Abstract
Background
Antipsychotic medication is a commonly prescribed drug class in individuals with autism spectrum disorder (ASD). However, the safety of these agents has not been fully assessed.
Objective
Our objective was to investigate the safety and tolerability profile of antipsychotics in individuals with ASD.
Methods
The Cochrane Library, MEDLINE, Embase and PsycINFO databases were searched up to January 2018. We included studies that reported adverse events (AEs) in participants with ASD taking first- or second-generation antipsychotic medication. The studies included in the analysis were randomized controlled trials (RCTs) and observational studies that were comparative or noncomparative and published as full text in the English language. The primary outcome of this review was AEs of any severity reported with antipsychotic use at any dose. Meta-analysis was performed on studies with child and adolescent participants to estimate the pooled prevalence of the overall AEs and the relative risk (RR) of AEs associated with antipsychotic use using a random-effects model. The Cochrane Collaboration tool and the modified Newcastle–Ottawa Scale (NOS) were used to assess the risk of bias of the included RCTs and observational studies, respectively.
Results
In total, 54 citations fulfilled the inclusion criteria, of which 40 were RCTs and 14 were observational studies; eight RCTs were included in the meta-analysis to estimate the RR of AEs associated with antipsychotic use and seven observational studies were included to estimate the pooled prevalence of AEs. The RR of AEs with antipsychotic treatment was 22% higher than with placebo (RR 1.22; 95% confidence interval [CI] 1.11–1.34; I2 = 30.6%; p = 0.184). The estimated pooled prevalence of AEs was 50.5% (95% CI 33–67). The most commonly reported AEs were increased appetite and weight gain, which were associated with discontinuation in many participants.
Conclusion
Antipsychotic-related AEs were common among patients with ASD. Further studies to investigate the implications of antipsychotic-related AEs on health and medication adherence are warranted. PROSPERO registration number: (CRD42018083632)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Antipsychotic medication increases the risk of developing adverse events by 22%. |
The prevalence of adverse events in people with autism receiving antipsychotics was 50%. |
The most commonly reported adverse events were increased appetite and weight gain. |
1 Introduction
Autism spectrum disorder (ASD) is a persistent neurodevelopmental condition characterized by impairment of social communication and stereotypical repetitive behavior patterns. The five diagnostic criteria used by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) for the diagnosis of ASD include (1) persistent deficits in social communication and social interaction across multiple contexts; (2) restricted, repetitive patterns of behavior, interests, or activities; (3) presentation of symptoms in the early developmental period; (4) symptoms cause clinically significant impairment in important areas of current functioning; and (5) these disturbances are not better explained by intellectual disability (intellectual developmental disorder) or global developmental delay. Furthermore, individuals who meet the DSM-IV diagnosis criteria of autistic disorder, Asperger’s disorder, or pervasive developmental disorder not otherwise specified should be given the diagnosis of ASD [1].
A systematic review of worldwide prevalence studies of ASD from 1990 to 2010 estimated the global burden of ASD to be 52 million cases, equal to 7.6 per 1000 individuals in 2010 [2]. Recent figures from the US Centers for Disease Control and Prevention (CDC) suggest an even higher prevalence of 1 in 59 of the population at the age of 8 years [3]. The prevalence rate also differs by ethnic group, with the estimated prevalence of ASD among white children greater than among black children by almost 30% and greater than among Hispanic children by approximately 50% [4]. ASD appears to affect males more than females, with an estimated male-to-female ratio of 4.62 [5]. In 2011, the lifetime cost of supporting an individual with ASD with intellectual disability was estimated to be £1.5 million in the UK and $US2.4 million in the USA [6].
ASD presents in childhood, although diagnosis is often delayed. Currently, there is no cure for ASD. Symptom management is required to improve the quality of life of affected individuals. Both pharmacological and nonpharmacological interventions are available for people with ASD. Nonpharmacological therapy includes educational, behavioral, and psychological therapies. Pharmacotherapy is reserved to treat some of the more challenging issues, such as irritability, aggression and self-injury [7].
Individuals with ASD may have other comorbid conditions such as attention-deficit/hyperactivity disorder (ADHD), depression, epilepsy and schizophrenia. Psychotropic medication such as antipsychotics, antidepressants, antiepileptic drugs and stimulants have been used for patients with ASD with associated comorbidities [8]. Evidence to guide psychotropic medication use in the ASD population is limited; however, a study conducted in a UK population identified that psychotropic drugs were prescribed to 29% of individuals with ASD [9]. International studies have reported the most prescribed drugs as being sleep medication, psychostimulants and antipsychotics [10, 11]. Antipsychotics have been prescribed for 7% of people with ASD in the UK [9]. Risperidone is the only antipsychotic that has been approved in the UK for the management of behavioral issues in people with ASD. In the USA, aripiprazole and risperidone were approved by the US FDA for the treatment of irritability associated with ASD in children [12, 13].
