Abstract
Introduction
The aim was to analyse data on the use of biochemical bone turnover markers (BTM) in postmenopausal osteoporosis.
Methods
We carried out a comparative analysis of the most important papers concerning BTM in postmenopausal osteoporosis that have been published recently.
Results
The BTM levels are influenced by several factors. They are moderately correlated with BMD and subsequent bone loss. Increased levels of bone resorption markers are associated with a higher risk of fracture. Changes in the BTM during the anti-osteoporotic treatment (including combination therapy) reflect the mechanisms of action of the drugs and help to establish their effective doses. Changes in the BTM during the anti-resorptive treatment are correlated with their anti-fracture efficacy.
Conclusion
Biological samples should be obtained in a standardised way. BTM cannot be used for prediction of the accelerated bone loss at the level of the individual. BTM help to detect postmenopausal women who are at high risk of fracture; however, adequate practical guidelines are lacking. BTM measurements taken during the anti-resorptive therapy help to identify non-compliers. They may improve adherence to the anti-resorptive therapy and the fall in the BTM levels that exceeds the predefined threshold improves patients’ persistence with the treatment. There are no guidelines concerning the use of BTM in monitoring anti-osteoporotic therapy in postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone metabolism is characterised by two opposing activities coupled in time and space at the level of a basic multicellular unit (BMU) [1]. Bone resorption consists of the dissolution of bone mineral and catabolism of the bone matrix constituents by osteoclasts, leading to formation of a resorptive cavity and release of bone matrix components. During bone formation, osteoblasts synthesise bone matrix, which fills up the resorption cavity and undergoes rapid primary mineralisation followed by the slow long-term secondary mineralisation.
Bone formation can be assessed by biochemical markers such as osteocalcin (OC), bone-specific alkaline phosphatase (BAP) as well as N-terminal and C-terminal propeptides of type I procollagen (P1NP, P1CP) [2]. Type I collagen is the most abundant protein of bone matrix. P1NP and P1CP are cleaved during the extracellular metabolism of procollagen and released into the blood, whereas the central part of the molecule is incorporated into the bone matrix. P1NP and P1CP are not specific to bone; however, bone has a faster metabolism than other tissues containing type I collagen and most serum P1NP and P1CP originates from bone. BAP is an enzyme located on the outer surface of osteoblasts, most probably involved in the regulation of the mineralisation of osteoid. OC is a vitamin K-dependent protein synthesised by osteoblasts and odontoblasts. It contains three gammacarboxyglutamic residues and its function has not been clearly established. Serum levels of bone formation markers correlate positively with histomorphometric parameters of bone formation [3–5]. They are also positively correlated with the radiotracer kinetic estimates of the bone mineral accretion; however, this association may depend on the bone mass [6, 7].
Bone resorption can be assessed by several biochemical markers, e.g. N-terminal and C-terminal crosslinking telopeptides of type I collagen (NTX-I and CTX-I), C-terminal crosslinking telopeptides of type I collagen generated by metalloproteinase (CTX-MMP, ICTP), helical peptide 620–633, deoxypyridinoline (DPD), hydroxylysine (HLys) glycosides, hydroxyproline (HPro) or isoform 5b of tartrate-resistant acid phosphatase (TRACP5b). NTX-I, CTX-I, ICTP and helical peptide 620–633 are mixtures of products of catabolism of type I collagen containing specific amino acid sequences, being antigenic epitopes. Then, they are catabolised to smaller molecules such as DPD, HLys and HPro. The catabolism of these peptides occurs partly in the tubular kidney cells. TRACP5b is an iron-containing enzyme synthesised by osteoclasts that can function as a phosphatase at acidic pH; however, its biological function is unknown [8]. Urinary excretion of DPD is correlated positively with histomorphometric parameters of bone resorption [9]. Estimates of bone resorption from radiotracer kinetic studies are correlated with baseline levels of bone resorption markers [10]. Also, during the anti-resorptive treatment, changes in the levels of bone resorption markers and in the radiotracer kinetic indices are strongly correlated [10, 11].
The histomorphometric and radiotracer studies show that bone turnover markers (BTM) levels are good measures of the corresponding metabolic processes at the tissular level in the entire skeleton. However, in the physiology and many pathologies, bone formation and resorption are coupled. Thus, bone resorption markers may be correlated with the histomorphometric and radiotracer kinetic indices of bone formation, whereas serum levels of the bone formation markers may be correlated with the histomorphometric and kinetic indices of bone resorption [3–11].
General limitations of BTM and pre-analytical variability
Clinical interpretation of biological measurements is more reliable if their analytical and pre-analytical variability is low. The analytical variability, assessed by using the interassay and intra-assay coefficients of variation, is critical for the accuracy of the BTM measurements. The analytical variability depends on the marker and on the method. This large technical subject will not be presented in this paper, which focusses on the clinical aspects. Pre-analytical variability comprises a large number of factors divided into two groups: controllable factors and factors that are not easily modified (Table 1).
Controllable factors comprise circadian, menstrual and seasonal variability, fasting and feeding status as well as exercise. Circadian rhythm has a very strong impact on the variability of some of the BTM levels. Bone resorption peaks in the second half of the night (between 3 and 7 a.m.) and has its nadir in the late afternoon [12–17]. The time of the peak is similar for different bone resorption markers whereas the amplitude is higher for CTX-I (for serum β-β-CTX-I and urinary CTX-I 40–60% of the average concentration) than for other markers, e.g. DPD and serum or urinary NTX-I (10–35%). Certain studies show circadian variation of bone formation; however, this variability is lower and the amplitude represents 5–25% of the average level [12, 13, 18]. Such low amplitude could not be detected in certain studies carried out in small, non-homogenous groups.
Several factors can modulate the circadian variability of the BTM levels. Certain factors, e.g. menopause or sex, strongly influence the average BTM levels, but only weakly their circadian pattern. Food intake has a strong effect on bone resorption, especially on serum CTX-I levels, whereas its effect on bone formation is weak or negligible [12, 19]. Fasting reduced (although did not abolish) the morning decrease in the serum CTX-I concentration [12]. This postprandial decrease in bone resorption is most probably mediated by glucagon-like peptide 2, the synthesis of which is stimulated by food intake [20]. Some factors have a limited effect on the amplitude of the BTM, e.g. the amplitude was increased in postmenopausal osteoporotic women (compared with the postmenopausal women with normal BMD), but lower during the treatment with bisphosphonates. Other factors, e.g. endogenous cortisol secretion, day–night cycle, bed rest, season (in healthy, normally nourished persons) or calcium intake, have a small and not clinically significant effect on the circadian variation of bone turnover. In postmenopausal women, leisure daily physical activity and light physical exercise have no significant effect on the BTM levels [21–23].
Bone turnover marker levels are also influenced by factors that are not easily modified. In postmenopausal and elderly women, the major uncontrollable factors are diseases and associated bed rest and immobility, medications, nutritional status and recent fractures. Several diseases can influence the BTM. Bone formation and resorption markers are increased in metabolic bone diseases characterised by an acceleration of bone turnover, such as Paget’s disease (BAP and bone resorption), primary hyperparathyroidism, thyrotoxicosis, acromegaly [3, 24–29]. Bone resorption markers are increased and bone formation markers are decreased in the diseases characterised by the dissociation of these processes, such as Cushing’s disease or multiple myeloma [30–33]. Bone formation and resorption are mildly decreased in diseases characterised by low bone turnover, such as hypoparathyroidism or hypopituitarism [34, 35]. Change in the BTM levels depends on the severity of the disease—the more severe the disease, the more abnormal the BTM levels [32, 34, 36]. During treatment, the clinical improvement is usually accompanied by normalisation of the BTM levels [26–30, 37].
Bone turnover marker levels are usually markedly increased (even several times the upper limit of the normal range) in patients with bone metastases, especially in postmenopausal women mainly with breast cancer [38, 39]. Practically all the BTM are elevated; however, ICTP and the native non-isomerised form of CTX-I (α-α-CTX-I) seem to be most strongly influenced by the bone involvement [38–40]. By contrast, the BTM levels can be close to the normal range in patients receiving an efficacious anticancer treatment [41, 42].
Increased BTM levels, especially bone resorption, are found in the institutionalised elderly and in patients with chronic diseases associated with prolonged bed rest and limited mobility such as dementia, Alzheimer’s disease, stroke, hemiplegia, and Parkinson’s disease [43–49].
Bone turnover marker levels are influenced by a recently sustained fracture. During the first hours after a fracture, the OC level decreases due to high cortisol secretion induced by stress [50]. Then, bone formation and resorption markers increase promptly and significantly (30–100%) reflecting the healing of the fracture [50, 51]. The BTM levels are markedly increased for at least 4 months after the fracture, then they decrease, but may remain elevated even 1 year after fracture [50–52].
Effect of drugs on BTM
Several groups of drugs influence BTM. The effect of drugs used in the treatment of metabolic bone diseases on the BTM levels will be described in the last part of this paper.
Corticosteroids increase the risk of osteoporosis. They promptly inhibit bone formation, a fall in the OC level being consistently most significant and most rapid followed by a delayed and milder decrease in P1CP, P1NP and BAP [53]. Bone resorption can increase, especially after treatment exceeding 3 months, but data are less consistent. Most studies focussed on long-term (>6 months) corticotherapy with high doses (>7.5 mg prednisone daily). Low-dose prednisone (5 mg daily) decreased bone formation but not bone resorption [54]. Inhaled corticosteroids induced a small but statistically significant decrease in the OC concentration [55, 56]. This effect was dose-dependent, but varied according to the corticosteroid [57, 58]. By contrast, their effect on other BTM was not significant [55, 59, 60]. In the analysis of the effect of corticosteroids on the BTM several points should be taken into account. Their effect seems to be stronger in postmenopausal than in premenopausal women [61]. Their effect depends on the underlying disease. In bronchial asthma, changes in the BTM reflect the undesirable effect of corticosteroids on bone turnover, while chronic inflammatory diseases such as rheumatoid arthritis may induce changes in bone turnover. In these patients, higher bone resorption may confound the effect of corticosteroids on BTM. Corticosteroid-treated patients usually have a more severe disease than patients who do not receive corticosteroids [62]. In the longitudinal studies, changes in the BTM reflect mainly the pharmacologic effect of corticosteroids and depend on the severity of the disease at baseline [63].
Inhibitors of aromatase are used in the treatment of breast cancer. They reduce the residual concentrations of oestrogens, which results in an additional acceleration of bone turnover and subsequent bone loss [64–67]. In treatment-naïve postmenopausal women with breast cancer, aromatase inhibitors increase the BTM levels by 10–35% in comparison with baseline (i.e. postmenopausal levels) [64–67]. This increase is not observed in the case of concomitant treatment with bisphosphonates or tamoxifen [64, 66, 67].
