Abstract
Background
Biochemical markers of bone turnover have been reported to predict fracture risk independent of bone mass in postmenopausal women. We investigated their use in predicting fractures in the frail elderly.
Methods
Cases were 151 low trauma fractures. For each case, a control was selected marched for sex, age, institution type and follow-up period. We measured two bone resorption markers (serum ICTP and serum CTX-I) and two bone formation markers (serum PINP and serum BAP). Quantitative Ultrasound (QUS) was measured in the calcaneus. Fractures were ascertained by x-ray reports.
Results
The mean age of subjects was 86.8 years (± 5.8 SD) and 86% were female. 76% had hypovitaminosis D (a serum 25 hydroxy vitamin D (25OHD) level < 39 nmol/L) and 81% had BUA < 67.4 dB/MHz (corresponding to a BMD T-score < −2.5). No significant differences in bone turnover markers were detected between fracture cases and their matched controls. In contrast, there was a significant difference between cases and controls for both broadband ultrasound attenuation (BUA) and velocity of sound (VOS) (both P < 0.05). These results remained the same after adjusting for weight, lower leg length and walking aids as well as the higher falls incidence in cases than controls (average 2.7 vs 0.9 falls respectively; P < 0.001) during the follow-up period.
Conclusion
In the frail elderly with vitamin D deficiency and high falls risk, calcaneal ultrasound but not markers of bone turnover were associated with fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Accurate assessment of fracture risk is the key step in identifying patients at “high risk” for osteoporosis. It is increasingly recognised that besides bone mineral density (BMD), other bone related variables, such as the rate of bone turnover, determine fracture susceptibility. In this context, it has been hypothesised that accelerated bone remodelling leads to a disruption of the trabecular bone network over time and, hence, to an increase in bone fragility.
Several independent studies have shown that in women and men, elevated levels of bone resorption markers are associated with an increased risk of vertebral and non-vertebral fractures independently of BMD [1–8]. For example, a rise in urinary deoxypyridinoline (a marker of bone resorption) by one standard deviation above the premenopausal mean has been associated with a two fold increase in hip fracture risk [1, 2, 8]. Importantly, the combined measurement of bone density and bone resorption markers is a stronger predictor of future fractures than the determination of one of these variables alone [2, 6]. Due to the high degree of variability in bone markers, these results are difficult to translate into the clinical setting facing the individual patient. Therefore, risk factors such as personal and maternal fracture history, low body weight and low bone mass are presently considered more feasible for the practical assessment of fracture risk than bone markers. However, if assessment of these risk factors gives equivocal results, measurement of bone turnover may be used to improve the estimate of future fracture risk.
Since vitamin D deficiency and the resulting secondary hyperparathyroidism are associated with disturbances of bone turnover, we examined whether the association of markers of bone turnover with incident fracture was altered in a population of predominantly vitamin D deficient elderly subjects.
Methods
Subjects
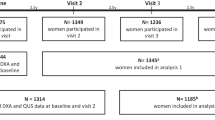
This paper reports a case-control study of 151 pairs of older people living in residential care facilities (RCF). The participants were a part of the ongoing Fracture Risk Epidemiology in the frail Elderly (FREE) Study in Sydney, Australia [9, 10]. All subjects gave informed consent or, if unable, their next of kin gave proxy consent. Ethics approval was given by the local institutional Human Research Ethics Committee. Cases were defined as the first 151 respondents who had sustained a low trauma fracture and in whom serum samples were available. Fracture data were collected by the research staff through regular liaison (every 6–12 weeks) with the residential care facilities and the five public hospitals and one private hospital. Prospective falls were ascertained from incident reports and nursing records and were defined as events that resulted in a person coming to rest unintentionally on the ground or other lower level, not as the result of a major intrinsic event or an overwhelming hazard [11]. The cases were validated by x-ray reports. The diagnosis of clinical vertebral fractures required subjects to have radiological evidence of vertebral fracture in association with acute back pain at a corresponding level (but a previous normal x-ray at that vertebral level was not required). Duration of follow-up for cases was calculated as the time from baseline to date of fracture. For each case, a control was selected marched for sex, age and institution type but with no incident fracture during a similar follow-up period. Clinical risk factors assessed at baseline included age, sex, type of RCF, use of walking aids, weight, lower leg length (an index of mature skeletal stature) and medications.