First- and second-generation antipsychotic medication is used for the treatment of behavioral problems in individuals with ASD [7]. Several randomized controlled trials (RCTs) have evaluated the efficacy of antipsychotics in improving some of the issues associated with ASD [14,15,16,17,18], but the evidence of antipsychotic safety in patients with autism is limited. Information on the adverse effects of antipsychotic medication used in the treatment of adult mental illness such as psychosis and mood disorders is extensive, but the population of people with ASD treated with antipsychotic medication is very different: treatment typically starts in childhood or adolescence, and the proportion of intellectual disability is high, as is the proportion of other comorbidities such as ADHD, epilepsy and sleep problems. Against this background, it was considered important to examine the evidence for adverse events (AEs) specifically in people with ASD treated with antipsychotic medication.
AEs associated with antipsychotic use regardless of the indication are common and include but are not limited to metabolic AEs such as weight gain, diabetes mellitus and hyperprolactinemia [19, 20] and movement disorders such as tardive dyskinesia, tremor and dystonia [20, 21]. Potentially serious AEs such as seizures are rare, and potentially fatal AEs such as rhabdomyolysis or neuroleptic malignant syndrome (NMS) have been reported [22,23,24]. The overall aim of this analysis was to provide a comprehensive review of the published evidence of the AEs associated with antipsychotic use in patients with ASD in different age groups. The main objective was to conduct a meta-analysis of the RCTs and observational studies focused on children and adolescents to investigate the prevalence of AEs and the relative risk (RR) of AEs associated with antipsychotic medication use.
2 Methods
This systematic review and meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [25] and registered with the International Prospective Register of Systematic Reviews, PROSPERO (CRD42018083632) by the Centre for Reviews and Dissemination.
2.1 Search Strategy
The Cochrane Library, MEDLINE, Embase and PsycINFO databases were searched from their inception dates until 15 January 2018 using appropriate medical subject heading terms and keywords. The literature retrieval was supplemented by manually searching the reference lists of all identified articles with regard to the inclusion criteria. See Appendix A in the Electronic Supplementary Material (ESM) for the complete search strategy.
2.2 Inclusion and Exclusion Criteria
Eligibility criteria were developed based on the PICOS (participant-intervention-comparison-outcome-study design) framework [26].
Studies were included if they were RCTs or observational studies. We only included studies in which the participants were diagnosed with ASD according to the DSM-IV, DSM-5 or the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) with no age restrictions on participants. The intervention of interest was first- or second-generation antipsychotic medication in any dose or frequency. The intervention could be compared with placebo, other medications or nonpharmacological therapy or could be without comparison. We included studies that reported AEs as the primary or secondary outcome. Only full-text papers published in English were included.
Exclusion criteria were studies published as case reports, narrative reviews, commentaries, editorials, book chapters, grey literature and other summaries. Studies carried out on animals were also excluded.
2.3 Study Selection Process
Two authors (BA and ZW) independently screened the titles, abstracts and full texts of the retrieved papers. Full-text exclusion was based on our inclusion/exclusion criteria, and inconsistent decisions were resolved through consensus.
2.4 Data Extraction and Management
Studies meeting the eligibility criteria were extracted independently by two investigators (BA and PM) using a predesigned extraction form. The following information was extracted: research design, location and setting, participants, intervention, outcome measures and quality assessments. Any discrepancies between two reviewers were resolved through discussion. Kappa statistics were calculated to assess the agreement between the two reviewers on the included studies. Kappa values ranged between zero and one, with zero reflecting complete inter-rater disagreement and one reflecting complete inter-rater agreement, and agreement can range between fair, good and excellent agreement (kappa values 0.40–0.59, 0.60–0.74 or ≥ 0.75, respectively) [27].
2.5 Assessment of Risk of Bias in Included Studies
Two authors (BA and PM) independently evaluated the risk of bias in each study using the Cochrane Collaboration tool for RCTs, which considers the following six domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. A judgment of “high,” “low” or “unclear” risk of bias was made for each paper. A study was considered to have a high risk of bias if one or more key domains were at high risk. A study was considered to have a low risk of bias if all key domains were at low risk. Otherwise, the study was regarded as having an unclear risk of bias [28].
The modified Newcastle–Ottawa Scale (NOS) was used for the methodological quality assessment of the observational studies. It consists of five domains of evaluation: methods for selecting study participants (i.e., selection bias), methods to control for confounding (i.e., performance bias), statistical methods (i.e., detection bias), methods of measuring outcome variables (i.e., information bias) and subject follow-up [29]. Each domain ranges between zero (high risk of bias) and three (low risk of bias). Based on the current authors’ judgment, the included observational studies were classified as high, moderate or low risk of bias if the overall scores were 0–1, > 1 and < 2 or 2–3, respectively.
