Abstract
The continuous increase in the energy demand has resulted in the gradual depletion of fossil fuel resources and an increase in greenhouse gas (GHG) emission. As an alternate, the emphasis has shifted towards green methods, i.e. biofuel generation using lignocellulosic plant biomass via microorganisms and its biomolecules (e.g. endo-xylanase). The lignocellulosic plant biomass serves as a suitable alternative for the fossil fuel resources. They are found abundantly on earth and can be considered as a renewable source for the suitable biorefinery process. Endo-xylanase is a crucial enzyme that effectively cleaves glycosidic linkages present in the complex structure of xylan which carry the most hemicellulosic part of the lignocellulosic plant cell wall. Using the enzymes individually or in combination with other enzymes or with multienzyme-producing microorganisms can be a suitable approach for developing advanced biorefinery processes. The present chapter deals with the involvement of xylanase in the biorefinery process and its advantages, limitations and future prospect.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Biorefining is the sustainable bioconversion of biomass (renewable resources) into a range of industrial products like chemical, food and feed and, similarly, bioenergy like electricity, heat and fuel (De Jong et al. 2009). Being a keystone of bioeconomy, the aim of completely revealing the potential of biomass from lignocellulosic plants (agricultural and forestry) in the economic method remains undefinable. The continuous increase in the consumption of energy and the decrease in the supply of fossil fuels have increased the researcher’s interest in developing sustainable methodologies for the production of biofuel (Yang et al. 2015). The biomass obtained from lignocellulosic plant is abundantly available in the environment and can be considered as a vital alternative of fossil fuels. The biomass can be found in the environment throughout the year in the bulk amount without being used in the form of agricultural and forestry waste/residues (Thomas et al. 2016). Most of the residues, e.g. rice and sugarcane cultivation, are burnt in the open fields mostly in Asian countries causing environmental pollution (Thomas et al. 2016).
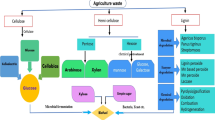
The composition of lignocellulosic plant biomass consists of three main components, i.e. lignin, hemicellulose and cellulose, which together make the recalcitrant structure of plant biomass (Singh et al. 2017). Due to this, the biorefinery process involves three major steps such as pretreatment, saccharification and hydrolysis for the complete bioconversion (Bhardwaj et al. 2020). Other important aspects such as the type of biomass to be used in the biorefinery process and biomass transportation are also a matter of concern along with the structure recalcitrance of the biomass to expose valuable sugars to be utilized in the biorefinery process to fulfil the bioenergy requirement of the world (Hassan et al. 2019).
The microbial hydrolytic enzymes play an important role in the bioconversion of biomass by converting it into fermentable sugar (Wei et al. 2012). Therefore, various strategies have been carried out till date such as isolation of new microbes and various optimization studies to improve the production of enzymes (Attri and Garg 2014; Haitjema et al. 2014; Nigam 2013). Enzymes are required in all the major steps of biorefinery processes, e.g. in the biological pretreatment method, using laccase for the removal of lignin which can help to reduce the recalcitrant nature of plant cell wall and making inner cellular parts, i.e. hemicellulose and cellulose, more accessible (Agrawal et al. 2019). Hemicellulases, e.g. xylanases and cellulases, are required in the hydrolysis and saccharification of plant residues which enhance the release of sugar molecules (Bala and Singh 2019a). These enzymes can be used either individually or as a cocktail (Bhardwaj et al. 2019). Although the commercially available enzyme cocktails are costly and affect the economy of the process, microbial enzyme can be considered as the best alternatives (Vaishnav et al. 2018). Along with the cost of the enzymes, another important factor to be considered is the amount/load of enzyme required for the process and futher study has to be done to identify suitable enzyme preparations to achieve enhanced saccharification rate (Cunha et al. 2017). Also getting microorganisms which can produce an enzyme cocktail that can act on multiple agricultural residues is another option to improve the economic viability of the process (Thomas et al. 2016). With the availability of a huge range of cellulases, lignocellulases can be utilized to allow the adaptation of such cocktails (Ang et al. 2015). This can be achieved by xylanase supplementation as endo-xylanase is known as one of the most suitable enzymes used in the hydrolysis process by breaking the internal glycosidic linkages present in the backbone of the complex structure of heteroxylan, resulting in the xylo-oligosaccharide formation (Thomas et al. 2014a, b). Later these xylo-oligosaccharides are converted into other fermentable sugars such as trimers (xylotriose), dimers (xylobiose) and monomers (xylose) (Brienzo et al. 2012).
Therefore, considering the importance of enzymatic system in the field of biorefinery, the main focus must be on finding new strains which can produce a large amount of xylanases along with other hydrolytic enzymes. Along with these, new methods should be found to enhance the production of fermentable sugar that can be further converted into biofuel. This chapter includes the brief overview of the process involved in the biorefinery system via microbial xylanases. A brief overview of the biorefinery process has been shown in Fig. 7.1.
7.2 Raw Material for Biorefinery
Residues obtained from agricultural industries such as wheat straw and bran, rice straw and husk, sugarcane bagasse, cotton stalk some of the most abundant lignocellulosic biomass. Lignocellulosic plant biomass have been recognized as an efficient raw material for the biorefinery processes which can replace huge sections of fossil resources (Maiti et al. 2018). The biorefinery process can produce three main end-products, i.e. biofuels, bioenergy and biochemicals. As compared to other renewable resources such as sun, wind and water, use of lignocellulosic biomass has some advantages as it contains carbon materials in addition to fossils (Pachapur et al. 2019). Biorefinery processes comprises of a broad range of methods which can separate plant biomass (cellulose, hemicellulose) resources, such as rice, wheat, wood, grass, corn, etc., into carbohydrates, triglycerides, proteins, etc, which can further be converted into value-added end-products such as biofuels and biochemicals (De Jong et al. 2009; Saba et al. 2015) via various physical, chemical or biological processes (Juodeikiene et al. 2011).
