Abstract
To examine current clinical research on the use of transcranial magnetic stimulation (TMS) in the treatment of pediatric and young adult autism spectrum disorder in intellectually capable persons (IC-ASD). We searched peer-reviewed international literature to identify clinical trials investigating TMS as a treatment for behavioral and cognitive symptoms of IC-ASD. We identified sixteen studies and were able to conduct a meta-analysis on twelve of these studies. Seven were open-label or used neurotypical controls for baseline cognitive data, and nine were controlled trials. In the latter, waitlist control groups were often used over sham TMS. Only one study conducted a randomized, parallel, double-blind, and sham controlled trial. Favorable safety data was reported in low frequency repetitive TMS, high frequency repetitive TMS, and intermittent theta burst studies. Compared to TMS research of other neuropsychiatric conditions, significantly lower total TMS pulses were delivered in treatment and neuronavigation was not regularly utilized. Quantitatively, our multivariate meta-analysis results report improvement in cognitive outcomes (pooled Hedges’ g = 0.735, 95% CI = 0.242, 1.228; p = 0.009) and primarily Criterion B symptomology of IC-ASD (pooled Hedges’ g = 0.435, 95% CI = 0.359, 0.511; p < 0.001) with low frequency repetitive TMS to the dorsolateral prefrontal cortex. The results of our systematic review and meta-analysis data indicate that TMS may offer a promising and safe treatment option for pediatric and young adult patients with IC-ASD. However, future work should include use of neuronavigation software, theta burst protocols, targeting of various brain regions, and robust study design before clinical recommendations can be made.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Since the first studies of repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder (MDD) in adults offered evidence of therapeutic response with excitatory high frequency rTMS (HF-rTMS) (≥ 10 Hz) of the left dorsolateral prefrontal cortex (LtDLPFC), (George, 1995; Milev, 2016; Pascual-Leone, 1996; Rossi, 2009) interest in the therapeutic opportunity of transcranial magnetic stimulation (TMS) has grown as have options for TMS modalities. Anti-depressant response to of inhibitory low frequency rTMS (LF-rTMS) (≤ 1 Hz) of the right dorsolateral prefrontal cortex (RtDLPFC) has been demonstrated, (Klein, 1999) though greater evidence supports MDD treatment via HF-rTMS of the LtDLPFC. (Lefaucheur, 2014) Theta burst stimulation (TBS) is another therapeutic TMS treatment with patterned pulses delivered in bursts of three at a higher frequency compared to rTMS (50 Hz). TBS requires less stimulation time, functions at a lower overall intensity compared to rTMS protocols, with promising safety profiles in pediatric patients based on a recent systematic review. (Elmaghraby et al., 2021) Two patterns of TBS are currently utilized, the inhibitory continuous (cTBS) and excitatory intermittent (iTBS). (Chung et al., 2015) Options for delivering “deep TMS” (dTMS) further into cortical tissue have been explored as well. Rather than the traditional figure eight coil, dTMS uses the double cone coil and the H-coil were developed to allow for greater depth of cortical penetration. (Carmi et al., 2018; Tofts & Branston, 1991) Safe and efficacious use of dTMS has been demonstrated, resulting in FDA approval to treat MDD as well as obsessive compulsive disorder (OCD) via stimulation of medial prefrontal cortex (mPFC) and the anterior cingulate cortex (ACC). (Berlim et al., 2014; Blomstedt et al., 2013; Carmi et al., 2018, 2019; Levkovitz et al., 2015) Given these robust findings, as well as emerging protocols documenting the safety, efficacy, non-invasive nature, and ease of administration of TMS as a neuromodulatory treatment in both adult and pediatric patients, (Allen et al., 2017; Connolly, 2012; Damji et al., 2013; Milev, 2016; Rajapakse & Kirton, 2013; Rossi, 2009) there is a growing interest regarding the possibility of using TMS for other neuropsychiatric conditions such as autism spectrum disorder (ASD).
ASD is a neurodevelopmental disorder which presents with deficits in social interaction and communication along with restricted/repetitive pattern of behaviors and interests (RRBs). (American Psychiatric Association, 2013) The condition is highly prevalent, affecting 1–2% of the population worldwide. (Lazoff et al., 2010) Individuals may present with or without intellectual disability, though substantial supports are often required in either case. (Howlin et al., 2004; Tillmann et al., 2019) Despite the burden of disease on individuals and health systems, (Becker et al., 2020) issues of diagnosis and treatment persist due to under-recognition, (Joshi et al., 2010) heterogeneity in clinical phenotypes, and the variability of symptom manifestation across development. (Jannati et al., 2020) Additionally, current pharmacologic interventions in ASD are limited to the management of co-occurring psychopathology and not for the core features of the disorder. (Hutton, 2008; Zhou et al., 2021).
Recent systematic reviews and meta-analytic research investigating the use of TMS and other neuromodulatory techniques in treatment and diagnosis (Jannati et al., 2021) of ASD regardless of age or intellectual capacities, suggest that TMS could be effective in the diagnosis of ASD, treatment of RRBs, and improving executive functioning (EF) deficits. However, heterogeneity in study design and possible publication bias are notable limiting factors in making clinical recommendations, points further highlighted in recent consensus statements by Oberman and Cole. (Barahona-Correa, 2018; Cole et al., 2019; Khaleghi et al., 2020; Oberman & Enticott, 2015) In this study, we aim to further advance current knowledge regarding the use of TMS by conducting a systematic review and meta-analysis of the literature with a focus on TMS as a therapeutic tool for intellectually capable individuals with ASD (IC-ASD) with the purpose of developing future TMS trials which would advance and optimize the therapeutic potential of TMS. We have chosen a younger patient population for this TMS review due to the developmental nature of the disorder (), increasing prevalence rates of IC-ASD in this patient population, (Maenner, 2020), potential for greater effects of TMS early in development when the brain is considered to be more plastic, (Oberman & Enticott, 2015) and to mitigate study design heterogeneity by focusing on a specific population of individuals with ASD. Our study also further advances the literature by including studies published since the seminal 2018 meta-analysis by Barahona-Correa and colleagues. (Barahona-Correa, 2018) To our knowledge, no systematic review or meta-analysis exists which specifically investigates TMS as a therapeutic tool in the management of pediatric and young adults with IC-ASD.
Methods
We conducted a systematic review of peer-reviewed international literature utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Our review was conducted via PubMed and EMBACE published through 03/22/2022 using the following search criteria: [Transcranial Magnetic Stimulation or TMS] AND [Autism Spectrum Disorder or Autism or ASD]. The authors screened, reviewed, and assessed the reference lists of the retrieved papers to ensure that all relevant articles were included in our review. Bibliographies were also cross referenced to ensure that no articles were missed. Our review was not registered. Following publication, our meta-analysis data and code will be publicly available on the Open Science Framework.
All articles were screened for predetermined inclusion and exclusion criteria by four authors. Authors worked collaboratively on each article selected, no automation tools were utilized Articles were included if they met the following criteria: (1) original research in a peer-reviewed journal, (2) the study sample included individuals below 18 years of age with IC-ASD, (3) investigated TMS as a therapeutic modality in the management of IC-ASD via open label trials, controlled trials, or cross over studies, and (4) devices used in the study were FDA-approved for sale in the United States. Articles were excluded if they met the following exclusion criteria: (1) ASD sample with intellectual disability (IQ < 65), (2) focused on other disorders that were not ASD, (3) studied transcranial direct current stimulation or utilized TMS practices other than rTMS or TBS, (4) published in a language other than English, (5) did not include interpretable data, (6) were purely diagnostic in study design or (7) performed a literature review or meta-analysis. Notably, studies were included which investigated individuals above 18 years of age if the study population also contained individuals below 18 years.
Statistical Methods
We performed two meta-analyses of standardized mean differences (SMD) in studies which conducted LF-rTMS to the DLPFC: one for behavioral outcomes and one for cognitive outcomes. To compute the SMDs, we extracted either the mean difference and standard deviation of the difference or the baseline and endpoint means and standard deviations, t-statistics, and p-values for each outcome. Given the small sample sizes of the included studies and the dependent nature of our comparison groups (pre-TMS vs. post-TMS), the standardized mean difference was calculated as Hedges’ g for pre-post scores using the following formula: \(g=\left(1-\frac{3}{4*df-1}\right)\times \left(\frac{{\overline{x} }_{post}-{\overline{x} }_{pre}}{{sd}_{within}}\right)\); and the accompanying sample variance was calculated as \(var\left(g\right)={\left(1-\frac{3}{4*df-1}\right)}^{2}\times \left(\left[\frac{1}{N}+ \frac{{\left(\frac{{\overline{x} }_{post}-{\overline{x} }_{pre}}{{sd}_{within}}\right)}^{2}}{2*N}\right]\times 2\times (1-r)\right)\) (Borenstein, 2009), where \({\overline{x} }_{pre}\) is the pre-treatment mean score, \({\overline{x} }_{post}\) is the post-treatment mean score, \({sd}_{within}\) is the within group standard deviation, df is the degrees of freedom used to estimate \({sd}_{within}\), and N is the sample size (# of pairs). The correlation coefficient, r, for pre-post scores was calculated as \(\frac{{sd}_{pre}^{2} +{ sd}_{post}^{2}-{sd}_{diff}^{2}}{2 \times {sd}_{pre}\times {sd}_{post}}\) (Barahona-Correa, 2018). For studies where insufficient information was available to calculate r, we imputed r using the average of all the calculated correlations. Only studies that provided sufficient data to make these calculations were included in the meta-analysis. Ten studies measuring behavioral outcomes (Casanova et al., 2012, 2020, 2021; Sokhadze et al., 2012, 2018) and eight studies measuring cognitive outcomes (Casanova et al., 2014, 2020; Sokhadze et al., 2009, 2010, 2016, 2018; Wang et al., 2016) possessed data for inclusion. As attempts to obtain unpublished data from the authors of the studies with insufficient data were unsuccessful, they were excluded from the meta-analysis.