Any discrepancy in bias assessment was resolved by discussion and group consensus among the authors. Kappa value was calculated to assess the agreement between the two reviewers on the quality assessment of the included papers. A table of the quality assessment of the included studies is provided in Appendix B in the ESM.
2.6 Data Synthesis
Study results were summarized by reporting the AEs as percentages; a systematic narrative synthesis was provided, with information presented in the text, tables and graphs to summarize and explain the characteristics and findings of the included studies.
We performed meta-analyses under the DerSimonian–Laird random-effects model to estimate the RR with 95% confidence intervals (CIs) for the risk of AEs in RCTs and pooled the prevalence of AEs across observational studies. Only studies conducted in children and adolescents were included in the meta-analyses (mean age of participants ≤ 18 years). RCTs that provided enough information to calculate the RR, i.e., the number of patients who had AEs in both intervention and placebo groups and number of patients who did not have AEs were included in the meta-analysis of the estimated pooled RR of AEs. Observational studies that reported the number of patients who had AEs and the total sample size number were included in the meta-analysis of the estimated pooled prevalence of AEs. To measure the degree of statistical heterogeneity between studies, we used the I2, which rates the heterogeneity between studies in percentages from 0 to 100%, where I2 < 25% indicates low, 25–75% moderate, and > 75% high heterogeneity [30]. To explore possible sources of heterogeneity, subgroup analyses were performed by medication. The results are presented using forest plots. All analyses were conducted using STATA, v14.1.
Publication bias was assessed for the observational studies used to pool the estimated prevalence of AEs and for the RCTs used to pool the estimated RR of antipsychotic use with funnel plots; Begg’s test and Egger’s test were used to test the significance.
3 Results
3.1 Search Results
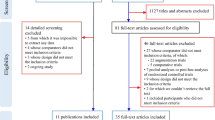
We identified 2805 citations in the database search (Fig. 1); 2620 citations were removed after identification of duplicates or after screening of titles and abstracts. In total, 185 full-text citations were assessed for eligibility. From those, 54 citations met our inclusion criteria and provided the data for our meta-analysis and narrative review. The kappa value of full-text screening was 0.72 (95% CI 0.54–0.88), which indicates good agreement.
3.2 Included Studies
From the 54 included studies (Tables 1 and 2), 14 were observational and 40 were RCTs, involving 3216 participants in total (2034 in the RCTs and 1182 in the observational studies). Males comprised ≥ 70% of the participants in most of the included studies. In one study, two of six participants were male [31]. The overall mean age of participants was 9.6 years. The sample size of the included studies ranged between 6 and 330 participants in RCTs and between 6 and 203 participants for observational studies. The shortest duration of follow-up was 6 weeks, and the longest duration was approximately 5.5 years in two studies; one of these was an open-label study and the other was a prospective cohort study. Detailed descriptions of the included studies are provided in Tables 1 and 2. Most of the participants were medication free for at least 1 week before the studies started; in some studies, anticonvulsants used for the treatment of a seizure disorder were permitted if the dose had been stable for at least 4 weeks and the patient was seizure free for at least 6 months. Stimulants were permitted in some studies for the management of ADHD if there was no change in the dose.
Of the included RCTs, 18 were blinded trials and 22 were open-label trials. Most of the observational studies comprised a treatment group only with no control group; only one observational study was a retrospective cohort study comparing the effect of risperidone and aripiprazole on body mass index (BMI) change [32]. In total, 51 studies examined the effect of second-generation antipsychotics (mainly risperidone and aripiprazole), whereas two studies examined the effect of a first-generation antipsychotic (haloperidol), and one study examined the effect of 14 different first- and second-generation antipsychotics [33].
3.3 Excluded Studies
The majority of excluded studies involved psychiatric patients in general (i.e., the population included multiple mental health diagnoses such as ADHD, schizophrenia, mood disorders and psychosis) in addition to those with ASD. Extraction of distinct safety information related to the ASD population in these studies was not feasible. In total, 36 citations were excluded because they were conference abstracts, and 13 had study designs irrelevant to our inclusion criteria, for example, reviews, case reports and letters to the editor. Nine studies did not meet the eligibility criteria because they used the DSM-III criteria for ASD diagnosis.
3.4 Quality Assessment
The included RCTs were assessed using the Cochrane Collaboration tool for assessing the risk of bias. Four RCTs were considered as at low risk of bias in all six domains [15, 34,35,36]. A total of 22 studies were considered as at high risk of bias in the study performance domain because of the open-label design [16, 37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. According to the selection bias domain, 12 studies were judged as having an unclear risk of bias because the random-sequence generation and allocation concealment were not clearly described [14, 17, 18, 58,59,60,61,62,63,64,65]. One included study was judged as having a high risk of bias because of a lack of blinding of the outcome assessment [66].