Some anti-epileptic drugs (AED) induce metabolic bone disease characterised by abnormal BTM levels. AED such as phenytoin, phenobarbital, carbamazepine, oxcarbazepine or primidone stimulate the activity of hepatic cytochrome P450 hydroxylase enzymes leading to increased synthesis of inactive 24-hydroxylated metabolites of vitamin D [68, 69]. Patients treated with these AED have decreased concentrations of bioavailable fractions of sex steroids [70]. In patients treated with the above AED, levels of markers of bone formation and bone resorption are higher [71–74]. Some non-enzyme-inducing AED (valproic acid, lamotrigine) can mildly increase serum OC and ICTP levels without influencing 25-hydroxycalciferol (25OHD) concentration [75, 76]. However, the mechanism of their action on bone metabolism is not known. Discordances between studies on AED may be due to the methodological problems. Groups are often small and treatment duration varies substantially. BTM levels can be also influenced by non-fasting status (for bone resorption markers) and season (especially for AED that interfere with vitamin D metabolism).
Statins (β-hydroxy-β-methylglutaryl-CoA reductase inhibitors) influence bone metabolism in experimental studies and moderately decrease the fracture risk [77]. However, in most studies the effect of the statins is not significant [78–81]. In other studies, their effect is weak (about 10%), limited to certain BTM and inconsistent [82–85]. The effect on the BTM levels did not differ between the lipophilic statins (e.g. atorvastatin, simvastatin) and hydrophilic pravastatin.
Thiazolidinediones (TZD), agonists of the perioxisome proliferators-activated receptor (PPAR), are used in the treatment of diabetes and other clinical conditions characterised by insulin resistance [86]. Currently, two TZD are commercially available (rosiglitazone and pioglitazone), whereas troglitazone has been withdrawn because of hepatotoxicity. TZD-induced activation of PPAR in mesenchymatous cells promotes their differentiation into adipocytes in preference to osteoblasts. The consequent deficiency of the osteoblast number results in moderately decreased concentrations of the bone formation markers [87]. BAP and P1NP decrease (5–15%) more than OC, probably because TZD act mainly on the young forms of cells of osteoblastic lineage [88–90]. TZD showed no consistent meaningful effect on the markers of bone resorption. However, data on the effect of TZD on BTM are limited to short-term studies and the effects of TZD on the BTM and BMD are somewhat disparate, e.g. troglitazone decreased BTM levels, but not BMD [89, 90].
Long-term treatment with unfractionated heparin decreased bone formation, increased bone resorption and resulted in development of osteoporosis [91]. Low molecular weight heparins have a more subtle effect on bone—they slightly inhibit bone formation and weakly decrease BMD. However, most data concerning the effect of various heparins on bone are based on experimental studies and there are few clinical data.
Vitamin K antagonists (oral anticoagulants) inhibit gamma carboxylation of OC and increase its under-carboxylated fraction [92–95]. As gamma carboxylation of OC is important for its secretion by osteoblasts, patients treated with these drugs have decreased concentration of total OC in some [92, 95], but not all [93, 94], studies. Other BTM are not influenced by oral anticoagulants [92].
In the elderly, bone metabolism and BTM levels are strongly influenced by vitamin D and calcium status, which itself is determined by seasonal changes and nutritional factors [96]. BTM levels are markedly increased mainly in the institutionalised and home-bound vitamin D-deficient elderly, who have lower concentrations of 25OHD and higher concentrations of parathyroid hormone (PTH) than the ambulatory ones [97, 98]. 25OHD is lower and PTH and BTM levels are higher during winter [98, 99].
The above effects vary by the factor, its intensity and the BTM, e.g. the circadian rhythm has a stronger effect on CTX-I than on other BTM. The circannual rhythm of BTM levels is more pronounced in the elderly than in young persons. The effect of the fasting–eating pattern on BTMs is stronger than that of physical activity. The effect of diseases on the BTM varies according to their severity, e.g. extension of bone metastases, gravity of renal failure. Drugs vary according to their effect, which is stronger for inhibitors of aromatase and weaker for heparin, TZD or AED. Their effect on BTM depends on the dose, duration of treatment and underlying disease.
How to deal with the pre-analytical variability in the clinical practice?
Thus, as pre-analytical variability has a strong effect on BTM levels, a detailed questionnaire is necessary to assess correctly their measurements [100]. Several factors may coexist in one person. In a patient with severe rheumatoid arthritis, BTM may be determined by the disease itself, long-term corticotherapy and limited mobility. A house-bound demented elderly person may have increased BTM levels due to poor mobility, malnourishment, and low exposure to sunlight. Certain factors may influence specifically certain BTM, e.g. inhaled corticosteroids and vitamin K antagonists may decrease the OC level, but have no effect on other BTM. Certain results have to be assessed cautiously, e.g. an expected increase in the BTMs during treatment with anti-aromatases may obscure development of bone metastases.
Collection of serum and urinary samples for the reference values and in the patients should be performed under standardised conditions, preferably in the fasting state in the morning. For urinary collection, the choice between a spot (usually second morning void [SMV]) and a 24-h collection is a trade-off between the biological interest and practical reliability. Twenty-four-hour urinary collection is representative of the overall bone metabolism, but difficult to perform in a controlled way. By contrast, the spot collection may be performed easily and repeated in a controlled way and most of the reference data on urinary bone resorption markers were obtained in the SMV urines. The correlation between the SMV urines and the 24-h urinary collection is significant (r = 0.28–0.72), but varies according to the marker and the cohort investigated [101] (and our unpublished data from the OFELY cohort).
Changes in bone turnover markers levels after the menopause
In young adults, the quantity of bone formed at a BMU is equal to that removed by resorption [1]. After the menopause, activation of bone resorption increases the number of BMUs and levels of bone resorption markers [102]. A slight increase in BTM in the perimenopausal period results from the early decrease in oestrogen secretion. BTM levels increase rapidly during the menopause, then remain relatively stable throughout life [102–104]. Bone formation increases to fill in the higher number of resorption cavities, which increases serum levels of bone formation markers. However, the quantity of bone formed is lower than the quantity of bone resorbed, which results in a net bone loss at the BMU level. Bone loss in a single BMU is small and the global bone loss is determined mainly by the increased number of BMUs, which itself is the principal determinant of postmenopausal BTM levels. After the menopause, BTM levels correlate negatively with BMD (in most studies, r = −0.35 to −0.40) [102, 105–107]. The negative correlation is observed regardless of the skeletal site, BTM, time elapsed after the menopause and the use of hormone replacement therapy (HRT). Thus, these data indicate the role played by high bone turnover as a determinant of BMD in postmenopausal women.
Association between the BTM levels and the rate of bone loss
In some studies baseline bone turnover rate is correlated significantly with the subsequent bone loss [108]. This association of variable degree for different skeletal sites was found both in perimenopausal and postmenopausal women [109–111]. However, for a given level of a bone marker, there is a large scatter of individual values of bone loss (Fig. 1) [112].
Spearman rank correlations between change in BMD expressed as a percentage change per year and markers of bone turnover in 60 postmenopausal women. The graphs show significant negative correlations between markers and change in bone mineral density (BMD). \({{\% \,{\text{change}}\,{\text{in}}\,{\text{BMD}}} \mathord{\left/ {\vphantom {{\% \,{\text{change}}\,{\text{in}}\,{\text{BMD}}} {{\text{year}} = \left( {{\text{BMD}}2 - {\text{BMD}}1} \right) \times 2 \times {{100} \mathord{\left/ {\vphantom {{100} {\left( {{\text{BMD}}2 + {\text{BMD}}1} \right) \times T}}} \right. \kern-\nulldelimiterspace} {\left( {{\text{BMD}}2 + {\text{BMD}}1} \right) \times T}}}}} \right. \kern-\nulldelimiterspace} {{\text{year}} = \left( {{\text{BMD}}2 - {\text{BMD}}1} \right) \times 2 \times {{100} \mathord{\left/ {\vphantom {{100} {\left( {{\text{BMD}}2 + {\text{BMD}}1} \right) \times T}}} \right. \kern-\nulldelimiterspace} {\left( {{\text{BMD}}2 + {\text{BMD}}1} \right) \times T}}}}\). T - duration of follow-up. (Reproduced with permission from [112]
Strength of the association between the BTMs and the rate of bone loss depends largely on the accuracy of their measurements. The estimation of bone loss depends on the skeletal site, BMD precision error in comparison with the yearly bone loss, follow-up duration and number of scans per person. Yearly rate of bone loss is small in comparison with the precision error of DXA. The precision is better for the distal forearm than for the hip. At the lumbar spine, rate of bone loss also depends on the progression of lumbar arthritis. Therefore, BTMs are better correlated with bone loss at the distal radius than with that at the spine, hip or calcaneum [104, 105, 108, 113–119]. The longer the follow-up and the more BMD measurements per person (on the same device), the more accurate is the estimation of bone loss and the stronger is its correlation with the BTM levels. Assessment of the rate of bone loss on the basis of two measurements is more influenced by the measurement error [120]. However, in long follow-ups other factors can intervene and influence the BTM levels and the rate of bone loss.
In the OFELY study, BMD was measured yearly for 4 years [111]. The BTM levels correlated negatively with the rate of bone loss at the distal radius where the precision error was comparable to the yearly bone loss. Conversely, the association between the BTM levels and bone loss at the hip was weaker in the SOF study, where BMD was measured twice and the coefficient of variation of DXA was higher than the average yearly bone loss [117].
Another approach is to define thresholds of the BTM levels that identify the fast bone losers, i.e. women who have a rate of bone loss that is faster than a predefined threshold, e.g. 3% per year or the highest tertile [121]. In some cohorts, women who had BTM levels above the predefined threshold had a higher risk of being a fast bone loser [111, 122]. However, at the individual level, the increased BTM levels identified only 40–55% of the fast bone losers [111, 121, 122]. It indicates that the BTM have limited value to identify fast bone losers.
Thus, the peri- and postmenopausal acceleration of bone turnover contributes to the subsequent bone loss. However, BTM cannot be used for prediction of accelerated bone loss at an individual level in clinical practice because of these limitations.
Association between the bone turnover rate and the risk of fracture
Increased BTM levels predict fragility fractures independently of age, BMD and prior fracture. This association has been assessed prospectively in several cohort studies (Table 2) [123–132] and in case-control studies [133–137]. This association to various degrees has been found both in postmenopausal and in elderly women, in an unselected population and in osteopenic women, but not in the frail elderly in whom incident falls were the strongest predictor of fractures [123–138]. BTM levels are predictive of all fractures, vertebral fractures, hip fracture and multiple fractures. This association was found, though not consistently, for bone resorption markers [124, 125, 131]. Bone formation markers are not associated with the fracture risk, except BAP, which predicted fragility fractures in some cohorts [124, 125, 131]. By contrast, less specific BTMs (total alkaline phosphatase, HPro) did not predict fractures during a 20-year follow-up [129]. In two cohorts of women, analysis of three major predictors, i.e. BMD T-score < −2.5, BTM level > 2 SDs above the mean in premenopausal women and prior fracture showed that each of them contributed independently to the prediction of hip fracture (Fig. 2) [139]. Higher urinary CTX-I level also remained a significant predictor of hip fracture after adjustment for heel broadband ultrasound attenuation (BUA); however, combining CTX with either femoral neck BMD or heel BUA increased weakly the global predictive power for hip fracture [136]. Importantly, osteopenic women with high BTM levels are at a risk of fracture similar to that of osteoporotic women, whereas osteopenic women with normal BTMs have a low fracture risk comparable to that of postmenopausal women with normal BMD (Fig. 3) [131]. Even more interestingly, increased urinary levels of total DPD was associated with a higher risk of hip fracture in selected elderly women with normal BMD from the EPIDOS cohort [140].