Given the dependencies among effect sizes, our analyses utilized random-effects multivariate meta-analysis models using the restricted maximum likelihood (REML) method with unstructured between-study covariance matrices as implemented in Stata (meta mvregress) (Statacorp, 2021). Due to the small number of effect sizes included in the meta-analysis, we applied Jackson-Riley adjustments to the standard errors of the regression coefficients, which are multivariate generalizations of the Knapp-Hartung adjustment in univariate meta-regression (Jackson & Riley, 2014; Statacorp, 2021). Our models utilized within-study standard errors and within-study correlations to define the within-study covariance matrices. Because our outcomes of interest were derived from only two rating scales (ABC and RBS-R) and one cognitive test (Kaniza Oddball Test), we assumed high within-study correlations between our outcomes and set the correlations to 0.8. We used the Cochran multivariate Q-test to assess the total heterogeneity across all effect sizes. A significant Q-test suggests that the effect sizes analyzed are not estimating the same population effect size. We quantified the amount of between-study variance using the Jackson-White-Riley I2 index (I2JWR) (Jackson et al., 2012).The value of this multivariate heterogeneity statistic lies between 0 and 100 and estimates the percentage of variation among effect sizes that can be attributed to heterogeneity. Additionally, since the multivariate meta-regression command (meta mvregress) in Stata provides pooled effect sizes for subdomains only and not an overall effect, we utilized the robumeta command which estimates an overall effect size by implementing meta-regression with robust variance estimation; (Hedges et al., 2010; Statacorp, 2021). When used in conjunction, the meta mvregress and robumeta commands are complement in terms of what the other is lacking. Thus, when reporting results, estimates for each subdomain were calculated using the meta mvregress command and overall estimates were calculated using the robumeta command.
We used the Egger method and funnel plots to assess for small-study effects and publication bias (Egger et al., 1997) and used selection models to correct for publication bias based on reported p-values (Iyengar & Greenhouse, 1988; Vevea & Hedges, 1995). The selection models were implemented using JASP (JASP Team, n.d.).While these statistics assessing and correcting for small-study effects and publication bias can provide some insight, they are univariate approaches and do not take into account the dependencies of effect sizes. Thus, the results should be interpreted with some caution.
Lastly, we estimated multivariate meta-analysis regression model with the effect sizes as the dependent variables and total number of pulses administered (standardized) as the independent variable. The variable for total pulses tested whether the magnitude of effect significantly differed as total pulses increased. All multivariate meta-analyses were two-tailed and performed at the 0.05 alpha level using Stata: Version 17 (Statacorp, 2021).
Results
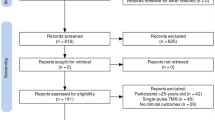
As outlined in Fig. 1, in our initial search 782 articles were screened. 329 articles remained for screening after duplicates were removed. Of these, 90 were not specific to IC-ASD, 123 investigated interventions other than TBS or rTMS, 55 were review or opinion articles, 4 were non-human, and 1 was not available in English. 56 articles were assessed for full text eligibility; 21 focused primarily on adults, 11 were purely diagnostic in their focus, and 8 included individuals with intellectual impairment (IQ < 65). Thus, 16 articles were included for final qualitative synthesis. Of those 16 articles, 12 utilized LF-rTMS targeting the DLPFC, incorporated waitlist control groups which had no statistically significant changes in symptoms over the study duration, had extractable base and endpoint data and were thus, included in the meta-analysis.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools
Qualitative Review Summary
Qualitative results of our review are outlined in Tables 1 and 2. Table 1 includes studies with waitlist control groups and sham TMS controls. Table 2 includes studies without a control group and those which included baseline neurotypical (NT) controls. In studies reviewed, sample sizes ranged from an n = 13 (Sokhadze et al., 2009) to n = 124. (Sokhadze et al., 2018) The maximum age of participants was 23 years with the single exception being the study by Ameis and colleagues which had a maximum age of 35 years and an average age of 22.6 years. (Ameis et al., 2020) Biologic sex was reported in all studies, with 105 females and 471 males with autism included in the research. Regarding assessment of intellectual capacities, 1/16 studies did not report quantitative measures of IQ, but identified patients as IC-ASD via DSM-IV. (Enticott, 2012) The diagnosis of IC-ASD was confirmed by clinical assessment and use of DSM-IV or DSM-V criteria based on the year the study was conducted in all studies. The autism diagnostic interview-revised (ADI-R) was incorporated in 14/16 studies (Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) and the autism diagnostic observation schedule (ADOS) in 2/9. (Ameis et al., 2020; Ni et al., 2021) See Tables 1 and 2 for ascertainment criteria of each study.
Study Design
As seen on Table 1, 9/16 studies incorporated control groups for the duration of the study which included waitlist groups in 6/9 (Casanova et al., 2012; Sokhadze et al., 2009, 2012, 2014a, b, 2018) and sham TMS in 3/9. (Ameis et al., 2020; Enticott, 2012; Ni et al., 2021) Randomization occurred in 7/9 studies; (Ameis et al., 2020; Casanova et al., 2012; Enticott, 2012; Ni et al., 2021; Sokhadze et al., 2012, 2014a, b, 2018) the two studies which were not randomized assigned patients to the waitlist control based on commitment to the research and feasibility of follow up. (Sokhadze et al., 2009, 2014a, b) 7/9 were unblinded trials. (Casanova et al., 2012; Enticott, 2012; Sokhadze et al., 2009, 2012, 2014a, b, 2018) Ni and colleagues conducted a randomized, single blind and sham controlled trial. (Ni et al., 2021) Ameis and colleagues conducted the lone randomized, parallel, double blind, and sham controlled trial. (Ameis et al., 2020).
As seen on Table 2, 7/16 studies did not use control groups for the duration of the study. (Casanova et al., 2020, 2021; Sokhadze et al., 2010, 2014a, b, 2016, 2017; Wang et al., 2016) 3/7 incorporated the use of NT controls. (Casanova et al., 2020, 2021; Sokhadze et al., 2016) Notably, NT controls were not exposed to TMS. Rather, they underwent cognitive testing to compare initial results in the IC-ASD group to NT controls; only IC-ASD participants were exposed to TMS. 3/7 studies also investigated autonomic dysregulation in response to LF-rTMS and found improvement in autonomic measures such as skin conductance level (SCL) and heart rate variability (HRV) which was positively correlated with improvements in behavioral measures. (Casanova et al., 2014; Sokhadze et al., 2017; Wang et al., 2016).
TMS Parameters
15/16 studies used rTMS (Ameis et al., 2020; Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) with 14/15 being LF-rTMS (0.5–1 Hz). (Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) Ameis and colleagues were the only study to investigate the effects of HF-rTMS, utilizing 20 Hz of stimulation. (Ameis et al., 2020) The DLPFC was targeted in 14/16 studies: (Ameis et al., 2020; Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) specifically the LtDLPFC in 13/16, RtDLPFC in 11/16, and bilateral DLPFC (BiDLPFC) in 9/16. The 2012 study by Enticott and colleagues represents the only study to modulate the left primary motor cortex (M1) and the supplemental motor area (SMA). The study was cross-over in design with participants receiving either Left M1, SMA, and sham TMS over three weekly sessions. (Enticott, 2012) Ni and colleagues conducted the only TBS study, using the excitatory iTBS protocol (Huang et al., 2005) to stimulate the posterior superior temporal sulcus (pSTS) bilaterally. Specific information regarding dosing schedules can be found in Tables 1 and 2.
Of studies which targeted the DLPFC, the site of TMS modulation was 5 cm anterior, and in the parasagittal plane, to site of first dorsal interossei muscle (FDI) maximal stimulation in 13/14 studies. (Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) Studies by Ameis and Ni incorporated MRI guided individual neuronavigation. (Ameis et al., 2020; Ni et al., 2021; Rusjan et al., 2010) Additionally, compared to other studies investigated, the studies by Ni and Ameis administered significantly more total TMS pulses over the study period; delivering 30,000 rTMS pulses over a 4 week course of treatment (Ameis et al., 2020) and 38,400 iTBS pulses over an 8 week period, respectively. (Ni et al., 2021) Comparatively, the next highest number of pulses recorded were 3,240 occurring over an 18-week period. This was done in 6/14 studies targeting the DLPFC. (Casanova et al., 2020, 2021; Sokhadze et al., 2014a, b, 2016, 2018) While no significant adverse effects or seizures were reported over all studies investigated, only the studies by Ni and Ameis reported specific adverse effects. Both studies reported mild and transient side effects, the most frequent of which was mild headache and pain at the TMS application site. (Ameis et al., 2020; Ni et al., 2021).
