Abstract
In our previous study on individuals with autism spectrum disorder (ASD) (Sokhadze et al., Appl Psychophysiol Biofeedback 34:37–51, 2009a) we reported abnormalities in the attention-orienting frontal event-related potentials (ERP) and the sustained-attention centro-parietal ERPs in a visual oddball experiment. These results suggest that individuals with autism over-process information needed for the successful differentiation of target and novel stimuli. In the present study we examine the effects of low-frequency, repetitive Transcranial Magnetic Stimulation (rTMS) on novelty processing as well as behavior and social functioning in 13 individuals with ASD. Our hypothesis was that low-frequency rTMS application to dorsolateral prefrontal cortex (DLFPC) would result in an alteration of the cortical excitatory/inhibitory balance through the activation of inhibitory GABAergic double bouquet interneurons. We expected to find post-TMS differences in amplitude and latency of early and late ERP components. The results of our current study validate the use of low-frequency rTMS as a modulatory tool that altered the disrupted ratio of cortical excitation to inhibition in autism. After rTMS the parieto-occipital P50 amplitude decreased to novel distracters but not to targets; also the amplitude and latency to targets increased for the frontal P50 while decreasing to non-target stimuli. Low-frequency rTMS minimized early cortical responses to irrelevant stimuli and increased responses to relevant stimuli. Improved selectivity in early cortical responses lead to better stimulus differentiation at later-stage responses as was made evident by our P3b and P3a component findings. These results indicate a significant change in early, middle-latency and late ERP components at the frontal, centro-parietal, and parieto-occipital regions of interest in response to target and distracter stimuli as a result of rTMS treatment. Overall, our preliminary results show that rTMS may prove to be an important research tool or treatment modality in addressing the stimulus hypersensitivity characteristic of autism spectrum disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In our previous study on individuals with autism spectrum disorder (ASD) (Sokhadze et al. 2009a) we reported abnormalities in the attention-orienting frontal event-related potentials (ERP) and the sustained-attention centro-parietal ERPs in a visual oddball experiment. The three stimulus oddball paradigm that we used in that study was aimed to test the hypothesis that individuals with autism abnormally orient their attention to novel distracters as compared to controls. At frontal regions-of-interest (ROI) the ASD group showed significantly higher amplitudes and prolonged latencies of early ERP components (e.g., 40–180 ms post-stimulus) to novel distracter stimuli in both hemispheres. The ASD group also showed prolonged latencies of several late ERP components (e.g., 240–500 ms post-stimulus) to novel distracter stimuli in both hemispheres. Our results indicated augmented and prolonged early frontal potentials and a delayed P3a component to novel stimuli, which suggest low selectivity in pre-processing and later-stage under-activation of integrative regions in the prefrontal cortices. At centro-parietal ROI the ASD group showed significantly prolonged N100 latencies and reduced amplitudes of the N2b component to target stimuli. Also, the latency of the P3b component was prolonged to novel distracters in the ASD group. In general, the autistic group showed prolonged latencies to novel stimuli especially in the right hemisphere. These results suggest that individuals with autism over-process information needed for the successful differentiation of target and novel stimuli.
In the present study we examined the effects of low-frequency, repetitive Transcranial Magnetic Stimulation (rTMS) on novelty processing as well as behavior and social functioning in individuals with ASD. Our hypothesis was that low-frequency rTMS application to dorsolateral prefrontal cortex (DLFPC) would result in an alteration of the cortical excitatory/inhibitory balance through the activation of select inhibitory GABAergic interneurons. rTMS offers a non-invasive method for altering excitability of the brain. It potentially induces a short-term functional reorganization in the human cortex. The character of rTMS-induced plasticity depends on the intensity, frequency, and number of magnetic pulses during stimulation sessions, and the excitability of the targeted cortex. Effects of rTMS are not limited to the stimulated target cortex because of functional and anatomical interconnections of cortical areas within a distributed functional network (Rossi and Rossini 2004; Ziemann 2004). TMS provides a non-invasive method for both the induction of focal currents and modulation of function in distinct isocortical parcellations. The lasting effects of rTMS offer new possibilities to study dynamic aspects of the pathophysiology of a variety of diseases and may have therapeutic potential in some psychiatric disorders. TMS is now used not only as a diagnostic tool (Pascual-Leone et al. 2000) but also as an alternative treatment for some psychopathologies such as depression, schizophrenia, obsessive–compulsive disorder, etc. (George and Belmaker 2007; George et al. 2000, 2003; Nahas et al. 2000; Wassermann et al. 1996). By convention, rTMS in the 0.3–1 Hz frequency range is referred to as “slow,” whereas “fast” rTMS refers to stimulation greater than 1 Hz. Hoffman and Cavus (2002) in their review of slow rTMS studies proposed long-term depression and long-term depotentiation as models for understanding the mechanism of slow rTMS. Isocortical long-term depression and changes in the cortical excitability induced by slow rTMS appear to accumulate in an additive fashion as the number of stimulations is increased over many days. Studies of both slow rTMS and long-term depression suggest additive efficacy when higher numbers of spaced, daily stimulations are administered. The reversal, or depotentiation, of previously enhanced synaptic transmission due to long-term potentiation may be the most relevant model for slow rTMS when used as a therapeutic tool.
Autism is associated with cortical cytoarchitectural abnormalities (Casanova 2005; Casanova et al. 2002a, b). In brief, the reduced neuropil space (periphery of the minicolumn) reported in autism is the compartment where lateral inhibition sharpens the borders of minicolumns and increases their definition (DeFelipe 1999, 2004; DeFelipe et al. 1990; Favorov and Kelly 1994a, b). The primary source of for this inhibitory effect may be derived from axon bundles of double-bouquet cells (DeFelipe et al. 1990; Favorov and Kelly 1994a). The axons of double bouquet cells arrange themselves in essentially repeatable patterns varying between 15 and 30 μm wide, depending on the cortical area examined (DeFelipe 1999). Double-bouquet cells in the peripheral neuropil space of minicolumns provide a “vertical stream of negative inhibition” (Mountcastle 1997, 2003) surrounding the minicolumnar core. Other GABAergic cells in the minicolumn, having collateral projections extending hundreds of microns tangentially, provide lateral inhibition of surrounding minicolumns on a macrocolumnar scale (Casanova 2006).
The value of each minicolumn’s output is insulated to a greater or lesser degree from the activity of its neighbors by GABAergic inhibition in its peripheral neuropil space. This allows for gradations in amplitude of excitatory activity across a minicolumnar field. Rubenstein and Merzenich (2003) have posited that reductions in GABAergic inhibitory activity may explain some symptomatology of autism, including increased incidence of seizures and auditory-tactile hypersensitivity (see also Casanova et al. 2003). This hypothesis is consistent with findings of reduced minicolumnar peripheral neuropil space in the neocortex of individuals with autism relative to controls (Casanova et al. 2002a, b). In this model, a reduction in the peripheral neuropil space would result in smaller minicolumns which would coalesce into discrete, isolated islands of coordinated excitatory activity. These islands could serve as potential ictal foci. Moreover, their autonomous activity would hinder the binding of associated cortical areas, arguably promoting focus on particulars as opposed to general features. Significantly, by puberty, one-third of autistic patients will have exhibited at least two unprovoked seizures (Volkmar and Nelson 1995). Anecdotal case reports have shown that anticonvulsants have ameliorated autistic traits in epileptic patients (Childs and Blair 1997; Hollander et al. 2001; Plioplys 1994; Uvebrant and Bauzienè 1994). Anticonvulsants may be of some benefit in treating autism, but at larger doses patients suffer from serious side effects including stupor and coma. These side effects are due to the non-selective nature of anticonvulsants whose mechanism of action (increasing GABAergic tone) is independent of cell type (e.g., double-bouquet, small and large basket, chandelier). The effects of anticonvulsants stand in contrast to the specificity of slow rTMS; this technique induces electricity in conductors at right angles to an expanding or collapsing magnetic field (law of electromagnetic induction). The effect of slow rTMS may be of benefit when selectively attempting to activate the inhibitory cells and fibers surrounding the minicolumn (peripheral neuropil space of Seldon) (Seldon 1981a, b); these anatomical elements have a geometric preference for being perpendicular to the cortical surface (Casanova et al. 2003). Ogawa et al. (2004) examined changes in high-frequency oscillations (HFOs) of somatosensory evoked potentials (SEPs) before and after slow rTMS over the right primary somatosensory cortex (0.5 Hz, 50 pulses, 80% motor threshold intensity). The HFOs, which represent the localized activity of intracortical inhibitory interneurons, were significantly increased after slow rTMS, while the SEPs were not changed. Their results suggest that slow rTMS affects cortical excitability by modulating the activity of the intracortical inhibitory interneurons beyond the time of the stimulation and that rTMS may have therapeutic effects on disorders defined by high cortical excitability. This is in line with our hypothesis indicating that slow rTMS will increase the activity of minicolumnar inhibitory cells, and as a result the minicolumnar spatial contrast needed for improved functional discrimination will be enhanced.