Ten-year probability of hip fracture in Swedish women according to age and relative risk. The symbols show the effect of risk factors on fracture probability derived from women aged 65 years (OFELY study) or 80 years (EPIDOS study). Data from the OFELY study are derived from information on all fractures. The following threshold values were used for the risk factors: low BMD—T-score < −2.5, high CTX—above premenopausal values. (Reproduced with permission from [139])
Survival probability without fracture in 671 postmenopausal women from the OFELY cohort according to the WHO criteria for BMD. Osteoporosis was defined by a T-score at the lumbar spine or hip of ≤ −2.5; osteopenia by the lowest T-score at the lumbar spine or hip of between −2.5 and −1; normal by a T-score at the spine and total hip of > −1. Among osteopenia, women were categorised in two groups: osteopenia with one or more risk factor(s) (RF+) and osteopenia without risk factor(s) (RF−). RF = low BMD (−2.5 < lowest T score ≤ −2.0), prior fracture, high level bone turnover markers (BTM). (Reproduced with permission from [131])
Data from the pharmaceutical trials show that the BTM measures improve the identification of women who will benefit the most from the anti-osteoporotic treatment, a way to improve the cost-effectiveness of the treatment. In the Fracture Prevention Trial, fracture risk was highest in women with increased BTM levels [141]. As teriparatide (recombinant human PTH-(1–34)) decreased the fracture incidence similarly regardless of the pre-treatment bone turnover rate, the number of avoided fractures was larger and the number of women needed to treat to avoid one fracture (NNT) was smaller in women with high BTMs [132]. Alendronate reduced the risk of fracture in osteopenic women with high BTMs, but not in those with low BTMs [142]. Statistical simulations show that use of BTMs may improve the identification of osteopenic women at high risk of fracture and the cost-effectiveness of their treatment with alendronate [143]. However, these data should be confirmed in prospective studies.
Thus, BTM measurement may improve the identification of women at high risk of fracture. However, practical guidelines for the use of BTMs for the prediction of osteoporotic fractures have not been updated since the publication of the working group from the International Osteoporosis Foundation in 2000 [144].
Mechanisms of the association between bone turnover and bone fragility
Several mechanisms relate accelerated bone turnover to increased bone fragility. During the high bone turnover, net bone loss is greater and BMD is lower, consistent with the negative correlation between BTM levels and BMD after menopause [102, 104–107]. Increased bone turnover is associated with faster bone loss and deterioration of bone microarchitecture, both in the trabecular compartment (trabecular perforations, loss of entire trabeculae, deterioration of the trabecular connectivity) and in the cortical compartment (cortical thinning, increased cortical porosity) [145–148]. The endosteal bone loss is not followed by a higher periosteal apposition (at least at the non-weight bearing distal radius), which could partly offset the decrease in bone strength. Thus, women with high bone turnover may lose more bone, experience more severe deterioration of bone microarchitecture and a more severe decrease in bone strength. However, they may also have experienced a higher bone loss and greater structural weakening of bone since achievement of their peak bone mass. Thus, remaining bone may sustain higher stress (even during everyday activities), which will result in more rapid fatigue of bone tissue and the deterioration of its material mechanical properties.
Furthermore, resorption cavities, especially two resorption cavities on the opposite side of a trabecula, trigger stress risers that result in the local weakening of the trabecula [148]. High bone turnover is associated with a higher fraction of recently synthesised bone. This bone is not fully mineralised and may have suboptimal mechanical resistance [149]. Shorter periods between metabolic cycles leave less time for the enzymatic post-translational modifications of bone matrix proteins, e.g. cross-linking and β-isomerisation of collagen type I. A lower degree of cross-linking and β-isomerisation may be associated with poor mechanical properties of bone [150–152]. Interestingly, postmenopausal women with a low degree of isomerisation of type I collagen, as assessed by the alpha/beta ratio of urinary CTX excretion, are at an increased risk of fracture independently of other predictors of fracture, including the rate of bone turnover. It suggests that the quality of bone matrix, e.g. type I collagen maturation, might play a role in skeletal fragility in postmenopausal women [127].
Biochemical bone turnover markers and anti-osteoporotic treatment
Bone turnover markers play an important role in pharmaceutical trials of new anti-osteoporotic drugs. They provide information on their metabolic effect on bone turnover, help to establish the lowest dose that induces the largest change in the BTM with a given medication (decrease with anti-resorptive drugs, increase with bone formation-stimulating ones), predict the increase in BMD and the decrease in the fracture risk, are helpful in the bridging studies and, according to preliminary data, their use may improve compliance to treatment.
However, BTM present several limitations in these studies. Pre-analytical and analytical variability has been mentioned above. They make no distinction between the bone resorption and the subsequent catabolism of the products of degradation of bone collagen. However, anti-resorptive drugs can have different effects on the enzymes involved in the catabolism of bone collagen resulting in variable changes in the levels of different bone resorption markers [153]. A part of urinary free DPD is produced in the renal tubular cells [154]. This fraction depends on the mass of DPD-containing peptides released from bone and on the renal function. Changes in the BTMs reflect only partly metabolic modifications occurring during the anti-osteoporotic treatment. BTM make no distinction between the cortical and the trabecular bone; however, drugs can have different effects in both compartments. The association between the changes in BMD, BTM and the fracture risk is not fully understood. It is not clear which degree of change in the BTM levels corresponds to the optimal bone turnover level necessary for the most efficient decrease in bone fragility. For instance, alendronate induces a larger decrease in BTM levels than risedronate, but both decrease the fracture risk to a similar extent [155].
Metabolic effect of the anti-osteoporotic treatment
Changes in the BTM levels during anti-osteoporotic therapy depend on the mechanism of action of the drugs. Inhibition of bone resorption by the anti-resorptive drugs given according to a clinically relevant dose regimen is accompanied by a decrease in the bone resorption markers [156–158]. As bone formation continues in BMUs activated prior to the therapy, bone formation markers are stable for several weeks and then decrease when osteoblasts fill in the lower number of BMUs formed after the beginning of the treatment. BMD increases most rapidly during the early period, when bone resorption is reduced, whereas bone formation remains at the baseline level.
Changes in the BTMs during the anti-resorptive therapy depend on the cellular mechanism of action of the drug, its potency (degree of inhibition of bone resorption) and the route of administration. Bisphosphonates inhibit the activity of mature osteoclasts [156], monoclonal anti-RANK ligand antibody (denosumab) inhibits both the osteoclastogenesis and the activity of mature osteoclasts [159], whereas inhibitors of cathepsin K bind to the enzyme and block its activity [160]. Thus, denosumab administered subcutaneously at clinically relevant doses inhibits bone resorption faster and more strongly than alendronate administered per os (p.o.) and the cathepsin K inhibitor lowers serum CTX-I level 10 h after p.o. administration [161, 162]. Bisphosphonates, at clinically relevant doses, inhibit bone resorption and decrease BTM levels more strongly than the selective oestrogen receptor modulator (SERM) raloxifene [157, 158, 163]. When intravenously (i.v.) administered, bisphosphonates inhibit bone resorption and decrease BTM levels faster than when administered p.o. [164–166].
During bone formation-stimulating treatment (teriparatide, PTH-(1–84)), an increase in bone formation is followed by an increase in bone resorption [167–170]. BMD increases rapidly during the early phase (mainly in the trabecular sites) [167, 171]. Increase in PINP induced by teriparatide is early and large, differing significantly from baseline by day 3 and followed by other BTM (Fig. 4) [172]. PTH stimulates the early osteoblastic cells, which express type I collagen, but not yet BAP or OC [173, 174]. The mass of P1NP released into the blood is equimolar to that of collagen incorporated into the bone matrix. By contrast, only a part of other bone formation markers is released into the blood. However, after 1 month of treatment, P1NP increases by about 60–70% above baseline [168, 169], whereas bone formation rate assessed by histomorphometry increases by factors of 4–5 according to the bone envelope [175]. P1NP increases for 3–6 months and then decreases. Other BTM are elevated for 12–18 months then decrease despite a continuous increase in BMD. Thus, the relationship between the changes in BMD, BTM and bone formation assessed by histomorphometry during the bone formation-stimulating therapy is not fully understood.
Changes in biochemical bone turnover markers levels during treatment with teriparatide. Changes in P1NP differed significantly from baseline by day 3. (Reproduced with permission from [172])
Strontium ranelate slightly increases BAP and slightly decreases serum CTX-I during the initial phase of the treatment [176], then both plateau throughout treatment. That apparent dissociation between resorption and formation has been reported as reflecting the “dual” mechanism of action.
Dose-finding studies—earlier and later
Bone turnover markers are used in dose-finding studies on anti-osteoporotic drugs. The treatment-induced changes in BTM are more rapid compared with BMD. Thus, preliminary short-term studies limited to BTM can contribute to establishing the appropriate dose.
Bone resorption decreases significantly after 3 weeks of the p.o. anti-resorptive treatment. Bone resorption attains the nadir after 3–6 months of treatment and then remains stable. This decrease is followed by a decrease in the bone formation markers (significant decrease after 6 weeks, nadir after 6–12 months). The higher the dose of the drug (in the clinically relevant range), the lower the steady-state BTM level and the higher the subsequent increase in BMD. Such trends are observed for transdermal 17β-estradiol, raloxifene, alendronate, risedronate, and ibandronate (daily, weekly, monthly) [158, 177–182].
Intravenous bisphosphonates and subcutaneous denosumab induces a rapid dose-dependent decrease in bone resorption (Fig. 5) [161, 164, 183]. Duration of the low bone resorption and residual depression of bone resorption before the next injection are dose dependent, whereas dose dependency of the decrease in bone resorption at the nadir is not consistently found [161, 165, 183]. Bone formation decreases with delay, progressively and dose-dependently. The steady state is attained after 3 months for denosumab and after 6 months for bisphosphonates. The higher the dose of the drug, the lower the levels of bone resorption markers before the next injection of the drug and the higher the increase in BMD.
Serum CTX-I (upper panel) and serum osteocalcin concentrations (lower panel) as a function of time in the three groups of postmenopausal non-osteoporotic women receiving either 1 mg (squares) or 2 mg of intravenous (triangles) ibandronate or no treatment (diamonds). The values are given as mean (±1 SEM) % of baseline values. (Reproduced with permission from [166])
The increase in BMD is proportionate to the lowest steady-state levels of BTM for the oral or transdermal anti-resorptive drugs and to the residual decrease in bone resorption markers before the next injection for the intravenous drugs. Bone resorption level at the nadir is predictive of the increase in BMD for the intravenous bisphosphonates.
Similarly, during treatment with the bone formation-stimulating parathyroid hormone, parallel dose-dependent increases in BMD and BTM levels have also been observed, especially for BMD of lumbar spine and serum concentrations of bone formation markers [170].