Biochemical Markers of Bone Turnover
At the baseline assessment, blood samples were collected in the late morning (non fasting) as it was impractical to collect fasting specimens in this cohort. All samples were stored at −40°C and analysed at the same time. Measurements for all of the following bone turnover markers were done only in the cases and their matched controls.
Serum concentrations of the carboxyterminal cross-linked telopeptide of type I collagen (ICTP) were measured by radioimmunoassay (Orion Diagnostica, Finland). This assay has a sensitivity of approximately 0.7 mg/L with an intra-assay precision of approximately 5.3% and an inter-assay precision of 7.6%. In healthy subjects aged 18–63, the manufacturer’s reference range is 1.4–5.2 μg/L for males and 1.6–5.3 μg/L for females.
Serum levels of the carboxyterminal telopeptide of type I collagen (CTX-I) were determined using an automated immunoassay (Elecsys 170 Roche Diagnostics). For the immunoassay, intra-assay precision was approximately 2.0%. The upper limit of the manufacturer’s reference range (calculated as mean ± 2SD) was 0.394 ± 0.460 ng/mL for normal adult males aged > 70 years and 0.556 ± 0.452 ng/mL for postmenopausal females.
Serum concentrations of the aminoterminal propeptide of type I procollagen (PINP) were determined using a specific mono-clonal antibody based radioimmunoassay (Orion Diagnostica, Finland). This assay has a sensitivity of approximately 2 mg/L with an intra-assay precision of 8.7% and an inter-assay precision of 5.1%. In healthy subjects aged 19–65, the manufacturer’s reference range is 21–78 μg/L for males and 19–102 μg/L for females.
Bone specific alkaline phosphatase (BAP) in serum was measured using a specific enzyme-immuno assay (Quidel Corp., USA) with a sensitivity of 0.7 U/L and an intra-assay and inter-assay precision of 5.8% and 7.6% respectively. The reference ranges are 15.0–41.3 U/L for normal male adults and 14.2–42.7 U/L for postmenopausal females.
Other Clinical Chemistry Measurements
Serum levels of 25-hydroxyvitamin D (25OHD) were measured using a radio immunoassay (Diasorin, USA). Sensitivity was measured at 4 nmol/L, intra-assay precision 7.6% and inter-assay precision 9.0%, with a laboratory reference range of 39–140 nmol/L.
Serum levels of intact parathyroid hormone (PTH) were determined by two-site chemiluminescent enzyme-linked immunometric assay on a DPC Immulite 1000 analyser. The assay has an intra-assay precision of 5.5% and an inter-assay precision of 7.9%. The reference range is 23.7–66.2 pg/mL (2.5–7.0 pmol/L).
A modified Jaffé (picric acid) kinetic colorimetric assay was used to measure serum creatinine with a normal range in males of 70–110 mmol/L and in females 50–90 mmol/L, manufacturer’s precision of 0.7% intra-assay and 2.3% inter-assay and a measured inter-assay CV of 3.0%. Creatinine clearance was calculated using the Cockcroft-Gault formula [12]. Only 131 subjects had creatinine results.
Quantitative Ultrasound
Broadband ultrasound attenuation (BUA) and velocity of sound (VOS) were measured in the calcaneus using a McCue Cuba II machine [10]. The precision with this machine was 3.1% for BUA and 0.3% for VOS [13]. Two left heel measurements with repositioning were obtained. We also calculated what BUA value using the McCue Cuba II corresponded to a BMD T-score of −2.5 measured by dual energy X-ray absorptiometry, using BMD and BUA data in a cohort of young normal females previously reported [14].
