Abstract
Lignocellulosic biomass (LCB) stands out as an abundant, inexpensive, and promising renewable source of energy that can be used to produce fuels and value-added products. LCB comprises of majorly 3 components: cellulose, hemicellulose, and lignin. These three components can be further transformed into commercially viable and sustainable products like ethanol, xylitol, acetic acid, glutamic acid, glucuronic acid, succinic acid, and vanillin and thus can contribute significantly towards developing cost-effective integrated biorefineries. Among these, xylitol has been a tremendously increasing area of interest. With having no petrochemical alternative, xylitol turns out to be one of the highest valued products which may be produced by utilizing lignocellulosic biomass. Its large-scale production is still carried out through chemical route by dehydrogenation of xylose under high pressure and temperature. Biotechnological route is the potential substitute for chemical route as it involves milder process conditions and can utilize both industrial and agricultural wastes thereby reducing the overall production cost. However, biological scheme has not been adopted yet at the industrial scale. This review focusses on the recent advances in production of xylitol using yeasts. Special emphasis is given on pretreatment and detoxification methods, critical growth parameters, various fermentation strategies, metabolic engineering, and product recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Xylitol is a pentose alcohol that has higher sweetening ability and fewer calories than sucrose. It has a wide application in pharmaceutical, food, nutraceuticals, and beverage industries [1, 2]. It is chemically less reactive than its corresponding ketose and aldose, thus preventing microbial degradation. It prevents dental and ear infection in kids. It is used as an alternative to sugar for patients suffering from diabetes, as it has lower glycemic index and does not trigger the insulin metabolism pathway [3, 4]. It has high solubility and cooling power, anticariogenic effect, and does not alter the food dietary values [5, 6]. It is also identified as one of the top 12 platform chemicals, as it serves as a building block for various industrially important chemicals like glycols, lactic acid, and xylaric acids [7]. The market of xylitol is expanding due to increase in awareness for health and naturally derived sugar-free products. It accounts for 12% share of the total sugar alcohol market and it is expected to grow threefold by 2025 [8].
Chemical route has been ongoing for nearly four decades for the industrial production of xylitol. This route involves the catalytic hydrogenation of pure d-xylose using Ni/Al2O3 as a catalyst under high temperature (80–140 °C) and pressure (up to 50 atm) [4]. Chemical route has several drawbacks like high energy requirements, high operating cost, extensive separation, and purification steps. Recently, biotechnological production of xylitol is garnering its importance due to the bioconversion of LCB with specific microbial strain to obtain xylitol at relatively milder conditions. The biological route is eco-friendly due to lower chemical discharge into the environment, lesser corrosion of the reactors, and lower carbon foot print [9]. It provides a better substitute in terms of energy requirements thereby may significantly reduce the overall process cost [10]. Moreover, this route can also be integrated with the second generation (2G) ethanol production plant where the hemicellulose fraction of LCB can be utilized to produce xylitol to compensate the cost of ethanol. Despite of having an edge over the chemical route, xylitol production from biotechnological route is not employed at the commercial scale. To make this process commercially feasible and bridge the gap between lab and industrial scale production, significant amount of research has been done in the previous decade.
This review is an overview of recent studies on biotechnological production of xylitol. It mainly focusses on the bioprocess aspects of xylitol production by fermentation using yeasts. Several critical parameters and different fermentation strategies that may significantly enhance xylitol productivity are discussed.
2 Applications of xylitol
Food and Drug Administration (FDA) USA has considered xylitol as “Generally Recognized as Safe” (GRAS) and “safe for teeth” since 1986 and 1994 respectively [11]. Xylitol is a potential substitute for sucrose in the food products like cookies [12], cupcakes [2], chewing gums [13], and chocolates [14] for reduction in calories intake. In pharmaceutical industries, its application has become widespread because of its anticariogenic effect, that can decrease tooth decay significantly [6]. It is very effective against bacterium that forms oral film like Streptococcus mutans [15], and other microorganisms that have an adverse effect upon oral health like Haemophilus influenzae and Streptococcus pneumoniae [16], Staphylococcus aureus [17], and Pseudomonas aerugionosa [18]. It is also used in the tooth calcification which is mainly caused by aging [19]. There are literatures which have reported it to be beneficial in treating many ailments like anemia [20], diabetes [21], acute otitis media [22], and osteoporosis [23].