7.3 Structure of Lignocellulosic Plant Biomass
In the complete structure of the plant cell wall, cellulose is the principal component which is present in a complex but systematic framework fibrous structure (Kumar et al. 2009). This fibrous structure is made up of approximately 500–15,000 anhydrous glucose units linked with β-1,4-glycosidic linkages which form a linear homo-polysaccharide with the series of small cellobiose units. Extremely crystalline structure of cellulose comprises inter- and intra-molecular H-bonds that are formed by β-1,4 arrangement of the glucoside bonds (Saini et al. 2015). Hemicellulose which is found in the upper layer of cellulose and below the lignin in the plant cell wall (Saini et al. 2015) contains a short polypeptide chain with 50–200 units of pentose and hexose sugar which is highly branched such as d-xylose, l-arabinose and d-mannose-galactose-glucose, respectively. The hemicellulose part also has an acetate group which is arranged randomly to the hydroxyl groups of the pentose sugar ring with ester linkages (Saini et al. 2015). Lignin is the third important component of the plant cell wall which is a highly crosslinked aromatic amorphous and heterogeneous polymer comprising trans-coniferyl, trans-sinapyl and trans-coumaryl alcohols. It forms a complex matrix arranged covalently linked to side groups of other diverse hemicelluloses and covers the cellulose microfibril. It occupies 2–40% of the plant cell wall in which C–C and C–O–C provide stability by protecting them from microbial attack (Mooney et al. 1998).
7.4 The Concept of Biorefinery
Biorefinery is classified into three different generations based on the use of different feedstock and the products (Azad et al. 2015). The raw materials used for first-generation biorefinery are corn, barley, sunflower, etc. Bio-based ethanol, diesel, biogas, methanol and vegetable oils come under this generation (Cherubini 2010). Due to the presence of high oil and sugar content, the bioconversion into biofuel is easy with this generation. Based on the previous reports of life cycle assessment analysis by Reinhardt et al. (2007) and Gasol et al. (2007), a remarkable decrease in the (GHG) emission has been observed as the consumption of bioethanol and biodiesel has efficiently replaced gasoline and diesel obtained from fossil resources. Apart from various benefits, this generation have a drawback of facing difficulties in feed and food industries as they use food resources and agricultural land (Cherubini 2010; Dutta et al. 2014).
In contrast to this, the second-generation biorefinery uses leftover residues from the food crops and cereals which are known as lignocellulosic plant biomass such as husks, bagasse, straws, animal fat and municipal solid wastes which can be used for biofuel production along with other value-added products (Azad et al. 2015; Geddes et al. 2011; Zanuso et al. 2017). Based on various literature of life cycle assessment analysis, it was concluded that the second generation is more advantageous than the first as it is more eco-friendly, economic and more socially feasible as compared to food-based resources and requirement of agricultural land (Dutta et al. 2014).
Whereas, in third generation of biorefinery, aquatic biomass, e.g. algae, rice in proteins, oil and carbohydrates has been used for biofuel production (Martín and Grossmann 2012). Aquatic biomass consists of three groups: microalgae, cyanobacteria, and macroalgae. Although it is not a seasonal feedstock, with high oil productivity and high tolerance rate, its processing cost is very high due to the high cultivation cost and energy input which eventually affects the economic viability of the process (Cervantes-Cisneros et al. 2017). Among all the three generations, the second generation has been considered more efficient, because the whole process can be considered economic from the use of waste products as resources till the production of value-added end-products.
7.5 Role of Enzymes in Biorefinery
7.5.1 In Biological Pretreatment
As discussed above, the biorefinery process involves three main steps, of which pretreatment of biomass is one of the important steps to enhance the production of fermentable sugar. Although pretreatment could be of three types, physical, chemical or biological, the biological pretreatment is more preferred as it is eco-friendly, easy, safe to use and involves the use of microbial enzymes and several microorganisms itself, e.g. white rot (Myrothecium verrucaria) and brown rot fungi (Trametes versicolor, Pleurotus ostreatus). It can be efficiently used in the delignification process without much requirement of energy (Kumar et al. 2009). Various enzymes, such as laccases, lignin peroxidases, manganese-dependent peroxidases, etc., have been employed for the delignification process (Agrawal et al. 2019). This process makes inner hemicellulose and cellulose part more accessible for the other hydrolytic enzymes such as endo-xylanases and cellulases, respectively, for the hydrolysis process (Bhardwaj et al. 2019). After this step, the accessibility of cellulose (carbon source) increases for efficient fermentation by microorganisms leading to the cost-effective enzyme production followed by hydrolysis of the same pretreated biomass. Therefore, it can be inferred that the rate of hydrolysis can be increased up to 90% after the pretreatment (Saini et al. 2015).
The pretreatment process via enzymes utilizes crude or purified enzymes or partially purified ligninolytic or hydrolytic enzymes. This may help to remove lignin via fungal pretreatment within less time period (Plácido and Capareda 2015). Although the complete efficiency of enzymatic pretreatment process is not yet studied properly as compared to thermal and chemical pretreatment process, treatment of sugarcane using alkaline (NaOH) and crude Anthracophyllum discolor enzyme extracts for the production of bioethanol resulted in 48.7% and 33.6% lignin removal by NaOH enzymatic methods, i.e. 31% lower than the enzymatic process alone (Asgher et al. 2013). However, in the study by Asgher et al. (2013) when sugarcane bagasse was treated enzymatically with the increased cellulose load, hydrolysis yeild of about 79% was obtained suggesting effectve treatmnet of the lignocellulosic biomass (Asgher et al. 2013). Hence, these results can be the examples of continuing new researches on the use of both ligninolytic and cellulolytic enzymes to disrupt the structure of lignocellulosic plant biomass for a better saccharification and hydrolysis process (Asgher et al. 2013). There are various reports in the enzymatic hydrolysis process such as a microalgal pretreatment for the biomethane gas production (Vanegas et al. 2015), production of biohydrogen (Mahdy et al. 2014), extraction of lipids for biodiesel generation (Fu et al. 2010) and production of bioethanol (Kim et al. 2014). Similarly, manganese peroxidase in the crude extract of Anthracophyllum discolor was used for the pretreatment of Botryococcus braunii for the production of biogas (Ciudad et al. 2014). Enzymatic pretreatment can be performed by using individual or cocktails of enzymes. Cocktails of enzymes are made by using either crude or partially purified enzymes. However, use of single enzymatic system has been reported with higher yield for the downstream processing of microalgal biomass (Vanegas et al. 2015); cocktails could be more hopeful for the hydrolysis of different biopolymers of plant biomass (Ehimen et al. 2013).