For observational studies, eight studies were judged as having a low risk of bias [33, 67,68,69,70,71,72,73], and six fell under the moderate risk of bias category [31, 32, 74,75,76,77]. Agreement between the two reviewers for the quality assessment of the included papers was good (kappa value 0.63; 95% CI − 0.025 to 1.000).
The papers included in the meta-analyses had either a low or moderate risk of bias; none of the papers with a high risk of bias was included. The details of the quality assessment are shown in Appendix B in the ESM.
3.5 Adverse Events (AEs) Based on Body Systems Classification
A total of 127 AEs were identified in the included studies. Central nervous system (CNS) events were the most frequently identified AEs, followed by endocrine disorders and gastrointestinal disorders (Fig. 2). In the observational studies, endocrine disorders were the most frequent AEs identified, followed by CNS events and gastrointestinal disorders.
3.6 AE Relative Risk and Prevalence
From the eight RCTs included in the meta-analysis to estimate the RR of AEs associated with antipsychotic use, we found that antipsychotic treatment increased the risk of developing AEs by 22% compared with placebo (RR 1.22; 95% CI 1.11–1.34; I2= 30.6%; p = 0.184) (Fig. 3a).
Seven observational studies reported the total number of participants who had AEs and were included in the meta-analysis to estimate the pooled prevalence of AEs, which was 50.5% (95% CI 33–67). However, there was significant heterogeneity in the results of the articles (I2 = 99.9%) (Fig. 3b).
3.7 Central Nervous System (CNS) AEs
A wide range of CNS AEs was reported. Appetite increase was the most frequently reported, followed by sedation, somnolence and headache. Extrapyramidal symptoms, including tremor, akathisia and tardive dyskinesia, were also reported frequently. Some AEs were infrequently reported but could potentially be serious. Examples included seizure reported by two patients and intentional self-injury and suicidal ideation, each of which were reported once [15, 35, 52, 63]. Moreover, CNS AEs caused many participants to withdraw from the study or discontinue the use of antipsychotic medication [16, 17, 35, 49, 54, 58, 61, 67, 78].
3.8 Endocrine AEs
Reporting of weight gain and hyperprolactinemia was prominent. Results from the meta-analysis of seven RCTs demonstrated that antipsychotic medication was associated with an increase in mean weight of 1.4 kg compared with placebo (I2 = 9.2%, p = 0.359). Weight increase was reported as one of the leading causes of study discontinuation for many participants [35, 47, 49, 54, 56, 75]. Hyperglycemia, hyperleptinemia and increased insulin resistance were prominent endocrine disorders. The mean serum prolactin increased by 17.7 ng/ml compared with placebo (I2 = 97.9%, p < 0.001). Forest plots of the meta-analysis of mean weight change and mean serum prolactin change are shown in Fig. 4.
3.9 Cardiovascular System and Other AEs
Cardiovascular AEs were identified less frequently. Change in heart rate and prolonged QT interval were reported in ten participants in RCTs looking for cardiac conduction effects of risperidone in children with ASD [65].
The other main AEs were vomiting, constipation, upper respiratory tract infection, nasopharyngitis, coughing, enuresis and fatigue.
3.10 Publication Bias
The assessment of publication bias of studies included in the meta-analysis of the RR resulted in a symmetrical funnel plot. In addition, the Begg’s test and Egger’s test showed no evidence of publication bias (p = 0.54 and 0.47, respectively). Findings were similar for the observational studies included in the meta-analysis of the prevalence of AEs (Appendix C in the ESM).
4 Discussion
4.1 Main Findings
This was an extensive systematic review and meta-analysis evaluating the safety and tolerability of antipsychotic medication in individuals with ASD. The meta-analysis of eight RCTs demonstrated that the RR of developing AEs was 22% higher with antipsychotics than with placebo. The overall RR was similar to the RR stratified by drug. The meta-analysis of seven observational studies resulted in an estimated overall AE prevalence of 50%. However, this estimated prevalence might be imprecise because of the high heterogeneity and the small number of included studies. The heterogeneity could be due to the different geographic locations of the included studies, drug type and variability of follow-up periods, which ranged from 1 to 68 months.
The frequently reported AEs identified in this review were weight gain, enuresis, somnolence, increased appetite and extrapyramidal symptoms. These findings are similar to those identified in a systematic review of antipsychotic use for challenging behaviors in people with learning disabilities [79].