3 Market trends
Since 1960s, the time when Finnish company initially produced xylitol, there has been a substantial expansion in its market [24]. In 2019, the market size of xylitol surpassed USD 880 million globally [25]. It is likely to increase to a projected value of USD 1404.31 million by 2026, thus observing a significant CAGR in the estimate period of 2019–2026 [26]. Application-wise xylitol is majorly employed for chewing gum production followed by confectionery products as they account for 70% of the xylitol consumption all over the world [11]. In Asia itself, 80–90% of xylitol is used for chewing-gum production [25].
China and the USA are the largest global suppliers and producers of industrial xylitol via catalysis using corn cobs and hardwoods like birch respectively [8]. Major global producers of xylitol are tabulated in Table 1. DuPont Danisco is currently the leading producer of xylitol with its three plants in China and the USA [25]. It utilizes xylose obtained from hardwood of paper and pulp plant to produce xylitol at commercial scale via catalysis [8]. The other major market participants are DFI Corporation, Shandong Futaste Co., Ltd., Xylitol Canada, Inc., Roquette Freres, and Zuchem Inc. Several commercial producers like Thomson Biotech (Xiamen) Co., Ltd. and ZuChem Inc. have begun to incorporate microbial fermentation processes in addition to the chemical processes for economical xylitol production [27]. Xylitol can be used as platform chemical in the making of glycol, xylaric acid, 1–2-propanediol, and hydroxyl furan. Thus, in the coming future, the increase in demands for these chemicals will eventually propel the demand of xylitol. Since xylitol can serve as the low-cost substitute for petrochemicals, it may lead to have a positive influence upon bio business market globally [27].
4 Chemical xylitol production
Industrially, production of xylitol is carried out via catalytic hydrogenation of pure xylose from hemicellulose hydrolysate [28]. This involves five major steps: The first one is acid hydrolysis to break the hemicellulosic fraction of lignocellulosic biomass [25]. The next step involves the purification of pentose sugar rich hydrolysate via activated carbon and ion-exchange methods to obtain pure xylose [26]. Third step involves the catalytic hydrogenation of pure xylose at elevated pressure (up to 50 atm) and temperature (80–140 °C). Fourth step is purification of obtained xylitol solution, and fifth is crystallization of xylitol [26, 27]. The effectiveness of chemical process from initial lignocellulosic biomass is 8–15%, from xylan is 50–60%, and from pure xylose is around 98% [28]. This process gives high yield and efficiency, but it poses several drawbacks like requirement of expensive and specialized equipment, wide ranging intermediate purification steps, deactivation of catalyst and its costly recovery, product recovery, and high energy demands, thus increasing the cost of overall process [11]. The manufacturing cost of xylose and xylitol after hydrogenation process is around $2300–2500/t and $350/t respectively [29]. Thus, production of xylose crystals represents more than 80% whereas hydrogenation represents less than 20% of the total xylitol production cost respectively [30]. The key reasons accountable for the higher production cost of xylose includes the following: First one is the requirement of tedious and complex purification steps for removal of different saccharides and non-sugar components from hemicellulosic hydrolysate. If hemicellulosic hydrolysate including saccharides undergoes hydrogenation, then all saccharides would result in their equivalent alcohols. The presence of alcohols would pose a challenge in xylitol recovery via crystallization. Second is the resemblances between physicochemical properties xylose and sugar impurities which might impede the xylose crystals formation. Around 20–30% of xylose remains in mother liquor after crystallization, and there is no commercially viable process of xylose recovery from it [29]. Thus, there is a tremendous scope for biotechnological route for addressing the challenges posed by chemical route in xylitol production. Figure 1 illustrates the schematic of key steps involved in xylitol production via chemical and biotechnological route.
5 Biotechnological xylitol production
Biotechnological production of xylitol is one of the prominent alternatives to the conventional chemical route as it requires milder pressure and temperature conditions. It can be based on lignocellulosic hydrolysates to save on substrate purification and energy costs. The biotechnological conversion is mainly carried out by employing the microorganisms or by using enzymes. In this review, microbial xylitol production using lignocellulosic biomass is mainly discussed. Production of xylitol via microorganisms broadly involves hydrolysis of lignocellulosic biomass, detoxification, fermentation of hemicellulosic hydrolysate, and recovery of the product.