A rapid decrease in the BTMs was observed with conventional doses of hormone replacement therapy (HRT), e.g. 1.0 mg/day 17β-estradiol or 0.625 mg/day conjugated equine oestrogen (CEE), which decrease the risk of fracture [184]. Interestingly, treatment with a low dose of 17β-estradiol or CEE decreased BTM levels and increased BMD in a similar manner to a higher dose with minimal adverse effects [185–187].
Decrease in BTM levels and anti-fracture efficacy of anti-resorptive treatment
The increase in BMD induced by anti-resorptive treatment has been perceived as a valid surrogate of their anti-fracture efficacy. However, there is a discrepancy between the magnitude of BMD increase (2% at 3 years for raloxifene and 6–8% for bisphosphonates) and their efficacy in reducing vertebral fracture risk. The association between changes in BMD induced by anti-resorptive therapy and their anti-fracture efficacy is significant but weak in meta-regression based on summary statistics of the pharmaceutical trials [188]. This association usually disappears when assessed on the basis of individual patient data [189]. In addition, for any change in BMD in comparison with baseline, the fracture risk is lower in the treated than in the placebo group [190]. Interestingly, women who lost up to 4% of BMD compared with baseline during the treatment with alendronate had a significant reduction in the vertebral fracture risk [191]. These data show that BMD is a poor surrogate parameter of the anti-fracture efficacy of the anti-resorptive treatment.
By contrast, the early decrease in the BTM levels is associated with a long-term anti-fracture efficacy of the anti-resorptive agent (Fig. 6) [192–196]. This association was found between the decrease in the spine fracture risk and the decrease in the serum BAP, P1NP and CTX-I levels in patients treated with alendronate [192], between the decrease in the spine fracture risk and the decrease in the urinary NTX-I and CTX-I levels in patients treated with risedronate [193, 194], and between the decrease in the spine fracture incidence and the decrease in the serum levels of OC, BAP and P1NP in patients treated with raloxifene [195, 196]. Moreover, in the alendronate-treated women, the fall in the BAP level was also predictive of the decrease in the incidence of the non-spine and hip fractures [192]. Similarly, during treatment with risedronate, a higher decrease in the urinary CTX-I and NTX-I was associated with a larger decrease in the incidence of the vertebral fractures [193].
One-year change in bone alkaline phosphatase and hip fracture risk among alendronate-treated women. Percentage change in bone alkaline phosphatase and predicted risk (log OR) of hip fracture (solid line) and 95% CI (dotted lines) from the logistic regression model. (Reproduced with permission from [194])
In the risedronate-treated women, the association between the decrease in the fracture risk and that in the BTM levels was similar for the decrease in the BTM level compared with baseline and for the BTM level during treatment [194]. For a given decrease in BTM levels and for a given BTM level during treatment, fracture incidence was similar in the active treatment and in the placebo-treated group [193–196]. Women with the greatest decrease in BTM levels had the lowest fracture incidence, whereas in women who had stable BTM, fracture incidence was similar to that in the placebo group [195, 196]. Thus, the rate of bone turnover and its change may be important determinants of the risk of fracture.
The association between the decrease in the BTM levels and the anti-fracture effect is found for spine fractures and for women with low baseline BMD [192]. This is plausible because spine fractures and fractures in individuals with very low BMD depend more on skeletal fragility, including the rate of bone turnover. By contrast, non-spine fractures and fractures in women with higher BMD may depend rather on the frequency and intensity of traumas.
BTM levels and anti-fracture efficacy of bone formation-stimulating treatment
Data on the relationship between changes in BTMs and anti-fracture efficacy during the bone formation-stimulating treatment are limited. A teriparatide-induced increase in BTM levels correlated positively with the later increase in BMD [168, 197]. As the number of BMUs was higher in the trabecular bone, the increase in volumetric BMD in the trabecular compartment was higher and correlated more strongly with the increase in the BTM levels [198]. However, the number of incident fractures in clinical trials is too low to assess a potential association between BTM changes and fracture risk during treatment with parathyroid hormone.
Changes in the bone turnover after discontinuation of the anti-osteoporotic treatment
Changes in the BTM levels after withdrawal of the anti-osteoporotic medications are related to the subsequent changes in BMD and probably to the decrease in anti-fracture efficacy.
Hormone replacement therapy (HRT) is active during its administration, is not accumulated in bone tissue and there is no residual effect of HRT on the bone turnover after the treatment has been stopped. Withdrawal of HRT results in a rapid increase in the BTM levels, which, after 6–12 months, returns to the pre-treatment level (Fig. 7) [199–202]. The increase in the BTM levels is followed by a significant decrease in BMD at most skeletal sites [199–202]. Some studies show a rapid increase in the BTM levels and accelerated bone loss directly after discontinuation of HRT that plateau thereafter [199, 201]. The changes in BTMs and BMD are faster than in postmenopausal women receiving placebo. These data suggest that the discontinuation of HRT mimics the early postmenopausal period. They are consistent with a decrease in the anti-fracture efficacy after withdrawal of HRT [203].
Serum CTX levels after HRT withdrawal over 3 years (white circles) and over the first 3 years from baseline in the group of women continuing with HRT (crosses), in the untreated early postmenopausal group (triangles), and in the untreated age-matched group (black circles). Results are shown as mean (SEM). (Reproduced with permission from [200])
By contrast, bisphosphonates have a strong affinity to bone, are accumulated in bone tissue, not metabolised and are released from the bone very slowly with estimated terminal half-lives of 1–10 years, presumably as a consequence of bone remodelling. It is assumed that after their discontinuation, osteoclasts are inhibited only by the drug released from the bone matrix. Consequently, the increase in the BTM and the decrease in BMD after withdrawal of the bisphosphonate appear to depend on its affinity with hydroxyapatite, the cumulative dose, the drug potency and the residual bone turnover [165, 201, 204–215].
A short treatment with 30 mg of risedronate daily for 2 weeks inhibited bone resorption [204]. Its withdrawal resulted in a rapid return to baseline, probably due to the low amount of the accumulated bisphosphonate. Ibandronate administered for 9–12 months inhibited bone turnover dose-dependently [165, 205]. After its withdrawal, bone resorption returned to the initial level followed by bone formation. The lower the cumulative dose of the drug, the sooner the BTM returned to baseline [205]. Withdrawal of risedronate after 3 years of treatment was followed by an increase in the BTM levels to baseline after 1 year [206].
After withdrawal of the long-term treatment with alendronate, BTM levels increase slightly and BMD decreases [178, 201, 207–208]. BTM levels increase slowly, with a plateau achieved 1 year after discontinuation of the treatment. However, even 5 years after withdrawal of the 5-year therapy, BTM do not attain the initial levels [207–210]. It is assumed that, after treatment withdrawal, osteoclasts resorb more bone; however, alendronate released from bone may inhibit osteoclasts, limiting the increase in the BTM levels. This pattern may explain why, after the withdrawal of alendronate, BTM increase less and BMD decreases more slowly than after withdrawal of HRT [199, 201].
Conclusions concerning the changes in the BTM levels in the long-term studies are limited by the methodological problems, such as the long storage of samples. Comparisons between the drugs from the same group are limited by different duration of treatment in various protocols. However, both after withdrawal of the 2-year and 4-year treatment with alendronate and after discontinuation of the 3-year treatment with risedronate, BTM returned to baseline (or to the level in the placebo group) after 1 year [199, 201, 206].
Data on the changes in the BTM after withdrawal of PTH are scanty. In a study by Black et al., withdrawal of PTH-(1–84) after 1 year of treatment was followed by a fall in the BTM levels to baseline [213]. The slowdown of bone turnover was accompanied by a decrease in the volumetric BMD of the trabecular bone. However, the BTM levels decreased after 12–18 months of treatment, despite continued administration of PTH. To our knowledge, none of the studies compared changes in the BTM levels after withdrawal of PTH and during long-term treatment with PTH.
Combination therapy and bone turnover markers
Three designs combining bisphosphonates and bone formation-stimulating therapy have been studied in postmenopausal women: both drugs administered jointly, anti-resorptive treatment followed by PTH, and PTH followed by the anti-resorptive treatment.
Alendronate and PTH-(1–84), administered jointly, rapidly decreased bone resorption (serum CTX-I) [214]. After 1 year, CTX-I was 50% lower than at baseline, much lower than in the PTH-(1–84)-treated group and higher than in the alendronate-treated group. Alendronate and PTH-(1–84) slightly increased bone formation (P1NP, BAP) during the first weeks of therapy, reflecting the direct effect of PTH-(1–84) on osteoblasts. Then, bone formation decreased and was slightly below the baseline levels. The time course of BTM levels during combination therapy was more strongly determined by alendronate consistent with the similar changes in BMD in the combination therapy group and in the group receiving alendronate alone.
During PTH treatment after anti-resorptive treatment, changes in the BTM levels depend on the degree of inhibition of bone turnover. After a strong suppression of bone resorption by alendronate, the increase in BTM levels induced by PTH-(1−34) was delayed and of lesser magnitude than after raloxifene therapy [215]. After long-term treatment with risedronate, BTM were higher and teriparatide-induced increases in BTMs were significantly greater and associated with a greater increase in bone volume (measured by QCT) than after long-term treatment with alendronate [216].
Alendronate administered after PTH-(1–84) decreases BTM levels that fall below the baseline values and are indistinguishable from those in the women treated with alendronate alone [213, 217]. This strong inhibition of bone turnover may prevent the resorption of the bone built under PTH-(1–84) treatment and prolongs its secondary mineralisation, which results in an additional increase in BMD. The increase in PINP after 1 month of PTH-(1–84) treatment was predictive of the increase in BMD after 24 months of sequential treatment [218].
Some studies compared HRT with the combination of bisphosphonate and HRT. Both alendronate and risedronate administered jointly with HRT decreased bone formation and bone resorption markers more than HRT alone [219–221].
Few studies assessed regimens combining oestrogens with progestins or androgens. The effect of progestins are generally weak. Changes in BTM levels induced by 17β-estradiol (17βE2) and levonorgestrel were similar to those induced by 17βE2 alone [222]. Changes in the BTM levels induced by medroxyprogesterone acetate with oestrogens (17βE2 or CEE) were similar to those induced by the respective oestrogen formulation alone [187, 223]. Postmenopausal women treated with 17βE2 and dydrogesterone had higher urinary DPD excretion, but lower serum concentrations of OC and P1CP [224]. Compared with CEE alone, the combination therapy of CEE with androgen (methyltestosterone) had no additional effect on bone resorption (DPD, HPro) [225]. By contrast, bone formation (OC, BAP, P1CP) was significantly higher in the women receiving the combination of CEE and methyltestosterone.
Potential utility of bone turnover markers for bridging studies
According to the Committee of Proprietary Medicinal Products of the European Agency for Evaluation of Medicines,
“For compounds having demonstrated anti-fracture efficacy and for which the indication ‘treatment of osteoporosis’ was granted for a specific dose, formulation or route of administration, an extension of the indication ‘treatment of osteoporosis’ could be given for a new dose, formulation or route of administration, on the basis of the demonstration of an equivalence in terms of BMD changes between the original and the new doses, formulations or routes of administration in a study of a minimum 2 years”
[226]. The aim of such studies (bridging studies), is to show that a new regimen is not less effective than a reference regimen through the use of a surrogate marker. BTMs are of interest because the treatment-induced reduction of the fracture risk and changes in BTM levels are correlated.