Event-related potentials (ERP) represent an effective way to address timing and topographic specifics of abnormalities of cognitive processes in pervasive neurodevelopmental disorders. Among all ERP components one of the most studied is the P300; it is elicited when a subject detects an unexpected (novel, rare) stimulus and consists of two components labeled P3a (fronto-central P300) and P3b (centro-parietal P300). The P3a (sometimes referred to as the novelty P300) is a frontocentral positive wave that reflects an aspect of the orienting response and has been related to evaluative attentional processes (Hruby and Marsalek 2003; Polich 2003). The P3b is a centro-parietal wave that has been linked to task-relevance and the decision-related character of the eliciting stimulus; it reflects memory-updating processes and/or processing closure (Picton 1992).
Studies in children and adolescents with autism have shown abnormalities in ERPs (Bomba and Pang 2004; Kemner et al. 1999). In one study the fronto-central N100 component has been found to be shorter in latency and higher in amplitude in an oddball task in children with autism compared to normal children (Oades et al. 1988). Other studies have reported a normal centro-parietal N100 in autistic children (Kemner et al. 1995; Lincoln et al. 1993), while still others interpret the same to be reduced and delayed (Bruneau et al. 1999). Children with autism have been found to differ from typical children in standard oddball tasks. In particular, Kemner et al. (1995, 1999) have reported an abnormally small occipital P300 in response to visual targets. Courchesne et al. (1989) also found a smaller frontal N450 to visual stimuli. Studies using simple, visual target detection have found delayed frontal P300 (P3a) but a relatively intact posterior P300 (P3b) in spatial, visual attention tasks (Townsend et al. 2001). Deficits in central-auditory processing in autism, as indexed by ERPs, have been described by different authors (Courchesne et al. 1989; Ciesielski et al. 1990, 1995; Ferri et al. 2003; Lincoln et al. 1993; Oades et al. 1988). In children with autism the most consistent and frequently reported abnormality is P3b amplitude attenuation with auditory stimulation (Bruneau et al. 1999; Seri et al. 1999), while P3b latency is spared (Courchesne et al. 1989; Lincoln et al. 1993; Oades et al. 1988). Reduced P3b amplitude has been explained as reflecting deficiencies in the allocation of attentional resources to stimuli (Dawson et al. 1988; Griffith et al. 1999; Holcomb et al. 1985; Bertone et al. 2005). P3b attenuation in autism is more specific to the auditory modality compared to the visual (Bomba and Pang 2004; Verbaten et al. 1991).
An autistic deficit in rapid attention shifting has been observed in behavioral tasks shifting between sensory modalities, spatial locations, and object features (Belmonte and Yurgelun-Todd 2003a, b; Burack 1994); these behavioral observations in autism are complemented by ERP results indicating that even when autistic individuals produce normal behavioral output, they tend to do so by abnormal physiological means. Frontal negativities associated with attention are reported to be reduced in the brains of individuals with autism (Ciesielski et al. 1995). Also, the frontal, late positive component in response to peripheral visual stimuli is delayed, and the visual P3b is highly variable (Courchesne et al. 1989) with a somewhat low average amplitude (Townsend et al. 2001). In addition to a failure in the normal modulation of ERP peaks, the brains of patients with autism are often inappropriately activated. The visual centro-parietal N200 (N2b) to novel stimuli is larger when a person with autism is performing a task in a passive mode (Kemner et al. 1994). Inappropriate activation occurs across modalities; for instance, when a response is required to an auditory stimulus, children with autism manifest an enhanced P3b at occipital sites overlying visual processing areas (Kemner et al. 1995). In general, perceptual filtering in autism seems to occur in an all-or-none manner, with little specificity for either the location of the stimulus, the behavioral relevance of the stimulus, or even for the sensory modality of the stimulus. The attention of patients with autism seems founded more on the control of general arousal level rather than on selective activation of specific perceptual systems.
Deficits in inhibitory functions under various experimental conditions during cognitive tasks are often found in various psychopathologies; for instance, in patients with schizophrenia it may be considered a core feature of the schizophrenic pathophysiology (Boutros et al. 2004; Mathalon et al. 2002). A disturbance in inhibitory function in response to irrelevant sensory input (i.e. an impaired ability to filter out irrelevant sensory information) may be related to inadequate sensory information processing and a result of a flooding of higher-level integrative cortical centers with task-irrelevant information (Boutros et al. 2004). Such impaired sensory filtering, shown by a failure to suppress evoked potentials elicited by task-irrelevant distracter, has also been observed in experiments on schizophrenic subjects using auditory-paired stimulus paradigms (so called “sensory gating”).
Our study examines the effects of low-frequency repetitive TMS (rTMS) on behavioral, and ERP outcomes in persons with ASD. rTMS was applied to the left DLPFC on a twice per week basis for 3 weeks in a group of 13 individuals with ASD. Our hypothesis was that low-frequency rTMS of dorsolateral prefrontal cortex (DLPFC) would result in strengthening of the inhibitory surround of minicolumns by activating GABAergic double bouquet interneurons. We expected to see post-TMS treatment differences in amplitude and latency of both early and late ERP components, specifically more differentiation of response to target, standard, and novel distracter stimuli along with positive behavioral changes. The instruments for evaluating social and behavioral functioning (Aman and Singh 1994; Bodfish et al. 1999, 2000; Constantino and Gruber 2005) were selected with the hypothesis that rTMS sessions would result in reduced irritability and hyperactivity, reduced obsessive–compulsive and stereotyped behavior. For functional outcome measures we selected a novelty test to assess changes in electrocortical measures, such as amplitudes and latencies of early and late frontal and parietal ERPs. We proposed that ERP evaluation in a repeated post-treatment novelty test could serve as a valuable outcome measurement for autistic patients.
Method
Participants
Participants with autism spectrum disorder (ASD) (age range 9–27 years) were recruited through the University of Louisville Weisskopf Child Evaluation Center (WCEC). Diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (American Psychiatric Association 2000) and further ascertained with the Autism Diagnostic Interview-Revised (ADI-R) (Le Couteur et al. 2003). Subjects had a medical evaluation performed by a developmental pediatrician and had normal hearing based on past hearing screens; they also had normal vision or wore corrective lenses. Participants with a history of seizure disorder, significant hearing or visual impairment, a brain abnormality conclusive from imaging studies or an identified genetic disorder were excluded. All participants were high-functioning persons with ASD with full-scale IQs >80 assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler 2003) or the Wechsler Abbreviated Scale of Intelligence (for adults) (WASI, Wechsler 2004).
Participating subjects and their parents (or legal guardians) were provided with all information regarding the study, including the purpose, requirements, responsibilities, risks, benefits, alternatives, and role of the Institutional Review Board (IRB). The consent and assent forms approved by the IRB were reviewed and explained to all subjects expressing an interest in participating. All questions were answered before a signature of consent was requested. If the individual agreed to participate, he or she signed and dated the consent form and received a copy countersigned by the investigator obtaining consent.