Statistical Analysis
Paired-sample t tests or Wilcoxon signed rank tests were used, as appropriate, to determine whether there were any differences between matched cases and controls in ICPT, CTX, PINP, BAP, 25OHD, PTH, BUA and VOS. The relationship between bone turnover markers or QUS parameters and fracture were adjusted for variables that were statistically significant (P < 0.05) or were an actual confounder (i.e. changed the effect of the variable under investigation by more than 10%) using conditional logistic regression. The effect of falls on these relationships was determined by adding falls into these models, categorised as 0, 1, or ≥ 2. Variables with positively skewed distributions, such as bone turnover markers and PTH, were transformed by natural logarithms in the regression analyses. Analyses were performed for all subjects combined and separately for females, but not for males, as there were only 23 pairs of male. Spearman’s rank correlation was used to estimate associations between bone markers and other variables.
Results
All 151 cases of fractures occurred within two years from baseline (mean 282 days, SD 197 days, range 1 to 730 days) (Table 1). There was a little difference in duration of follow-up between a case and their matched control with a median difference of 5 days (inter-quartile range of 1 to 14 days). Fractures comprised 50 hip fractures, 27 clinical vertebral fractures, 15 wrist fractures, 14 pelvic fractures, 12 rib fractures, 11 humeral fractures and 22 ‘other site’ fractures.
Cases and controls were institutionalised elderly subjects with a mean (± SD) age of 86.8 (± 5.8) years. The majority (86%) of the subjects were female, 67% were frail (as defined by the use of a walking aid) and 35% lived in nursing homes. Baseline characteristics of cases and controls are shown in Table 1. In the follow-up period, cases were much more likely to sustain falls than controls (93% vs 36%, P < 0.001) with an average of 2.7 vs 0.9 falls respectively, indicating a strong association between falls and fractures in this population. 85% of total fractures and 94% of hip fractures were associated with a fall.
Table 2 presents results of biochemical measures and QUS parameters by fracture status. 76% of all subjects were considered to have hypovitaminosis D (using a cut-off of 39 nmol/L) and 47% had PTH levels above the laboratory reference (>66.2 pg/mL). For 131 subjects who had serum creatinine measurements, 56% had a creatinine clearance between 30–60 ml/min and 32% were ≤ 30 ml/min. The above results were consistent in both cases and controls. All the bone turnover markers were significantly positively correlated with age and PTH and negatively correlated with BUA (data not shown).
Table 2 also contains the mean differences between matched pairs and 95% confidence interval for bone turnover markers, serum PTH, 25OHD, creatinine and QUS parameters. There were no significant differences between matched cases and controls in bone turnover markers (all P > 0.65), 25OHD (P = 0.77), PTH (P = 0.77) or creatinine clearance (P = 0.17). These results were the same for those with hip fracture. The difference between hip fracture cases and their matched controls was 3.2 μg/L (95% CI: −8.8 to 15.3; P = 0.62) for PINP, −2.3 U/L (95% CI: −8.8 to 4.2; P = 0.51) for BAP, −0.6 μg/L (95% CI: −3.1 to 1.9; P = 0.85) for ICTP, 0.02 ng/mL (95% CI: −0.12 to 0.16; P = 0.61) for CTX, −19.7 pg/mL (95% CI: −54.6 to 15.3; P = 0.99) for PTH, and −1.8 ml/min (95%CI: −10.8 to 7.2; P = 0.43) for creatinine clearance.
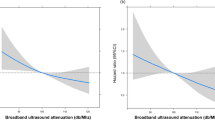
In contrast, significant differences between matched cases and controls were shown for BUA and VOS for total fractures, but not for hip fractures. BUA was on average 5.7 dB/MHz (95% CI: 1.7 to 9.8; P = 0.006) and 3.5 dB/MHz (95% CI: −3.6 to 10.6; P = 0.33) lower in cases than in their matched controls for all fractures and hip fracture respectively. For VOS, the difference between cases and their matched controls were −10 m/s (95% CI: −1 to −19; P = 0.03) for all fractures and −4 m/s (95% CI: −19 to 10; P = 0.58) for hip fracture. In 81% of all subjects, BUA was less than 67.4 dB/MHz (the value corresponding to a BMD T-score of −2.5 of young healthy females). However, only 75% of controls had a BUA value below 67.4 dB/MHz compared to 88% of cases. This difference was also statistically significant (P = 0.005).