Examples of bridging studies include those comparing once-weekly vs daily oral regimens of bisphosphonates. Both regimens of alendronate and risedronate induced similar decreases in BTM levels and increases in BMD [227–229]. Monthly and intermittent oral ibandronate were at least as effective as the daily regimen with regard to both BMD and BTM levels [230–232]. The same concept has been used successfully to gain approval for once-weekly and once-monthly dosing regimens of bisphosphonates.
Comparison of BTM levels during oral and intravenous treatment or for different intravenous doses is more complex. Intravenous treatment rapidly decreases the bone resorption rate [161, 165, 166]. The degree and the duration of this decrease are dose dependent. The decrease in bone formation starts later and is progressive. The temporal pattern of BTM changes should repeat those observed with the original regimen in order to induce the same effect on BMD and bone strength [226]. It has been suggested that:
“The magnitude of the reduction of the rate of bone resorption at the end of the drug- free interval rather than the pattern of its fluctuation following administration of bisphosphonate determine antifracture efficacy, provided that these fluctuations occur within the range of values of premenopausal women”
[233].
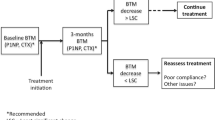
Association between BTM levels and adherence with anti-resorptive treatment
Low compliance is a serious problem during anti-osteoporotic treatment, limiting the decrease in the fracture risk [234]. BTM can be used to assess compliance because of rapid and considerable changes during the treatment. Analyses from the IMPACT study show that BTM change reflects the degree of compliance to risedronate therapy in osteoporotic women [235]. The better the compliance, the greater the average decrease in bone turnover.
Measurement of the BTM levels improved adherence to the treatment with risedronate regardless of the result, whereas the persistence (risk of treatment discontinuation) depended on the result of the BTM measurement [236, 237]. The persistence was better in women who received positive information corresponding to a decrease in the NTX-I level exceeding the predefined threshold [207, 208]. By contrast, risk of discontinuation was higher (lower persistence) in women who received negative information (lack of predefined decrease in the NTX-I level, an increase in NTX-I excretion of more than 30%). Such an association was not found in women who had not been informed about the change in the NTX-I level. Thus, measurement of BTM can improve the persistence with anti-resorptive treatment and is not only a method of identification of the non-compliant patients. Obviously, in clinical practice, women who do not have the expected decrease in the BTM levels need an individual approach. These data need to be confirmed before BTM can be recommended in the clinical monitoring of patients receiving anti-osteoporotic treatment.
Areas of investigation
Two principal areas of investigation of BTM are pathophysiology of bone metabolism and the relationship between bone turnover and bone fragility, especially during the different strategies of the anti-osteoporotic treatment.
Bone turnover markers could be useful in making a distinction between the number and the activity of bone cells. TRACP5b reflects the osteoclast number and total DPD reflects the mass of resorbed bone. However, it is uncertain if their ratio reflects the activity of osteoclasts. No data are available for osteoblasts (markers of the osteoblast number, markers of the mass of synthesised bone matrix). We need more BTM reflecting the action of enzymes involved in the catabolism of bone matrix, e.g. cathepsin K, metalloproteinases. They would be useful to assess enzymatic mechanisms of bone matrix degradation and the efficacy of drugs acting on certain metabolic pathways, e.g. cathepsin K inhibitors. We need markers reflecting structural aspects of bone matrix related to its strength. Such potential candidates are the isomerised or racemised forms of CTX-I and bone-specific advanced glycation end-products.
The relationship between the change in the BTM levels and the decrease in the fracture risk needs more study. What is the proportion of the anti-fracture effect explained by the fall in the BTM level? How should the BTM levels be expressed in relation to the anti-fracture efficacy during the anti-osteoporotic treatment (absolute value during the treatment, decrease relative to baseline)? Are BTM equally predictive of vertebral and non-vertebral fracture incidence? Is the lack of decrease in BTM levels during anti-resorptive therapy indicative of a lack of anti-fracture efficacy? BTM may help to understand the clinical relevance (if any) of the “frozen bone” attributed to the potent bisphosphonates. Are the changes in BTM levels induced by the bone formation-stimulating drugs related to their anti-fracture efficacy? Data on the use of BTM to improve compliance during anti-osteoporotic treatment are preliminary and limited to risedronate. We need more data, especially long-term studies and studies concerning other anti-osteoporotic medications.
Bone turnover markers—clinical studies versus clinical practice: a summary
Bone turnover markers are a useful tool in clinical studies on osteoporosis. They show that the increased bone turnover rate is associated with a higher fracture risk. Conversely, its decrease induced by anti-resorptive drugs is associated with a decrease in the risk of fracture. Thus, BTM levels reflect the relationship among the bone turnover rate, bone fragility and the effect of anti-resorptive treatment, but they cannot explain the pathophysiological mechanism of this association. Increased BTM levels are associated with faster bone loss, but this phenomenon has not been found consistently. In pharmaceutical studies BTM provide a lot of data on the effect of medications on bone turnover; in particular, they help to understand the effect of oral vs intravenous anti-resorptive therapy, of combination and sequential therapies. They also help to understand changes in the BTM and BMD after discontinuation of the treatment. From the economic point of view, preliminary estimations suggest that the use of BTMs may improve the cost-effectiveness of anti-osteoporotic treatment in women. They can be useful for bridging studies, which may decrease the costs of the evaluation of the more convenient formulations of anti-osteoporotic drugs.
By contrast, the practical use of BTM in the clinical management of osteoporosis is very limited. Practical guidelines were established for the use of BTM to predict the increase in BMD during anti-resorptive therapy [238]. However, interest in them decreased because the change in BMD during anti-resorptive treatment poorly predicts the change in fracture risk. BTM levels are influenced by several factors that are more or less easy to control. In order to limit pre-analytical variability as much as possible, biological samples should be obtained in a standardised way, e.g. for serum BTM, in the fasting state in the morning. Urinary measurements and their reference values should be obtained under standardised conditions, e.g. during the second morning void. BTM measurements may help to exclude other bone pathologies characterised by high bone turnover, e.g. Paget’s disease, osteomalacia, multiple myeloma, bone metastases. Results of BTM should be interpreted cautiously in patients with determinants of pre-analytical variability, e.g. in patients who sustained a fracture several months before biological examination. BTM cannot be used for the prediction of the accelerated bone loss in postmenopausal women (nor in any other group investigated until now). Epidemiological studies suggest that BTM measurements may help to detect postmenopausal women at high risk of fracture. However, adequate practical guidelines are still lacking.
Concerning the anti-osteoporotic treatment, there are no data suggesting that BTM may help to choose the appropriate anti-osteoporotic treatment. It is assumed that BTM measurements during anti-resorptive therapy help to identify non-compliers. Preliminary data show that BTM measurements may improve adherence to anti-resorptive treatment and the fall in BTM levels exceeding the predefined threshold (proof of therapeutical efficacy) may improve persistence with anti-resorptive treatment. Although the lack of compliance is probably the most frequent reason for the lack of decrease in the BTM during treatment, other causes should be analysed in an individual patient, e.g. circadian variation (mainly CTX-I), seasonal variation (mainly in the elderly), analytical variability (measurement error, change in the immunoassay), rapid development of another metabolic bone disease (multiple myeloma, bone metastases). Currently, there are no guidelines concerning the use of BTM in monitoring anti-osteoporotic therapy. Thus, the practical use of BTM in the clinical management of postmenopausal osteoporosis still remains an open area for investigation.
References
Dempster DW (2006) Anatomy and functions of the adult skeleton. In: Favus MJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism. American Society for Bone and Mineral Research, Washington, DC, pp 7–11
Garnero P, Delmas PD (2001) Biochemical markers of bone turnover in osteoporosis. In: Marcus M, Feldman D, Kelsey J (eds) Osteoporosis, vol 2. Academic Press, New York, pp 459–477
Delmas PD, Demiaux B, Malaval L, Chapuy MC, Edouard C, Meunier PJ (1986) Serum bone gamma carboxyglutamic acid-containing protein in primary hyperparathyroidism and in malignant hypercalcemia comparison with bone histomorphometry. J Clin Invest 77:985–991
Ureña P, Hruby M, Ferreira A, Ang KS, de Vernejoul MC (1996) Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol 7:506–512
Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J (1993) Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J Bone Miner Res 8:127–132
Eastell R, Delmas PD, Hodgson SF, Eriksen EF, Mann KG, Riggs BL (1988) Bone formation rate in older normal women: concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J Clin Endocrinol Metab 67:741–748
Charles P, Poser JW, Mosekilde L, Jense FT (1985) Estimation of bone turnover evaluated by 47Ca-kinetics. Efficiency of serum bone gamma-carboxyglutamic acid-containing protein, serum alkaline phosphatase, and urinary hydroxyproline excretion. J Clin Invest 76:2254–2258
Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Väänänen HK (2006) Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 52:499–509
Roux JP, Arlot ME, Gineyts E, Meunier PJ, Delmas PD (1995) Automatic-interactive measurement of resorption cavities in transiliac bone biopsies and correlation with deoxypyridinoline. Bone 17:153–156
Eastell R, Colwell A, Hampton L, Reeve J (1997) Biochemical markers of bone resorption compared with estimates of bone resorption from radiotracer kinetic studies in osteoporosis. J Bone Miner Res 12:59–65
Denk E, Hillegonds D, Hurrell RF et al (2007) Evaluation of 41calcium as a new approach to assess changes in bone metabolism: effect of a bisphosphonate intervention in postmenopausal women with low bone mass. J Bone Miner Res 22:1518–1525
Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C (2002) Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 31:57–61
Eastell R, Simmons PS, Colwell A et al (1992) Nyctohemeral changes in bone turnover assessed by serum bone Gla-protein concentration and urinary deoxypyridinoline excretion: effects of growth and ageing. Clin Sci 83:375–382