ERP Data Acquisition, and Signal Processing
Electroencephalographic (EEG) data were acquired using a 128 channel Electrical Geodesics Inc. (EGI) system (v. 200), consisting of Geodesic Sensor Net electrodes, Net Amps and Net Station software (Electrical Geodesics Inc., Eugene, OR) running on a Macintosh G4 computer. EEG data are sampled at 500 Hz and 0.1–200 Hz analog filtered. Impedances were kept <50 kΩ, and according to the EGI Technical Manual (2003) impedances <50 kΩ are sufficient for recording quality EEG data; Ferree et al. (2001) have suggested that modern high input impedance amplifiers and accurate digital filters for power noise provide excellent EEG signals in conjunction with scalp impedances of approximately 40 kΩ.
The Geodesic Sensor Net is a lightweight elastic thread structure containing Ag/AgCl electrodes housed in a synthetic sponge on a pedestal. The sponges are soaked in a KCl solution to render them conductive. EEG data are recorded continuously. EEG channels with high impedance or visually detectable artifacts (e.g., channel drift, gross movement, etc.) were marked in ‘on-line’ mode using Net Station’s event-marker tools and further removal was performed in ‘off-line’ mode using the Net Station Waveform Tool (NSWT).
Stimulus-locked EEG data are segmented off-line into 1,000 ms epochs spanning 200 ms pre-stimulus to 800 ms post-stimulus around the critical stimulus events resulting in three conditions: (1) rare target, (2) rare non-target distracter (novel), and (3) frequent non-target (standard). Data are digitally screened for artifacts (eye blinks, movements), and contaminated trials are removed using artifact rejection tools. The Net Station Waveform Tools’ Artifact Detection module in “off-line” mode rejects EEG channels if the fast average amplitude exceeds 200 μV, the differential average amplitude exceeds 100 μV, or if the channel has zero variance. Segments are rejected if they contain more than 10 bad channels or if eye blinks or movement are detected (>70 μV). After the detection of ‘rejected’ channels, the NSWT’s “Bad channel replacement” function is used to replace rejected channel data with data interpolated from the remaining acceptable channels (or segments); this process uses spherical splines (for more information on the interpolation methods used in EGI Net Station systems refer to Fletcher et al. 1996; Luu et al. 2001; Perrin et al. 1987; Srinivasan et al. 1998).
The remaining data are digitally filtered using 60 Hz Notch and 0.3–20 Hz bandpass filters and are then segmented by condition and averaged to create ERPs. Averaged ERP data are baseline corrected and re-referenced into an average reference frame. All stimulus presentation and behavioral response collection is controlled by a PC computer running E-prime software (Psychology Software Tools Inc., PA). Visual stimuli are presented on a 15” display. Manual responses are collected with a 5-button keypad (Serial Box, Psychology Software Tools, Inc, PA).
Three-Stimuli Visual Oddball with Novel Distracters
This test represents a traditional visual three-stimuli oddball task. Stimuli letters “X”, “O”, and novel distracters (“v,” “^,” “>,” and “<”signs) are presented on the screen after a fixation mark “+”. One of the stimuli (“O”) is presented on 50% of the trials (frequent standard); the novel stimuli stimulus (e.g., “>“) is presented on 25% of the trials (rare distracter), whereas the third (“X”) is presented on the remaining 25% of the trials and represents the target. Subjects are instructed to press a key when they see the target letter on the screen. Each stimulus is presented for 250 ms, with an 1,100 ms inter-trial interval. There are 480 trials in total, with a break every 240 trials. The complete sequence takes 20 min.
Behavioral Measures
Behavioral response measures were mean reaction time (in ms) and response accuracy (percent of correct hits).
Event-Related Potentials (ERP)
ERP dependent measures were: adaptive mean amplitude and latency of the ERP peak (e.g., P3a, P3b) within a temporal window across a region-of-interest (ROI); each ROI contained at least 4 electrodes. ERP dependent variables included stimulus-averaged amplitudes and latencies of frontal ERP components: P50 (40–80 ms post-stimulus), N100 (80–180 ms), P200 (180–320 ms), N200 (220–350 ms), and P3a (300–520 ms); and posterior ERP components: P50 (40–100 ms), N100 (120–180 ms), P200 (160–250 ms), and centro-parietal N200 (N2b, 180–320 ms) and P3b (320–560 ms). The frontal ROIs for the P100, N100, N200 and P3a components included the following EGI channels: left ROI—EGI channel 12, F1, F3, FC1; midline ROI—FCz, Fz); right ROI—EGI channel 5, F2, F4, FC2. The anterior-frontal ROI for the P2a component had more anterior scalp locations, including AF3, AF4, FPz, AFz and 4 neighboring EGI channels: 18, 19, 9, 10. The centro-parietal ROI for N2b and P3b components included the following EGI channels: left ROI—EGI channel 32, CP1, P1, P3, EGI channel 54; midline ROI—CPz, Pz; right ROI—CP2, P2, P4, EGI channels 80 and 81. The early and middle latency ERP components (P50, N100, P200, N200) were analyzed as well for parieto-occipital and occipital ROIs (left—PO7, O1, EGI channels 65,71; right—PO8, O2, EGI channels 84,91). Frontal negativities (N100, N200) were analyzed separately for midline frontal and fronto-central ROIs (Fz, FCz, EGI channels 12, 5) and lateral frontal and fronto-central ROIs (left—F1, FC1, FC3, EGI channel 29; right—F2, FC2, FC4, EGI channel 118).
TMS Procedure
A trained electrophysiologist delivered rTMS using a Magstim Rapid (Model 220) instrument (Magstim Corporation, Sheffield, England) with a 70-mm wingspan figure-eight coil. Motor threshold (MT) was determined in all individuals by gradually increasing the output power of TMS device by 5% until a 50 μV deflection or a visible twitch in the First Dorsal Interosseous (FDI) hand muscle was identified in 2 out of 3 trials. The cortical representation site for the FDI muscle is 5–8 cm lateral to the cranial vertex (Garvey and Mall 2008).
Electromyographic responses were monitored on a continuous basis with a C-2 (J&J Engineering, Inc.) physiological monitor (Poulsbo, WA). Motor-evoked potentials were recorded from the contralateral FDI muscle using the C-2 system with USE-2 Physiodata software applications. The physiological monitor had 4 channels for physiological recording. We recorded on the hand ipsilateral to TMS stimulation: EMG of the left FDI, skin conductance and skin temperature. EMG and other physiological recordings were stored for later analysis.
All subjects with autism were initially enrolled in a training session to comply with EEG/ERP recording during the psychophysiological assessments, cognitive tests, and TMS procedure. Autistic patients had at least one session for EEG/ERP net conditioning to get familiar with the experimental room and laboratory setting and one additional visit to get familiar with the TMS procedure.
The TMS treatment course was administered two times per week for 3 weeks (a total of six 0.5 Hz rTMS treatments) over the left DLPFC. The stimulation site of the DLPFC was located by placing the center of the coil 5 cm anterior to the site of maximal FDI stimulation in the parasagital plane. The figure-eight coil was kept flat over the scalp, and subjects were wearing a swimming cap to aid in coil placement. Stimulation was done at 0.5 Hz and 90% MT, with a total of 150 pulses/day (fifteen 10 s trains with a 20–30 s interval between trains).
The selection of 0.5 Hz was determined by two factors: (1) we preferred to start the TMS trial at the lower frequency (0.5 Hz instead of 1.0 Hz) as a safety precaution: as the lower the rTMS frequency the lower the risk for seizures; and (2) we used the enhanced mode of the Magstim Rapid device which in our configuration allows stimuli to be delivered at 0.5 Hz even when stimulation threshold (MT) is relatively high. The selection of 90% of MT was based on prior studies where rTMS was used for prefrontal stimulation in different psychiatric and neurological disorders (Daskalakis et al. 2002; Gershon et al. 2003; Greenberg 2007; Loo and Mitchell 2005; Rosenberg et al. 2002; Wassermann and Lisanby 2001). The number of TMS pulses during a session and the frequency of sessions was selected according to a current review conclusions (Helmich et al. 2006) that rTMS applied with less than 100 pulses/per session and with a frequency of less than twice per week was not very promising in terms of therapeutic efficacy.
Pre- and Post-TMS Behavioral Measures
Social and behavioral functioning for participants was evaluated utilizing caregiver questionnaires and clinician ratings of improvement. Participants were evaluated prior to receiving TMS and 2 weeks following treatment. Measures included.