Outcome Measures
9/16 studies measured cognitive and behavioral outcomes of TMS intervention; (Casanova et al., 2012, 2020, 2021; Sokhadze et al., 2009, 2010, 2014a, b, 2016, 2018) 3/16 exclusively investigated cognitive measures (Ameis et al., 2020; Enticott, 2012; Sokhadze et al., 2012) and 4/16 exclusively behavioral measures. (Casanova et al., 2014; Ni et al., 2021; Sokhadze et al., 2017; Wang et al., 2016) Behaviorally, the social responsiveness scale (SRS-2) (Constantino & Gruber, 2012) was included in 6/16 behavioral studies (Casanova et al., 2012; Ni et al., 2021; Sokhadze et al., 2014a, b, 2017) and reported in 4/6, (Casanova et al., 2012; Ni et al., 2021; Sokhadze et al., 2010; G. E. Sokhadze et al., 2017) repetitive behavioral scale-revised (RBS-R) (Lam & Aman, 2007) was used in all thirteen studies, and the aberrant behavioral checklist (ABC) (Aman et al., 1985) was used in 12/13. (Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2014a, b, 2017; Wang et al., 2016) Of those which reported SRS-2 results, Sokhadze 2010 and Casanova et al., 2012 found no significant changes in SRS-2 measures in response to LF-rTMS of the LtDLPFC or of the LtDLPFC and RtDLPFC, respectively. (Casanova et al., 2012; Sokhadze et al., 2010) Sokhadze 2017 reported statistically significant reductions in the SRS-2 domains of social awareness and social cognition at the end of the 18-week trial. (Sokhadze et al., 2017) Ni and colleagues, observed statistically significant improvement in the total SRS-2 score at 8 weeks following iTBS treatments to the bilateral STS following the open-label phase of the study in the active-active group (8 weeks of active treatment), but not in sham-active group (4 weeks of active treatment). Baseline SRS-2 score in the active-active group 107.3 (24.0) at week 1 to 98.5 (28.7) at week 8. Notably, when divided into subdomains, statistically significant improvement in the SRS-2 scale was observed only in the domains of autistic mannerisms and social communication in the active-active group. (Ni et al., 2021) Post-TMS results from the RBS-R were reported in all thirteen studies with only one study not reporting a reduction in total score. (Casanova et al., 2021) Regarding subscales, reductions in stereotypic, ritualistic, and compulsive subscales were reported in 8/13, 6/13, and 4/13 studies, respectively. (Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2014a, b, 2016, 2017; Wang et al., 2016) Notably, similar to their SRS-2 findings, Ni and colleagues reported reductions in total RBS-R score by week 8 in the active-active group of their study; improvements were not observed in the sham-active group. (Ni et al., 2021) Results from the ABC were reported in 11/13 studies; statistically significant reductions in hyperactivity, irritability, social withdrawal / lethargy, stereotypic behavior, and inappropriate speech were reported in 10/13, 7/13, 5/13, 1/13, and 1/13 studies, respectively. (Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2009, 2014a, b, 2016, 2017, 2018; Wang et al., 2016).
12/16 studies investigated cognitive outcomes (Ameis et al., 2020; Casanova et al., 2012, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2018) with 10/12 incorporating use of event related potentials (ERP) via the Kanizsa oddball task. (Casanova et al., 2012, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2018) The task involves the presentation of targets, non-targets, and distracters for the participant to identify. Behavioral response changes which occur during the task are then measured. They include the following: ERP, reaction time, error rates, and accuracy. (Kanizsa, 1976) In these studies, the rate of commission, omission, and total errors was measured pre- and post-TMS after subjects were exposed to the Kanizsa oddball task. Improvements in the rate of omission, commission, and total errors were reported in 3/10, 5/10, and 8/10 studies, respectively. (Casanova et al., 2012, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2018) In their assessment of cognitive outcomes, Ameis and colleagues were the lone study (Ameis et al., 2020) to use the Behavioral Rating Inventory for Executive Function (BRIEF)-Self Report (SR) Version or BRIEF-Adult, (Rosenthal et al., 2013) the CANTAB spatial working memory task, (CANTAB Cognitive Research Software, n.d.) and Vineland Adaptive Behavior Scale–II (VABS-II), a standardized measure of daily functioning. (Sparrow & Cicchetti, 1985) Overall, they found no significant difference between active and sham rTMS on EF, defined as the higher order cognitive functions necessary for flexibly shifting focus, regulating and controlling behavior, and working memory. (Pellicano, 2012). However, individuals with lower baseline adaptive functioning per the VABS-II experienced significant improvement in the active vs sham rTMS group. (Ameis et al., 2020) Lastly, the 2013 study by Enticott and colleagues which investigated the use of LF-rTMS to the left primary motor cortex and SMA sought to improve movement-related cortical potentials (MRCP) often impaired in IC-ASD. (Enticott, 2012; Rinehart et al., 2006) rTMS to the SMA and left primary motor strip was found to be associated with a gradient increase to the early component and late component of MRCPs respectively. Overall, they noted that this improvement in movement related electrophysiological activity may be due to LF-rTMS influence on cortical inhibitory processes. (Enticott, 2012).
Meta-analysis Results
Of the 16 articles identified, twelve had extractable data for meta-analysis of behavioral or cognitive outcomes in patients administered TMS treatment. (Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2016, 2018; Wang et al., 2016).
Behavioral Outcomes
Fifty-two behavioral measures from ten studies were included in the meta-analysis of behavioral outcomes and the results are reported in Tables 1, 2, 3 and Fig. 2A, B. The multivariate meta-analysis with robust variance estimation showed an overall significant improvement in behavioral outcomes after treatment with TMS (pooled Hedges’ g = 0.435, 95% CI = 0.359, 0.511; p < 0.001). The Q-test was significant (p < 0.001) and the joint I2JWR was 98.92%, indicating high heterogeneity and suggesting the outcomes were not estimating a common Hedges’ g. There was significant evidence of small-study effects as determined by Egger’s test (p < 0.001) in Stata and publication bias as determined by selection modeling in JASP (p < 0.001). Selection modeling indicated that the pooled effect size after adjusting for publication bias was 0.443 (95% CI: 0.385, 0.500; p < 0.001). However, this result should be interpreted with caution because there was no way to account for dependencies among effect sizes.
Stratified multivariate analyses by behavioral scale showed similar patterns (Tables 1, 2, 3 and Fig. 2A, B). For all seven scales (ABC Hyperactivity, Irritability, and Lethargy/Social Withdrawal; RBS-R Compulsive Behavior, Ritualistic/Sameness Behavior, Stereotypic Behavior, and Total), the pooled Hedges’ g effect sizes, while small to moderate in size, indicated significant improvement with TMS treatment (all p < 0.001) and ranged from 0.297 for ABC Hyperactivity to 0.638 for RBS-R Total. Heterogeneity was low for the all three ABC scales and the RBS-R Compulsive Behavior and Ritualistic/Sameness Behavior with I2JWR statistics ranging from 0.00% to 5.42% (Table 3). Heterogeneity for the remaining two RBS-R scales was considerably higher with I2JWR = 37.14% for Stereotypic Behavior and I2JWR = 69.89% for Total. Furthermore, when we included total pulses in the model there was a significant association between total pulses and the effect of TMS when looking at the ABC Hyperactivity scale (p = 0.02). The effect of TMS on ABC Hyperactivity significantly increased as total pulses increased. There was no association between total pulses and the effect of TMS for any of the other scales.
Cognitive Outcomes
Eleven cognitive measures from eight studies were included in the meta-analysis of cognitive outcomes and the results are reported in Tables 1, 2, 3 and Fig. 2C. The multivariate meta-analysis with robust variance estimation showed an overall significant improvement in cognitive outcomes after treatment with TMS (pooled Hedges’ g = 0.735, 95% CI = 0.242, 1.228; p = 0.009). The Q-test was significant (p < 0.001) and the joint I2JWR was 97.39%, indicating high heterogeneity and suggesting the outcomes were not estimating a common Hedges’ g. There was significant evidence of small-study effects as determined by Egger’s test in Stata (p < 0.001) and publication bias as determined by selection modeling in JASP (p < 0.001). Selection modeling indicated that the pooled effect size after adjusting for publication bias was 1.671 (95% CI: 1.334, 2.009; p < 0.001). However, this result should be interpreted with caution because there was no way to account for dependencies among effect sizes.
Stratified multivariate analyses by cognitive task showed similar patterns (Tables 1, 2, 3 and Fig. 2C). The pooled Hedges’ g effect sizes for Commission Error Rate (0.759; p = 0.007) and Total Error Rate (0.777; p = 0.008) indicated significant improvement with TMS treatment. Heterogeneity was high for both scales with I2JWR = 78.37% for Commission Error Rate and I2JWR = 80.16% for Total Error Rate. When we included total pulses in the model there was no significant association between total pulses and the effect of TMS for either of the tasks (both p > 0.05).
Discussion
Our review and meta-analysis found some consistencies within current TMS research for the treatment of IC-ASD in this patient population. Regarding TMS parameters, inhibitory TMS dosing was used in 14/16 studies, (Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) with studies by Ameis and Ni being the only excitatory TMS treatments.(Ameis et al., 2020; Ni et al., 2021) In recent work on TMS in ASD, LF-rTMS is more often researched compared to HF-rTMS or iTBS based on the neurobiological hypothesis of parvalbumin (PV) deficiency attributed to ASD. (Hashemi et al., 2017; Lee et al., 2017; Steullet et al., 2017) PV containing cells are susceptible to oxidative injury and make up the largest subgroup of cortical inhibitory interneurons. Reduced numbers of PV-expressing cells have been reported in human postmortem brain samples (Hashemi et al., 2017) and animal models of ASD. (Lee et al., 2017) Additionally, reduced levels of PV expression are associated with ASD-like behavioral deficits and sensory-motor symptoms associated with ASD. In animal models, long term reversal of PV deficits by pharmacologic or cell type specific gene rescue normalizing or diminishes these symptoms.(Lee et al., 2017; Mukherjee et al., 2019; Selimbeyoglu et al., 2017) Thus, researchers have identified a possible excitatory/inhibitory (E/I) imbalance in IC-ASD, also termed “electrophysiological endophenotype” as a target of intervention in the treatment of ASD. (Rojas & Wilson, 2014; Sokhadze et al., 2009; Steullet et al., 2017) The E/I imbalance may represent glutaminergic cortical excitotoxicity, (Rojas, 2014) hyperplasticity due to dysfunction of N-methyl-D-aspartate receptor mediated long-term depression and potentiation-like plasticity mechanisms, or inhibitory GABAnergic dysfunction; all of which may be modulated by specific TMS protocols and monitored via electroencephalogram (EEG) guided ERP. (Buzsáki & Wang, 2012; Jeste & Nelson, 2009) Abnormal oscillations in high gamma band have been observed in this patient population, as well as changes in TMS derived biomarkers of cortical inhibition in response to the inhibitory cTBS; (Casanova et al., 2020, 2021; Jannati et al., 2020; Kirkovski et al., 2022; Oberman et al., 2016; Oberman et al., 2014a, b) adding further evidence of an abnormal E/I balance in IC-ASD which may be modified by inhibitory TMS protocols. (Buzsáki & Wang, 2012; Casanova et al., 2020; Jeste & Nelson, 2009) Encouragingly, multiple studies have reported normalization of gamma wave activity with use of LF-rTMS in IC-ASD persons. (Brown et al., 2005; Casanova et al., 2020; Floris et al., 2016; Rippon et al., 2007; Snijders et al., 2013; Sokhadze et al., 2009, 2014a, b, 2016).