Wichers M, Schmidt E, Bidlingmaier F, Klingmüller D (1999) Diurnal rhythm of crosslaps in human serum. Clin Chem 45:1858–1860
Gertz BJ, Clemens JD, Holland SD, Yuan W, Greenspan S (1998) Application of a new serum assay for type I collagen cross-linked N-telopeptides: assessment of diurnal changes in bone turnover with and without alendronate treatment. Calcif Tissue Int 63:102–106
Aoshima H, Kushida K, Takahashi M et al (1998) Circadian variation of urinary type I collagen crosslinked C-telopeptide and free and peptide-bound forms of pyridinium crosslinks. Bone 22:73–78
Bollen AM, Martin MD, Leroux BG, Eyre DR (1995) Circadian variation in urinary excretion of bone collagen cross-links. J Bone Miner Res 10:1885–1890
Schlemmer A, Hassager C (1999) Acute fasting diminishes the circadian rhythm of biochemical markers of bone resorption. Eur J Endocrinol 140:332–337
Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30:886–890
Henriksen DB, Alexandersen P, Bjarnason NH et al (2003) Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res 18:2180–2189
Brooke-Wavell K, Jones PRM, Hardman AE, Tsuritani I, Yamada Y (2001) Commencing, continuing and stopping brisk walking: effects on bone mineral density, quantitative ultrasound of bone and markers of bone metabolism in postmenopausal women. Osteoporos Int 12:581–587
Hla MM, Davis JW, Ross PD, Yates AJ, Wasnich RD (2001) The relation between lifestyle factors and biochemical markers of bone turnover among early postmenopausal women. Calcif Tissue Int 68:291–296
Suleiman S, Nelson M, Li F, Buxton-Thomas M, Moniz C (1997) Effect of calcium intake and physical activity level on bone mass and turnover in healthy, white, postmenopausal women. Am J Clin Nutr 66:937–943
Seibel MJ, Gartenberg F, Silverberg SJ, Ratcliffe A, Robins SP, Bilezikian JP (1992) Urinary hydroxypyridinium cross-links of collagen in primary hyperparathyroidism. J Clin Endocrinol Metab 74:481–486
Alvarez L, Guanabens N, Peris P et al (1995) Discriminative value of biochemical markers of bone turnover in assessing the activity of Paget's disease. J Bone Miner Res 10:458–463
Garnero P, Gineyts E, Schaffer AV, Seaman J, Delmas PD (1998) Measurement of urinary excretion of nonisomerized and beta-isomerized forms of type I collagen breakdown products to monitor the effects of the bisphosphonate zoledronate in Paget's disease. Arthritis Rheum 41:354–360
Pantazi H, Papapetrou PD (2000) Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab 85:1099–1106
Siddiqi A, Burrin JM, Noonan K et al (1997) A longitudinal study of markers of bone turnover in Graves’ disease and their value in predicting bone mineral density. J Clin Endocrinol Metab 82:753–759
Parkinson C, Kassem M, Heickendorff L, Flyvbjerg A, Trainer PJ (2003) Pegvisomant-induced serum insulin-like growth factor-I normalization in patients with acromegaly returns elevated markers of bone turnover to normal. J Clin Endocrinol Metab 88:5650–5655
Kristo C, Jemtland R, Ueland T, Godang K, Bollerslev J (2006) Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing’s syndrome: a prospective, long-term study. Eur J Endocrinol 154:109–118
Chiodini I, Carnevale V, Torlontano M et al (1998) Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: study in eumenorrheic patients with Cushing’s syndrome. J Clin Endocrinol Metab 83:1863–1867
Alaxandrakis MG, Passam FH, Malliaraki N, Katachanakis C, Kyriakou DS, Margioris N (2002) Evaluation of bone disease in multiple myeloma: a correlation between biochemical markers of bone metabolism and other clinical parameters in untreated multiple myeloma patients. Clin Chim Acta 325:51–57
Corso A, Arcaini L, Mangiacavalli S et al (2001) Biochemical markers of bone disease in asymptomatic early stage multiple myeloma. A study on their role in identifying high risk patients. Haematologica 86:394–398
Colao A, di Somma C, Pivonello R et al (1999) Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab 84:1919–1924
Mizunashi K, Furukawa Y, Miura R, Yumita S, Sohn HE, Yoshinaga K (1988) Effects of active vitamin D3 and parathyroid hormone on the serum osteocalcin in idiopathic hypoparathyroidism and pseudohypoparathyroidism. J Clin Invest 82:861–865
Engler H, Oettli RE, Riesen WF (1999) Biochemical markers of bone turnover in patients with thyroid dysfunctions and in euthyroid controls: a cross-sectional study. Clin Chim Acta 289:159–172
White HD, Ahmad AM, Durham BH et al (2006) Effect of active acromegaly and its treatment on parathyroid circadian rhythmicity and parathyroid target-organ sensitivity. J Clin Endocrinol Metab 91:913–919
Voorzanger-Rousselot N, Juillet F, Mareau E, Zimmermann J, Kalebic T, Garnero P (2006) Association of 12 serum biochemical markers of angiogenesis, tumour invasion and bone turnover with bone metastases from breast cancer: a cross-sectional and longitudinal evaluation. Br J Cancer 95:506–514
Leeming DJ, Koizumi M, Byrjalsen I, Li B, Qvist P, Tanko LB (2006) The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol Biomarkers Prev 15:32–38
Cloos PA, Lyubimova N, Solberg H et al (2004) An immunoassay for measuring fragments of newly synthesized collagen type I produced during metastatic invasion of bone. Clin Lab 50:279–289
Costa L, Demers LM, Gouveia-Oliveira A et al (2002) Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol 20:850–856
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935
Chen JS, Cameron ID, Cumming RG et al (2006) Effect of age-related chronic immobility on markers of bone turnover. J Bone Miner Res 21:324–331
Nilsson K, Gustafson L, Isaksson A, Hultberg B (2005) Plasma homocysteine and markers of bone metabolism in psychogeriatric patients. Scand J Clin Lab Invest 65:671–680
Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K (2005) Abnormal bone and calcium metabolism in immobilized Parkinson’s disease patients. Mov Disord 20:1598–1603
Sato Y, Asoh T, Oizumi K (1998) High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone 23:555–557
Sato Y, Oizumi K, Kuno H, Kaji M (1999) Effect of immobilization upon renal synthesis of 1,25-dihydroxyvitamin D in disabled elderly stroke patients. Bone 24:271–275
Sato Y, Kuno H, Kaji M, Ohshima Y, Asoh T, Oizumi K (1998) Increased bone resorption during the first year after stroke. Stroke 29:1373–1377
Iwamoto J, Takeda T, Ichimura S (2001) Relationships between physical activity and metacarpal cortical bone mass and bone resorption in hemiplegic patients. J Orthop Sci 6:227–233
Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ (2007) Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res 22:1155–1164
Stoffel K, Engler H, Kuster M, Riesen W (2007) Changes in biochemical markers after lower limb fractures. Clin Chem 53:131–134
Obrant KJ, Ivaska KK, Gerdhem P, Alantalo SL, Pettersson K, Väänänen HK (2005) Biochemical markers of bone turnover are influenced by recently sustained fracture. Bone 36:786–792
Lems WF, van Veen GJM, Gerrits MI et al (1998) Effect of low-dose prednisone (with calcium and calcitriol supplementation) on calcium and bone metabolism in healthy volunteers. Br J Rheumat 37:27–33
Ton FJ, Gunawardene SC, Lee H, Neer RM (2005) Effects of low-dose prednisone on bone metabolism. J Bone Miner Res 20:464–470
Richy F, Bousquet J, Ehrlich GE et al (2003) Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int 14:179–190
Scanlon PD, Connett JE, Wise RA et al (2004) Loss of bone density with inhaled triamcinolone in Lung Health Study. Am J Resp Crit Care Med 170:1302–1309
Fardon TC, Lee DKC, Haggart K, McFarlane LC, Lipworth BJ (2004) Adrenal suppression with dry powder formulations of fluticasone propionate and mometasone furoate. Am J Resp Crit Care Med 170:960–966
Martin RJ, Szefler SJ, Chinchilli VM et al (2002) Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Resp Crit Care Med 165:1377–1383
Egan JJ, Maden C, Kalra S, Adams JE, Eastell R, Woodcock AA (1999) A randomized, double-blind study comparing the effects of beclomethasone and fluticasone on bone density over two years. Eur Resp J 13:1267–1275
Malerba M, Bossoni S, Radaeli A et al (2005) Growth hormone response to growth hormone-releasing hormone is reduced in adult asthmatic patients receiving long-term inhaled corticosteroid treatment. Chest 127:515–521
Fujita K, Kasayama S, Hashimoto J et al (2001) Inhaled corticosteroids reduce bone mineral density in early postmenopausal but not premenopausal asthmatic women. J Bone Miner Res 16:782–787
Hall GM, Spector TD, Delmas PD (1995) Markers of bone metabolism in postmenopausal women with rheumatoid arthritis. Arthritis Rheum 38:902–906
Pearce G, Ryan PFJ, Delmas PD, Tabensky DA, Seeman E (1998) The deleterious effects of low-dose corticosteroids on bone density in patients with polymyalgia rheumatica. Br J Rheum 37:292–299
Brufsky A, Harker WG, Beck JT et al (2007) Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol 25:829–836
Gonnelli S, Cadirni A, Caffarelli C et al (2007) Changes in bone turnover and in bone mass in women with breast cancer switched from tamoxifen to exemestane. Bone 40:205–210
Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD (2007) Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone 41:346–352
Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE (2006) Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the anastrozole, tamoxifen, alone or in combination (ATAC) trial (18233230). J Bone Miner Res 21:1215–1223
Fitzpatrick LA (2004) Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav 5:S3–S15
Pack AM, Morrell MJ (2004) Epilepsy and bone health in adults. Epilepsy Behav 5:S24–S29
Pack A, Shane E, McMahon D, Randall A, Morell M (2007) Antiepileptic drugs affect bone loss via reproductive hormones. J Bone Miner Res 22 [Suppl 1]:S467, Abstract W415
Mintzer S, Boppana P, Toguri J, DeSantis A (2006) Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine and oxcarbazepine. Epilepsia 47:510–515
Pack AM, Morrell MJ, Marcus R et al (2005) Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol 57:252–257
Verrotti A, Greco R, Morgese G, Chiarelli F (2000) Increased bone turnover in epileptic patients treated with carbamazepine. Ann Neurol 47:385–388
Lau KHW, Nakade O, Barr B, Taylor AK, Houchin K, Baylink DJ (1995) Phenytoin increases markers of osteogenesis for human species in vitro and in vivo. J Clin Endocrinol Metab 80:2347–2353
Kim SH, Lee JW, Choi KG, Chung HW, Lee HW (2007) A 6-month longitudinal study of bone mineral density with antiepileptic drug monotherapy. Epilepsy Behav 10:291–295
Sato Y, Kondo I, Ishida S et al (2001) Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology 57:445–449
Toh S, Hernandez-Diaz S (2007) Statins and fracture risk. A systematic review. Pharmacoepidemiol Drug Saf 16(6):627–640
Bone HG, Kiel DP, Lindsay RS et al (2007) Effects of atorvastatin on bone in postmenopausal women with dyslipidemia: a double-blind, placebo-controlled, dose-ranging trial. J Clin Endocrinol Metab 92:4671–4677
Berthold HK, Unverdorben S, Zittermann A et al (2004) Age-dependent effects of atorvastatin on biochemical bone turnover markers: a randomized controlled trial in postmenopausal women. Osteoporos Int 15:459–467
Braatvedt GD, Bagg W, Gamble G, Davidson J, Reid IR (2004) The effect of atorvastatin on markers of bone turnover in patients with type 2 diabetes. Bone 35:766–770
Rejnmark L, Buus NH, Vestergaard P et al (2004) Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res 19:737–744
Rosenson RS, Tangney CC, Langman CB, Parker TS, Mevine DM, Gordon BR (2005) Short-term reduction in bone markers with high-dose simvastatin. Osteoporos Int 16:1272–1276
Montagnani A, Gonnelli S, Cepollaro C et al (2003) Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone 32:427–433