Biotechnological route has potential to alleviate the cost of xylitol production. Unlike chemical route, this involves only one step of crystallization (xylitol recovery from fermentation broth).
Although hemicellulosic hydrolysate requires detoxification before fermentation, however organic acids and inorganic elements present in hydrolysate are utilized by the microorganisms as nutrients. Thus, overall cost of purification of fermentation broth to xylitol crystal is lesser than the cost of production of pure xylose crystals required in chemical route. In fermentation process, complete consumption of glucose and xylose promotes purification of xylitol even in the occurrence of other impure monosaccharides (arabinose and galactose) whereas the chemical route employs the complex separation process for segregating xylitol and xylose.
Table 2 summarizes the key differences between the chemical and microbial xylitol production. Enzymatic production process has also gained much attention as it has the potential of converting 100% of xylose to xylitol, as there is no requirement for any metabolic diversion for cell maintenance [31]. However, due to high cofactor requirements, its large-scale production may not be feasible and cost effective [11].
6 Hydrolysis of lignocellulosic biomass
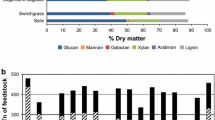
Pretreatment is the one of the most important steps for fractionating out of the three components of lignocellulosic biomass, i.e., cellulose, hemi-cellulose, and lignin which are further processed to produce biofuels and value-added chemicals. On large scale, cost-effective pretreatment technologies are not yet fully developed due to which transformation of lignocellulosic biomass into biochemicals is restricted [32]. Pretreatment methods should be selected in such a way that they are of low cost, preserve the hemi-cellulose fractions, and restrict the generation of inhibitors. One of the key holdups that affects techno-economic feasibility of xylitol production is lack of inexpensive pretreatment methods for efficient recovery of xylose. In the recent years, efforts are being continuously made in the direction of reducing the cost, enhancing the efficiency of current pretreatments, and in developing economically viable methods. Biorefineries may play a vital part in decreasing the effect of this step on overall manufacturing cost by incorporating different bioprocesses. Due to the complex structure of hemicellulose along with xylose, multiple compounds like phenolic or aliphatic acids, furaldehydes, and weak acids are also produced. These act as inhibitors to microorganism during the fermentation process. In this review, the pretreatment methods targeting high recovery of xylose from lignocellulosic biomass are only being discussed. Table 3 summarizes different compositions of sugars obtained from the hydrolysis of hemicellulose.
6.1 Acid hydrolysis
Acid hydrolysis is one of the most popular pretreatment methods reported in literatures for producing xylitol [10, 42, 43]. It is rapid and simple method which can be done by dilute acid or highly concentrated acid. Generally, dilute acid hydrolysis is preferred over highly concentrated acid due to low cost, effectiveness in disruption of lignocellulosic matrix to give hemicellulosic sugar, and high reaction rate. For acid hydrolysis process, sulfuric acid is mainly used among the other acids as it is comparatively lesser corrosive to equipment at low concentration, cost-effective, and less volatile. Parameters like reaction time, pressure, solid–liquid loading, temperature, and varied concentrations of acid must be adjusted to enhance the xylose fraction in hydrolysate and to decrease the generation of inhibitors. Recently, Unrean and Ketsub [33] treated the sugarcane bagasse with 2.5% (w/v) H2SO4, 20% (w/v) solid loading for 30 min and obtained 20.04 gL−1 of xylose and 4.01 gL−1 of glucose. Zahed et al. [38] studied the effect of varying sulfuric acid concentration (w/v) (2%, 3.5%, and 5%) at 100 °C for the hydrolysis of rice straw and found out that xylose and glucose were obtained at the optimum acid concentration of 3.5%, 15.05, and 2.3 gL−1 respectively.