The majority of the articles reported weight gain. Both short-term and long-term studies reported a greater mean weight increase with risperidone than with placebo [17, 34, 36, 60, 66]. We noted that long-term therapy was associated with more weight gain than was short-term therapy. Furthermore, weight gain led to study discontinuation for many participants. Psychiatrists are encouraged to consider weight gain evaluation in individuals on antipsychotic therapy. Although hyperprolactinemia was one of the frequently identified AEs, elevated serum prolactin was not reported in any of the studies on aripiprazole. This finding is consistent with what has been published previously regarding the relationship between hyperprolactinemia and aripiprazole compared with other antipsychotic therapy [80]. Reports of elevated prolactin with risperidone being decreased by the addition of aripiprazole have been published [81, 82].
NMS is an uncommon but potentially fatal AE that may occur with antipsychotic treatment. In this systematic review, we were unable to identify any cases of NMS, probably because there are no published observational studies or RCTs investigating the association between antipsychotic medication use in individuals with ASD and the risk of developing NMS, and this serious AE appears to be rare. The implication is that very large numbers would be required to yield valid frequency data.
4.2 Strengths and Limitations
This is the first systematic review to assess the safety of both first- and second-generation antipsychotics in individuals with ASD. It provides an evidence-based overview of the prevalence and type of AEs associated with antipsychotic medication use in people with ASD. The publications included in this review were identified through electronic searches of four different databases using a comprehensive search strategy to provide the best chance of identifying all relevant citations. In addition, our review follows the standard methodology of systematic review and meta-analysis, which is recommended by the Cochrane and PRISMA guidelines [25, 26].
The potential limitations of our systematic review include the following. (1) One of the major limitations in most of the included studies was that the safety of the antipsychotic medication was a secondary outcome, and the primary outcome was its efficacy. This reflects on the quality and completeness of safety data. (2) The quality of included studies was questionable. First, most of the observational studies comprised one group (intervention arm), which did not allow us to draw any comparisons. Second, almost half of the included RCTs were open-label studies, which increases the risk of bias in outcome measurement. Third, the sample sizes were very small in many reviewed studies and could be unrepresentative: eight studies had a sample size of fewer than 20 participants. However, we decided to include these studies because well-designed clinical trials investigating the safety of antipsychotics are lacking in the ASD population. Fourth, even though the agreement between the two reviewers on the quality of the included papers was good, the CIs were wide, hence this value may not provide enough information to make a decision and should be interpreted with caution. (3) The overall I2 values were markedly high for the meta-analysis of the prevalence of AEs and mean serum prolactin change, 99% and 97%, respectively. This indicates high heterogeneity between the studies included in the meta-analysis. (4) Although no evidence of publication bias was identified by the Begg’s and Egger’s tests, these tests could be underpowered because of the small number of studies included in the analysis. (5) Only studies published in English were included in this review, which may lead to language bias. However, Moher et al. [83] reported that the exclusion of trials published in non-English language had no significant effect on meta-analyses results. Furthermore, over the past two decades, the number of RCTs published in languages other than English has been declining, which may diminish the extent of language bias introduced [84].
5 Conclusions
AEs are highly prevalent in individuals who take antipsychotic medication. The RR of developing AEs with antipsychotics was 22% higher than with placebo. The most frequent AEs involved the CNS. Weight gain and hyperprolactinemia were particularly associated with antipsychotics, although hyperprolactinemia depends on the antipsychotic, being common with risperidone but apparently not associated with aripiprazole. Aripiprazole and risperidone are the only antipsychotics licensed for children with ASD. Several studies have been carried out using other antipsychotics, which indicates the off-license use of antipsychotics in ASD management. Currently, the available evidence on the association between antipsychotic use in individuals with ASD and the risk of developing AEs is limited. The findings of this review highlight the need for well-designed safety and tolerability studies to investigate the association between antipsychotics in individuals with ASD and AEs.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–13. https://doi.org/10.1017/s003329171400172x.
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1.
Investigators Autism Developmental Disabilities Monitoring Network Surveillance Year Principal. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morb Mortal Wkl Rep Surveill Summ. 2014;63(2):1–21.
Watkins EE, Zimmermann ZJ, Poling A. The gender of participants in published research involving people with autism spectrum disorders. Res Autism Spectr Disord. 2014;8(2):143–6.
Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721–8. https://doi.org/10.1001/jamapediatrics.2014.210.
Posey DJ, McDougle CJ. Pharmacotherapeutic management of autism. Expert Opin Pharmacother. 2001;2(4):587–600. https://doi.org/10.1517/14656566.2.4.587.
National Collaborating Centre for Mental Health. Autism recognition, referral, diagnosis and management of adults on the autism spectruM. Leicester: The British Psychological Society and The Royal College of Psychiatrists; 2012.