Table 3 shows the results of conditional logistic regression analyses for bone turnover markers and QUS parameters with all fractures as the outcome variable. In multivariate regression analyses, weight and lower leg length were identified as independently significant risk factors and use of walking aids was a confounder, although it was not a significant risk factor. Other variables, such as 25OHD, PTH, creatinine clearance and number of medications were neither significant nor confounding. Falls were an extremely important predictor of fractures in all models (see Table 3). After allowing for falls, weight was no longer significant (P = 0.55) but lower leg length and use of walking aids were significant independent risk factors (last column, Table 3). However, falls were neither significant nor confounding when falls that occurred on the date of fracture were excluded. No significant interactions were found between bone turnover markers or QUS parameters and factors, such as sex, age, creatinine clearance and falls. The results from the analyses of females and of hip fractures were similar to those in Table 3, with higher P values because of reduced sample size.
Discussion
We have found in the frail elderly with vitamin D deficiency, calcaneal ultrasound but not markers of bone turnover were associated with fractures. Quantitative ultrasound of the calcaneus has been shown to predict fracture in other studies [15, 16]. The lack of ability of biochemical markers to discriminate fractures cases from controls may be because in this vitamin deficient population, falls risk plays a dominant role in determinating fracture incidence. We observed that falls were an extremely important risk factor for fractures and falls incidences were much higher in cases than in controls during the follow-up period. Also, the high degree of variability in markers of bone turnover would reduce the power of detecting a significant difference between cases and controls. Less variability in QUS measures than in bone markers might explain why QUS parameters are the only positive finding.
Most studies of the relationship between markers of bone turnover and fracture have been performed in postmenopausal women who have generally been more vitamin D replete than our population. For example in the OFELY study, the mean serum 25OHD was 79 nmol/l in the fracture cases [3]. In a study of 178 elderly women (all aged >75) who had sustained fractures [4] only 26% had serum 25OHD levels below 30 ng/ml (personal communication). However in the EPIDOS study, mean serum 25OHD values were only 40 nmol/L in the fracture cases [2].
The frail elderly with vitamin D deficiency are considered to be at high risk of falling [17, 18]. Vitamin D deficiency is thought to be a contributing factor for falls risk via effects on neuromuscular function [18–21]. Some studies have demonstrated the association between vitamin D deficiency and muscle weakness and abnormal gait [19, 22]; others have shown an improvement in muscle strength, walking distance and functional ability after supplementation with vitamin D therapy in the frail elderly [22–24]. On the other hand, severe immobility reduces exposure to falling and produces a lower impact of the fall (i.e. falling from a chair rather than from an upright position), but contributes to the state of accelerated bone turnover in the elderly [25]. Increased fracture risk due to elevated levels of bone turnover markers would likely be masked by high and uneven fall incidences in this population.
Vitamin D deficiency results in a reduction in serum calcium and stimulation of the parathyroid gland to release PTH which acts to increase circulating calcium levels by increasing renal reabsorption of calcium in the kidney, increasing absorption of calcium from the intestine, and in bone increasing bone resorption. PTH-driven increased bone resorption may be a confounding factor in the present cohort. PTH levels have been positively correlated with bone turnover in other studies [3]. Recently Sahota et al. [26] examined bone turnover markers in 421 women with vertebral fractures of whom 39% had low vitamin D levels. They identified increased bone turnover in those with elevated PTH and our results are consistent with this finding.
This study has several strengths and limitations. It is unlikely our study is underpowered for observing a clinically meaningful effect of bone markers on fracture. Our cohort comprised 151 fractures in total and 50 hip fractures. An effect of bone markers was seen in the Rotterdam study with 17 fracture cases [8], in the OFELY study with 55 fracture cases [3] and in Malmo in 178 fractures cases [4] and in Dubbo with 50 fracture cases [5]. Our samples were non fasting, but they were taken at a relatively standard time of day. Although serum CTX is affected by food, we also measured serum ICTP which is little affected by food intake, as are PINP and BAP. Moreover serum ICTP, BAP and PINP show diurnal amplitudes of less than 20%. In clinical practice, obtaining fasting samples in the frail elderly is likely to be difficult so in this sense our results represent the ‘real world’.
In conclusion, the rate of bone turnover was not associated with fractures in frail older people living in residential care with a high prevalence of vitamin D deficiency. Bone turnover markers may not be useful to determine the risk of fracture in elderly populations with vitamin D deficiency and high falls risk.