Mostaza JM, de la Piedra C, Curiel MD, Pena R, Lahoz C (2001) Pravastatin therapy increases procollagen I N-terminal propeptide (PINP), a marker of bone formation in post-menopausal women. Clin Chim Acta 308:133–137
Ho-Ming Chan M, Wing-Lai M, Wai-Kwun Chiu R, Chow CC, Hiu-Shuen I, Wai-Kei Lam C (2001) Simvastatin increases serum osteocalcin concentration in patients treated for hypercholesterolaemia. J Clin Endocrinol Metab 86:4556–4559
Yki-Jarvinen H (2004) Thiazolidinediones. N Engl J Med 351:1106–1118
Grey A, Bolland M, Gamble G et al (2007) The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92:1305–1310
Berberoglu Z, Gursoy A, Bayraktar N, Yazici AC, Tutuncu NB, Demirag NG (2007) Rosiglitazone decreases serum bone-specific alkaline phosphatase activity in postmenopausal diabetic women. J Clin Endocrinol Metab 92:3523–3530
Watanabe S, Takeuchi Y, Fukumoto S, Fujita H, Nakano T, Fujita T (2003) Decrease in serum leptin by troglitazone is associated with preventing bone loss in type 2 diabetic patients. J Bone Miner Metab 21:166–171
Okazaki R, Miura M, Toriumi M et al (1999) Short-term treatment with troglitazone decreases bone turnover in patients with type 2 diabetes mellitus. Endocr J 46:795–801
Rajgopal R, Bear MK, Butcher MK, Shaugnessy SG (2007) The effects of heparin and low molecular weight heparins on bone. Thromb Res http://dx.doi.org/10.1016/j.thromres.2006.10.025
Knapen MHJ, Hellemons-Boode BSP, Langenberg-Ledeboer M et al (2000) Effect of oral anticoagulant treatment on markers for calcium and bone metabolism. Haemostasis 30:290–297
Obrant KJ, Käkönen SM, Astermark J et al (1999) The proportion of carboxylated to total or intact osteocalcin in serum discriminates warfarin-treated patients from control subjects. J Bone Miner Res 14:555–560
Menon RK, Gill DS, Thomas M, Kernoff PBA, Dandona P (1987) Impaired carboxylation of osteocalcin in warfarin-treated patients. J Clin Endocrinol Metab 64:59–61
Pietschmann P, Woloszczuk W, Panzer S, Kyrle P, Smolen J (1988) Decreased serum osteocalcin levels in phenprocoumon-treated patients. J Clin Endocrinol Metab 66:1071–1074
Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ (1996) Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. J Clin Endocrinol Metab 81:1129–1133
Theiler R, Stähelin HB, Kräzlin M, Tyndall A, Bischoff HA (1999) High bone turnover in the elderly. Arch Phys Med Rehabil 80:485–489
Theiler R, Stähelin HB, Kräzlin M et al (2000) Influence of physical mobility and season on 25-hydroxyvitamin D-parathyroid hormone interaction and bone remodelling in the elderly. Eur J Endocrinol 143:673–679
Rapuri PB, Kinaymu HK, Gallagher JC, Haynatzka V (2002) Seasonal changes in calciotropic hormones, bone markers, and bone mineral density in elderly women. J Clin Endocrinol Metab 87:2024–2032
Hannon R, Eastell R (2000) Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int 11 [Suppl 6]:30–44
Smith SM, Dillon EL, DeKerlagand DE, Davis-Street JE (2004) Variablity of collagen crosslinks: impact of sample collection period. Calcif Tissue Int 74:336–341
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Garnero P, Mulleman D, Munoz F, Sornay-Rendu E, Delmas PD (2003) Long-term variability of markers of bone turnover in postmenopausal women and implications for their clinical use: the OFELY study. J Bone Miner Res 18:1789–1794
Chaki O, Yoshikata I, Kikuchi R et al (2000) The predictive value of biochemical markers of bone turnover for bone mineral density in postmenopausal Japanese women. J Bone Miner Res 15:1537–1544
Löfman O, Magnusson P, Toss G, Larsson L (2005) Common biochemical markers of bone turnover predict future bone loss: a 5-year follow-up study. Clin Chim Acta 356:67–75
Schneider DL, Barrett-Connor EL (1997) Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med 157:1241–1245
Sherman SS, Tobin JD, Hollis BW, Gundberg CM, Roy TA, Plato CC (1992) Biochemical parameters associated with low bone density in healthy men and women. J Bone Miner Res 7:1123–1130
Stepan JJ (2000) Prediction of bone loss in premenopausal women. Osteoporos Int 11 [Suppl 6]:S45–S54
Iki M, Morita A, Ikeda Y et al (2006) Biochemical markers of bone turnover predict bone loss in perimenopausal women but not in postmenopausal women—the Japanese Population-based Osteoporosis (JPOS) cohort study. Osteoporosis Int 17:1086–1095
Chapurlat RD, Garnero P, Sprnay-Rendu E, Arlot ME, Claustrat B, Delmas PD (2000) Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int 11:493–498
Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD (1999) Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 14:1614–1621
Rogers A, Hannon RA, Eastell R (2000) Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res 15:1398–1404
Cheng S, Suominen H, Väänänen K, Käkönen SM, Pettersson K, Heikkinen E (2002) Serum osteocalcin in relation to calcaneal bone mineral density in elderly men and women: a 5-year follow-up. J Bone Miner Metab 20:49–56
Melton LJ III, Atkinson EJ, O’Connor MK, O’Fallon W, Riggs BL (2000) Determinants of bone loss from the femoral neck in women of different ages. J Bone Miner Res 15:24–31
Yoshimura N, Hashimoto T, Sakata K, Morioka S, Kasamatsu T, Copper C (1999) Biochemical markers of bone turnover and bone loss at the lumbar spine and femoral neck: the Taiji study. Osteoporos Int 65:198–202
Dennison E, Eastell R, Fall CHD, Kellingray S, Wood PJ, Cooper C (1999) Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int 10:384–391
Bauer DC, Sklarin PM, Stone KL et al (1999) Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res 14:1404–1410
Slemenda C, Longcope C, Peacock M, Hui S, Johston CC (1996) Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest 97:14–21
Keen RW, Nguyen T, Sobnack R, Perry LA, Thompson PW, Spector TD (1996) Can biochemical markers predict bone loss at the hip and spine ? A 4-year prospective study of 141 early postmenopausal women. Osteoporos Int 6:399–406
Cummings SR, Palermo L, Browner W (2000) Monitoring osteoporosis therapy with bone densitometry. Misleading changes and regression to the mean. JAMA 283:1318–1321
Christiansen C, Riis BJ, Rodbro P (1987) Prediction of rapid bone loss in postmenopausal women. Lancet I:1105–1108
Ross PD, Knowlton W (1998) Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res 13:297–302
Akesson K, Ljunghall S, Jonsson B et al (1995) Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res 10:1823–1829
Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536
Ross PD, Kress BC, Parson RE, Wasnich RD, Armour KA, Mizrahi IA (2000) Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int 11:76–82
Tromp AM, Ooms ME, Popp-Snijders C, Roos JC, Lips P (2000) Predictors of fractures in elderly women. Osteoporos Int 11:134–140
Garnero P, Cloos P, Sornay-Rendu E, Qvist P, Delmas PD (2002) Type I collagen racemization and isomerization and the risk of fracture in postmenopausal women: the OFELY prospective study. J Bone Miner Res 17:826–833
Bruyère O, Collette J, Delmas P et al (2003) Interest of biochemical markers of bone turnover for long-term prediction of new vertebral fracture in postmenopausal women. Maturitas 44:259–265
Melton LJ III, Crowson CS, O’Fallon WM, Wahner HW, Riggs BL (2003) Relative contribution of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res 18:312–318
Gerdhem P, Ivaska KK, Alatalo SL et al (2004) Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res 19:386–393
Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 20:1813–1819
Ivaska KK, Gerdhem P, Akesson K, Obrant KJ (2007) Bone turnover markers and prediction of fracture: nine-year follow-up study of 1040 elderly women. J Bone Miner Res 22 [Suppl 1]:S21, Abstract 1073
Garnero P, Hausher E, Chapuy MC et al (1996) Markers of bone resorption predict hip fracture in elderly women: the Epidos prospective study. J Bone Miner Res 11:1531–1538
Chapurlat RD, Garnero P, Bréart G, Meunier PJ, Delmas PD (2000) Serum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS study. Bone 27:283–286
Chen JS, Seibel MJ, Zochling J et al (2006) Calcaneal ultrasound but not bone turnover predicts fractures in vitamin D deficient frail elderly at high risk of falls. Calcif Tissue Int 79:37–42
Garnero P, Dargent-Molina P, Hans D et al (1998) Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int 8:563–569
Weel AEAM, Seibel MJ, Hofman A, Leeuwen JPTM, Pols HAP (1999) Which fractures are associated with high bone resorption in elderly women: the Rotterdam study. J Bone Miner Res 14 [Suppl 1]:S160, Abstract 1110
Dobnig H, Piswanger-Sölkner JC, Obermayer-Pietsch B et al (2007) Hip and nonvertebral fracture prediction in nursing home patients: role of bone ultrasound and bone marker measurements. J Clin Endocrinol Metab 92:1678–1686
Johnell O, Oden A, de Laet C, Garnero P, Delmas PD, Kanis JA (2002) Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int 13:523–526
Robbins JA, Schott AM, Garnero P, Delmas PD, Hans D, Meunier PJ (2005) Risk factors for hip fracture in women with high BMD: EPIDOS study. Osteoporos Int 16:149–154
Delmas PD, Licata AA, Reginster JY et al (2006) Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 39:237–243
Bauer DC, Garnero P, Hochberg MC et al DM (2006) Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21:292–299
Schousboe JT, Bauer DC, Nyman JA, Kane RL, Melton LJ, Ensrud KE (2007) Potential for bone turnover markers to cost-effectively identify and select post-menopausal osteopenic women at high risk of fracture for bisphosphonate therapy. Osteoporos Int 18:201–210
Garnero P (2000) Markers of bone turnover for the prediction of fracture risk. Osteoporos Int 11 [Suppl 6]:S55–S65
Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD (2004) Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 34:736–746
Szulc P, Seeman E, Dubeouf F, Sornay-Rendu E, Delmas PD (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21:1856–1863
Seeman E, Delmas PD (2006) Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261
Dempster DW (2000) The contribution of trabecular architecture to cancellous bone quality. J Bone Miner Res 15:20–23
Follet H, Boivin G, Rumelhart C, Meunier PJ (2004) The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone 34:783–789
Viguet-Carin S, Roux JP, Arlot ME et al (2006) Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone 39:1073–1079
Oxlund H, Barckman M, Ortoft G, Andreassen TT (1995) Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 17 [Suppl 4]:365S–371S
Banse X, Sims TJ, Bailey AJ (2002) Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermollecular cross-links. J Bone Miner Res 17:1621–1628
Garnero P, Gineyts E, Arbault P, Christiansen C, Delmas PD (1995) Different effects of bisphosphonate and estrogen therapy on free and peptide-bound cross-links excretion. J Bone Miner Res 10:641–649
Colwell A, Eastell R (1996) The renal clearance of free and conjugated pyridinium cross-links of collagen. J Bone Miner Res 11:1976–1980
Bonnick S, Saag KG, Kiel DP et al (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endcrinol Metab 91:2631–1637
Reszka AA, Rodan GA (2003) Mechanism of action of bisphosphonates. Curr Osteoporos Rep 1:45–52
Liberman UM, Weiss SR, Broll J (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med 333:1437–1443
Harris ST, Watts NB, Genant HK (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. A randomized controlled trial. JAMA 282:1344–1352
Roodman D (2006) Regulation of osteoclast differentiation. Ann N Y Acad Sci 1068:100–109
Kim MK, Kim HD, Park JH et al (2006) An orally active cathepsin K inhibitor, furan-2-carboxylic acid, 1-{1-[4-flouro-2-(2-oxo-pyrrolidin-1-yl)-phenyl]-3-oxo-piperidin-4-ylcarbamoyl}-cyclohexyl)-amide (OST-4077), inhibits osteoclast activity in vitro and bone loss in ovariectomized rats. J Pharmacol Exp Ther 318:555–562