Murray ML, Hsia Y, Glaser K, Simonoff E, Murphy DG, Asherson PJ, et al. Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology. 2014;231(6):1011–21. https://doi.org/10.1007/s00213-013-3140-7.
Hsia Y, Wong AY, Murphy DG, Simonoff E, Buitelaar JK, Wong IC. Psychopharmacological prescriptions for people with autism spectrum disorder (ASD): a multinational study. Psychopharmacology. 2014;231(6):999–1009.
Wong A, Hsia Y, Chan EW, Murphy DG, Simonoff E, Buitelaar JK, et al. The variation of psychopharmacological prescription rates for people with autism spectrum disorder (ASD) in 30 countries. Autism Res. 2014;7(5):543–54.
Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634–41. https://doi.org/10.1542/peds.2003-0264-F.
Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–40. https://doi.org/10.1542/peds.2008-3782.
Ichikawa H, Mikami K, Okada T, Yamashita Y, Ishizaki Y, Tomoda A, et al. Aripiprazole in the treatment of irritability in children and adolescents with autism spectrum disorder in Japan: a randomized, double-blind, placebo-controlled study. Child Psychiatry Hum Dev. 2017;48(5):796–806.
Loebel A, Brams M, Goldman R, Silva R, Hernandez D, Deng L, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016. https://doi.org/10.1007/s10803-015-2628-x.
Stigler K, Mullett J, Erickson C, Posey D, McDougle C. Paliperidone for irritability in adolescents and young adults with autistic disorder. Psychopharmacology. 2012. https://doi.org/10.1007/s00213-012-2711-3.
Pandina G, Bossie C, Youssef E, Zhu Y, Dunbar F. Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007. https://doi.org/10.1007/s10803-006-0234-7.
Hollander E, Wasserman S, Swanson E, Chaplin W, Schapiro M, Zagursky K, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006. https://doi.org/10.1089/cap.2006.16.541.
Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15(2):139–50. https://doi.org/10.1007/s40272-013-0016-6.
Almandil NB, Wong IC. Review on the current use of antipsychotic drugs in children and adolescents. Arch Dis Child Educ Pract Edn. 2011;96(5):192–6. https://doi.org/10.1136/archdischild-2011-300054.
Fleischhaker C, Heiser P, Hennighausen K, Herpertz-Dahlmann B, Holtkamp K, Mehler-Wex C, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol. 2006;16(3):308–16. https://doi.org/10.1089/cap.2006.16.308.
Rani FA, Byrne P, Cranswick N, Murray ML, Wong IC. Mortality in children and adolescents prescribed antipsychotic medication: a retrospective cohort study using the UK general practice research database. Drug Saf. 2011;34(9):773–81. https://doi.org/10.2165/11591120-000000000-00000.
Sheehan R, Horsfall L, Strydom A, Osborn D, Walters K, Hassiotis A. Movement side effects of antipsychotic drugs in adults with and without intellectual disability: UK population-based cohort study. BMJ Open. 2017;7(8):e017406. https://doi.org/10.1136/bmjopen-2017-017406.
Star K, Iessa N, Almandil NB, Wilton L, Curran S, Edwards IR, et al. Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis. J Child Adolesc Psychopharmacol. 2012;22(6):440–51. https://doi.org/10.1089/cap.2011.0134.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Cochrane Collab. 2011;5:83–4.
Cooper H, Hedges LV, Valentine JC. Evaluating coding decisions. In: The handbook of research synthesis and meta-analysis. Russell Sage Foundation; 2009. p. 187–201.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Kroon FP, van der Burg LR, Ramiro S, Landewé RB, Buchbinder R, Falzon L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.cd010952.pub2.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327(7414):557.
Beherec L, Lambrey S, Quilici G, Rosier A, Falissard B, Guillin O. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341–4.
Wink LK, Early M, Schaefer T, Pottenger A, Horn P, McDougle CJ, et al. Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J Child Adolesc Psychopharmacol. 2014;24(2):78–82.
Wink LK, Pedapati EV, Horn PS, McDougle CJ, Erickson CA. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91–4.
Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J Child Neurol. 2006;21(6):450–5.
Owen R, Sikich L, Marcus R, Corey-Lisle P, Manos G, McQuade R, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009. https://doi.org/10.1542/peds.2008-3782.
Kent J, Kushner S, Ning X, Karcher K, Ness S, Aman M, et al. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J Autism Dev Disord. 2013. https://doi.org/10.1007/s10803-012-1723-5.
Findling RL, Maxwell K, Wiznitzer M. An open clinical trial of risperidone monotherapy in young children with autistic disorder. Psychopharmacol Bull. 1997;33(1):155–9.
McDougle CJ, Holmes JP, Bronson MR, Anderson GM, Volkmar FR, Price LH, et al. Risperidone treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Am Acad Child Adolesc Psychiatry. 1997;36(5):685–93.