References
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538
Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536
Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, Pettersson K, Vaananen HK, Akesson K, Obrant KJ (2004) Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res 19:386–393
Meier C, Nguyen TV, Center JR, Seibel MJ, Eisman JA (2005) Bone resorption and osteoporotic fractures in elderly men: the dubbo osteoporosis epidemiology study. J Bone Miner Res 20:579–587
Ross PD, Kress BC, Parson RE, Wasnich RD, Armour KA, Mizrahi IA (2000) Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int 11:76–82
Seibel MJ, Naganathan V, Barton I, Grauer A (2004) Relationship between pretreatment bone resorption and vertebral fracture incidence in postmenopausal osteoporotic women treated with risedronate. J Bone Miner Res 19:323–329
Van Daele PL, Seibel MJ, Burger H, Hofman A, Grobbee DE, Van Leeuwen JP, Birkenhager JC, Pols HA (1996) Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. BMJ 312:482–483
Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, Zochling J, Sitoh YY, Lau TC, Schwarz J, Seibel MJ (2004) Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab 89:1572–1576
Zochling J, Sitoh YY, Lau TC, Cameron ID, Cumming RG, Lord SR, Schwarz J, Trube A, March LM, Sambrook PN (2002) Quantitative ultrasound of the calcaneus and falls risk in the institutionalized elderly: sex differences and relationship to vitamin D status. Osteoporos Int 13:882–887
Gibson MJ, Andres RO, Isaacs B, Radebaugh T, Worm-Petersen J (1987) The Prevention Of Falls In Later Life. A Report Of The Kellogg International Work Group on the Prevention of Falls by the Elderly. Dan Med Bull 34:1–24
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Zochling J, Nguyen TV, March LM, Sambrook PN (2004) Quantitative ultrasound measurements of bone: measurement error, discordance, and their effects on longitudinal studies. Osteoporos Int 15:619–624
Naganathan V, March L, Hunter D, Pocock NA, Markovey J, Sambrook PN (1999) Quantitative heel ultrasound as a predictor for osteoporosis. Med J Aust 171:297–300
Bauer DC, Gluer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, Black DM (1997) Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 157:629–634
Khaw KT, Reeve J, Luben R, Bingham S, Welch A, Wareham N, Oakes S, Day N (2004) Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study. Lancet 363:197–202
Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ (2002) Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res 17:891–897
Flicker L, Mead K, MacInnis RJ, Nowson C, Scherer S, Stein MS, Thomasx J, Hopper JL, Wark JD (2003) Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc 51:1533–1538
Mowe M, Haug E, Bohmer T (1999) Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc 47:220–226
Janssen HC, Samson MM, Verhaar HJ (2002) Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 75: 611–615
Pfeifer M, Begerow B, Minne HW (2002) Vitamin D and muscle function. Osteoporos Int 13:187–194
Bischoff HA, Stahelin HB, Urscheler N, Ehrsam R, Vonthein R, Perrig-Chiello P, Tyndall A, Theiler R (1999) Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil 80:54–58
Gloth FM III, Smith CE, Hollis BW, Tobin JD (1995) Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D-deficient older people. J Am Geriatr Soc 43:1269–1271
Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA (2000) Muscle strength, functional mobility and vitamin D in older women. Aging (Milano.) 12:455–460
Chen JS, Cameron ID, Cumming RG, Lord SR, March LM, Sambrook PN, Simpson JM, Seibel MJ (2006) Effect of age-related chronic immobility on markers of bone turnover. J Bone Miner Res 21:324–331
Sahota O, Mundey MK, San P, Godber IM, Lawson N, Hosking DJ (2004) The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone 35:312–319
Acknowledgments
This study was supported by an Australian NHMRC grant, Arthritis Australia and the Osteoporosis Australia Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J.S., Seibel, M.J., Zochling, J. et al. Calcaneal Ultrasound but Not Bone Turnover Predicts Fractures in Vitamin D Deficient Frail Elderly at High Risk of Falls. Calcif Tissue Int 79, 37–42 (2006). https://doi.org/10.1007/s00223-005-0287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-005-0287-1