Abstract
The purpose of the present study was to examine of the current role of bone turnover markers (BTMs) in the management of osteoporosis. Perusal of the literature examines the available evidence for the utility of BTMs for decision to treat and for the monitoring of treatment for osteoporosis. There is no evidence for the use of BTMs for fracture risk calculation, decision to treat or for treatment selection. A very abnormal BTM value may be a clue to the presence of bone pathology other than uncomplicated osteoporosis. Whilst changes to BTMs following various osteoporosis treatments are well defined, their utility in monitoring individual patients has been less well established. Some fracture outcome-based data exist for the use of u-NTX target of <21 nmol BCE/mmol for antiresorptive therapy; the equivalent s-CTX level is ~250 ng/L. Suboptimal BTM response to treatment may indicate non-compliance or the presence of secondary causes of osteoporosis which may need addressing. Studies are needed to establish treatment targets based on fracture outcomes for commonly used BTMs for each established osteoporosis therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biochemical markers of bone turnover (BTMs) are released during bone formation (bone formation markers) or bone resorption (bone resorption markers) and can be measured in blood and/or urine. They are considered to provide a surrogate measure of the rate of bone formation and resorption, respectively [1]. The utility of BTMs for the diagnosis and monitoring of Paget’s disease of bone has been clearly demonstrated and generally accepted [2]. However, the use of BTMs in the management of osteoporosis is more controversial. A joint working group of the International Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine have examined the literature and proposed serum procollagen type I N propeptide (s-PINP) and serum C-terminal crosslinking telopeptide of type I collagen (s-CTX) as reference markers of bone formation and bone resorption, respectively, and suggested that they be included in future studies in order to accumulate adequate evidence for their utility in the management of osteoporosis [3]. Standardisation of their measurements is also required in order for results from studies to be collated. It should be noted that EDTA plasma (rather than serum) is the optimum sample for CTX stability [3]. Studies are currently under way to establish reference intervals stratified by age, sex and, in women, menopausal state [4]. In the meantime, until these occur, what is the current role of BTMs in the management of osteoporosis, if any?
Large studies have shown that BTMs are inversely related to bone mineral density (BMD) and associated with fracture risk in postmenopausal cohorts [5, 6]. Their predictive value for fracture in an individual subject is less clear. A recent meta-analysis showed a moderate but significant association between s-PINP and s-CTX and the risk of future fractures not adjusted for BMD [7]. The increase in fracture risk was approximately 20 % per standard deviation increase in s-PINP or s-CTX. This gradient of risk is substantially lower than those reported for the use of femoral neck BMD in the prediction of fracture where each SD reduction is associated with a doubling of fracture risk. The meta-analysis was not able to determine if fracture risk prediction by BTMs was independent of BMD, and if so, to what extent [7]. Fracture risk is now calculated by the use of algorithms such as the FRAX calculator which incorporates a number of risk factors including age and BMD, but not BTMs [8]. The inclusion of BTMs in fracture risk algorithms will have to await the evaluation of the role of BTMs in fracture risk prediction independent of other risk factors currently included in the fracture risk calculation.
However, BTMs may still be useful in the initial assessment of subjects for osteoporosis. Subjects whose BTMs lie outside the 95 % limits defined by population reference intervals may have bone remodelling occurring at an unusual rate due to a pathological process. Although studies to examine the utility of reference limits to identify subjects with secondary causes of osteoporosis are lacking, pathological processes should be considered in patients presenting with osteoporosis as a possible explanation for BTMs that lie outside the reference limits [1, 9]. As to how extensive the investigations should be to seek a pathological mechanism for the BTM abnormality, this would depend on the degree of suspicion which in turn would depend on the degree of BTM abnormality (a Z score ≥3 has been suggested [1]), the BMD Z score (a Z score less than −2.0 indicating a statistical outlier for age) as well as any unusual clinical presentations such as the presence of a pathological fracture or unusual minimal-trauma-fracture for age. On the other hand, it should be noted that an acute fracture may lead to an increase in bone resorption and formation markers for 6–12 months, respectively.
The availability of both antiresorptive and anabolic therapies for the treatment of osteoporosis raises the question of selecting the treatment modality for individual patients based on their baseline bone turnover. However, no evidence exists to support this in practice. Although theoretically one would envisage choosing an anabolic agent to treat osteoporosis in low-turnover state and an antiresorptive agent when bone turnover is high, no study has stratified patients according to bone turnover in order to examine the utility of tailoring therapy based on bone turnover rate. Results of post hoc analysis of some, but not all, studies with antiresorptive agents do suggest that treatment benefit may be increased when baseline bone turnover is high [10] but the converse is not true for anabolic agents. For teriparatide, fracture risk reduction was shown to be independent of pre-treatment bone turnover; however, since subjects with the highest pre-treatment BTM concentrations had the greatest fracture risk, absolute risk reduction was greatest for women with higher, rather than lower, pre-treatment bone turnover [11]. Any expectation that patients with low bone turnover would benefit most from anabolic therapy has not been fulfilled. The issue of pre-treatment BTM levels in predicting the response to teriparatide treatment is difficult as patients suitable for teriparatide are generally not treatment naive and often will have previous exposure to potent antiresorptive agents with residual effects on BTMs.
