ulusoy N, Ergen N, Cevdet EA, Vidin A (1996) Synthesis and anticonvulsant activity of new 3-[(2-furyl) carbonyl] amino-4-thiazolidinone and 2-[(2-furyl) carbonyl] hydrazono-4-thiazoline derivatives. Farmaco 11:729–732
Ergenc N, Capan G, Gunay NS, Ozkirimli S, Gungor M, Ozbey S, Kendi E (1999) Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4, 5-imidazolidinedione derivatives. Arch Pharm 10:343–347
Gautam V, Chawla V, Sonar PK, Saraf SK (2010) Syntheses, characterization and antimicrobial evaluation of some 1,3,5-trisubstituted pyrazole derivatives. E-J Chem 7:1190–1195
Gursoy A, Terzioglu N (2005) Synthesis and isolation of new regioisomeric 4-thiazolidinones and their anticonvulsant activity. Turk J Chem 29:247–254
Jarrahpour AA, Khalili D, Clercq ED, Salmi C (2007) Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules 12:1720–1730
Ottana R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Paola RD, Sautenbin L, Cuzzocrea S, Vigorita G (2005) 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem 13:4243–4252
Pai NR, Suryanvansi JP (2006) Synthesis and antibacterial screening of N-[naphtho[1,2-b]pyrano[3,4-d]thiazol-8-yl] spiroindoloazetidin-2-ones/thiazolidin-4-ones. Indian J Chem 45B:1226–1230
Pal M, Sharma NK, Priyanka, Jha KK (2011) Synthetic and biological multiplicity of isatin: a review. J Adv Sci Res 2(2):35–44
Pandey V, Chawla V, Saraf SK (2011) Comparative study of conventional and microwave-assisted synthesis of some Schiff bases and their potential as antimicrobial agents. Med Chem Res. doi:10.1007/s00044-011-9592-6
Pooja C, Singh R, Saraf SK (2011a) Synthesis and evaluation of 2, 5-disubstituted 4-thiazolidinone analogues as antimicrobial agents. Med Chem Res. doi:10.1007/s00044-011-9730-1
Pooja C, Singh R, Saraf SK (2011b) Effect of chloro and fluoro groups on the antimicrobial activity of 2, 5-disubstituted 4-thiazolidinones: a comparative study. Med Chem Res. doi:10.1007/s00044-011-9864-1
Rao A, Carbone A, Chimirri A, Clercq ED, Monforte AM, Monforte P, Pannecouque C, Zappala M (2003) Synthesis and anti-HIV activity of 2, 3-diaryl-1, 3-thiazolidin-4-ones. Farmaco 58:115–120
Rao A, Balzarini J, Carbone A, Chimirri A, Clercq ED (2004) 2-(2,6-Dihalophenyl)-3-(pyrimidin-2-yl)-1,3-thiazolidin-4-ones as non-nucleoside HIV-1 reverse transcriptase inhibitors. Antiviral Res 63:79–84
Rawal RK, Prabhakar YS, Katti SB, Clercq ED (2005) 2-(Aryl)-3-furan-2-yl methyl-thiazolidin-4-ones as selective HIV-RT inhibitors. Bioorg Med Chem 13:6771–6776
Rawal RK, Tripathi R, Katti SB, Pannecouque C, Clercq ED (2007a) Synthesis and evaluation of 2-(2, 6-dihalophenyl)-3-pyrimidinyl-1,3-thiazolidin-4-one analogues as anti-HIV-1 agents. Bioorg Med Chem 15:3134–3142
Rawal RK, Tripathi R, Katti SB, Pannecouque C, Clercq ED (2007b) Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1, 3-thiazolidin-4-ones as anti-HIV agents. Bioorg Med Chem 15:1725–1731
Rawal RK, Katti SB, Basu KN, Arora P, Pan Z (2008a) Non-nucleoside inhibitors of the hepatitis C virus NS5B RNA-dependant RNA polymerase: 2-aryl-3-heteroaryl-1, 3-thiazolidin-4-one derivatives. Bioorg Med Chem Lett 18:6110–6114
Rawal RK, Tripathi R, Katti SB, Pannecouque C, Clercq ED (2008b) Design and synthesis of 2-(2, 6-dibromophenyl)-3-heteroaryl-1, 3-thiazolidin-4-ones as anti-HIV agents. Eur J Med Chem 43:2800–2806
Sridhar SK, Saravanana M, Ramesh A (2001) Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Med Chem 36:615–625
Verma A, Saraf SK (2008) 4-Thiazolidinone–a biologically active scaffold. Eur J Med Chem 43:897–905
Vicini P, Geronikaki A, Anastasia K, Incerti M, Zani F (2006) Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg Med Chem 14:3859–3864
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrawal, O.P., Sonar, P.K. & Saraf, S.K. 4-Thiazolidinone and 1-thia-3,4,9-triaza fluorene conjugates: synthesis, characterization and antimicrobial screening. Med Chem Res 22, 1972–1978 (2013). https://doi.org/10.1007/s00044-012-0200-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0200-1