7.5.2 In Enzymatic Hydrolysis
For the economic generation of ethanol from cellulosic plant biomass, enzyme-based hydrolysis is an advantageous process as it is a very cost-effective method, with a probably vast yield when compared to chemical treatment. Long chain of carbohydrate present in the plant cell wall can be deconstructed by hydrolysis method with the help of enzyme catalysis process. By forming a physical barrier, hemicellulose restricts the cellulase accessibility to cellulose (Zhang et al. 2012). Hence, supplying enzymes such as xylanases which can degrade them can be the most suitable method to enhance the release of overall fermentable sugar from various pretreated lignocellulosic plant biomass (Kumar et al. 2009; Öhgren et al. 2007). Xylanases, e.g. endo-β-1,4-xylanases (EC 3.2.1.8) and β-xylosidase (EC 3.2.1.37), can act in the main chains along with the side chain residues of the complex structure of xylan. Endo-β-1,4-xylanase disrupts the long chain of xylan into smaller ones (Aditiya et al. 2016); similarly, xylopyranose is produced by β-xylosidase which is a pyranose unit made up of xylose monomers which are formed by continuous cleaving of oligosaccharide. Other xylanolytic accessory enzymes such as feruloyl esterase (EC 3.1.139) and acetyl xylan esterase (EC 3.1.1.72) cleave the outer chains (Aditiya et al. 2016). Due to their more amorphous nature, hemicelluloses are quite different from celluloses, and also hemicellulolytic enzymes are more complicated but with very particular actions. Hence, it can be confirmed that destruction of xylan by enzymatic hydrolysis may remove the cellulose covering and also it can help in the improvement of cellulase performance (Zhang et al. 2012).
7.6 Enzyme Synergy: A Conceptual Strategy
Synergistic action of enzymes can be stated as the combination of pretreatment and hydrolysis steps to convert most of the polymeric components to fermentable sugar (Ang et al. 2015). In this process, some attention must be taken that the process should not degrade or irreversibly transform the sugars, which will eventually lead to the loss in fermentable sugar. Further, the slurries generated after the pretreatment may have some unwanted physical and chemical characteristics which may hinder the catalysis process of enzymatic proteins. Thus, to avoid the extent of degradation, less severe pretreatment methodologies must be selected, e.g. biological pretreatment via enzymes and microorganisms like fungi (Teter et al. 2014; Zhang et al. 2012).
In order to avoid the loss of fermentable sugar, all the three major steps, i.e. pretreatment, hydrolysis and fermentation, of biomass conversion can be incorporated together which will lead to the reduction in multistep process. Hence, different enzymes can be mixed together in sufficient ratio to prepare the suitable enzyme cocktail (Bhardwaj et al. 2019). These enzymes will work synergistically and will lead to the enhanced biomass conversion and release of maximum sugar as compared to other physical and chemical methods (Chaturvedi and Verma 2013). Later the released sugar in the slurry can further be converted into bioethanol by the use of ethanologenic microorganisms such as Saccharomyces cerevisiae (Bhardwaj et al. 2019). Although bio-based methods have various advantages such as high specificity, no formation of toxic and inhibitory chemicals and expensive and sophisticated instruments are not required, they have some limitations also like high enzyme cost, limited temperature and pH stability (Bala and Singh 2019a).
A study has been reported on the use of thermo-alkali-stable ligno-hemicellulolytic enzyme laccase from Myrothecium verrucaria (Agrawal et al. 2019), xylanase from Aspergillus oryzae (Bhardwaj et al. 2017) and cellulase from Schizophyllum commune (Kumar et al. 2018) cocktails (crude, partially purified) in combination with Saccharomyces cerevisiae MTCC-173, by using simultaneous delignification, saccharification and fermentation (SDSF) in combination with Saccharomyces cerevisiae MTCC-173 (Bhardwaj et al. 2019). Various forms of xylanase were produced by some thermophilic fungi such as Malbranchea cinnamomea (Mahajan et al. 2014), Pyrenophora phaeocomes (Rastogi et al. 2016) and Trametes versicolor, Pleurotus ostreatus and Piptoporus betulinus (Valášková and Baldrian 2006). Similarly, thermophilic mould such as T. aurantiacus was found capable of producing xylanase and cellulases by using agricultural biomass (Jain et al. 2015).
Similarly, in coculturing method, combination of enzyme produced by Aspergillus niger and Trichoderma reesei resulted in a three-fold higher hydrolysis rate of unwashed pretreated sugarcane bagasse with only 0.7 FPU activity/g glucan enzyme load when compared to 5–15 times enzyme loading (Florencio et al. 2016). Therefore, it can be stated that cocktails of various enzymes and coculture of microorganisms could be a better approach to enhance the fermentable sugar production (Kolasa et al. 2014).
7.7 Factors Affecting Biological Pretreatment
In order to get highest yield via enzymatic pretreatment, it is required to understand the factor affecting the microbial growth and metabolism (Wan and Li 2012). The factors which may affect the process are nature, moisture content and particle size of the biomass or substrates, microorganism type and inoculum concentration, enzyme type and conditions like time, pH and temperature. Biomass surface contains internal and external area where the particle size and shape is important for the maintenance of biomass component capillary structure (Maurya et al. 2015). Further, particles with small sizes are more preferred due to increased digestibility and total yield, although the use of small-size particles is difficult in the downstream processing (Bolado-Rodríguez et al. 2016). On the other hand, the small size of particles affects the efficiency of the pretreatment as it affects the proper microbial growth and metabolism by reducing the aeration rate (Sharma et al. 2019), whereas larger particle size affects the pretreatment process by reducing the penetration of microorganisms into the substrates and reducing the uniform air diffusion. Similarly, time is another important factor which varies according to the microorganism and microbial enzymes. Taniguchi et al. (2005) reported highest sugar yield with rice straw after hydrolysis using P. ostreatus when pretreated for 60 days (Taniguchi et al. 2005) whereas Salvachúa et al. (2011) reported less sugar concentration in wheat bran pre-treated with P. chrysosporium-after 14 days. Further, an increased sugar yield was reported for wood chips pretreatment by T. versicolor (Hwang et al. 2008). Another important factor required for the treatment of the biomass is moisture content as it is required in specific amount for proper microbial growth and biodegradation (Gervais and Molin 2003), although this also varies on the basis of type of strain and biomass (Mustafa et al. 2016). Physical parameter such as temperature has also been found to be another important parameter in enzymatic pretreatment process which is necessary for the optimum microbial growth and cells’ metabolic activities. Based on various microorganisms, the temperature optima also varied from 25 to 30 °C. Fungi from ascomycetes group can grow at a higher temperature nearly up to 39 °C, whereas, in the case of basidiomycetes, the required temperature optima is 15 and 35 °C (Sindhu et al. 2016). This is because of the difference in the physiology of fungus substrate type and microbial strains (Isroi et al. 2011). The WRF metabolism in solid-state system generates heat, which eventually enhances the bioreactors’ gradient temperature (Wan and Li 2012), and plays as an important challenge for the researchers while designing the bioreactor for the solid-state pretreatment application in large scale. Similarly, pH in culture medium also affects the microbial growth, enzyme secretion and hydrolysis (Sharma et al. 2019).