6.2 Autohydrolysis and steam explosion
Autohydrolysis and steam explosion pretreatments are operated at high pressure and temperature conditions. In autohydrolysis, fractionation takes place by using compressed hot water which is subjected to the temperature ranging from 150 to 230℃ [24]. The hydronium ions cleave the acetyl linkages from the hemicellulose to release acetic acid. The acetic acid acts as catalyst and facilitates the removal of sugars and oligosaccharides from hemicellulose [44, 45]. This process simultaneously solubilizes hemicellulose and removes water soluble extracts. After the autohydrolysis of the biomass, the total sugar released can be increased by subjecting it further to acid or enzymatic hydrolysis [46]. Santucci et al. [47] reported that the hydrothermal pretreatment favored the conversion of hemicellulose into sugars and oligomers when it was conducted at high temperature for 15 min as it reduced the generation of degraded products in hydrolysate. The conversion efficiency of hemicellulose to sugars and oligomers when subjected to autohydrolysis at 170℃ for 90 min was 61.7%. Koo et al. [48] proposed the modified two-stage autohydrolysis where they achieved 83.9% of total sugar recovery from sweet sorghum in comparison to the one-stage autohydrolysis where the recovery was 68.1%.
Steam explosion pretreatment involves the exposure of lignocellulosic biomass to high pressured steam for brief amount of time, and then subsequently rapid release of pressure [49]. Due to the process of flash evaporation, a thermochemical force is exerted by the water causing the biomass to rupture [50]. Liu and Chen [51] stated that the combined steam explosion and enzymatic digestion of corn stover resulted in 62.8% yield of xylose.
7 Detoxification of hemicellulosic hydrolysate
Fermentative xylitol production does not necessarily require xylose purification as this process is biologically selective. However, pretreatment may give rise to many toxic compounds which may hamper the growth of microorganisms; thus, there is a need of detoxification step for effective conversion of xylose to xylitol.
The degree of tolerance of inhibitors is dependent on inhibitor’s concentration, microorganism used and its degree of adaption, the presence of other inhibitors, and the fermentation process employed [25]. Phenolics which are formed due to the partial degradation of lignin are major inhibitors during acid hydrolysis. They damage the integrity of biological membrane [10]. Some common phenols include dihydroconiferyl alcohol, 4-hydroxybenzoic acid, coniferyl aldehyde, syringaldehyde, 4-hydroxybenzaldehyde, vanillin, and syringic acid [52]. There is an inverse relationship between the molecular weight of phenols and toxicity on yeasts. The toxicity of phenols is higher in comparison to carboxylic acids and furans as they can easily penetrate cell membrane because of their low molecular weight [53].
During acid hydrolysis, high temperature, pressure, and longer residence time result in degradation of xylose and hexose, which then form furfural and 5-HMF respectively. Both compounds alter the biological and enzymatic activities of microorganism, damages DNA, and restrict the synthesis of proteins and RNA [10]. Acetic acid formation takes place due to the dehydration of acetyl group in hemicellulose without any exception; therefore, there is a need of neutralization or detoxification before further steps [52]. Acetic acid enters the cell in undissociated form. It is further dissociated due to intracellular pH, which in turn increases acetate ions and protons concentration, and thus affects the proton gradient involved in energy production and nutrient transport [54, 55]. Apart from acetic acid, other acids like levulinic, acrylic acid and formic acid are generated by either HMF breakdown or by deacetylation of hemicellulose [52].
Besides the inhibitors generated from sugars and lignin, trace elements like iron, chromium, copper, and nickel may be present, from either soil, pretreatment reactor, or biomass and that may inhibit the growth of xylitol-producing microorganisms [54].
Various methods can be employed to reduce the concentration of toxic compounds depending upon raw materials, microorganisms employed, and on the hydrolytic process [10]. Detoxification methods like activated charcoal adsorption, neutralization, liquid, pH adjustment, overlimiting, biological detoxification, membrane separation, nanofiltration, extraction, electrochemical detoxification, and ion-exchange resins have been recently reviewed [10, 52].
Activated charcoal detoxification method is low cost and widely used since centuries. There have been several literatures which have reported the use of activated charcoal for various purification processes.
Sago trunk hydrolysate was detoxified via activated charcoal which reduced furfural and phenolic compounds by 53% and 78% respectively and improved the yield of xylitol [56]. Another recent study also reported that when acid hydrolysed corn cob was treated with activated charcoal, xylose content loss was less than 1% [57]. Combined pH adjustment and activated charcoal adsorption resulted in removal of 72.9% of 5-HMF, 89.3% of furfural, 35.6% of acetic acid, and 34.3% of phenolic compounds [58]. Kumar et al. [59] studied the effect of ion-exchange resin detoxification method on corncob hydrolysate and reported that resin treatment effectively removed 70% of phenolic content and 5-HMF and 70% of nitrate salt.