Regarding the two studies with excitatory TMS dosing, Ameis and colleagues utilized 20 Hz HF-rTMS in an effort to improve EF in IC-ASD individuals. (Ameis et al., 2020) Their rationale was driven by previous rTMS research in schizophrenia which used similar rTMS parameters and demonstrated improvements in cognitive and functional impairments in schizophrenia comparable to those observed in IC-ASD. (Maxwell et al., 2015) While improvements across the entire group were negligible in their study, individuals with significant adaptive functional impairment demonstrated robust EF improvements compared to sham. (Ameis et al., 2020) This is of clinical interest given that, based on the excitotoxic PV hypothesis of ASD, it may be assumed that HF-rTMS would have minimal clinical effect. However, these findings by Ameis and colleagues suggest that phenotypic expression of low adaptive functioning abilities in IC-ASD may be an indicator of clinical response in the realm of EF to HF-rTMS compared to IC-ASD individuals with higher adaptative functioning. Furthermore, guided by previous neuroimaging research demonstrating pSTS hypofunction in ASD, (Yang et al., 2015) Ni and colleagues utilized the iTBS protocol in their work. They found that significant clinical response was more likely for individuals with baseline higher intellectual functioning, better social cognitive performance, and less attention deficit hyperactivity disorder symptomology, and when treatment occurred over the course of 8 weeks rather than 4 weeks. (Ni et al., 2021) The direct correlation between time in treatment and clinical response was observed by Sokhadze in 2018 as well. (Sokhadze et al., 2018) Thus, future research is warranted regarding greater clarification and identification of possible ASD subtypes, their neurobiologic underpinnings, outcome measures specific to a site of TMS modulation, (Cole et al., 2019) as well as the possible correlation between clinical response and time in TMS treatment.
TMS coil placement technique was investigated as well. 14/16 studies targeted the DLPFC (Ameis et al., 2020; Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) with 13/14 using the traditional “5 cm rule” of coil placement at 5 cm anterior, and in the parasagittal plane, to the site of FDI maximal stimulation. (Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) Current TMS literature suggests that when the “5 cm rule” is used, the DLPFC is not accurately targeted in 33% (George, 2010) to 68% of individuals, (Herwig et al., 2001) leading.
In our review, Ameis and Ni conducted the only two studies to incorporate use MRI guided TMS targeting. (Ameis et al., 2020; Ni et al., 2021) In light of this information and regular use neuronavigation in other TMS research areas, (Cole et al., 2020; Li et al., 2020) consideration of either MRI guided or Beam F3 targeting in future research is warranted.
The results of our meta-analysis were divided into two sections, behavioral and cognitive. In the behavioral realm, the ABC and RBS-R results were indicative of mild to moderate clinical improvements. Cognitively, the Kanizsa oddball task was used most often. (Kanizsa, 1976) The results of our meta-analysis indicate large and significant improvements in rates of total errors and commission errors with LF-rTMS treatment. However, our study was limited quantitatively as we were able to retrieve only published and statistically significant data which primarily characterized criterion B symptoms of ASD. Additionally, only 12/16 studies were able to be included in the meta-analysis due to availability of data, TMS protocols, and target of treatment. However, reported improvements in RRB via this treatment modality is encouraging in light of the fewer pharmacological options in treatment of criterion B symptoms. (American Psychiatric Association, 2013; Zhou et al., 2021).
Limitations
Our study has several limitations. In controlled studies, blinding was done inconsistently, randomization did not occur in 2/9 studies due to feasibility concerns, (Sokhadze et al., 2009, 2014a, b) and waitlist controls were often utilized over sham TMS. Waitlist controls demonstrate IC-ASD symptom stability over time rather than fully investigating for a placebo response as would be observed with sham controls. 4/16 (Sokhadze et al., 2010, 2016, 2017; Wang et al., 2016) studies had no control group and 3/16 incorporated NT individuals as controls only for baseline data. (Casanova et al., 2020, 2021; Sokhadze et al., 2016) Thus, only 2/16 studies in our systematic review were randomized control trials, (Ameis et al., 2020; Ni et al., 2021) and all studies included in the meta-analysis were open label in design. This is of significant importance in pediatric TMS research design as recent literature reports a lack of separation from sham TMS when investigating the use of TMS in the treatment of adolescent refractory MDD. (Croarkin et al., 2021) In our review, Ameis and colleagues conducted the only double blinded, sham controlled, and parallel study; a design urgently needed in future research. (Ameis et al., 2020) We were also limited in our investigation into the core social features of IC-ASD. The RBS-R well characterizes a wide breath of Criterion B symptomology (Lam & Aman, 2007) and was measured in all reviewed behavioral studies. Significant improvements in the ABC subscales of hyperactivity and irritability were also frequently reported, though are likely influenced primarily by Criterion B symptomology. In contrast, improvements in socially mediated symptoms were not as well characterized. Significant improvements in the ABC subdomain of lethargy/social withdrawal were reported in 5/13 behavioral studies. (Casanova et al., 2021; Sokhadze et al., 2016, 2014a, b, 2017, 2018) The SRS-2 well characterizes social symptom burden, (Constantino & Gruber, 2012) but was only used in 6/16 behavioral studies (Casanova et al., 2012; Ni et al., 2021; Sokhadze et al., 2009, 2010, 2014a, b, 2017) and results were reported in 4/6. (Casanova et al., 2012; Ni et al., 2021; Sokhadze et al., 2010, 2017) Moreover, 2/4 found no statistically significant improvement in SRS-2 scores. (Casanova et al., 2012; Sokhadze et al., 2009) Additionally, because we relied on data reported by authors, we were restricted by what investigators chose to present. There are also very few studies with small sample sizes that could lead to inflated estimates of effect sizes due to a publication bias which favors the publication of positive over negative studies. While attempts were made to adjust for publication bias using selection models, the results need to be interpreted with caution given there was not a way to correct for dependencies among effect sizes. Likewise, the small number of studies and lack of control for dependencies among effect sizes also limits the confidence we can place on Egger’s test for small-study effects.
Other limitations include a lack of reporting on patient sociodemographic factors, an absence of multi-center trials, limited involvement of other research groups as M. F. Casanova or E.M. Sokhadze were authors on 13/16 studies. (Casanova et al., 2012, 2014, 2020, 2021; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) Moreover, as observed in other areas of IC-ASD research, biologically female IC-ASD patients were underrepresented. (Mo et al., 2021) In studies investigated, the ratio of male to female was nearly 4.5:1 rather than 3:1 as reported in recent literature. (Loomes et al., 2017) In addition, while the average age of the study by Ameis and colleagues was 22, their maximum age was 35; serving as an outlier in our systematic review. (Ameis et al., 2020) Lastly, results from behavioral measures may be limited by informant- vs self-reporting. Close family members are often used as informants. However, many family members of individuals with ASD carry the diagnosis or demonstrate autistic traits without meeting criteria of the disorder (Rubenstein et al., 2019) and often under report symptoms in others which they experience. (De la Marche et al., 2015) Additionally, due to interpersonal and social deficits observed in ASD, self-appraisal of social/emotional symptoms can be uniquely challenging. (Rankin et al., 2016).
Conclusions
Overall, the results of our review and meta-analysis indicate that TMS and TBS may be a safe therapeutic option for pediatric and young adult individuals with IC-ASD. Additionally, that RRBs as well as cognitive and EF deficits may be therapeutically targeted via TMS pulses to the DLPFC. Notably, the study by Ni and colleagues shows promise for improvement in social symptoms via targeted TBS pulses to the pSTS. (Ni et al., 2021) However, the results of our study are limited by a lack of randomized sham-controlled trials, an inability to include randomized control trials in our meta-analysis, likely TMS pulse underdosing, inconsistent use of neuronavigation, and under-representation of biologically female individuals. Regardless, given current limitations and side effect profiles associated with psychopharmacologic treatment of ASD, (Alfageh et al., 2019; Hutton, 2008; Smith & Pierce, 2022; Zhou et al., 2021) continued research into TMS as a therapeutic option in IC-ASD is warranted. Moreover, it is possible that future research investigating modulation of other neural networks and cortical regions may result in improvement to other IC-ASD symptom domains. (Guo et al., 2019; Joshi et al., 2019; Williams, 2016) Specifically, attempts to target the default mode, salience, and affective networks may be of benefit as they are commonly associated with complex cognitive and emotional tasks which may be challenging for individuals with ASD. (Lavin et al., 2013; Singh et al., 2020; Smith et al., 2019; Williams, 2016) Given the high prevalence of suicidality, mood and anxiety disorders in autistic youth, future research should also consider targeting these co-morbidities via TMS. (Hollocks et al., 2019; O’Halloran et al., 2022; Schwartzman et al., 2021) Lastly, relative to current TMS research on other neuropsychiatric conditions, the number of TMS pulses administered in 14/16 IC-ASD TMS studies were significantly lower; (Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) raising concern for underdosing. (Carmi et al., 2019; Cole et al., 2020; Li et al., 2020).