7.8 Advantages of Xylanases from Thermophilic Microorganisms in Biorefinery
Various thermophilic microorganisms have been reported for the production of different enzymes such as hemicellulases, amylases, cellulases, phosphatases, proteases, laccases, lipases, etc., which have various applications in different industries like food, textile and detergent, dairy, pharmaceutical and others (Singh 2016). The similarity of thermophilic microorganisms in their phylogenetic analysis and their enzymes showed common origin with other mesophiles (Zeldes et al. 2015). Thus, cellulases and xylanases were obtained from thermophilic origin, and their mode of action was found to be similar except only with some specific features which indicate their advantage at various industries. Thermophiles are found to be a good source of different enzymes as they can produce thermostable enzymes. As compared to mesophilic enzymes, thermophiles have high resistance for denaturing agents and high-pressure tolerance. Hence, they may be considered as the valuable domain for the production of biofuels at higher temperatures (Haki and Rakshit 2003), because high temperature may enhance the penetration of enzymes via cell wall of lignocellulosic plant biomass and can behave as a physical factor for the disorganization of the cell wall of lignocellulosic biomass (Paës and O’Donohue 2006). Among various pretreatment methods, enzymatic degradation of lignocellulosic biomass using cellulase and xylanase is found to be the most suitable and specific with no other toxic effects or product formation and no loss of substrate. Thermostable xylanases and cellulases play a very important role in the pharmaceutical, chemical, food and paper and pulp industries. Xylanases have been found to be an alternative of chlorine in paper and pulp industry due to their involvement in the leaching of xylan from carbohydrate-lignin complex. This way xylanase can be useful in the replacement of chlorine and in pulp bleaching process and can reduce the environmental pollution caused by them. A thermostable xylanase obtained from Myceliophthora thermophila was found suitable as compared to a thermolabile xylanase obtained from Trichoderma reesei in paper and pulp industry. A thermostable xylanase from Bacillus sp. NCIM5 was utilized in the bagasse pulp pre-bleaching by simultaneously reducing the demand of chlorine (Kulkarni and Rao 1996). Various bacterial strains such as Bacillus sp. and Dictyoglomus sp. were successful at commercial scale (Rani and Nand 2000). Although, for many xylanolytic and cellulolytic enzymes, the temperature and pH optima were found to be below 50 °C and acidic or neutral pH (Gessesse 1998), various thermophilic fungi are found to be the good producers of xylanases and cellulases which were successfully used in the lignocellulosic biomass saccharification (Kaur and Satyanarayana 2004).
7.9 The Products of Biorefinery
A list of some recent xylanases involved in the biorefinery process has been shown in Table 7.1 and discussed as follows.
7.9.1 Bioethanol
Bioethanol produced from lignocellulosic plant biomass is ecological process that can be enhanced by using suitable enzymes and microorganisms. Previous studies have reported that thermophilic microorganism can produce more amount of bioethanol via simultaneous delignification, saccharification and fermentation process. Thermal stability has been found to be an important and desirable property for cellulolytic and xylanolytic enzymes required for successful saccharification. The hydrolysis rate of Trichoderma is low as it has less β-glucosidase level (Mohanram et al. 2013). Hence, thermophilic fungi can serve as a suitable alternative of this. Various moulds, e.g. Sporotrichum thermophile, Thermoascus aurantiacus and Scytalidium thermophilum (Berka et al. 2011; Kaur et al. 2004), which are thermophilic in nature have shown sufficient enzymatic system for the lignocellulosic plant biomass bioconversion process for enhanced bioethanol production. Saccharomyces cerevisiae and Pichia stipites have been used for the production of bioethanol with high yield at 30 °C after 72 h (Bala and Singh 2019b). Similar reports with the rice straw and waste tea cup paper hydrolysis are there in the literature using partially purified cellulases and xylanase obtained from S. thermophile BJAMDU5, resulting in the high yield of reducing sugars (Bala and Singh 2016). Various thermophilic bacteria, such as Clostridium, Caldanaerobacter and Thermoanaerobacter, were reported for high ethanol production (Taylor et al. 2009).
7.9.2 Biobutanol
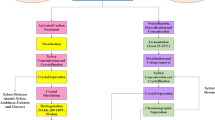
Another product obtained from biorefinery and has attracted the attention of scientists as an efficient alternative for gasoline (Bhandiwad et al. 2014) (Fig. 7.2) is biobutanol. Microorganisms, such as Clostridium spp., C. saccharoperbutylacetonicum, Clostridium acetobutylicum and C. beijerinckii, are example of microorganims capabable of produding biobutanol by using sugars from agricultural residues (Bhandiwad et al. 2014; Nakayama et al. 2011). Similarly, Thermoanaerobacterium thermosaccharolyticum showed 1.8–5.1 mM n-butanol production from the overexpression of thl, hbd, crt, bcd, etfA and etfB genes of bcs operon required for butyryl-CoA formation (Bhandiwad et al. 2014). 7.9 g/L of n-butanol was produced by coculture of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum (Nakayama et al. 2011). 7.7 g/L of acetoin and 14.5 g/L of 2,3-butanediol were reported from Geobacillus strain XT15 from corn steep liquor at 55 °C (Yang et al. 2015).