The use of BTMs in osteoporosis management is most accepted for monitoring treatment. Some, but not all, studies have suggested that monitoring therapy with measurement of BTMs may improve adherence to treatment although improvement in patient outcome has not been specifically related to the use of BTMs rather than other aspects of monitoring [12, 13]. Drug trials have generally been analysed based on intention to treat and not based on adherence. However, where examined, the degree of reduction in BTMs following antiresorptive therapy has been shown to relate to fracture risk reduction [14–16]. The analysis of the HORIZON study was performed to examine if very low BTM levels attained after zoledronic acid therapy were associated with an increase in fracture risk, which was shown not to be the case. In fact, the authors found that “clinical fracture risk was lower in those with lower levels of PINP at 1 year” [16]. With the anabolic agent teriparatide, an early increase in BTMs has been shown to be predictive of a subsequent increase in BMD [17].
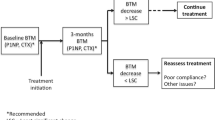
With the above caveats, treatment targets need to be defined for the use of BTMs in monitoring therapy. Urine NTX and s-CTX are the most responsive BTMs following antiresorptive treatment [1, 18]. They are two of the most widely used BTMs in clinical practice. Confirmation of a treatment effect is shown by a decrease in BTMs greater than the reference change value (previously known as least significant change) which, since the direction of change is known, is √2 × 1.65 × CV = 2.33 × CV equating to ~25 % for serum markers and ~50 % for urine markers [19]. However, a statistically significant change in a marker may indicate a treatment effect only, not optimal effect. Optimal target values have been defined by experts as < the mean (not median or the mid-point that are different due to the skewed distribution) of the pre-menopausal reference interval [3, 5, 14], which are 27 nmol BCE/mmol for u-NTX [20] and ~300 ng/L for s-CTX but assay dependent due to inter-assay variations for CTX measurement [21]. The BTM values in the vast majority of bisphosphonate-treated osteoporosis patients would be expected to decrease below these targets, and in those patients that do not, an explanation for inadequate response such as non-compliance or a secondary cause should be sought, and can be found in most [8, 22].
However, targets based on clinical outcomes (fracture in this instance) would have a higher level of evidence, although few studies of such outcomes are available. An analysis of the fracture intervention trial (FIT) data showed that alendronate-treated women with >30 % reduction in bone alkaline phosphatase (B-ALP) at 12 months had a lower risk of non-spine and hip fractures compared to those with reductions <30 % [15]. The relationships between the change in S-CTX and subsequent fracture were similar to those seen with B-ALP, but did not reach statistical significance. Unfortunately, 80 % of the baseline samples and most follow-up specimens in that study were obtained in the non-fasting state, compromising the results for s-CTX which is significantly affected by food intake [15]. This lack of attention to pre-analytical factors may well have contributed to the apparent large biological variation and this could be revisited with more carefully collected specimens in future studies. A secondary analysis of the IMPACT study demonstrated that incidence of all fractures following risedronate therapy was lower in patients with >30 % reduction in u-NTX or s-CTX compared with patients who experienced a <30 % BTM reduction [23].
Eastell et al. have established an absolute threshold for u-NTX associated with the lowest vertebral fracture risk following treatment with risedronate as 21 nmol BCE/mmol [14]. This is the only evidence-based treatment target for antiresorptive therapy currently available. Similar data for s-CTX are lacking; however, we have established the equivalent to u-NTX of 21 nmol BCE/mmol as s-CTX of ~250 ng/L [24], but it is assay dependent due to inter-assay variations [21]. Studies directly examining s-CTX targets based on fracture outcomes are needed. The targets may be lower for other antiresorptive agents such as alendronate, zoledronic acid and denosumab which are associated with lower post-treatment BTM values than risedronate therapy [25–27]; studies examining fracture outcomes with post-treatment BTM for each therapy are needed. For patients with a low pre-treatment BTM, a decrease greater than the reference change value in addition to below the target value would be useful in confirming treatment effect [9, 19]. With anabolic therapy, the bone formation marker PINP is the earliest BTM to show an increase. The treatment target for s-PINP following teriparatide therapy has been established as an increase of >10 μg/L from baseline within 1–3 months, based on subsequent BMD change [28, 29]; again, fracture-based targets are needed.