Aberrant Behavior Checklist (ABC)
The ABC (Aman and Singh 1994) is a clinician administered rating scale assessing five problem areas: Irritability, Lethargy/Social Withdrawal, Stereotypy, Hyperactivity, and Inappropriate Speech, and is based on caregiver reports. Each area contains multiple items receiving a rating from 0 to 3. Items are summed and high scores for each area reflect severity of the problem area. The ABC has been shown to be effective in assessing behavioral changes in autism (Aman 2004).
Social Responsiveness Scale (SRS)
The SRS (Constantino and Gruber 2005) is a caregiver completed rating scale assessing social interest and interaction. The scale provides a dimensional measure of social interaction allowing the rating of social skills in autism as well as non-autistic individuals.
Repetitive Behavior Scale: Revised (RBS)
The RBS (Bodfish et al. 1999) is a caregiver completed rating scale assessing repetitive and restricted behavior patterns. The RBS is a measure of different behaviors: stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted range (Bodfish et al. 2000). Items from scales are summed to obtain a measure of the severity of repetitive behavior.
For each behavioral measure a Group (waiting-list vs. treatment) × Time (pre- vs. post-TMS) ANOVA was completed to determine changes associated with TMS.
Statistical Data Analysis
Statistical analyses were performed on subject-averaged behavioral and ERP data with subject averages being observations. The primary analysis model was the repeated measures ANOVA, with dependent variables being reaction time (RT), error rate and specific ERP components’ amplitudes and latencies at selected ROIs. The data of each ERP dependent variable for each relevant ROI was analyzed using ANOVA with the following factors (all within-participants): Stimulus (Target, Novel, Standard), Hemisphere (Left, Right), and Time (pre-, post-TMS). Post-hoc analysis was conducted where appropriate. A-priori hypotheses were tested with Student’s t-tests. In all ANOVAs Greenhouse-Geisser corrected P-values were employed where appropriate. SPSS v.14 and Sigma Stat 3.1 packages were used for statistical analysis.
Results
Subjects
The mean age of the 13 participants enrolled in the ASD rTMS trial group was 15.6 ± (standard deviation) 5.8 years (range 8–27 years, 12 males, 1 female). The mean full-scale IQ score for the patients with ASD was 94.3 ± 16.6. Full-scale IQ scores were determined using either the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler 2003), the Stanford-Binet Intelligence Test (Roid 2003), or the Wechsler Abbreviated Scale of Intelligence (for adults) (WASI, Wechsler 2004).
Behavioral Responses
Reaction time to targets did not show any statistically significant changes due to the TMS treatment, but there was a significant improvement in error percentage: pre-TMS, 11.0 ± 12.34% versus post-TMS, 3.29 ± 3.17% (t = 2.43, df = 11, P = 0.039).
Event-Related Potentials
Frontal ERPs
Data from one subject were excluded from analysis due to excessive ocular artifacts at lateral frontal ROIs; this subject was also excluded from analysis at posterior ROIs.
P50
Amplitude of the left frontal P50 to targets increased after rTMS (0.01 ± 1.61 to 1.42 ± 1.62 μV, F 1, 23 = 4.36, P = 0.047) and showed a Stimulus (target, novel) × Time (pre-, post-TMS) interaction (F 1, 23 = 5.39, P = 0.03) expressed as a significant increase in amplitude to targets and a decrease to novels. The effect was most visible over the left frontal ROI (Hemisphere × Time, F 1, 23 = 7.33, P = 0.013) (Fig. 1).
Latency of P50 to targets after rTMS treatment significantly increased at the midline frontal ROI (Fz-FCz: from 53.0 ± 16.1 to 73.3 ± 23.1 ms, F 1, 23 = 5.73, P = 0.026) and the left frontal ROI (53.8 ± 17.2 to 76.5 ± 31.1 ms, F 1, 23 = 4.91, P = 0.037). ANOVA yielded a Stimulus (target, standard, novel) × Time (pre-TMS, post-TMS) interaction (F 2, 46 = 4.89, GG corrected P = 0.013) at the midline ROI; this effect can be described as increased latency of P50 to targets, a slight latency increase to novels, and no changes of latency to standard stimuli (Fig. 2).
N100 and P200
The frontal N100 and P200 ERPs did not show any statistically significant amplitude or latency post-TMS changes.
N200
Latency of the lateral frontal N200 component to target and novel distracter stimuli showed a Stimulus (target, novel) × Time (pre-, post-TMS) interaction (F 1, 23 = 4.49, P = 0.046), where N200 latency decreased more to novels than targets (from 341 to 311 ms to novels; and from 325 to 312 ms to targets).
P3a
A Stimulus (target, novel) × Hemisphere (left, right) × Time (pre-, post-TMS) interaction was significant for frontal P3a amplitude (F 1, 23 = 5.10, P = 0.034). The effect manifested as a more significant post-TMS increase of P3a amplitude to novels than targets and a higher amplitude of P3a across the stimuli at the left hemisphere. There were no significant P3a latency changes after rTMS (Fig. 3).
Posterior (Centro-Parietal and Parieto-Occipital) ERPs
Parieto-Occipital P50
Amplitude of the modality-specific P50 component to novel stimuli decreased at the left hemisphere (from 3.57 ± 3.13 to 1.25 ± 1.92 μV, F 1, 23 = 5.24, P = 0.033). A comparison of the left P50 amplitude in response to target and novel stimuli revealed a Stimulus × Time interaction (F 1, 23 = 7.37, P = 0.013) where P50 amplitude to novels significantly decreased, while to targets decreased but not significantly (from 2.7 to 3.35 μV, n.s.). There were no post-TMS effects detected for P50 latency except a decrease to targets at the right ROI (from 47. 2 ± 6.1 to 37.2 ± 12.3 ms, F 1, 23 = 6.32, P = 0.02) (Fig. 4).
Centro-Parietal P50
Amplitude of P50 (50–100 ms post-stimulus) across all stimuli at centro-parietal ROIs showed a marginal Stimulus (target, standard, novel) × Time interaction (F 2, 22 = 3.54, P = 0.037, GG corrected P = 0.052). The effect was expressed as an increase in amplitude to targets and a decrease to standards without any changes to novels. The increase in amplitude to targets was accompanied by significant latency increase at the midline centro-parietal ROI (F 1, 23 = 4.60, P = 0.043).
Centro-Parietal N100 and N200 (N2b)
There were no significant amplitude or latency changes in the N100 or N200 components following rTMS treatment.
Centro-Parietal P200
Latency of the midline centro-parietal P200 component showed a tendency toward a Stimulus (target, standard) × Time (pre- post-TMS) interaction (F 1, 23 = 4.26, GG corrected P = 0.05). Specifically, the latency to targets increased (17.0 ± 36.8 ms) without any notable changes to standards (Fig. 5).
Centro-Parietal P3b
Analysis of centro-parietal P3b amplitude yielded significant differences for target and standard stimuli after rTMS. A Stimulus × Time interaction indicated an increase in amplitude to targets with a decrease to standards (F 1,23 = 6.01, P = 0.023). There were no significant centro-parietal latency changes found for the P3b component (Fig. 6).
Clinical Evaluations After TMS
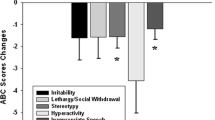
Clinical evaluation results are presented in Table 1. Following rTMS subjects were reported to have reduced repetitive-ritualistic behavior as measured by the Repetitive Behavior Scale; this change was primarily due to reduced obsessive–compulsive behaviors reported by caregivers. No changes in social awareness, irritability, or hyperactivity were observed.
It is worthy to note that one reason we may not see changes in irritability and hyperactivity is that the subjects in this study were high functioning autistic (HFA) individuals with minimal behavioral problems. In future studies we plan to also include low functioning subjects with significant behavioral problems, and we may see improvement in these behaviors. In other words, the HFA subjects in our current study did not have many behavior problems other than OCD symptoms, so we can’t expect much change following rTMS. In another study underway we combine rTMS with Applied Behavioral Analysis (ABA) to address social awareness and social skills training. One subject excluded for excess ocular and gross movement artifacts was also excluded from behavioral measures, thus reported are the changes for the same 12 subject with complete ERP data.