7.9.3 Hydrogen
It is a carrier of energy having a high potential of being considered as an alternative for fossil fuel. As it is a clean fuel, it can be used as an internal fuel for combustion engines in combination with oxygen (Koskinen et al. 2008). Thermophilic microorganisms, e.g. Pyrococcus furiosus, Thermococcus kodakarensis and all Thermotoga and Caldicellulosiruptor species, have been found to be the good producers of hydrogen with only the water vapour emission (Verhaart et al. 2010). adhE and aldH genes are not present in these microorganisms; therefore, they do not produce ethanol; hence due to hydrogenase, hydrogen production increases. However, Clostridium uzonii strain AK15 and Thermoanaerobacterium aciditolerans AK17 isolated from Iceland during geothermal springs showed good hydrogen production along with bioethanol (Koskinen et al. 2008).
7.10 Molecular Aspects of Enzymes in Biorefinery
The advances of effective hydrolysis enzymes with advanced properties, e.g. better interaction with cheap substrates, higher specific activity and higher stability, are important factors for the industrial production of biofuel. As discussed above, lignocellulosic plant biomass degradation into their monomeric sugars comprises two important constituents, i.e. hemicellulose and cellulose (Balat 2011; Pareek et al. 2013; Ulaganathan et al. 2017), and the composite hemicellulose structure needs the synergistic action of different enzymes, and endo-1,4-β-xylanase plays an important role to degrade the complex polymer of xylan into oligosaccharides and other monomeric sugars (Madadi et al. 2017). Naturally hemicellulolytic enzymes are not sufficient for the complete hydrolysis of recalcitrance lignocellulosic biomass (Himmel et al. 2007). Hence, there is a requirement of enzymes, and they are commercially expensive which will eventually lead to the product loss (Visser et al. 2015). The only solution for this problem is the efficiency of the enzymes should be increased (Morone and Pandey 2014) along with the exploitation of accessory enzymes, e.g. xylanase and β-glucosidase, which can be synergistically act with cellulases (Berlin et al. 2005). Recently, various reports are found in the literature based on the improvements of hydrolytic enzymes which has only considered the cellulase and their synergy with hemicellulases (Diogo et al. 2015; Quiñones et al. 2015; Yang et al. 2018), but very few reports are there focusing on xylanases individually. Molecular biology aspects which include directed evolution, library construction strategies, mutagenesis and gene recombination have gained researchers’ interest to improve the genetic variations on enzymes (McLachlan et al. 2009). The increased hydrolysis of pretreated sugarcane bagasse was reported with xylanase (Ribeiro et al. 2014). Two xylanase genes (GH10 and GH11) from Malbranchea cinnamomea, i.e. XYN10A_MALCI and XYN11A_MALCI, respectively, that were expressed in P. pastoris X33 showed improved hydrolysis of substituted arabinoxylan and unsubstituted xylan. The synergistic action of recombinant xylanase with commercial cellulase resulted in the better hydrolysis of acid- and alkali-treated rice straw (Basotra et al. 2018). Similarly, Geobacillus thermodenitrificans JK1 showed the production of isoforms of xylanase, i.e. XynA1 and XynA2, acting synergistically with β-xylosidases and arabinofuranosidase for the improved birchwood xylan hydrolysis (Huang et al. 2017).
7.11 Conclusion
Advancement in the enzyme efficiency and effective hydrolysis is highly required in the world of biorefinery; for that, scientists must focus on the economic and eco-friendly processes. Xylanase plays a key role in the biorefinery process; hence, its production and hydrolytic efficiency must be enhanced by finding new microorganisms which can produce isoforms of xylanases. Overexpression of new genes from novel xylanases from different microorganisms can be explored for future applications. Hence, using the advantage of gene editing and synthetic biological techniques in the future, with improved characteristics like thermostability, can be a fruitful contribution towards the high demand of biorefinery.
References
Aditiya H, Mahlia TMI, Chong W, Nur H, Sebayang A (2016) Second generation bioethanol production: a critical review. Renew Sust Energ Rev 66:631–653
Agrawal K, Bhardwaj N, Kumar B, Chaturvedi V, Verma P (2019) Process optimization, purification and characterization of alkaline stable white laccase from Myrothecium verrucaria ITCC-8447 and its application in delignification of agroresidues. Int J Biol Macromol 125:1042–1055
Ang SK, Abd-Aziz S, MS, M. (2015) Potential uses of xylanase-rich lignocellulolytic enzymes cocktail for oil palm trunk (OPT) degradation and lignocellulosic ethanol production. Energy Fuel 29(8):5103–5116
Asgher M, Ahmad Z, Iqbal HMN (2013) Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind Crop Prod 44:488–495
Attri S, Garg G (2014) Isolation of microorganisms simultaneously producing xylanase, pectinase and cellulase enzymes using cost effective substrates. J Innov Biol 1(1):45–50
Azad AK, Rasul M, Khan MMK, Sharma SC, Hazrat M (2015) Prospect of biofuels as an alternative transport fuel in Australia. Renew Sustain Energy Rev 43:331–351
Bala A, Singh B (2016) Cost-effective production of biotechnologically important hydrolytic enzymes by Sporotrichum thermophile. Bioprocess Biosyst Eng 39(1):181–191
Bala A, Singh B (2019a) Cellulolytic and xylanolytic enzymes of thermophiles for the production of renewable biofuels. Renew Energy 136:1231–1244
Bala A, Singh B (2019b) Development of an environmental-benign process for efficient pretreatment and saccharification of Saccharum biomasses for bioethanol production. Renew Energy 130:12–24
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energ Conver Manage 52(2):858–875
Basotra N, Joshi S, Satyanarayana T, Pati PK, Tsang A, Chadha BS (2018) Expression of catalytically efficient xylanases from thermophilic fungus Malbranchea cinnamomea for synergistically enhancing hydrolysis of lignocellulosics. Int J Biol Macromol 108:185–192