Recently, studies have been performed to entirely remove the detoxification step from the biotechnological route. This can be achieved either by improving the pretreatment methods in a way that the generation of toxic compounds is negligible, or by immobilization and adaptive evolution. Mishra and Ghosh [60] proposed modified fractional hydrolysis method, which yielded the maximum fermentable sugars in a separate fraction with minimum toxics generation. Four acids were selected (HNO3, H2SO4, H3PO4, and HCl) for 8-stage fractional hydrolysis. H2SO4 resulted in maximum extraction of pentose and hexose sugars with minimum toxic compounds generation. Jiang et al. [61] stated that after cell adaption and multiple recycle of the strain Candida maltose, high xylitol yield and productivity (0.73 gg−1 and 2 gl−1 h−1 respectively) were obtained from corncob. Pereira et al. [62] in their studies reported that the presence of ferulic acid, acetic acid, and syringaldehyde may influence the metabolism of Candida guilliermondii; however, their toxicity depended upon their concentration, hence complete removal of these compounds may not be required for effective xylitol production. Perna et al. [63] conducted a study about the tolerance of Meyerozyma guilliermondii towards inhibitors, and they found that the acetic acid (initial concentration 5 gL−1) was consumed as a substrate with fermenting sugars even in the presence of furfural. Immobilization of Candida tropicalis using calcium alginate could enhance the bioconversion of untreated and nondetoxified corncob hydrolysate to xylitol (yield 0.73 gg−1 and productivity 0.43 gL−1 h−1) [64]. For industrial production, the inclusion of detoxification step be a trade-off between cost of detoxification and profit on xylitol production efficiency.
8 Microorganisms and metabolic pathway
Xylitol production from the biological route is based on xylose assimilation in those microorganisms that have ability to ferment pentose. Microorganisms like fungi, bacteria, and yeast can convert both types of xylose either synthetic or the one in hydrolysate from lignocellulosic biomass to xylitol. Xylitol production by fungi and bacteria is comparatively less reported than that of yeast. A few bacteria, like Corynebacterium, Enterobacter liquefaciens sp., Gluconobacter oxydans, and Mycobacterium smegmatis, have been reported that convert xylose to xylitol [65]. Seventeen cultures of bacteria of genera Cellulomonas, Serratia, and Cornynebacterium were screened by Rangaswamy and Agblevor [66] for xylitol production. Corynebacterium sp. strain produced maximum xylitol with the yield of 0.57 gg−1 within 24 h from 75 gL−1 initial xylose concentration. Studies with filamentous fungi are very few. However, some researchers have reported that fungi like Hypocrea jecorina [67] and Trichoderma reesei [68] produced some amount xylitol. Among 11 fungi of genera Penicillium and Aspergillus, Penicillium crustosum resulted in maximum xylitol yield of 0.045 gg−1 [69].
Yeasts are considered as one of the best xylitol-producing organisms because of high assimilation rate of pentose sugars and high productivity when compared to bacteria and fungi. Xylitol is an intermediate product in the xylose metabolic pathway of the yeasts. Reduction and oxidation are two steps involved in xylose metabolism (Fig. 2). Firstly, D-xylose is transported across the cell membrane and subsequently gets reduced to xylitol via xylose reductase which is NAD(P)H-dependent enzyme (XR; EC 1.1.1.21). It further gets oxidized into xylulose via NAD+-dependent xylitoldehydrogenase (XDH; EC 1.1.1.9) before undergoing phosphorylation into xylulose-5-phosphate via xylulokinase (XK) [11]. Both steps are the rate-determining steps. Xylose reductase and xylitoldehydrogenase are the main enzymes that are involved in fermentation of xylose and xylitol production. Oxygen availability and its transfer rate play a vital role in regeneration of the cofactors. At higher oxygen level, oxidation of NADH to NAD+ takes place that results in the formation of xylulose. At lower oxygen level, NADH cannot be completely oxidized by electron transport system. Thus, restricting the activity of XDH eventually promotes the accumulation of xylitol but at the same time it affects cell growth and energy production. The accumulation of xylitol leads to the reduction in carbon flow through the pentoses phosphate pathway, which is the central metabolic pathway responsible for NADH regeneration and subsequently affecting xylose reductase [11]. Thus, it can be concluded that supply of oxygen plays an important role in xylitol production.