Discussion
Our study applied low-frequency rTMS to the DLPFC for 3 weeks to individuals with ASD at frequencies known to increase local and transynaptic inhibition. Behavioral responses did not show post-TMS differences in RT, but the accuracy of responses improved significantly. We found post-TMS treatment changes in ERP components at the frontal, centro-parietal, and parieto-occipital ROIs. The amplitude of the early parieto-occipital P50 component decreased to novel distracters but not to targets. A similar response was observed for the frontal P50 where both amplitude and latency to targets increased, while the magnitude of P50 to non-target stimuli decreased. The simultaneous increase in amplitude and latency of P50 to targets is a positive effect of rTMS considering our previous study (Sokhadze et al. 2009b) showed higher early frontal positivity to standard and novel stimuli in the autism group compared to controls. This study also showed increased amplitude of the frontal P3a component to rare novel stimuli and can be interpreted as more effective orienting of attention to distracters after TMS; this late frontal component is supposed to be higher to novel rather than target stimuli. Also, the centro-parietal P3b showed increased reactivity post-TMS to targets and decreased reactivity to frequent standards. These post-treatment changes represent a positive development in the ERP profiles of individuals with ASD at both early and late stages of processing visual stimuli. Rare novels in a three-category oddball paradigm elicit a fronto-central P3a, whereas rare target stimuli elicit a parietally distributed P3b (Katayama and Polich 1998; Polich 2003). In this task, the P3a response corresponds to an “orienting” activity, while P3b indexes ability to sustain attention to target stimuli (Wijers et al. 1996; Potts et al. 2004). Children and adolescents with autism show abnormal ERPs (Bomba and Pang 2004; Kemner et al. 1999), and notably differ from typical children with respect to the P3b in standard oddball tasks. Studies using simple visual target detection have found dissociations of frontal (delayed) and posterior P300 (intact) responses in visual spatial attention tasks (Townsend et al. 2001).
Several studies have found support for the “functional disconnectivity” hypothesis of autism (Belmonte et al. 2004a, b; Casanova et al. 2006a, b; Chandana et al. 2005; Courchesne and Pierce, 2005; Welchew et al. 2005). The progress of information transfer and deficits of functional connections between brain areas can be followed by an analysis of specific electrocortical processes reflected in ERP measures. In our prior study (Sokhadze et al. 2009a) we outlined several novelty processing abnormalities in children with autism compared to typically developing children using the same visual oddball task. The abnormalities we found can primarily be interpreted as inefficient functioning of the fronto-parietally distributed attention system along with a lower integrative capacity relative to controls. Our own neuropathological findings of minicolumnar abnormalities and a resulting disruption in the ratio between cortical excitation and inhibition in autism (Casanova et al. 2002a, b, 2006a, b), along with similar studies (e.g., Rubenstein and Merzenich 2003), provide additional support for the “functional disconnectivity” hypothesis. Furthermore, the findings of our study show that abnormalities in the cortical ratio between excitation and inhibition in autism could be assessed using ERP methods and that repetitive TMS may ameliorate these deficient functional connections in autism.
Cortical activity during different stages of visual information processing and changes induced by rTMS in autism can be detected with ERPs; ERPs represent stimulus-driven corticoelectric field potentials and can be categorized as short-latency (e.g., P50), middle latency (e.g., P200) or long-latency (endogenous, e.g. P300). Modality-specific exogenous ERPs (e.g., P50) reflect mostly early-stages of processing, whereas middle-latency and late-stage (e.g., P300) polymodal ERP components reflect associative processing respectively. In a novelty oddball task a rare target must be distinguished from a rare novel distracter stimulus, and both are presented randomly within a sequence of a common, high-frequency stimulus. The early and middle latency ERP components are a series of potentials that are recorded at the scalp following sensory stimulation that usually occur between 40 and 200 ms post-stimulation. These components are also characterized by being exogenous in nature (i.e. they are predictably generated by delivering sensory stimulation without a need for the subject to perform any mental operations). This characteristic differentiates the early and middle latency ERPs from endogenous ERPs (e.g., P300), which require the performance of a cognitive task such as a novelty task used in our study at pre- and post-TMS tests.
The initial processing of an incoming visual stimulus during an oddball task is a multistage operation; it is reflected not only in the P50 ERP component (a positive component seen approximately 40–80 ms after the presentation of a visual stimulus at occipital, parietal and parieto-occipital areas), but also in the subsequent N100 and P200 responses. The N100 is a negative component seen approximately 100–120 ms following a given stimulus, while the P200 component is a positive wave occurring approximately 200 ms post-stimulus (Kisley et al. 2004). The later component has been related to relatively higher-order cognitive processes (Kisley and Cornwell 2006). Boutros et al. (2004) suggested that P50, N100 and P200 responses reflected different stages of information processing (pre-attentive: reflected by the P50; early attentive: reflected by the N100; later attentive: reflected by the P200). Dysfunctional selective filtering of visual stimuli may occur not only at the P50 “sensory gating” stage, but at the levels of the N100 and P200 responses. P50 sensory gating is based conceptually on selective attention, as attention towards one stimulus requires the automatic concurrent inhibition of attention towards another stimulus. Habituation to irrelevant sensory input is an important function in information-processing, and a failure to do so may be associated with mental disturbances. Usually sensory gating is studied in a paired-stimuli paradigm using auditory evoked potentials (e.g., P50), and amplitudes generally become lower with repetitive stimuli of short intervals. The relationship between P50 sensory gating and the hypersensitivity of individuals with autism to sensory stimulation remains understudied especially outside the auditory modality, and the clinical correlates of the visual P50, N100 and P200 components in autism are yet to be examined.
Sensory gating indices derived from the early and mid-latency evoked potential responses during pre-attentive (P50) and attentive (P200, N200, P3b) phases of information processing collected from our patients with autism show significant changes following rTMS treatment; in particular changes were found reflecting enhanced processing of the task-relevant targets and lowered reactivity to the task-irrelevant standard and novel stimuli. A number of potentially interesting correlations were found between N100 and P200 sensory gating measures and P300 variables in schizophrenia (Boutros et al. 2004). Among these correlations, the positive correlation between the N100 and P300 (P3b) latency is of interest, as it is relevant to the findings of our previous (Sokhadze et al. 2009a) and current study. The study of Boutros et al. (2004) suggests that decreased gating at the P50-N100 phase of information processing negatively impacts the speed of information processing as measured by P3b latency. The further observation that this correlation was stronger in schizophrenia patients (Boutros et al. 2004) further supports the possible deleterious effects of abnormal P50 and/or N100 level gating on information processing. This finding raises the possibility that the P50-N100 gating deficit may also impact the resource allocation capacity of the cortex as measured by P3b amplitude. Similar correlations were found for the P200 component, also suggesting that a sensory gating deficit at this stage could also have a detrimental effect on information-processing capacity. We found significant pre- versus post-TMS differences in response to target, standard, and novel stimuli in frontal, occipital, parietal, and centro-parietal ERP components. All of the detected post-TMS ERP changes pointed toward normalization of response patterns to task-relevant stimuli (targets) and decreased reactivity to task-irrelevant stimuli (both novel distracters and standards).
There may be several mechanisms resulting in the normalization of sensory over-reactivity (seen in ERPs) following low-frequency (inhibitory) rTMS treatment in autism. In general, perceptual filtering of incoming stimulation in autism is thought to occur in an “all-or-none” mode without relevance to task-specificity for the stimulus (Belmonte and Yurgelun-Todd 2003a, b). The attention of patients with autism seems founded more on the coarse control of general arousal than on selective activation of specific perceptual systems. It is reasonable to suggest that active inhibition of irrelevant distracters is not properly functioning and allows both task-relevant and task-irrelevant stimuli to pass through earlier filtering processes creating an overload on later stages of stimulus processing. It is unsurprising that an increased ratio of excitation/inhibition in key neural systems and high “cortical noise” have been considered core abnormalities in autism (Casanova et al. 2003; Rubenstein and Merzenich 2003).