Berka RM, Grigoriev IV, Otillar R, Salamov A, Grimwood J, Reid I, Ishmael N, John T, Darmond C, Moisan M-C (2011) Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat Biotechnol 29(10):922–927
Berlin A, Gilkes N, Kilburn D, Bura R, Markov A, Skomarovsky A, Okunev O, Gusakov A, Maximenko V, Gregg D (2005) Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates–evidence for the role of accessory enzymes. Enzyme Microb Technol 37(2):175–184
Bhandiwad A, Shaw AJ, Guss A, Guseva A, Bahl H, Lynd LR (2014) Metabolic engineering of Thermoanaerobacterium saccharolyticum for n-butanol production. Metab Eng 21:17–25
Bhardwaj N, Chanda K, Kumar B, Prasad HK, Sharma GD, Verma P (2017) Statistical optimization of nutritional and physical parameters for xylanase production from newly isolated aspergillus oryzae LC1 and its application in the hydrolysis of lignocellulosic agro-residues. Bioresources 12(4):8519–8538
Bhardwaj N, Kumar B, Agrawal K, Verma P (2019) Bioconversion of rice straw by synergistic effect of in-house produced ligno-hemicellulolytic enzymes for enhanced bioethanol production. Bioresour Technol Rep 10:100352
Bhardwaj N, Kumar B, Verma P (2020) Microwave-assisted pretreatment using alkali metal salt in combination with orthophosphoric acid for generation of enhanced sugar and bioethanol. Biomass Convers Biorefin 2:1–8
Bolado-Rodríguez S, Toquero C, Martín-Juárez J, Travaini R, García-Encina PA (2016) Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour Technol 201:182–190
Boonchuay P, Techapun C, Leksawasdi N, Seesuriyachan P, Hanmoungjai P, Watanabe M, Takenaka S, Chaiyaso T (2018) An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresour Technol 256:399–407
Brienzo M, Monte J, Milagres AMF (2012) Induction of cellulase and hemicellulase activities of Thermoascus aurantiacus by xylan hydrolyzed products. World J Microbiol Biotechnol 28(1):113–119
Cervantes-Cisneros DE, Arguello-Esparza D, Cabello-Galindo A, Picazo B, Aguilar CN, Ruiz HA, Rodríguez-Jasso RM (2017) Hydrothermal processes for extraction of macroalgae high value-added compounds. In: Hydrothermal processing in biorefineries. Springer, Cham, pp 461–481
Chaturvedi V, Verma P (2013) An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 3(5):415–431
Cherubini F (2010) The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energ Conver Manage 51(7):1412–1421
Ciudad G, Rubilar O, Azócar L, Toro C, Cea M, Torres Á, Ribera A, Navia R (2014) Performance of an enzymatic extract in Botryococcus braunii cell wall disruption. J Biosci Bioeng 117(1):75–80
Cunha FM, Badino AC, Farinas CS (2017) Effect of a novel method for in-house cellulase production on 2G ethanol yields. Biocatal Agric Biotechnol 9:224–229
Damis SIR, Murad AMA, Bakar FDA, Rashid SA, Jaafar NR, Illias RM (2019) Protein engineering of GH11 xylanase from Aspergillus fumigatus RT-1 for catalytic efficiency improvement on kenaf biomass hydrolysis. Enzyme Microb Technol 131:109383
De Jong E, van Ree R, Kwant IK (2009) Biorefineries: adding value to the sustainable utilisation of biomass. IEA Bioenergy 1:1–16
Diogo JA, Hoffmam ZB, Zanphorlin LM, Cota J, Machado CB, Wolf LD, Squina F, Damásio AR, Murakami MT, Ruller R (2015) Development of a chimeric hemicellulase to enhance the xylose production and thermotolerance. Enzyme Microb Technol 69:31–37
Dutta K, Daverey A, Lin J-G (2014) Evolution retrospective for alternative fuels: first to fourth generation. Renew Energy 69:114–122
Ehimen EA, Holm-Nielsen J-B, Poulsen M, Boelsmand J (2013) Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew Energy 50:476–480
Florencio C, Cunha FM, Badino AC, Farinas CS, Ximenes E, Ladisch MR (2016) Secretome analysis of Trichoderma reesei and aspergillus Niger cultivated by submerged and sequential fermentation processes: enzyme production for sugarcane bagasse hydrolysis. Enzyme Microb Technol 90:53–60
Fu C-C, Hung T-C, Chen J-Y, Su C-H, Wu W-T (2010) Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour Technol 101(22):8750–8754
Gasol CM, Gabarrell X, Anton A, Rigola M, Carrasco J, Ciria P, Solano M, Rieradevall J (2007) Life cycle assessment of a Brassica carinata bioenergy cropping system in southern Europe. Biomass Bioenergy 31(8):543–555
Geddes CC, Nieves IU, Ingram LO (2011) Advances in ethanol production. Curr Opin Biotechnol 22(3):312–319
Gervais P, Molin P (2003) The role of water in solid-state fermentation. Biochem Eng J 13(2–3):85–101
Gessesse A (1998) Purification and properties of two thermostable alkaline xylanases from an Alkaliphilic bacillus sp. Appl Environ Microbiol 64(9):3533–3535
Haitjema CH, Solomon KV, Henske JK, Theodorou MK, O'Malley MA (2014) Anaerobic gut fungi: advances in isolation, culture, and cellulolytic enzyme discovery for biofuel production. Biotechnol Bioeng 111(8):1471–1482
Haki G, Rakshit S (2003) Developments in industrially important thermostable enzymes: a review. Bioresour Technol 89(1):17–34
Hassan SS, Williams GA, Jaiswal AK (2019) Moving towards the second generation of lignocellulosic biorefineries in the EU: drivers, challenges, and opportunities. Renew Sustain Energy Rev 101:590–599
Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315(5813):804–807
Huang D, Liu J, Qi Y, Yang K, Xu Y, Feng L (2017) Synergistic hydrolysis of xylan using novel xylanases, β-xylosidases, and an α-l-arabinofuranosidase from Geobacillus thermodenitrificans NG80-2. Appl Microbiol Biotechnol 101(15):6023–6037
Hwang S-S, Lee S-J, Kim H-K, Ka J-O, Kim K-J, Song H-G (2008) Biodegradation and saccharification of wood chips of Pinus strobus and Liriodendron tulipifera by white rot fungi. J Microbiol Biotechnol 18:1819–1825
Isroi I, Millati R, Niklasson C, Cayanto C, Taherzadeh MJ, Lundquist K (2011) Biological treatment of lignocelluloses with white-rot fungi and its applications. Bioresources 6(4):5224–5259
Jain KK, Dey TB, Kumar S, Kuhad RC (2015) Production of thermostable hydrolases (cellulases and xylanase) from Thermoascus aurantiacus RCKK: a potential fungus. Bioprocess Biosyst Eng 38(4):787–796