The potential use of BTMs has been advocated for managing drug holidays which are now utilised to minimise the risk of development of osteonecrosis of the jaw (ONJ) and atypical fractures; however, examination of data from the fracture intervention trial long-term extension (FLEX) did not demonstrate a significant association between either baseline (at discontinuation of alendronate) u-NTX or B-ALP or a 1- or 3-year change in either marker with the risk of fracture after discontinuation [30]. Similarly, there is no adequate evidence for the suggested use [31] of s-CTX measurement to identify bisphosphonate-treated patients at increased risk of developing osteonecrosis of the jaw (ONJ) following dental procedures [32]. A recent publication that is the largest series in which s-CTX was used in the setting of dental extraction showed that a threshold of <150 ng/L (Roche Diagnostics assay) achieved a PPV of 2 % for ONJ, higher than baseline but clearly still very low [33].
In conclusion, although BTMs currently do not contribute to absolute fracture calculations for osteoporosis treatment decision, very abnormal BTM may be a pointer to secondary causes of bone loss or a pathological bone process. Whilst the evidence for the utility of BTMs in monitoring therapy is thin, there is evidence for the use of the target of <21 nmol BCE/mmol for u-NTX equivalent to s-CTX of less than ~250 ng/L for antiresorptive therapy. Further studies are needed to establish authoritative treatment targets for each BTM with each therapeutic agent based on fracture outcomes.
References
P.D. Delmas, R. Eastell, P. Garnero, M.J. Seibel, J. Stepan, The use of biochemical markers of bone turnover in osteoporosis. Osteoporos. Int. 11, 2–17 (2000)
T. Cundy, I.R. Reid, Paget’s disease of bone. Clin. Biochem. 45(1–2), 43–48 (2012)
S. Vasikaran, R. Eastell, O. Bruyère, A.J. Foldes, P. Garnero, A. Griesmacher, M. McClung, H.A. Morris, S. Silverman, T. Trenti, D.A. Wahl, C. Cooper, J.A. Kanis, IOF-IFCC Bone Marker Standards Working Group, Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos. Int. 22, 391–420 (2011)
D. Bauer, J. Krege, N. Lane, E. Leary, C. Libanati, P. Miller, G. Myers, S. Silverman, H.W. Vesper, D. Lee, M. Payette, S. Randall, National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos. Int. 23, 2425–2433 (2012)
P. Garnero, E. Sornay-Rendu, B. Claustra, P.D. Delmas, Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J. Bone. Miner. Res. 15(8), 1526–1536 (2000)
P. Garnero, E. Hausherr, M.C. Chapuy, C. Marcelli, H. Grandjean, C. Muller, C. Cormier, G. Bréart, P.J. Meunier, P.D. Delmas, Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J. Bone Miner. Res. 11(10), 1531–1538 (1996)
H. Johansson, A. Odén, J.A. Kanis, E.V. McCloskey, H.A. Morris, C. Cooper, S. Vasikaran, IFCC-IOF Joint Working Group on Standardisation of Biochemical Markers of Bone Turnover, A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif. Tissue Int. 94(5), 560–567 (2014)
J.A. Kanis, O. Johnell, A. Oden, H. Johansson, E. McCloskey, FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 19, 385–397 (2008)
I. Baxter, A. Rogers, R. Eastell, N. Peel, Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos. Int. 24(3), 941–947 (2013)
D.C. Bauer, P. Garnero, M.C. Hochberg, A. Santora, P. Delmas, S.K. Ewing, D.M. Black, Fracture Intervention Research Group, Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J. Bone Miner. Res. 21(2), 292–299 (2006)
P.D. Delmas, A.A. Licata, J.Y. Reginster, G.G. Crans, P. Chen, D.A. Misurski, R.B. Wagman, B.H. Mitlak, Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 39(2), 237–243 (2006)
J.A. Clowes, N.F. Peel, R. Eastell, The impact of monitoring on adherence and persistence with antiresorptive treatment for Osteoporos Int postmenopausal osteoporosis: a randomized controlled trial. J. Clin. Endocrinol. Metab. 89, 1117–1123 (2004)
P.D. Delmas, B. Vrijens, R. Eastell, C. Roux, H.A. Pols, J.D. Ringe, A. Grauer, D. Cahall, N.B. Watts, Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators, Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 92, 1296–1304 (2007)