Our previous work on postmortem material suggests that the brains of autistic patients have a defect in the inhibitory surround (peripheral neuropil space) of minicolumns (Casanova et al. 2002a, b, 2006a, b; Casanova 2005). The effect of loss of surround inhibition results in local signal amplification and a loss of information transfer to distal cortical areas. The behavioral consequences of signal/sensory amplification and a low signal-to-noise ratio is increased physiological stress, impulsive actions, and over-responding to environmental stimuli. It could also be proposed that excessive signal amplification provides for a hyperexcitable cortex and the kindling of adjacent minicolumns that may result in a nidus for seizures.
In this study we used slow rTMS over the dorsolateral prefrontal cortex (DLPFC) of autistic patients in an attempt to increase the inhibitory surround of minicolumns in this prefrontal area. Due to the phenomenon of diaschisis and the connectivity of this brain region we expected the intervention not to be limited only to the site of magnetic stimulation but rather to generalize to other cortical areas. A focal electrical current induced by rTMS orthogonal to the pial surface results in a short-term functional reorganization of cortical activity. Since the effects of rTMS are not limited to the stimulated target cortex but give rise to functional changes in anatomically and functionally interconnected cortical areas, rTMS can be used to affect the strength of functional connectivity between cortical areas. Low-frequency or “slow” rTMS (in the 0.3–1 Hz frequency range) has been proposed to operate via long-term depression of cortical activity (Hoffman and Cavus 2002), which we hypothesize preferentially activates radially-oriented double-bouquet axons.
Since this is apparently the central assumption of the study, probably a more extensive explanation should be given why this type of rTMS was expected to affect selectively only inhibitory activity. The study uses a figure-eight coil that focuses the magnetic flux under the intersection of the coils that goes down to by up to 25 mm (Barker 1999). Considering the skull thickness and width of DLPFC it is not probable that rTMS has significant effects in the neural elements of sulci; rather, effects seem circumscribed to gyral crests. The DLPFC was selected for our rTMS study based on the topographical analysis of minicolumnar morphometry in cortices varying in cytoarchitectural differentiation (Casanova et al. 2006a, b). Results indicate that minicolumnar abnormalities in autism are more typical for high-order association areas, while idiotypic areas apparently are relatively sparred. TMS-based normalization of the prefrontal area known to be interconnected with distributed cortical networks may provide beneficial cascading effects at secondary sites (Walsh and Pascual-Leone 2003).
This is one of the first studies (see also Sokhadze et al. 2009b) where rTMS to the DLPFC at frequencies known to enhance local cortical inhibition was applied in individuals with autism. It was very important to ensure safety in this novel intervention. There were two main safety concerns proceeding with this study: (1) risks associated with applying TMS in children and (2) the risk of inducing a seizure with TMS given the increased incidence of spontaneous seizures in autistic disorders. Quintana (2005) evaluated studies using TMS in persons younger than 18. Repetitive TMS has also been applied in children with psychiatric disorders such as ADHD, ADHD comorbid with Tourette’s, and depression (Garvey and Mall 2008). Only one study in the review by Quintana (2005) reported applying rTMS to the left dorsal lateral prefrontal cortex in children and adolescents with depression. Although there are limited studies using rTMS (in 34 children), these studies did not report any significant adverse effects or seizures. There were no adverse side effects or any negative complications reported in our study. Results of the clinical evaluations showed that following rTMS patients with autism were reported to have reduced repetitive-ritualistic behavior as measured by the Repetitive Behavior Scales. This change was primarily due to reduced obsessive–compulsive behaviors reported by parents. We could not find any changes in social awareness or irritability. The reduced number of errors reported in our study points to better inhibitory control and lower impulsivity following rTMS treatment. We believe that a combination of rTMS with behavioral therapy (e.g., social skills training) may exert positive effects on social awareness, and we plan to use integrated rTMS-behavioral training treatment in our future clinical trials. All high functioning autistic individuals tolerated ERP recording and the rTMS procedure.
This study describes a potential therapeutic intervention that targets a putative neuropathological finding in autism and uses TMS for neuromodulation. There are several limitations that should be mentioned: the design of the project does not incorporate a control group (e.g., sham rTMS, waiting-list group) and represents a case series study rather than a controlled-study; the conclusions are limited by the small number of patients and relatively wide range of age groups. Only the left hemisphere was stimulated with rTMS. However, the study is open for enrollment for another 6 sessions of rTMS. Most of the patients and their parents/caregivers expressed willingness to return for a 6 month follow-up evaluation and ERP test, and to continue rTMS for another 6 sessions. This will allow a relatively longitudinal follow-up assessing the lasting effects of the described behavioral and ERP changes.
The neuroanatomical evidence of a lack of minicolumnar inhibition (Casanova et al. 2002a, b; Casanova et al. 2006a, b) and the findings of our previous study (Sokhadze et al. 2009a) indicating higher early frontal positivity to standard and novel stimuli adds to a growing body of knowledge of cortical inhibitory deficits in autism. Our current study validated the use of low-frequency rTMS as a modulatory tool that altered the disrupted ratio of cortical excitation to inhibition in autism. After rTMS the parieto-occipital P50 amplitude decreased to novel distracters but not to targets; also the amplitude and latency to targets increased for the frontal P50 while decreasing to non-target. These results clearly indicate a statistically significant change in early ERP measurements of sensory gating in response to target and distracter visual stimuli as a result of rTMS. Consequently, the centro-parietal P3b showed increased reactivity to targets and decreased reactivity to frequent standards after rTMS. Also, the amplitude of the frontal P3a component to rare novel stimuli normalized after TMS. Low-frequency rTMS minimized early cortical responses to irrelevant stimuli and increased responses to relevant stimuli. Improved selectivity in early cortical responses lead to better stimulus differentiation at later-stage responses as was made evident by our P3b and P3a component findings. Overall, more research needs to be done on sensory processing abnormalities in autism spectrum disorders, but rTMS may prove to be an important research tool or treatment modality in addressing the stimulus hypersensitivity characteristic of the disorder.
References
Aman, M. G. (2004). Management of hyperactivity and other acting-out problems in patients with autism spectrum disorder. Seminars in Pediatric Neurology, 11(3), 225–228.
Aman, M. G., & Singh, N. N. (1994). Aberrant behavior checklist—Community. Supplementary manual. East Aurora, NY: Slosson Educational Publications.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (DSM-IV TR) (text revised) (4th ed.). D.C: Washington.
Barker, A. T. (1999). The history and basic principles of magnetic nerve stimulation. Electroencephalography and Clinical Neurophysiology, 51, 3–21.
Belmonte, M. K., Allen, G., Beckel-Mitchener, A., Boulanger, L., Carper, R., & Webb, S. J. (2004a). Autism and abnormal development of brain connectivity. Journal of Neuroscience, 24, 9228–9231.
Belmonte, M. K., Cook, E. H., Anderson, G. M., Rubenstein, J. L. R., Greenhough, W. T., Beckel-Mitchener, A., et al. (2004b). Autism as a disorder of neural information processing: Directions for research and targets for therapy. Molecular Psychiatry, 9, 646–663.
Belmonte, M. K., & Yurgelun-Todd, D. A. (2003a). Functional anatomy of impaired selective attention and compensatory processing in autism. Cognitive Brain Research, 17, 651–664.
Belmonte, M. K., & Yurgelun-Todd, D. A. (2003b). Anatomic dissociation of selective and suppressive processes in visual attention. Neuroimage, 19, 180–189.
Bertone, A., Mottron, L., Jelenic, P., & Faubert, J. (2005). Enhanced and diminished visuo-spatial information processing in autism depend on stimulus complexity. Brain, 128, 2430–2441.
Bodfish, J. W., Symons, F. J., & Lewis, M. H. (1999). Repetitive Behavior Scale. Western Carolina Center Research Reports.
Bodfish, J. W., Symons, F. S., Parker, D. E., & Lewis, M. H. (2000). Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30, 237–243.
Bomba, M. D., & Pang, E. W. (2004). Cortical auditory evoked potentials in autism: A review. International Journal of Psychophysiology, 53, 161–169.
Boutros, N. N., Korzyukov, O., Jansen, B., Feingold, A., & Bell, M. (2004). Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Research, 126, 203–215.