Regarding use of specific TMS modalities, 15/16 studies in our review utilized rTMS. (Ameis et al., 2020; Casanova et al., 2012, 2014, 2020, 2021; Enticott, 2012; Sokhadze et al., 2009, 2010, 2012, 2014a, b, 2016, 2017, 2018; Wang et al., 2016) However, given the promising work from Ni and colleagues (Ni et al., 2021) and that TBS has been safely used in similar patient populations, (Elmaghraby et al., 2021) can be rapidly administered in accelerated protocols, has been researched as a diagnostic tool to detect IC-ASD in pediatric patients, (Cole et al., 2020; Huang et al., 2005; Jannati et al., 2020; Pedapati et al., 2016) use of TBS should be strongly considered in future work. Moreover, given the absence of seizure activity uncovered in our review and very low frequency reported in others, (Elmaghraby et al., 2021) further research into TBS and dTMS as a therapeutic option in IC-ASD is warranted. (Cole et al., 2020) Although, known ethical considerations must be taken into account. (Davis, 2014; Maslen et al., 2014).
In summary, the field of IC-ASD TMS research would undoubtably benefit from greater use of neuronavigation software, (Herwig et al., 2001; Nauczyciel et al., 2011) robust study design, (Croarkin et al., 2021) gender matching, use of agreed upon and consistent TMS protocols, as well as increased reporting of sociodemographic factors. Additionally, incorporation, focus, and reporting on outcomes measures related to criterion A of IC-ASD as well as adaptive functioning is needed to obtain a greater understanding of phenotypic expression of IC-ASD and response to treatment. Based on our review, meta-analysis, and previous meta-analysis work, (Barahona-Correa, 2018) further investigation into the use of TMS targeting various networks and cortical regions for the treatment of ASD related social impairments and RRBs, cognitive deficits, common psychopathologic co-morbidities, and executive functioning deficits is warranted.
Availability of Data and Materials
Data regarding systematic review is attached to the submission. Further meta-analytic data than presented in this manuscript is available upon request.
Abbreviations
- rTMS :
-
Repetitive transcranial magnetic stimulation
- MDD :
-
Major depressive disorder
- HF-rTMS :
-
High frequency rTMS
- LtDLPFC :
-
Left dorsolateral prefrontal cortex
- TMS :
-
Transcranial magnetic stimulation
- LF-rTMS :
-
Low frequency rTMS
- RtDLPFC :
-
Right dorsolateral prefrontal cortex
- TBS :
-
Theta burst stimulation
- cTBS :
-
Continuous theta burst stimulation
- iTBS :
-
Inhibitory theta burst stimulation
- dTMS :
-
Deep TMS
- OCD :
-
Obsessive compulsive disorder
- mPFC :
-
Medial prefrontal cortex
- ACC :
-
Anterior cingulate cortex
- ASD :
-
Autism spectrum disorder
- RRB :
-
Restricted/repetitive behaviors
- EF :
-
Executive functioning
- IC-ASD :
-
Autism spectrum disorder in intellectually capable persons
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SMD :
-
Standardized mean differences
- REML :
-
Restricted maximum likelihood
- I2 JWR :
-
Jackson-White-Riley I2 index
- NT :
-
Neurotypical
- ADI-R :
-
Autism diagnostic interview-revised
- ADOS :
-
Autism diagnostic observation schedule
- SCL :
-
Skin conductance level
- HRV :
-
Heart rate variability
- BiDLPFC :
-
Bilateral DLPFC
- M1:
-
Primary motor cortex
- SMA :
-
Supplemental motor area
- pSTS :
-
Posterior superior temporal sulcus
- FDI :
-
First dorsal interossei muscle
- SRS-2:
-
Social responsiveness scale
- RBS-R :
-
Repetitive behavioral scale-revised
- ABC:
-
Aberrant behavioral checklist
- ERP :
-
Event related potentials
- BRIEF:
-
Behavioral Rating Inventory for Executive Function
- SR:
-
Self-Report
- VABS-II:
-
Vineland Adaptive Behavior Scale–II
- MRCP:
-
Movement-related cortical potentials
- PV:
-
Parvalbumin
- E/I:
-
Excitation-to-inhibition
- EEG:
-
Electroencephalogram
References
Alfageh, B. H., Wang, Z., Mongkhon, P., Besag, F. M. C., Alhawassi, T. M., Brauer, R., & Wong, I. C. K. (2019). Safety and Tolerability of Antipsychotic Medication in Individuals with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Pediatric Drugs, 21(3), 153–167. https://doi.org/10.1007/s40272-019-00333-x
Allen, C. H., Kluger, B. M., & Buard, I. (2017). Safety of Transcranial Magnetic Stimulation in Children: A Systematic Review of the Literature. Pediatric Neurology, 68, 3–17.
Aman, M. G., Singh, N. N., Stewart, A. W., & Field, C. J. (1985). The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89(5), 485–491.
Ameis, S. H., Blumberger, D. M., Croarkin, P. E., Mabbott, D. J., Lai, M.-C., Desarkar, P., Szatmari, P., & Daskalakis, Z. J. (2020). Treatment of Executive Function Deficits in autism spectrum disorder with repetitive transcranial magnetic stimulation: A double-blind, sham-controlled, pilot trial. Brain Stimulation, 13(3), 539–547. https://doi.org/10.1016/j.brs.2020.01.007
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing Arlington. https://doi.org/10.1176/appi.books.9780890425596
Barahona-Correa, J. B. (2018). Repetitive Transcranial Magnetic Stimulation for Treatment of Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Frontiers in Integrative Neuroscience, 12, 27.
Becker, J. E., Smith, J. R., & Hazen, E. P. (2020). Pediatric Consultation-Liaison Psychiatry: An Update and Review. Psychosomatics, 61(5), 467–480. https://doi.org/10.1016/j.psym.2020.04.015
Berlim, M. T., Van den Eynde, F., Tovar-Perdomo, S., Chachamovich, E., Zangen, A., & Turecki, G. (2014). Augmenting antidepressants with deep transcranial magnetic stimulation (DTMS) in treatment-resistant major depression. The World Journal of Biological Psychiatry: THe Official Journal of the World Federation of Societies of Biological Psychiatry, 15(7), 570–578. https://doi.org/10.3109/15622975.2014.925141
Blomstedt, P., Sjöberg, R. L., Hansson, M., Bodlund, O., & Hariz, M. I. (2013). Deep Brain Stimulation in the Treatment of Obsessive-Compulsive Disorder. World Neurosurgery, 80(6), e245–e253. https://doi.org/10.1016/j.wneu.2012.10.006
Borenstein, M. (2009). Effect Sizes for Continuous Data. In H. Cooper, L. V. Hedges, & J. C. Valentine (Eds.), The Handbook of Research Synthesis and Meta-Analysis (2nd ed.). Russell Sage Foundation.
Brown, C., Gruber, T., Boucher, J., Rippon, G., & Brock, J. (2005). Gamma abnormalities during perception of illusory figures in autism. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 41(3), 364–376. https://doi.org/10.1016/s0010-9452(08)70273-9
Buzsáki, G., & Wang, X.-J. (2012). Mechanisms of Gamma Oscillations. Annual Review of Neuroscience, 35(1), 203–225. https://doi.org/10.1146/annurev-neuro-062111-150444
CANTAB Cognitive Research Software. (n.d.). Retrieved May 14, 2021, from https://www.cambridgecognition.com/cantab/
Carmi, L., Alyagon, U., Barnea-Ygael, N., Zohar, J., Dar, R., & Zangen, A. (2018). Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimulation, 11(1), 158–165. https://doi.org/10.1016/j.brs.2017.09.004
Carmi, L., Tendler, A., Bystritsky, A., Hollander, E., Blumberger, D. M., Daskalakis, J., Ward, H., Lapidus, K., Goodman, W., Casuto, L., Feifel, D., Barnea-Ygael, N., Roth, Y., Zangen, A., & Zohar, J. (2019). Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. American Journal of Psychiatry, 176(11), 931–938. https://doi.org/10.1176/appi.ajp.2019.18101180
Casanova, M. F., Baruth, J. M., El-Baz, A. S., Tasman, A., Sears, L. L., & Sokhadze, E. M. (2012). Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Event-Related Potential (ERP) Indices of Attention in Autism. Translational Neuroscience, 3(2), 170–180. https://doi.org/10.2478/s13380-012-0022-0
Casanova, M. F., Hensley, M. K., Sokhadze, E. M., El-Baz, A. S., Wang, Y., Li, X., & Sears, L. L. (2014). Effects of weekly low-frequency rTMS on autonomic measures in children with autism spectrum disorder. Frontiers in Human Neuroscience, 8, 851. https://doi.org/10.3389/fnhum.2014.00851
Casanova, M. F., Shaban, M., Ghazal, M., El-Baz, A. S., Casanova, E. L., Opris, I., & Sokhadze, E. M. (2020). Effects of Transcranial Magnetic Stimulation Therapy on Evoked and Induced Gamma Oscillations in Children with Autism Spectrum Disorder. Brain Sciences, 10(7), 423. https://doi.org/10.3390/brainsci10070423
Casanova, M. F., Shaban, M., Ghazal, M., El-Baz, A. S., Casanova, E. L., & Sokhadze, E. M. (2021). Ringing Decay of Gamma Oscillations and Transcranial Magnetic Stimulation Therapy in Autism Spectrum Disorder. Applied Psychophysiology and Biofeedback, 46(2), 161–173. https://doi.org/10.1007/s10484-021-09509-z
Chung, S. W., Hoy, K. E., & Fitzgerald, P. B. (2015). Theta-burst stimulation: A new form of TMS treatment for depression? Depression and Anxiety, 32(3), 182–192.