Nicolson R, Awad G, Sloman L. An open trial of risperidone in young autistic children. J Am Acad Child Adolesc Psychiatry. 1998;37(4):372–6.
Masi G, Cosenza A, Mucci M. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. J Child Adolesc Psychopharmacol. 2001;11(4):389–94.
Masi G, Cosenza A, Mucci M, Brovedani P. Open trial of risperidone in 24 young children with pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2001;40(10):1206–14.
Masi G, Cosenza A, Mucci M, De Vito G. Risperidone monotherapy in preschool children with pervasive developmental disorders. J Child Neurol. 2001;16(6):395–400.
Kemner C, Willemsen-Swinkels SH, de Jonge M, Tuynman-Qua H, van Engeland H. Open-label study of olanzapine in children with pervasive developmental disorder. J Clin Psychopharmacol. 2002;22(5):455–60.
Malone RP, Maislin G, Choudhury MS, Gifford C, Delaney MA. Risperidone treatment in children and adolescents with autism: short- and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry. 2002;41(2):140–7.
Gagliano A, Germano E, Pustorino G, Impallomeni C, D’Arrigo C, Calamoneri F, et al. Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. J Child Adolesc Psychopharmacol. 2004;14(1):39–47.
Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McCracken JT, et al. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361–9.
Troost PW, Lahuis BE, Steenhuis M-P, Ketelaars CE, Buitelaar JK, Engeland HV, et al. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1137–44.
Malone RP, Delaney MA, Hyman SB, Cater JR. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779–90.
Troost PW, Lahuis BE, Hermans MH, Buitelaar JK, Van Engeland H, Scahill L, et al. Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol. 2007;27(1):52–7.
Capone GT, Goyal P, Grados M, Smith B, Kammann H. Risperidone use in children with Down syndrome, severe intellectual disability, and comorbid autistic spectrum disorders: a naturalistic study. J Dev Behav Pediatr. 2008;29(2):106–16.
Gencer O, Emiroglu F, Miral S, Baykara B, Baykara A, Dirik E. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder: an open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217–25.
Stigler KA, Diener JT, Kohn AE, Li L, Erickson CA, Posey DJ, et al. Aripiprazole in pervasive developmental disorder not otherwise specified and asperger’s disorder: a 14-week, prospective, open-label study. J Child Adolesc Psychopharmacol. 2009;19(3):265–74.
Hellings JA, Cardona AM, Schroeder SR. Long-term safety and adverse events of risperidone in children, adolescents, and adults with pervasive developmental disorders. J Ment Health Res Intellect Disabil. 2010;3(3):132–44.
Marcus RN, Owen R, Manos G, Mankoski R, Kamen L, McQuade RD, et al. Safety and tolerability of aripiprazole for irritability in pediatric patients with autistic disorder: a 52-week, open-label, multicenter study. J Clin Psychiatry. 2011;72(9):1270–6.
Kent J, Hough D, Singh J, Karcher K, Pandina G. An open-label extension study of the safety and efficacy of risperidone in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2013. https://doi.org/10.1089/cap.2012.0058.
Ichikawa H, Hiratani M, Yasuhara A, Tsujii N, Oshimo T, Ono H, et al. An open-label extension long-term study of the safety and efficacy of aripiprazole for irritability in children and adolescents with autistic disorder in Japan. Psychiatry Clin Neurosci. 2017;23:23.
Nikvarz N, Alaghband-Rad J, Tehrani-Doost M, Alimadadi A, Ghaeli P. Comparing efficacy and side effects of memantine vs. risperidone in the treatment of autistic disorder. Pharmacopsychiatry. 2017;50(1):19–25.
Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: a double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol. 2001;21:440–4.
McCracken J, McGough J, Shah B, Cronin P, Hong D, Aman M, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002. https://doi.org/10.1056/nejmoa013171.
Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61(4):545–50.
Marcus R, Owen R, Kamen L, Manos G, McQuade R, Carson W, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009. https://doi.org/10.1097/chi.0b013e3181b76658.
Findling RL, Mankoski R, Timko K, Lears K, McCartney T, McQuade RD, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22–30.
Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. 2014;45(2):185–92.
Scahill L, Jeon S, Boorin S, McDougle C, Aman M, Dziura J, et al. Weight gain and metabolic consequences of risperidone in young children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2016. https://doi.org/10.1016/j.jaac.2016.02.016.
Vo L, Snyder C, McCracken C, McDougle C, McCracken J, Aman M, et al. No apparent cardiac conduction effects of acute treatment with risperidone in children with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2016. https://doi.org/10.1089/cap.2016.0090.