Bruneau, N., Roux, S., Adrien, J. L., & Bathelemy, C. (1999). Auditory associative cortex dysfunction in children with autism: Evidence from late auditory evoked potentials (N 1 wave- T Complex). Clinical Neurophysiology, 110, 1927–1934.
Burack, J. A. (1994). Selective attention deficits in persons with autism: Preliminary evidence for inefficient attentional lens. Journal of Abnormal Psychology, 103, 515–543.
Casanova, M. F. (2005). Minicolumnar pathology in autism. In M. F. Casanova (Ed.), Recent developments in autism research (pp. 133–144). New York: Nova Biomedical Books.
Casanova, M. F. (2006). Neuropathological and genetic findings in autism: The significance of a putative minicolumnopathy. Neuroscientist, 12(5), 435–441.
Casanova, M. F., Buxhoeveden, D., & Gomez, J. (2003). Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. The Neuroscientist, 9, 496–507.
Casanova, M. F., Buxhoeveden, D. P., Switala, A. E., & Roy, E. (2002a). Minicolumnar pathology in autism. Neurology, 58, 428–432.
Casanova, M. F., Buxhoeveden, D. P., Switala, A. E., & Roy, E. (2002b). Neuronal density and architecture (gray level index) in the brains of autistic patients. Journal Child Neurology, 17, 515–521.
Casanova, M. F., van Kooten, I., Switala, A. E., van England, H., Heinsen, H., Steinbuch, H. W. M., et al. (2006a). Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clinical Neuroscience Research, 6(3–4), 127–133.
Casanova, M. F., van Kooten, I., van Engeland, H., Heinsen, H., Steinbursch, H. W. M., Hof, P. R., et al. (2006b). Minicolumnar abnormalities in autism II. Neuronal size and number. Acta Neuropathologica, 112, 287–303.
Chandana, S. R., Behen, M. E., Juhász, C., Muzik, O., Rothermel, R., Mangner, T. J., et al. (2005). Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. International Journal of Developmental Neuroscience, 23, 171–182.
Childs, J. A., & Blair, J. L. (1997). Valproic acid treatment of epilepsy in autistic twins. Journal Neuroscience Nursing, 29, 244–248.
Ciesielski, K. T., Courchesne, E., & Elmasian, R. (1990). Effects of focused selective attention tasks on event-related potentials in autistic and normal individuals. Electroencephalography Clinical Neurophysiology, 75, 207–220.
Ciesielski, K. T., Knoght, J. E., Prince, R. J., Harris, R. J., & Handmaker, S. D. (1995). Event-related potentials in cross-modal divided attention in autism. Neuropsychologia, 33, 225–246.
Constantino, J. N., & Gruber, C. P. (2005). The Social Responsiveness Scale (SRS) manual. Los Angeles, CA: Western Psychological Services.
Courchesne, E., Lincoln, A. J., Yeung-Courchesne, R., Elmasian, R., & Grillon, C. (1989). Pathophysiologic findings in nonretarded autism and receptive developmental disorder. Journal of Autism and Developmental Disorders, 19, 1–17.
Courchesne, E., & Pierce, K. (2005). Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. International Journal Developmental Neuroscience, 23, 153–170.
Daskalakis, Z. J., Christensen, B. K., Fitzgerald, P. B., & Chen, R. (2002). Transcranial magnetic stimulation: A new investigational and treatment tool in psychiatry. Journal of Neuropsychiatry and Clinical Neurosciences, 14, 406–415.
Dawson, G., Finley, C., Phillips, S., Galpert, L., & Lewy, A. (1988). Reduced P3 amplitude of the event-related brain potential: Its relationship to language ability in autism. Journal of Autism and Developmental Disorders, 18, 493–504.
DeFelipe, J. (1999). Chandelier cells and epilepsy. Brain, 122, 1807–1822.
DeFelipe, J. (2004). Cortical microanatomy and human brain disorders: Epilepsy. Cortex, 40, 232–233.
DeFelipe, J., Hendry, S. H. C., Hashikawa, T., Molinari, M., & Jones, E. G. (1990). A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons.
Favorov, O. V., & Kelly, D. G. (1994a). Minicolumnar organization within somatosensory cortical segregates, I: Development of afferent connections. Cerebral Cortex, 4, 408–427.
Favorov, O. V., & Kelly, D. G. (1994b). Minicolumnar organization within somatosensory cortical segregates, II: Emergent functional properties. Cerebral Cortex, 4, 428–442.
Ferree, T. C., Luu, P., Russell, G. S., & Tucker, D. M. (2001). Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology, 112, 444–536.
Ferri, R., Elia, M., Agarwal, N., Lanuzza, B., Musumeci, S. A., & Pennisi, G. (2003). The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology, 114, 1671–1680.
Fletcher, E. M., Kussmaul, C. L., & Mangun, G. R. (1996). Estimation of interpolation errors in scalp topographic mapping. Electroctoencephalography and Clinical Neuraphysiology, 98, 422–434.
Garvey, M. A., & Mall, V. (2008). Transcranial magnetic stimulation in children. Clinical Neurophysiology, 119, 973–984.
George, M. S., & Belmaker, R. H. (2007). Transcranial magnetic stimulation in clinical psychiatry. Arlington, VA: American Psychiatry Publishing Inc.
George, M. S., Nahas, J., Kozol, F. A., Li, X., Yamanaka, K., Mishory, A., et al. (2003). Mechanisms and the current state of transcranial magnetic stimulation. CNS Spectrums, 8(7), 496–514.
George, M. S., Nahas, Z., Molloy, M., Speer, A. M., Oliver, N. C., Li, X.-B., et al. (2000). A controlled trial of daily prefrontal cortex TMS for treating depression. Biological Psychiatry, 48, 962–970.
Gershon, A. A., Dannon, P. N., & Grunhaus, L. (2003). Transcranial magnetic stimulation in the treatment of depression. American Journal of Psychiatry, 160, 835–845.
Greenberg, B. D. (2007). Transcranial magnetic stimulation in anxiety disorders. In M. S. George & R. H. Belmaker (Eds.), Thanscranial magnetic stimulation in clinical psychiatry (pp. 165–178). Washington, DC: American Psychiatric Publishing Inc.
Griffith, E. M., Pennington, B. F., Wehner, E. A., & Rogers, S. J. (1999). Executive functions in young children with autism. Child Development, 70, 817–832.
Helmich, R. C., Siebner, H. R., Bakker, M., Munchau, A., & Bloem, B. R. (2006). Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson’s disease. Journal of Neurological Sciences, 248, 84–96.
Hoffman, R. E., & Cavus, I. (2002). Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. American Journal of Psychiatry, 159, 1093–1102.
Holcomb, P. J., Ackerman, P. T., & Dykman, R. A. (1985). Cognitive event-related brain potentials in children with attention and reading deficits. Psychophysiology, 22, 656–667.
Hollander, E., Dolgoff-Kaspar, R., Cartwright, C., Rawitt, R., & Novotny, S. (2001). An open trial of divalproex sodium in autism spectrum disorders. Journal of Clinical Psychiatry, 62, 530–534.
Hruby, T., & Marsalek, P. (2003). Event-related potentials—The P3 wave. Acta Neurobiologiae Experimentalis (Wars), 63, 55–63.
Katayama, J., & Polich, J. (1998). Stimulus context determines P3a and P3b. Psychophysiology, 35, 23–33.
Kemner, C., van der Gaag, R. J., Verbaten, M., & van Engeland, H. (1999). ERP differences among subtypes of pervasive developmental disorders. Biological Psychiatry, 46, 781–789.
Kemner, C., Verbaten, M. N., Cuperus, J. M., Camfferman, G., & Van Engeland, H. (1994). Visual and somatosensory event-related brain potentials in autistic children and three different control groups. Electroencephalography and Clinical Neurophysiology, 92, 225–237.
Kemner, C., Verbaten, M. N., Cuperus, J. M., Camfferman, G., & Van Engeland, H. (1995). Auditory event-related potentials in autistic children and three different control groups. Biological Psychiatry, 38, 150–165.