Juodeikiene G, Basinskiene L, Vidmantiene D, Makaravicius T, Bartkiene E, Schols H (2011) The use of β-xylanase for increasing the efficiency of biocatalytic conversion of crop residues to bioethanol. Catal Today 167(1):113–121
Kamat S, Khot M, Zinjarde S, Ravi Kumar A, Gade WN (2013) Coupled production of single cell oil as biodiesel feedstock, xylitol and xylanase from sugarcane bagasse in a biorefinery concept using fungi from the tropical mangrove wetlands. Bioresour Technol 135:246–253
Kaur G, Satyanarayana T (2004) Production of extracellular pectinolytic, cellulolytic and xylanolytic enzymes by thermophilic mould Sporotrichum thermophile Apinis in solid state fermentation. Indian J Biotechnol 3:552–557
Kaur G, Kumar S, Satyanarayana T (2004) Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresour Technol 94(3):239–243
Kim KH, Choi IS, Kim HM, Wi SG, Bae H-J (2014) Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour Technol 153:47–54
Kolasa M, Ahring BK, Lübeck PS, Lübeck M (2014) Co-cultivation of Trichoderma reesei RutC30 with three black aspergillus strains facilitates efficient hydrolysis of pretreated wheat straw and shows promises for on-site enzyme production. Bioresour Technol 169:143–148
Koskinen PE, Beck SR, Örlygsson J, Puhakka JA (2008) Ethanol and hydrogen production by two thermophilic, anaerobic bacteria isolated from Icelandic geothermal areas. Biotechnol Bioeng 101(4):679–690
Kulkarni N, Rao M (1996) Application of xylanase from alkaliphilic thermophilic bacillus sp. NCIM 59 in biobleaching of bagasse pulp. J Biotechnol 51(2):167–173
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729
Kumar B, Bhardwaj N, Alam A, Agrawal K, Prasad H, Verma P (2018) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Express 8(1):173
Madadi M, Tu Y, Abbas A (2017) Recent status on enzymatic saccharification of lignocellulosic biomass for bioethanol production. Electron J Biol 13(2):135–143
Mahajan C, Chadha B, Nain L, Kaur A (2014) Evaluation of glycosyl hydrolases from thermophilic fungi for their potential in bioconversion of alkali and biologically treated Parthenium hysterophorus weed and rice straw into ethanol. Bioresour Technol 163:300–307
Mahdy A, Mendez L, Ballesteros M, González-Fernández C (2014) Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energ Conver Manage 85:551–557
Maiti S, Gallastegui G, Suresh G, Pachapur VL, Brar SK, Le Bihan Y, Drogui P, Buelna G, Verma M, Galvez-Cloutier R (2018) Microwave-assisted one-pot conversion of agro-industrial wastes into levulinic acid: an alternate approach. Bioresour Technol 265:471–479
Martín M, Grossmann IE (2012) Optimal synthesis of sustainable biorefineries. In: Integrated biorefineries design analysis and optimization. CRC, New York, pp 325–347
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5(5):597–609
McLachlan MJ, Sullivan RP, Zhao H (2009) Directed enzyme evolution and high throughput screening. In: Biocatalysis for the pharmaceutical industry-discovery, development, and manufacturing. Wiley, Singapore, pp 45–64
Mohanram S, Amat D, Choudhary J, Arora A, Nain L (2013) Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustain Chem Process 1(1):15
Mooney CA, Mansfield SD, Touhy MG, Saddler JN (1998) The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresour Technol 64(2):113–119
Morone A, Pandey R (2014) Lignocellulosic biobutanol production: gridlocks and potential remedies. Renew Sustain Energy Rev 37:21–35
Mustafa AM, Poulsen TG, Sheng K (2016) Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl Energy 180:661–671
Nakayama S, Kiyoshi K, Kadokura T, Nakazato A (2011) Butanol production from crystalline cellulose by cocultured Clostridium thermocellum and clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microbiol 77(18):6470–6475
Nigam PS (2013) Microbial enzymes with special characteristics for biotechnological applications. Biomol Ther 3(3):597–611
Öhgren K, Bura R, Saddler J, Zacchi G (2007) Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour Technol 98(13):2503–2510
Pachapur VL, Brar SK, Le Bihan Y (2019) Wood hydrolysate biorefinery: improvements and tailor-made two-step strategies on hydrolysis techniques. Bioresour Technol 299:122632
Paës G, O’Donohue MJ (2006) Engineering increased thermostability in the thermostable GH-11 xylanase from Thermobacillus xylanilyticus. J Biotechnol 125(3):338–350
Pandey AK, Edgard G, Negi S (2016) Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew Energy 98:51–56
Pareek N, Gillgren T, Jönsson LJ (2013) Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour Technol 148:70–77
Plácido J, Capareda S (2015) Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Bioresour Bioprocess 2(1):23
Prasertsan P, Khangkhachit W, Duangsuwan W, Mamimin C, Sompong O (2017) Direct hydrolysis of palm oil mill effluent by xylanase enzyme to enhance biogas production using two-steps thermophilic fermentation under non-sterile condition. Int J Hydrogen Energy 42(45):27759–27766
Quiñones TS, Retter A, Hobbs PJ, Budde J, Heiermann M, Plöchl M, Ravella SR (2015) Production of xylooligosaccharides from renewable agricultural lignocellulose biomass. Biofuels 6(3–4):147–155
Rani DS, Nand K (2000) Production of thermostable cellulase-free xylanase by clostridium absonum CFR-702. Process Biochem 36(4):355–362
Rastogi S, Soni R, Kaur J, Soni SK (2016) Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour Technol 222:458–469
Reinhardt GA, Rettenmaier N, Gärtner SO, Pastowski A (2007) Rain forest for biodiesel?: ecological effects of using palm oil as a source of energy. Wuppertal Institut für Klima, Wuppertal
Ribeiro LF, De Lucas RC, Vitcosque GL, Ribeiro LF, Ward RJ, Rubio MV, Damásio AR, Squina FM, Gregory RC, Walton PH (2014) A novel thermostable xylanase GH10 from Malbranchea pulchella expressed in aspergillus nidulans with potential applications in biotechnology. Biotechnol Biofuels 7(1):115