Cole, E. J., Enticott, P. G., Oberman, L. M., Gwynette, M. F., Casanova, M. F., Jackson, S. L. J., Jannati, A., McPartland, J. C., Naples, A. J., & Puts, N. A. J. (2019). The Potential of Repetitive Transcranial Magnetic Stimulation for Autism Spectrum Disorder: A Consensus Statement. Biological Psychiatry, 85(4), e21–e22. https://doi.org/10.1016/j.biopsych.2018.06.003
Cole, E. J., Stimpson, K. H., Bentzley, B. S., Gulser, M., Cherian, K., Tischler, C., Nejad, R., Pankow, H., Choi, E., Aaron, H., Espil, F. M., Pannu, J., Xiao, X., Duvio, D., Solvason, H. B., Hawkins, J., Guerra, A., Jo, B., Raj, K. S., & Williams, N. R. (2020). Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. American Journal of Psychiatry, 177(8), 716–726. https://doi.org/10.1176/appi.ajp.2019.19070720
Connolly, K. R. (2012). Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: Results observed with the first 100 consecutive cases of depression at an academic medical center. Journal of Clinical Psychiatry, 73(4), 567–573.
Constantino, J. N., & Gruber, C. P. (2012). Social Responsiveness Scale (SRS-2): Manual (2nd ed.). Western Psychological Services.
Croarkin, P. E., Elmaadawi, A. Z., Aaronson, S. T., Schrodt, G. R., Holbert, R. C., Verdoliva, S., Heart, K. L., Demitrack, M. A., & Strawn, J. R. (2021). Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: A double-blind, randomized, sham-controlled trial. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 46(2), 462–469. https://doi.org/10.1038/s41386-020-00829-y
Damji, O., Roe, J., Shinde, S., Kotsovsky, O., & Kirton, A. (2013). Effects of paired associative stimulation on developmental motor plasticity in children. Clinical Neurophysiology, 124(10), e147–e148. https://doi.org/10.1016/j.clinph.2013.04.251
Davis, N. J. (2014). Transcranial stimulation of the developing brain: A plea for extreme caution. Frontiers in Human Neuroscience, 8, 600. https://doi.org/10.3389/fnhum.2014.00600
De la Marche, W., Noens, I., Kuppens, S., Spilt, J. L., Boets, B., & Steyaert, J. (2015). Measuring quantitative autism traits in families: Informant effect or intergenerational transmission? European Child & Adolescent Psychiatry, 24(4), 385–395. https://doi.org/10.1007/s00787-014-0586-z
Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ : British Medical Journal, 315(7109), 629–634.
Elmaghraby, R., Sun, Q., Ozger, C., Shekunov, J., Romanowicz, M., & Croarkin, P. E. (2021). A Systematic Review of the Safety and Tolerability of Theta Burst Stimulation in Children and Adolescents. Neuromodulation: Technology at the Neural Interface, ner.13455. https://doi.org/10.1111/ner.13455
Enticott, P. G. (2012). Repetitive transcranial magnetic stimulation (rTMS) improves movement-related cortical potentials in autism spectrum disorders. Brain Stimulation, 5(1), 30–37.
Floris, D. L., Barber, A. D., Nebel, M. B., Martinelli, M., Lai, M.-C., Crocetti, D., Baron-Cohen, S., Suckling, J., Pekar, J. J., & Mostofsky, S. H. (2016). Atypical lateralization of motor circuit functional connectivity in children with autism is associated with motor deficits. Molecular Autism, 7, 35. https://doi.org/10.1186/s13229-016-0096-6
George, M. S. (1995). Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience.
George, M. S. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Archives of General Psychiatry, 67(5), 507–516.
Guo, B., Chen, J., Chen, Q., Ren, K., Feng, D., Mao, H., Yao, H., Yang, J., Liu, H., Liu, Y., Jia, F., Qi, C., Lynn-Jones, T., Hu, H., Fu, Z., Feng, G., Wang, W., & Wu, S. (2019). Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nature Neuroscience, 22(8), 1223–1234. https://doi.org/10.1038/s41593-019-0445-9
Hashemi, E., Ariza, J., Rogers, H., Noctor, S. C., & Martínez-Cerdeño, V. (2017). The number of parvalbumin-expressing interneurons is decreased in the prefrontal cortex in autism. Cerebral Cortex (New York, N.Y.: 1991), 27(3), 1931–1943. https://doi.org/10.1093/cercor/bhw021
Hedges, L. V., Tipton, E., & Johnson, M. C. (2010). Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods, 1(1), 39–65. https://doi.org/10.1002/jrsm.5
Herwig, U., Padberg, F., Unger, J., Spitzer, M., & Schönfeldt-Lecuona, C. (2001). Transcranial magnetic stimulation in therapy studies: Examination of the reliability of “standard” coil positioning by neuronavigation. Biological Psychiatry, 50(1), 58–61. https://doi.org/10.1016/S0006-3223(01)01153-2
Hollocks, M. J., Lerh, J. W., Magiati, I., Meiser-Stedman, R., & Brugha, T. S. (2019). Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychological Medicine, 49(4), 559–572. https://doi.org/10.1017/S0033291718002283
Howlin, P., Goode, S., Hutton, J., & Rutter, M. (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45(2), 212–229. https://doi.org/10.1111/j.1469-7610.2004.00215.x
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45, 201–206.
Hutton, J. (2008). New-onset psychiatric disorders in individuals with autism. Autism, 12(4), 373–390.
Iyengar, S., & Greenhouse, J. B. (1988). Selection Models and the File Drawer Problem. Statistical Science, 3(1), 109–117. https://doi.org/10.1214/ss/1177013012
Jackson, D., & Riley, R. D. (2014). A refined method for multivariate meta-analysis and meta-regression. Statistics in Medicine, 33(4), 541–554. https://doi.org/10.1002/sim.5957
Jackson, D., White, I. R., & Riley, R. D. (2012). Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Statistics in Medicine, 31(29), 3805–3820. https://doi.org/10.1002/sim.5453
Jannati, A., Block, G., Ryan, M. A., Kaye, H. L., Kayarian, F. B., Bashir, S., Oberman, L. M., Pascual-Leone, A., & Rotenberg, A. (2020). Continuous Theta-Burst Stimulation in Children With High-Functioning Autism Spectrum Disorder and Typically Developing Children. Frontiers in Integrative Neuroscience, 14, 13. https://doi.org/10.3389/fnint.2020.00013
Jannati, A., Ryan, M. A., Kaye, H. L., Tsuboyama, M., & Rotenberg, A. (2021). Biomarkers Obtained by Transcranial Magnetic Stimulation in Neurodevelopmental Disorders. Journal of Clinical Neurophysiology, Publish Ahead of Print. https://doi.org/10.1097/WNP.0000000000000784
JASP Team. (n.d.). JASP (0.16.3) [Computer software].
Jeste, S. S., & Nelson, C. A. (2009). Event Related Potentials in the Understanding of Autism Spectrum Disorders: An Analytical Review. Journal of Autism and Developmental Disorders, 39(3), 495. https://doi.org/10.1007/s10803-008-0652-9
Joshi, G., Gönenç, A., Lukas, S., Wozniak, J., Dallenbach, N., Hoskova, B., Castle, R., & Biederman, J. (2019). Anterior Cingulate Cortex Glutamate Activity in Autism Spectrum Disorder with and without Emotional Dysregulation (S19.008). Neurology, 92(15 Supplement). https://n.neurology.org/content/92/15_Supplement/S19.008
Joshi, G., Petty, C., Wozniak, J., Henin, A., Fried, R., Galdo, M., Kotarski, M., Walls, S., & Biederman, J. (2010). The Heavy Burden of Psychiatric Comorbidity in Youth with Autism Spectrum Disorders: A Large Comparative Study of a Psychiatrically Referred Population. Journal of Autism and Developmental Disorders, 40(11), 1361–1370. https://doi.org/10.1007/s10803-010-0996-9
Kanizsa, G. (1976). Subjective contours. Scientific American, 234(4), 48–52. https://doi.org/10.1038/scientificamerican0476-48
Khaleghi, A., Zarafshan, H., Vand, S. R., & Mohammadi, M. R. (2020). Effects of Non-invasive Neurostimulation on Autism Spectrum Disorder: A Systematic Review. Clinical Psychopharmacology and Neuroscience, 18(4), 527–552. https://doi.org/10.9758/cpn.2020.18.4.527
Kirkovski, M., Hill, A. T., Rogasch, N. C., Saeki, T., Fitzgibbon, B. M., Yang, J., Do, M., Donaldson, P. H., Albein-Urios, N., Fitzgerald, P. B., & Enticott, P. G. (2022). A single- and paired-pulse TMS-EEG investigation of the N100 and long interval cortical inhibition in autism spectrum disorder. Brain Stimulation, 15(1), 229–232. https://doi.org/10.1016/j.brs.2021.12.010
Klein, E. (1999). Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: A double-blind controlled study. Archives of General Psychiatry, 56(4), 315–320.