Luby J, Mrakotsky C, Stalets M, Belden A, Heffelfinger A, Williams M, et al. Risperidone in preschool children with autistic spectrum disorders: an investigation of safety and efficacy. J Child Adolesc Psychopharmacol. 2006. https://doi.org/10.1089/cap.2006.16.575.
Masi G, Cosenza A, Millepiedi S, Muratori F, Pari C, Salvadori F. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders: a retrospective study. CNS Drugs. 2009;23(6):511–21.
Aman M, Rettiganti M, Nagaraja HN, Hollway JA, McCracken J, McDougle CJ, et al. Tolerability, safety, and benefits of risperidone in children and adolescents with autism: 21-month follow-up after 8-week placebo-controlled trial. J Child Adolesc Psychopharmacol. 2015;25(6):482–93.
Hellings JA, Jadhav M, Jain S, Jadhav S, Genovese A. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618–24.
Hongkaew Y, Ngamsamut N, Puangpetch A, Vanwong N, Srisawasdi P, Chamnanphon M, et al. Hyperprolactinemia in Thai children and adolescents with autism spectrum disorder treated with risperidone. Neuropsychiatr Dis Treat. 2015;11:191–6.
Ngamsamut N, Hongkaew Y, Vanwong N, Srisawasdi P, Puangpetch A, Chamkrachangpada B, et al. 9-Hydroxyrisperidone-Induced hyperprolactinaemia in thai children and adolescents with autism spectrum disorder. Basic Clin Pharmacol Toxicol. 2016;119(3):267–72.
Nuntamool N, Ngamsamut N, Vanwong N, Puangpetch A, Chamnanphon M, Hongkaew Y, et al. Pharmacogenomics and efficacy of risperidone long-term treatment in thai autistic children and adolescents. Basic Clin Pharmacol Toxicol. 2017;121(4):316–24.
Srisawasdi P, Vanwong N, Hongkaew Y, Puangpetch A, Vanavanan S, Intachak B, et al. Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin Biochem. 2017;50(12):678–85.
Masi G, Cosenza A, Mucci M, Brovedani P. A 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidone. J Clin Psychiatry. 2003;64(9):1039–47.
Corson AH, Barkenbus JE, Posey DJ, Stigler KA, McDougle CJ. A retrospective analysis of quetiapine in the treatment of pervasive developmental disorders. J Clin Psychiatry. 2004;65(11):1531–6.
Boon-Yasidhi V, Jearnarongrit P, Tulayapichitchock P, Tarugsa J. Adverse effects of risperidone in children with autism spectrum disorders in a naturalistic clinical setting at siriraj hospital, Thailand. Psychiatry J Print. 2014;2014:136158.
Vanwong N, Srisawasdi P, Ngamsamut N, Nuntamool N, Puangpetch A, Chamkrachangpada B, et al. Hyperuricemia in children and adolescents with autism spectrum disorder treated with risperidone: the risk factors for metabolic adverse effects. Front Pharmacol. 2017;7:527.
McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633–41.
Brylewski J, Duggan L. Antipsychotic medication for challenging behaviour in people with learning disability. Cochrane Database Syst Rev. 2004. https://doi.org/10.1002/14651858.CD000377.pub2.
Kirino E. Serum prolactin levels and sexual dysfunction in patients with schizophrenia treated with antipsychotics: comparison between aripiprazole and other atypical antipsychotics. Ann Gen Psychiatry. 2017;16(1):43. https://doi.org/10.1186/s12991-017-0166-y.
Yasui-Furukori N, Furukori H, Sugawara N, Fujii A, Kaneko S. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596–9.
Chen J-X, Su Y-A, Bian Q-T, Wei L-H, Zhang R-Z, Liu Y-H, et al. Adjunctive aripiprazole in the treatment of risperidone-induced hyperprolactinemia: a randomized, double-blind, placebo-controlled, dose–response study. Psychoneuroendocrinology. 2015;58:130–40.
Moher D, Pham B, Lawson M, Klassen T. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7(41):1–90.
Galandi D, Schwarzer G, Antes G. The demise of the randomised controlled trial: bibliometric study of the German-language health care literature, 1948 to 2004. BMC Med Res Methodol. 2006;6(1):30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
IW has received educational grants for projects unrelated to the current study from pharmaceutical companies that manufacture antipsychotic medicines and received research grants from the European Commission and Hong Kong Research Grant Council for the evaluation of antipsychotic drugs use in patients. BA, ZW, PM, FB, TA and RB have no conflicts of interest that are directly relevant to the content of this article.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alfageh, B.H., Wang, Z., Mongkhon, P. et al. Safety and Tolerability of Antipsychotic Medication in Individuals with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Pediatr Drugs 21, 153–167 (2019). https://doi.org/10.1007/s40272-019-00333-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-019-00333-x