Saba N, Jawaid M, Hakeem K, Paridah M, Khalina A, Alothman O (2015) Potential of bioenergy production from industrial kenaf (Hibiscus cannabinus L.) based on Malaysian perspective. Renew Sustain Energy Rev 42:446–459
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5(4):337–353
Sakthiselvan P, Madhumathi R, Partha N (2015) Eco friendly bio-butanol from sunflower oil sludge with production of xylanase. Eng Agric Environ Food 8(4):212–221
Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez ÁT, Martínez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour Technol 102(16):7500–7506
Sanjivkumar M, Silambarasan T, Balagurunathan R, Immanuel G (2018) Biosynthesis, molecular modeling and statistical optimization of xylanase from a mangrove associated actinobacterium Streptomyces variabilis (MAB3) using Box-Behnken design with its bioconversion efficacy. Int J Biol Macromol 118:195–208
Shariq M, Sohail M (2019) Application of Candida tropicalis MK-160 for the production of xylanase and ethanol. J King Saud Univ-Scii 31(4):1189–1194
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valoriz 10(2):235–251
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass–an overview. Bioresour Technol 199:76–82
Singh B (2016) Myceliophthora thermophila syn. Sporotrichum thermophile: a thermophilic mould of biotechnological potential. Crit Rev Biotechnol 36(1):59–69
Singh A, Patel AK, Adsul M, Mathur A, Singhania RR (2017) Genetic modification: a tool for enhancing cellulase secretion. Biofuel Res J 4(2):600–610
Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T (2005) Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J Biosci Bioeng 100(6):637–643
Taylor MP, Eley KL, Martin S, Tuffin MI, Burton SG, Cowan DA (2009) Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol 27(7):398–405
Terrone CC, de Freitas C, Terrasan CRF, de Almeida AF, Carmona EC (2018) Agroindustrial biomass for xylanase production by Penicillium chrysogenum: purification, biochemical properties and hydrolysis of hemicelluloses. Electron J Biotechnol 33:39–45
Teter S, Sutton KB, Emme B (2014) Enzymatic processes and enzyme development in biorefining. In: Advances in biorefineries. Elsevier, Amsterdam, pp 199–233
Thomas L, Joseph A, Gottumukkala LD (2014a) Xylanase and cellulase systems of Clostridium sp.: an insight on molecular approaches for strain improvement. Bioresour Technol 158:343–350
Thomas L, Ushasree MV, Pandey A (2014b) An alkali-thermostable xylanase from Bacillus pumilus functionally expressed in Kluyveromyces lactis and evaluation of its deinking efficiency. Bioresour Technol 165:309–313
Thomas L, Parameswaran B, Pandey A (2016) Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew Energy 98:9–15
Ulaganathan K, Goud S, Reddy M, Kayalvili U (2017) Genome engineering for breaking barriers in lignocellulosic bioethanol production. Renew Sustain Energy Rev 74:1080–1107
Vaishnav N, Singh A, Adsul M, Dixit P, Sandhu SK, Mathur A, Puri SK, Singhania RR (2018) Penicillium: the next emerging champion for cellulase production. Bioresour Technol Rep 2:131–140
Valášková V, Baldrian P (2006) Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res Microbiol 157(2):119–124
Vanegas C, Hernon A, Bartlett J (2015) Enzymatic and organic acid pretreatment of seaweed: effect on reducing sugars production and on biogas inhibition. Int J Ambient Energy 36(1):2–7
Verhaart MR, Bielen AA, Oost Jvd, Stams AJ, Kengen SW (2010) Hydrogen production by hyperthermophilic and extremely thermophilic bacteria and archaea: mechanisms for reductant disposal. Environ Technol 31(8–9):993–1003
Visser EM, Leal TF, de Almeida MN, Guimarães VM (2015) Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling. Biotechnol Biofuels 8(1):5
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30(6):1447–1457
Wei W, Wu S, Liu L (2012) Enzymatic saccharification of dilute acid pretreated eucalyptus chips for fermentable sugar production. Bioresour Technol 110:302–307
Wood IP, Cook NM, Wilson DR, Ryden P, Robertson JA, Waldron KW (2016) Ethanol from a biorefinery waste stream: saccharification of amylase, protease and xylanase treated wheat bran. Food Chem 198:125–131
Xin F, He J (2013) Characterization of a thermostable xylanase from a newly isolated Kluyvera species and its application for biobutanol production. Bioresour Technol 135:309–315
Xue D, Zeng X, Lin D, Yao S (2019) Thermostable ethanol tolerant xylanase from a cold-adapted marine species Acinetobacter johnsonii. Chin J Chem Eng 27(5):1166–1170
Yang M, Zhang J, Kuittinen S, Vepsäläinen J, Soininen P, Keinänen M, Pappinen A (2015) Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone–butanol–ethanol fermentation. Bioresour Technol 189:131–137
Yang Y, Yang J, Liu J, Wang R, Liu L, Wang F, Yuan H (2018) The composition of accessory enzymes of Penicillium chrysogenum P33 revealed by secretome and synergistic effects with commercial cellulase on lignocellulose hydrolysis. Bioresour Technol 257:54–61
Zanuso E, Lara-Flores AA, Aguilar DL, Velazquez-Lucio J, Aguilar CN, Rodríguez-Jasso RM, Ruiz HA (2017) Kinetic modeling, operational conditions, and biorefinery products from hemicellulose: depolymerization and solubilization during hydrothermal processing. In: Hydrothermal processing in biorefineries. Springer, Cham, pp 141–160
Zeldes BM, Keller MW, Loder AJ, Straub CT, Adams MW, Kelly RM (2015) Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals./ Front Microbiol 6:1209
Zhang J, Tang M, Viikari L (2012) Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour Technol 121:8–12
Competing Interests
All the authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhardwaj, N., Verma, P. (2021). Xylanases: A Helping Module for the Enzyme Biorefinery Platform. In: Srivastava, M., Srivastava, N., Singh, R. (eds) Bioenergy Research: Revisiting Latest Development. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-33-4615-4_7
Download citation
DOI: https://doi.org/10.1007/978-981-33-4615-4_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-4614-7
Online ISBN: 978-981-33-4615-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)