Lam, K. S. L., & Aman, M. G. (2007). The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(5), 855–866. https://doi.org/10.1007/s10803-006-0213-z
Lavin, C., Melis, C., Mikulan, E., Gelormini, C., Huepe, D., & Ibañez, A. (2013). The anterior cingulate cortex: An integrative hub for human socially-driven interactions. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2013.00064
Lazoff, T., Zhong, L., Piperni, T., & Fombonne, E. (2010). Prevalence of Pervasive Developmental Disorders among Children at the English Montreal School Board. The Canadian Journal of Psychiatry, 55(11), 715–720. https://doi.org/10.1177/070674371005501105
Lee, E., Lee, J., & Kim, E. (2017). Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders. Biological Psychiatry, 81(10), 838–847. https://doi.org/10.1016/j.biopsych.2016.05.011
Lefaucheur, J.-P. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS. Clinical Neurophysiology, 125(11), 2150–2206.
Levkovitz, Y., Isserles, M., Padberg, F., Lisanby, S. H., Bystritsky, A., Xia, G., Tendler, A., Daskalakis, Z. J., Winston, J. L., Dannon, P., Hafez, H. M., Reti, I. M., Morales, O. G., Schlaepfer, T. E., Hollander, E., Berman, J. A., Husain, M. M., Sofer, U., Stein, A., & Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64–73. https://doi.org/10.1002/wps.20199
Li, X., Hartwell, K. J., Henderson, S., Badran, B. W., Brady, K. T., & George, M. S. (2020). Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: A double-blind, sham-controlled, randomized clinical trial. Brain Stimulation, 13(5), 1271–1279. https://doi.org/10.1016/j.brs.2020.06.007
Loomes, R., Hull, L., & Mandy, W. P. L. (2017). What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(6), 466–474. https://doi.org/10.1016/j.jaac.2017.03.013
Maenner, M. J. (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR. Surveillance Summaries, 69. https://doi.org/10.15585/mmwr.ss6904a1
Maslen, H., Earp, B. D., Cohen Kadosh, R., & Savulescu, J. (2014). Brain stimulation for treatment and enhancement in children: An ethical analysis. Frontiers in Human Neuroscience, 8, 953. https://doi.org/10.3389/fnhum.2014.00953
Maxwell, C. R., Villalobos, M. E., Schultz, R. T., Herpertz-Dahlmann, B., Konrad, K., & Kohls, G. (2015). Atypical laterality of resting gamma oscillations in autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 292–297. https://doi.org/10.1007/s10803-013-1842-7
Milev, R. V. (2016). Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 4. Neurostimulation Treatments. Can J Psychiatry, 61(9), 561–575.
Mo, K., Sadoway, T., Bonato, S., Ameis, S. H., Anagnostou, E., Lerch, J. P., Taylor, M. J., & Lai, M.-C. (2021). Sex/Gender differences in the human autistic brains: a systematic review of 20 years of neuroimaging research. NeuroImage: Clinical, 102811. https://doi.org/10.1016/j.nicl.2021.102811
Mukherjee, S., Bhattacharjee, A., Naha, S., Majumdar, T., Debbarma, S. K., Kaur, H., Dutta, S., & Basu, S. (2019). Molecular characterization of NDM-1-producing Klebsiella pneumoniae ST29, ST347, ST1224, and ST2558 causing sepsis in neonates in a tertiary care hospital of North-East India. Infection, Genetics and Evolution : Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 69, 166–175. https://doi.org/10.1016/j.meegid.2019.01.024
Nauczyciel, C., Hellier, P., Morandi, X., Blestel, S., Drapier, D., Ferre, J. C., Barillot, C., & Millet, B. (2011). Assessment of standard coil positioning in transcranial magnetic stimulation in depression. Psychiatry Research, 186(2–3), 232–238. https://doi.org/10.1016/j.psychres.2010.06.012
Ni, H.-C., Chen, Y.-L., Chao, Y.-P., Wu, C.-T., Wu, Y.-Y., Liang, S.H.-Y., Chin, W.-C., Chou, T.-L., Gau, S.S.-F., Huang, Y.-Z., & Lin, H.-Y. (2021). Intermittent theta burst stimulation over the posterior superior temporal sulcus for children with autism spectrum disorder: A 4-week randomized blinded controlled trial followed by another 4-week open-label intervention. Autism. https://doi.org/10.1177/1362361321990534
Oberman, L. M., & Enticott, P. G. (2015). Editorial: The safety and efficacy of noninvasive brain stimulation in development and neurodevelopmental disorders. Frontiers in Human Neuroscience, 9, 544. https://doi.org/10.3389/fnhum.2015.00544
Oberman, L. M., Ifert-Miller, F., Najib, U., Bashir, S., Heydrich, J. G., Picker, J., Rotenberg, A., & Pascual-Leone, A. (2016). Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome. Journal of Child and Adolescent Psychopharmacology, 26(7), 617–624. https://doi.org/10.1089/cap.2015.0166
Oberman, L. M., Pascual-Leone, A., & Rotenberg, A. (2014a). Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Frontiers in Human Neuroscience, 8, 627. https://doi.org/10.3389/fnhum.2014.00627
Oberman, L. M., Rotenberg, A., & Pascual-Leone, A. (2014b). Aberrant Brain Plasticity in Autism Spectrum Disorders. In J. I. Tracy, B. M. Hampstead, & K. Sathian (Eds.), Cognitive Plasticity in Neurologic Disorders (pp. 176–196). Oxford University Press. https://doi.org/10.1093/med/9780199965243.003.0008
O’Halloran, L., Coey, P., & Wilson, C. (2022). Suicidality in autistic youth: A systematic review and meta-analysis. Clinical Psychology Review, 93, 102144. https://doi.org/10.1016/j.cpr.2022.102144
Pascual-Leone, A. (1996). Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. The Lancet, 348(9022), 233–237.
Pedapati, E. V., Gilbert, D. L., Erickson, C. A., Horn, P. S., Shaffer, R. C., Wink, L. K., Laue, C. S., & Wu, S. W. (2016). Abnormal Cortical Plasticity in Youth with Autism Spectrum Disorder: A Transcranial Magnetic Stimulation Case-Control Pilot Study. Journal of Child and Adolescent Psychopharmacology, 26(7), 625–631. https://doi.org/10.1089/cap.2015.0183
Pellicano, E. (2012). The Development of Executive Function in Autism. Autism Research and Treatment, 2012, 1–8. https://doi.org/10.1155/2012/146132
Rajapakse, T., & Kirton, A. (2013). Non-invasive brain stimulation in children: Applications and future directions. Translational Neuroscience, 4(2). https://doi.org/10.2478/s13380-013-0116-3
Rankin, J. A., Weber, R. J., Kang, E., & Lerner, M. D. (2016). Parent- and Self-Reported Social Skills Importance in Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 46(1), 273–286. https://doi.org/10.1007/s10803-015-2574-7
Rinehart, N. J., Tonge, B. J., Bradshaw, J. L., Iansek, R., Enticott, P. G., & Johnson, K. A. (2006). Movement-related potentials in high-functioning autism and Asperger’s disorder. Developmental Medicine and Child Neurology, 48(4), 272–277. https://doi.org/10.1017/S0012162206000594
Rippon, G., Brock, J., Brown, C., & Boucher, J. (2007). Disordered connectivity in the autistic brain: Challenges for the “new psychophysiology.” International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 63(2), 164–172. https://doi.org/10.1016/j.ijpsycho.2006.03.012
Rojas, D. C. (2014). The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. Journal of Neural Transmission, 121(8), 891–905. https://doi.org/10.1007/s00702-014-1216-0
Rojas, D. C., & Wilson, L. B. (2014). γ-band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine, 8(3), 353–368. https://doi.org/10.2217/bmm.14.15
Rosenthal, M., Wallace, G. L., Lawson, R., Wills, M. C., Dixon, E., Yerys, B. E., & Kenworthy, L. (2013). Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology, 27(1), 13–18. https://doi.org/10.1037/a0031299
Rossi, S. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12).