McClung MR, Lewiecki EM, Cohen SB et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
Papanastasiou P, Ortmann CE, Olson M, Vigneron A, Trechsel U (2006) Effect of three month treatment with the cathepsin K inhibitor, balicatib, on biochemical markers of bone turnover in postmenopausal women. Evidence for uncoupling of bone resorption and bone formation. J Bone Miner Res 21 [Suppl 1]:S59, Abstract 1223
Delmas PD, Bjarnason NH, Mitlak BH (1997) Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 337:1641–1647
Ravn P, Clemmesen B, Riis BJ, Christiansen C (1996) The effect on bone mass and bone markers of different doses of ibandronate: a new bisphosphonate for prevention and treatment of postmenopausal osteoporosis: a 1-year, randomized, double-blind, placebo-controlled dose-finding study. Bone 19:527–533
Thiébaud D, Burckhardt P, Kriegbaum H et al (1997) Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med 103:298–307
Christiansen C, Tanko LB, Warming L et al (2003) Dose dependent effects on bone resorption and formation of intermittently administered intravenous ibandronate. Osteoporos Int 14:609–613
Greenspan SL, Bone HG, Ettinger MP et al (2007) Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis. Ann Intern Med 146:326–339
Chen P, Satterwhite JH, Licata AA et al (2005) Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 20:962–970
Dobnig H, Sipos A, Jiang Y et al (2005) Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab 90:3970–3977
Hodsman AB, Hanley DA, Ettinger MP et al (2003) Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mienral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88:5212–5220
Lindsay R, Nieves J, Formica C et al (1997) Randomised controlled study of effect of parathyroid hormone on vertebral bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555
Eastell R, McCloskey EV, Glover S et al (2007) Rapid and robust biochemical response to teriparatide therapy for osteoporosis. J Bone Miner Res 22 [Suppl 1]:S322, Abstract T366
Jilka RL (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446
Aubin JE, Lian JB, Stein GS (2006) In: Favus M (ed) Bone formation: maturation and functional activities of osteoblast lineage cells. Primer on the metabolic bone diseases and disorders of mineral metabolism, 6th edn. American Society for Bone and Mineral Research, Washington, DC, pp 20–29
Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB (2007) Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res 2007 22:495–502
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Delmas PD, Pornel B, Felsenberg D et al (1999) A dose-ranging trial of a matrix transdermal 17β-estradiol for the prevention of bone loss in early postmenopausal women. Bone 24:517–523
McClung M, Clemmensen B, Daifotis A (1998) Alendronate prevents postmenopausal bone loss in women without osteoporosis. A double-blind, randomized, controlled trial. Ann Intern Med 128:253–261
Harris ST, Gertz BJ, Genant HK et al (1993) The effect of short term treatment with alendronate on vertebral density and biochemical markers of bone remodelling in early postmenopausal women. J Clin Endocrinol Metab 76:1399–1406
Reginster JY, Adami S, Lakatos P et al (2006) Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 65:654–661
McClung M, Wasnich R, Recker R et al (2004) Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res 19:11–18
Tanko LB, Felsenberg D, Czerwinski E et al (2003) Oral weekly ibandronate prevents bone loss in postmenopausal women. J Intern Med 254:159–167
Reid IR, Brown JP, Burckhardt P (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359:2018–2026
Prestwood KM, Kenny AM, Unson C, Kulldorff M (2000) The effect of low dose micronized 17β-estradiol on bone turnover, sex hormone levels, and side effects in older women: a randomized, double blind, placebo-controlled study. J Clin Endocrinol Metab 85:4462–4469
Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M (2003) Ultralow-dose micronized 17β-estradiol and bone density and bone metabolism in older women. A randomized controlled trial. JAMA 290:1042–1048
Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH (2002) Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 287:2668–2676
Delmas PD, Seeman E (2004) Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 34:599–604
Delmas PD, Li Z, Cooper C (2004) Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res 19:330–337
Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD (2002) Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 17:1–10
Chapurlat RD, Palermo L, Ramsay P, Cummings SR (2005) Risk of fracture among women who lose bone density during treatment with alendronate. The fracture intervention trial. Osteoporos Int 16:842–848
Bauer DC, Black DM, Garnero P et al (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 19:1250–1258
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD (2007) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 22:1656–1660
Reginster JY, Sarkar S, Zegels B et al (2004) Reduction in PINP, a marker of bone metabolism, with raloxifene treatment and its relationship with vertebral fracture risk. Bone 34:344–351
Bjarnasson NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C (2001) Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 12:922–930
Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM (1998) Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34). A randomized control trial. JAMA 280:1067–1073
Bauer DC, Garnero P, Bilezikian JP et al (2006) Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 91:1370–1375
Wasnich RD, Bagger YZ, Hosking DJ et al (2004) Changes in bone density and turnover after alendronate or estrogen withdrawal. Maturitas 11:622–630
Sornay-Rendu E, Garnero P, Munoz F, Duboeuf F, Delmas PD (2003) Effect of withdrawal of hormone replacement therapy on bone mass and bone turnover: the OFELY study. Bone 33:159–166
Greenspan SL, Emkey RD, Bone HG (2002) Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 137:875–883
Gallagher JC, Rapuri PB, Haynatzki G, Detter JR (2002) Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 87:4914–4923
Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black DM, Cummings SR (1995) Estrogen replacement therapy and fractures in older women. Study of osteoporotic fractures research group. Ann Intern Med 122:9–16
Raisz L, Smith JA, Trahiotis M et al (2000) Short-term risedronate treatment in postmenopausal women: effects on biochemical markers of bone turnover. Osteoporos Int 11:615–620
Ravn P, Christensen JO, Baumann M, Clemmensen B (1998) Changes in biochemical markers and bone mass after withdrawal of ibandronate treatment: prediction of bone mass changes during treatment. Bone 22:559–564
Watts NB, Chines A, Olszynski WP et al (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 19:365–372
Bone HG, Hosking D, Devolelaer JP et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Tonino RP, Meunier PJ, Emkey R et al (2000) Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. J Clin Endocrinol Metab 85:3109–3115
Ravn P, Bidstrup M, Wasnich RD et al (1999) Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med 131:935–942
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C (2003) Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone 33:301–307
Ensrud KE, Barrett-Connor EL, Schwartz A et al (2004) Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the fracture intervention trial long-term extension. J Bone Miner Res 19:1259–1269
Black DM, Bilezikian JP, Ensrud KE et al (2005) One year alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565
Black DM, Greenspan SL, Ensrud KE et al (2003) The effects of parathyroid hormone and alendronate alone or in comination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Delmas PD, Watts N, Miller P, Cahall D, Bilezikian J, Lindsay R (2007) Bone turnover markers demonstrate greater earlier responsiveness to teriparatide following treatment with risedronate compared with alendronate: the OPTAMISE study. J Bone Miner Res 22 [Suppl 1]:S27, Abstract 1092
Rittmaster RS, Bolognese M, Ettinger MP et al (2000) Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85:2129–2134
Black DM, Bauer DC, Rosen CJ, Greenspan SL, Bilezikian JP (2007) Prediction of 24 month change in BMD on PTH followed by alendronate: the PaTH study. J Bone Miner Res 22 [Suppl 1]:S26, Abstract 1090
Eviö S, Tiitinen A, Laitinen K, Ylikorkala O, Välimäki MJ (2004) Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. J Clin Endocrinol Metab 89:626–631
Lindsay R, Cosman F, Lobo RA et al (1999) Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomized, controlled clinical trial. J Clin Endocrinol Metab 84:3076–3081
Harris ST, Eriksen EF, Davidson M et al (2001) Effect of combined risedronate and hormone replacement therapies on bone mineral density in postmenopausal women. J Clin Endocrinol Metab 86:1890–1897
Warming L, Ravn P, Christiansen C (2005) Levonorgestrel and 17β-estradiol given transdermally for the prevention of postmenopausal osteoporosis. Maturitas 50:78–85
Liu JH, Muse KN (2005) The effects of progestins on bone density and bone metabolism in postmenopausal women: a randomized controlled trial. Am J Obstet Gynecol 192:1316–1324
Tobias JH, Clarke S, Mitchell K, Robins S, Amer H, Fraser WD (2001) Analysis of the contribution of dydrogesterone to bone turnover changes in postmenopausal women commencing hormone replacement therapy. J Clin Endocrinol Metab 86:1194–1198
Raisz LG, Wiita B, Artis A et al (1996) Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab 81:37–43
Committee for Medicinal Products for Human Use—Guideline on the evaluation of medicinal products in the treatment of primary osteoporosis. European Medicinal Agency CPMP/EWP/552/95 Rev. 2
Rizzoli R, the Alendronate Once-weekly Study Group (2002) Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res 17:1988–1996
Watts NB, Lindsay R, Li Z, Kasibhatla C, Brown J (2003) Use of matched historical controls to evaluate the anti-fracture efficacy of once-a-week risedronate. Osteoporos Int 14:437–441
Brown JP, Kendler DL, McClung MR et al (2002) The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 71:103–111
Chesnut CH III, Skag A, Christiansen C et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal women. J Bone Miner Res 19:1241–1249
Delmas PD, Recker RR, Chesnut CH III et al (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15:792–798
Miller PD, McClung MR, Macovei L et al (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 20:1315–1322
Papapoulos S, Schimmer R (2007) Changes in bone remodeling and antifracture efficacy of intermittent bisphosphonate therapy: implications from clinical studies with ibandronate. Ann Rheum Dis 66:853–838
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Eastell R, Garnero P, Vrijens B et al (2003) Influence of patient compliance with risedronate therapy on bone turnover marker and bone mineral density response: the IMPACT study. Calcif Tissue Int 72:408, Abstract P-297
Clowes JA, Peel NFA, Eastell R (2004) The impact of monitoring on adherence and persistence with anti-resorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89:1117–1123
Delmas PD, Vrijens B, Eastell R et al (2007) Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 92:1296–1304
Delmas PD (2000) Markers of bone turnover for monitoring treatment of osteoporosis with antiresorptive drugs. Osteoporos Int 11 [Suppl 6]:S66–S76
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szulc, P., Delmas, P.D. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int 19, 1683–1704 (2008). https://doi.org/10.1007/s00198-008-0660-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0660-9