Kisley, M. A., & Cornwell, Z. M. (2006). Gamma and beta neural activity evoked during a sensory gating paradigm: Effects of auditory, somatosensory and cross-modal stimulation. Clinical Neurophysiology, 11, 2549–2563.
Kisley, M. A., Noecker, T. L., & Guinther, P. M. (2004). Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology, 41, 604–612.
Le Couteur, A., Lord, C., & Rutter, M. (2003). The autism diagnostic interview—Revised (ADI-R). Los Angeles, CA: Western Psychological Services.
Lincoln, A. J., Courchesne, E., Harms, L., & Allen, M. (1993). Contextual probability evaluation in autistic, receptive developmental disorder and control children: Event-related potential evidence. Journal of Autism and Developmental Disorders, 23, 37–58.
Loo, C., & Mitchell, P. (2005). A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. Journal of Affective Disorders, 88, 255–267.
Luu, P., Tucker, D. M. L., Englander, R., Lockfeld, A., Lutsep, H., & Oken, B. (2001). Localizing acute stroke-related EEC changes: Assessing the effects of spatial undersampling. Journal of Clinical Neurophysiology, 18, 302–317.
Mathalon, D. H., Fedor, M., Faustman, W. O., Gray, M., Askari, N., & Ford, J. M. (2002). Response-monitoring dysfunction in schizophrenia: An event-related brain potential study. Journal of Abnormal Psychology, 111, 22–41.
Mountcastle, V. B. (1997). The columnar organization of the neocortex. Brain, 120, 701–722.
Mountcastle, V. B. (2003). Introduction: Computation in cortical columns. Cerebral Cortex, 13, 2–4.
Nahas, Z., DeBrux, C., Chandler, V., Lorberbaum, J. P., Speer, A. M., et al. (2000). Lack of significant changes on magnetic resonance scans before and after 2 weeks of daily left prefrontal repetitive transcranial magnetic stimulation for depression. The Journal of ECT, 16(4), 380–390.
Oades, R. D., Walker, M. K., Geffen, L. B., & Stern, L. M. (1988). Event-related potentials in autistic and healthy children on an auditory choice reaction time task. International Journal of Psychophysiology, 6, 25–37.
Ogawa, A., Ukai, S., Shinosaki, K., Yamamoto, M., Kawaguchi, S., Ishii, R., et al. (2004). Slow repetitive transcranial magnetic stimulation increases somatosensory high-frequency oscillations in humans. Neuroscience Letters, 358, 193–196.
Pascual-Leone, A., Walsh, V., & Rothwell, J. (2000). Transcranial magnetic stimulation in cognitive neuroscience—Virtual lesion, chronometry, and functional connectivity. Current Opinions in Neurobiology, 10, 232–237.
Perrin, E., Pernier, J., Bertrand, O., Giard, M., & Echallier, J. F. (1987). Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology, 66, 75–81.
Picton, T. W. (1992). The P300 wave of the human event-related potential. Journal Clinical Neurophysiology, 9, 456–479.
Plioplys, A. V. (1994). Autism: Electroencephalogram abnormalities and clinical improvement with valproic acid. Archives of Pediatrics and Adolescent Medicine, 148, 220–222.
Polich, J. (2003). Theoretical overview of P3a a nd P3b. In J. Polich (Ed.), Detection of change: Event-related potential and fMRI Findings (pp. 83–98). Boston: Kluwer Academic Press.
Potts, G. F., Patel, S. H., & Azzam, P. N. (2004). Impact of instructed relevance on the visual ERP. International Journal of Psychophysiology, 52, 197–209.
Quintana, H. (2005). Transcranial magnetic stimulation in persons younger than the age of 18. The Journal of ECT, 21, 88–95.
Roid, G. H. (2003). Stanford-Binet Intelligence Scales, fifth edition, technical manual. Itasca, IL: Riverside Publishing.
Rosenberg, P. B., Mehndiratta, R. B., Mehndiratta, Y. P., Wamer, A., Rosse, R. B., & Balish, M. (2002). Repetitive magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. Journal of Neuropsychiatry and Clinical Neurosciences, 14, 270–276.
Rossi, S., & Rossini, P. M. (2004). TMS in cognitive plasticity and the potential for rehabilitation. Trends in Cognitive Sciences, 86, 273–279.
Rubenstein, J. L., & Merzenich, M. M. (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behavior, 2, 255–267.
Seldon, H. L. (1981a). Structure of human auditory cortex, I: Cytoarchitectonics and dendritic distributions. Brain Research, 229, 277–294.
Seldon, H. L. (1981b). Structure of human auditory cortex, II: Axon distributions and morphological correlates of speech perception. Brain Research, 229, 295–310.
Seri, S., Cerquiglini, A., Pisani, F., & Curatolo, P. (1999). Autism in tuberous sclerosis: Evoked potential evidence for a deficit in auditory sensory processing. Clinical Neurophysiology, 110, 1825–1830.
Sokhadze, E., Baruth, J., Tasman, A., Sears, L., Mathai, G., El-Baz, A., et al. (2009a). Event-related potential study of novelty processing abnormalities in autism. Applied Psychophysiology and Biofeedback, 34, 37–51.
Sokhadze, E. M., Singh, S., El-Baz, A., Baruth, J., Mathai, G., Sears, L., et al. (2009b). Effect of a low-frequency repetitive transcranial magnetic stimulation (rTMS) on induced gamma frequency oscillations and event-related potentials during processing of illusory figures in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 619–634.
Srinivasan, R., Tucker, D. M., & Murias, M. (1998). Estimating the spatial Nyquist of the human EEC. Behavior Research Methods, Instruments, and Computers, 30, 8–19.
Townsend, J., Westerfield, M., Leaver, E., Makeig, S., Jung, T., et al. (2001). Event-related brain response abnormalities in autism: Evidence for impaired cerebello-frontal spatial attention networks. Brain Research: Cognitive Brain Research, 11, 127–145.
Uvebrant, P., & Bauzienè, R. (1994). Intractable epilepsy in children: The efficacy of lamotrigine treatment, including non-seizure-related benefits. Neuropediatrics, 25, 284–289.
Verbaten, M. N., Roelofs, J. W., van Engeland, H., Kenemans, J. K., & Slangen, J. L. (1991). Abnormal visual event-related potentials of autistic children. Journal of Autism and Developmental Disorders, 21(4), 449–470.
Volkmar, F. R., & Nelson, D. S. (1995). Seizure disorders in autism. Journal of American Academy of Child Adolescent Psychiatry, 29, 127–129.
Walsh, V., & Pascual-Leone, A. (2003). Transcranial magnetic stimulation: A neurochronometrics of mind. Cambridge, Massachusetts: MIT Press.
Wassermann, E. M., Grafman, J., Berry, C., Hollnagel, C., Wild, K., Clark, K., et al. (1996). Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalography Clinical Neurophysiology, 101, 412–417.
Wassermann, E. M., & Lisanby, S. H. (2001). Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clinical Neurophysiology, 112, 1367–1377.
Wechsler, D. (2003). Wechsler Intelligence Scale for children (4th ed.). San Antonio, TX: Harcourt Assessment Inc.
Wechsler, D. (2004). Wechsler Abbreviated Scale for intelligence. San Antonio, TX: Harcourt Assessment Inc.
Welchew, D. E., Ashwin, C., Berkouk, K., Salvador, R., Suckling, J., Baron-Cohen, S., et al. (2005). Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biological Psychiatry, 57, 991–998.
Wijers, A. A., Mulder, G., Gunter, T. C., & Smid, H. G. O. M. (1996). Brain potential analysis of selective attention. In O. Neumann & A. F. Sanders (Eds.), Handbook of perception and action. Vol. 3: Attention (pp. 333–387). Tullamore, Ireland: Academic Press.
Ziemann, U. (2004). TMS induced plasticity in human cortex. Reviews Neuroscience, 15(4), 253–266.
Acknowledgments
The project was partially supported by R01 Eureka grant from the National Institutes of Health to Manuel Casanova.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sokhadze, E., Baruth, J., Tasman, A. et al. Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Affects Event-Related Potential Measures of Novelty Processing in Autism. Appl Psychophysiol Biofeedback 35, 147–161 (2010). https://doi.org/10.1007/s10484-009-9121-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-009-9121-2