R. Eastell, I. Barton, R.A. Hannon, A. Chines, P. Garnero, P.D. Delmas, Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J. Bone Miner. Res. 18, 1051–1056 (2003)
D.C. Bauer, D.M. Black, P. Garnero, M. Hochberg, S. Ott, J. Orloff, D.E. Thompson, S.K. Ewing, P.D. Delmas, Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J. Bone Miner. Res. 19, 1250–1258 (2004)
P.D. Delmas, F. Munoz, D.M. Black, F. Cosman, S. Boonen, N.B. Watts, D. Kendler, E.F. Eriksen, P.G. Mesenbrink, R. Eastell, The HORIZON-PFT Research Group, Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J. Bone Miner. Res. 24, 1544–1551 (2009)
P. Chen, J.H. Satterwhite, A.A. Licata, E.M. Lewiecki, A.A. Sipos, D.M. Misurski, R.B. Wagman, Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J. Bone Miner. Res. 20, 962–970 (2005)
E. Fink, C. Cormier, P. Steinmetz, C. Kindermans, Y. Le Bouc, J.C. Souberbielle, Differences in the capacity of several biochemical bone markers to assess high bone turnover in early menopause and response to alendronate therapy. Osteoporos. Int. 1, 295–303 (2000)
P. Bergmann, J.J. Body, S. Boonen, Y. Boutsen, J.P. Devogelaer, S. Goemaere, J.M. Kaufman, J.Y. Reginster, V. Gangji, Members of the Advisory Board on Bone Markers, Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int. J. Clin. Pract. 63, 19–26 (2009)
R. Eastell, P. Garnero, C. Audebert, D. Cahall, Reference intervals of bone turnover markers in healthy premenopausal women: results from a cross-sectional European study. Bone 50(5), 1141–1147 (2012)
S.A. Chubb, C.D. Mandelt, S.D. Vasikaran, Comparison of results from commercial assays for plasma CTX: the need for harmonization. Clin. Biochem. 48, 519–524 (2015)
D.A. Eekman, I.E. Bultink, A.C. Heijboer, B.A. Dijkmans, W.F. Lems, Bone turnover is adequately suppressed in osteoporotic patients treated with bisphosphonates in daily practice. BMC Musculoskelet. Disord. 12, 167 (2001)
R. Eastell, B. Vrijens, D.L. Cahall, J.D. Ringe, P. Garnero, N.B. Watts, Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J. Bone Miner. Res. 26(7), 1662–1669 (2011)
S.A. Chubb, C. Mandelt, S. Vasikaran, Comparison of clinical cut-points and treatment targets for urine NTX and plasma βCTX-I in osteoporosis. Clin. Biochem. (2015). doi:10.1016/j.clinbiochem.2015.12.002
A.I. Sebba, S.L. Bonnick, R. Kagan, D.E. Thompson, C.S. Skalky, E. Chen, A.E. de Papp, Fosamax Actonel Comparison Trial Investigators, Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosis. Curr. Med. Res. Opin. 20(12), 2031–2041 (2004)
K. Saag, R. Lindsay, A. Kriegman, E. Beamer, W. Zhou, A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40(5), 1238–1243 (2007)
D.L. Kendler, C. Roux, C.L. Benhamou, J.P. Brown, M. Lillestol, S. Siddhanti, H.S. Man, J. San Martin, H.G. Bone, Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone. Miner. Res. 25, 72–78 (2010)
R. Eastell, J.H. Krege, P. Chen, E.V. Glass, J.Y. Reginster, Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr. Med. Res. Opin. 22, 61–66 (2006)
M. Tsujimoto, P. Chen, A. Miyauchi, H. Sowa, J.H. Krege, PINP as an aid for monitoring patients treated with teriparatide. Bone 48, 798–803 (2009)
D.C. Bauer, A. Schwartz, L. Palermo, J. Cauley, M. Hochberg, A. Santora, S.R. Cummings, D.M. Black, Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern. Med. 174(7), 1126–1134 (2014)
R.E. Marx, J.E. Cill Jr, J.J. Ulloa, Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 65(12), 2397–2410 (2007)
S.L. Ruggiero, T.B. Dodson, J. Fantasia, R. Goodday, T. Aghaloo, B. Mehrotra, F. O’Ryan, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J. Oral Maxillofac. Surg. 72(10), 1938–1956 (2014)
A. Hutcheson, A. Cheng, R. Kunchar, B. Stein, P. Sambrook, A. Goss, A C-terminal crosslinking telopeptide test-based protocol for patients on oral bisphosphonates requiring extraction: a prospective single-center controlled study. J. Oral Maxillofac. Surg. 72(8), 1456–1462 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vasikaran, S.D., Paul Chubb, S.A. The use of biochemical markers of bone turnover in the clinical management of primary and secondary osteoporosis. Endocrine 52, 222–225 (2016). https://doi.org/10.1007/s12020-016-0900-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0900-2