Rubenstein, E., Wiggins, L. D., Schieve, L. A., Bradley, C., DiGuiseppi, C., Moody, E., Pandey, J., Pretzel, R. E., Howard, A. G., Olshan, A. F., Pence, B. W., & Daniels, J. (2019). Associations between parental broader autism phenotype and child autism spectrum disorder phenotype in the Study to Explore Early Development. Autism, 23(2), 436–448. https://doi.org/10.1177/1362361317753563
Rusjan, P. M., Barr, M. S., Farzan, F., Arenovich, T., Maller, J. J., Fitzgerald, P. B., & Daskalakis, Z. J. (2010). Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Human Brain Mapping, NA-NA. https://doi.org/10.1002/hbm.20964
Schwartzman, J. M., Smith, J. R., & Bettis, A. H. (2021). Safety Planning for Suicidality in Autism: Obstacles, Potential Solutions, and Future Directions. Pediatrics, e2021052958. https://doi.org/10.1542/peds.2021-052958
Selimbeyoglu, A., Kim, C. K., Inoue, M., Lee, S. Y., Hong, A. S. O., Kauvar, I., Ramakrishnan, C., Fenno, L. E., Davidson, T. J., Wright, M., & Deisseroth, K. (2017). Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Science Translational Medicine, 9(401), eaah6733. https://doi.org/10.1126/scitranslmed.aah6733
Singh, A., Erwin-Grabner, T., Sutcliffe, G., Paulus, W., Dechent, P., Antal, A., & Goya-Maldonado, R. (2020). Default mode network alterations after intermittent theta burst stimulation in healthy subjects. Translational Psychiatry, 10(1), 75. https://doi.org/10.1038/s41398-020-0754-5
Smith, J. R., & Pierce, D. L. (2022). Letter to the Editor: Aripiprazole-Induced Hypersexuality in an Autistic Child. Journal of Child and Adolescent Psychopharmacology, 32(1), 70–71. https://doi.org/10.1089/cap.2021.0130
Smith, R., Ahern, G. L., & Lane, R. D. (2019). The role of anterior and midcingulate cortex in emotional awareness: A domain-general processing perspective. In Handbook of Clinical Neurology (Vol. 166, pp. 89–101). Elsevier. https://doi.org/10.1016/B978-0-444-64196-0.00006-6
Snijders, T. M., Milivojevic, B., & Kemner, C. (2013). Atypical excitation-inhibition balance in autism captured by the gamma response to contextual modulation. NeuroImage. Clinical, 3, 65–72. https://doi.org/10.1016/j.nicl.2013.06.015
Sokhadze, E. M., Baruth, J. M., Sears, L. L., Sokhadze, G. E., El-Baz, A. S., & Casanova, M. F. (2012). Prefrontal Neuromodulation Using rTMS Improves Error Monitoring and Correction Function in Autism. Applied Psychophysiology and Biofeedback, 37(2), 91–102. https://doi.org/10.1007/s10484-012-9182-5
Sokhadze, E. M., Baruth, J. M., Tasman, A., Mansoor, M., Ramaswamy, R., Sears, L. L., Mathai, G., El-Baz, A. S., & Casanova, M. F. (2010). Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Applied Psychophysiology and Biofeedback, 35(2), 147–161. https://doi.org/10.1007/s10484-009-9121-2
Sokhadze, E. M., Casanova, M. F., El-Baz, A. S., Farag, H. E., Li, X., & Wang, Y. (2016). TMS-based neuromodulation of evoked and induced gamma oscillations and event-related potentials in children with autism. NeuroRegulation, 3(3), 101–126. https://doi.org/10.15540/nr.3.3.101
Sokhadze, E. M., El-Baz, A. S., Baruth, J. M., Mathai, G., Sears, L. L., & Casanova, M. F. (2009). Effects of Low Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) on Gamma Frequency Oscillations and Event-Related Potentials During Processing of Illusory Figures in Autism. Journal of Autism and Developmental Disorders, 39(4), 619–634. https://doi.org/10.1007/s10803-008-0662-7
Sokhadze, E. M., El-Baz, A. S., Sears, L. L., Opris, I., & Casanova, M. F. (2014a). RTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Frontiers in Systems Neuroscience. https://doi.org/10.3389/fnsys.2014.00134
Sokhadze, E. M., El-Baz, A. S., Tasman, A., Sears, L. L., Wang, Y., Lamina, E. V., & Casanova, M. F. (2014b). Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: An exploratory study. Applied Psychophysiology and Biofeedback, 39(3–4), 237–257. https://doi.org/10.1007/s10484-014-9264-7
Sokhadze, E. M., Lamina, E. V., Casanova, E. L., Kelly, D. P., Opris, I., Tasman, A., & Casanova, M. F. (2018). Exploratory Study of rTMS Neuromodulation Effects on Electrocortical Functional Measures of Performance in an Oddball Test and Behavioral Symptoms in Autism. Frontiers in Systems Neuroscience, 12, 20. https://doi.org/10.3389/fnsys.2018.00020
Sokhadze, G. E., Casanova, M. F., Kelly, D., Casanova, E. L., Russell, B., & Sokhadze, E. M. (2017). Neuromodulation Based on rTMS Affects Behavioral Measures and Autonomic Nervous System Activity in Children with Autism. NeuroRegulation, 4(2), 65–78. https://doi.org/10.15540/nr.4.2.65
Sparrow, S. S., & Cicchetti, D. V. (1985). Diagnostic Uses of the Vineland Adaptive Behavior Scales. Journal of Pediatric Psychology, 10(2), 215–225. https://doi.org/10.1093/jpepsy/10.2.215
Statacorp. (2021). Stata Statistical Software (Release 17) [Statacorp]. StataCorp LLC.
Steullet, P., Cabungcal, J.-H., Coyle, J., Didriksen, M., Gill, K., Grace, A. A., Hensch, T. K., LaMantia, A.-S., Lindemann, L., Maynard, T. M., Meyer, U., Morishita, H., O’Donnell, P., Puhl, M., Cuenod, M., & Do, K. Q. (2017). Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Molecular Psychiatry, 22(7), 936–943. https://doi.org/10.1038/mp.2017.47
Tillmann, J., San José Cáceres, A., Chatham, C. H., Crawley, D., Holt, R., Oakley, B., Banaschewski, T., Baron-Cohen, S., Bölte, S., Buitelaar, J. K., Durston, S., Ham, L., Loth, E., Simonoff, E., Spooren, W., Murphy, D. G., Charman, T., EU-AIMS LEAP group. (2019). Investigating the factors underlying adaptive functioning in autism in the EU-AIMS Longitudinal European Autism Project. Autism Research: Official Journal of the International Society for Autism Research, 12(4), 645–657. https://doi.org/10.1002/aur.2081
Tofts, P., & Branston, N. (1991). The measurement of electric field, and the influence of surface charge, in magnetic stimulation. Electroencephalography and Clinical Neurophysiology/evoked Potentials Section, 81(3), 238–239.
Vevea, J. L., & Hedges, L. V. (1995). A general linear model for estimating effect size in the presence of publication bias. Psychometrika, 60(3), 419–435. https://doi.org/10.1007/BF02294384
Wang, Y., Hensley, M. K., Tasman, A., Sears, L. L., Casanova, M. F., & Sokhadze, E. M. (2016). Heart Rate Variability and Skin Conductance During Repetitive TMS Course in Children with Autism. Applied Psychophysiology and Biofeedback, 41(1), 47–60. https://doi.org/10.1007/s10484-015-9311-z
Williams, L. M. (2016). Precision psychiatry: A neural circuit taxonomy for depression and anxiety. The Lancet Psychiatry, 3(5), 472–480. https://doi.org/10.1016/S2215-0366(15)00579-9
Yang, D.Y.-J., Rosenblau, G., Keifer, C., & Pelphrey, K. A. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience & Biobehavioral Reviews, 51, 263–275. https://doi.org/10.1016/j.neubiorev.2015.01.020
Zhou, M. S., Nasir, M., Farhat, L. C., Kook, M., Artukoglu, B. B., & Bloch, M. H. (2021). Meta-analysis: Pharmacologic Treatment of Restricted and Repetitive Behaviors in Autism Spectrum Disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 60(1), 35–45. https://doi.org/10.1016/j.jaac.2020.03.007
Funding
This work is funded in part by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under award numbers T32HD007475 in the Eunice Kennedy Shriver National Institute of Child Health and Human Development, K23MH100450, and the Alan and Lorraine Bressler Clinical and Research Program for Autism Spectrum Disorder. Dr. Joshi is specifically supported by the NIMH of the NIH under Award Number K23MH100450. Dr. Anteraper receives grant support from NIMH of the NIH under award number 1R03MH121879-01A1. Dr. Croarkin receives funding from the NIH under grants R01 MH113700 and R01 MH124655. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NIMH or the NIH.
Author information
Authors and Affiliations
Contributions
JS: Conceptualization, Methodology, Investigation, Data Curation, Formal Analysis, Writing—Original Draft. MD: Conceptualization, Methodology, Investigation, Data Curation, Formal Analysis, Writing – Review and Editing. AG: Conceptualization, Methodology, Investigation, Writing – Review and Editing. AC: Conceptualization, Methodology, Investigation, Data Curation, Writing – Review and Editing. SA: Conceptualization, Writing – Review and Editing. PC: Conceptualization, Supervision, Writing – Review and Editing. GJ: Conceptualization, Methodology, Investigation, Data Curation, Formal Analysis, Supervision, Writing – Review and Editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors provide consent for publication.
Competing Interests
JRS is employed by Vanderbilt University Medical Center as a child and adolescent psychiatrist with no other financial relationships to disclose. AG and MD do not have financial relationships to disclose. AC reports personal fees from Massachusetts Department of Mental Health, non-financial support from NIH, personal fees from Pamlab, Inc., personal fees from US Department of Defense, non-financial support from Neurocentria, Inc., personal fees from Food and Drug Administration, non-financial support from Pfizer Pharmaceuticals, Inc., personal fees from Sunovion Pharmaceuticals, Inc., non-financial support from Roche TCRC, Inc. SA is employed by the Carle Foundation Hospital in Urbana, Illinois with no other financial relationships to disclose. PC has received research support from Pfizer, Inc. and equipment support from Neuronetics, Inc. and MagVenture, Inc. He has received grant in kind support from AssureRX for supplies and genotyping. He has been the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc. He has served as a paid consultant for Engrail Therapeutics, Myriad Neuroscience, Procter and Gamble Company, and Sunovion. In the last year, GJ has received research support from the Demarest Lloyd, Jr. Foundation as a primary investigator (PI) for investigator-initiated studies. Additionally, GJ receives research support F. Hoffmann-La Roche Ltd. as a site PI for multi-site trials. In the past three years, GJ has received research support from Pfizer and the Simons Center for the Social Brain. In addition, GJ has received honorarium from the Governor's Council for Medical Research and Treatment of Autism in New Jersey and from NIMH for grant review activities. Finally, he received speaker’s honorariums from the American Academy of Child and Adolescent Psychiatry, The Israeli Society of ADHD, the Canadian Academy of Child and Adolescent Psychiatry, and the University of Jülich.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smith, J.R., DiSalvo, M., Green, A. et al. Treatment Response of Transcranial Magnetic Stimulation in Intellectually Capable Youth and Young Adults with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Neuropsychol Rev 33, 834–855 (2023). https://doi.org/10.1007/s11065-022-09564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-022-09564-1