Xylose metabolic pathway in yeasts (based on [4])
Among the different xylitol-producing yeasts, the best xylitol producers are from genus Candida. Literatures have reported the conversion efficiencies of wild type Candida nearly 86 ± 1% for xylitol production. With this type of organism, the availability of oxygen is the key factor from D-xylose. Yablochkova et al. [70] evaluated the activity of XDH and XR in 11 species of yeast that included Pichia, Candida, Kluyveromyces, Pacchysolen, and Torulopsis. They reported that Candida tropicalis Y456 showed the highest specific activity (6.57 μmol min−1mgprotein−1) of XR. Misra et al. [71] screened 18 strains of yeast for xylitol production and found out that the Candida yeasts were the better producer of xylitol. More reported examples related to yield and productivity will be cited in the following sections.
9 Growth conditions
There are many factors that may affect the growth of microorganisms. To optimize these factors, certain conditions specific to each microorganism are needed to be adjusted. pH, temperature, inoculum concentration, aeration rate, and nutrients (nitrogen, carbon, and salts, etc.) are some of the widely studied factors that may influence the microbial growth. Effective control of these parameters plays a significant role in obtaining a good quality product. Many studies have focused upon optimal growth conditions for effective xylitol production and some are discussed below.
9.1 pH and temperature
Yeasts usually have a wide range of tolerance for pH variation from pH 2.5 to 8.0 [72]. pH plays a vital role in transport of xylose and maintains the protonation state of XR for its smooth catalytic activity [11]. Most of the xylitol-producing yeasts are reported to grow within the temperature range of 30–38℃. However, in few cases, there may be the requirement of high temperatures. One such yeast is Kluyveromyces marxianus which is highly thermotolerant that makes it different from other organisms involved in the fermentation studies. This is due to the faster enzymatic reactions and comparatively lower risk of contamination by other organisms [73]. (Rodrussamee et al. [74] studied the effect of temperature (30 °C, 40 °C, and 45 °C) on Kluyveromyces marxianus DMKU3-1042 for ethanol and xylitol production. Growth of cell and consumption of sugar were seen at all the temperatures. At 40 °C, production of xylitol was more than at 30 °C (7.0 g L−1 and 2.5 g L−1 respectively). From Indian distillery Wilkins et al. [75], isolated strains of K. marxianus (IMB2, IMB3, and IMB4)). They conducted experiments to observe the effect of varying pH values (4.5, 5.0, and 5.5) and high temperatures (40–45 °C) on the growth of three strains. It was found that at 45 °C and initial pH of 4.5 or 5, yeast strains IMB2 and IMB5 gave the highest yield of xylitol. da Silveiraet al. [76] conducted pH values (3–10) and temperatures (25–45 °C) study for xylitol production by newly isolated M. guilliermondii UFV-1. The optimum growth conditions for the strain were pH 8 and temperature 30 °C, although it continued to grow over the wide pH range till 45 °C. The optimal growth temperature and pH of Candida kefyr (ATCC 38,296) were studied using response surface methodology and obtained the values as 28.15ºC and 6.05 respectively [77]. Morais Junior et al. [72] selected 7 new Candida strains out of which C. tropicalis JA2 produced maximum xylitol. Using this strain, pH studies were done by adjusting pH of media to 4.6, 5.5, 6.0, 6.4, and 7.0. It was observed that all the xyloses were consumed at pH 6.4 after 42 h, whereas 87.5% of the sugar was consumed at pH 5.5, 6, and 7. There was reduction in xylose consumption by 22% at pH 4.6. After the optimization of all the factors, the yield for xylitol production was 0.86 and xylitol concentration was 109.5 gL−1. Many studies regarding xylitol production with Candida tropicalis have employed the temperature of 30–36 °C. Ping et al. [34] used C. tropicalis CCTCC M2012462 at 35 °C to produce xylitol from corncob with the productivity of 0.461 gL−1 h−1. Misra et al. [78] also used C.tropicalis to produce xylitol (11.89 gL−1) from corncob hydrolysate at 30 °C.
9.2 Carbon source
Lignocellulosic materials offer renewable, cheap, and abundant supply of carbon sources for manufacturing commercially valuable products. Sugarcane bagasse, corncobs, rice straw, cashew bagasse, sorghum, and sawdust are generally used as cheap substrate for microorganisms. The xylose obtained after the hydrolyses of hemicellulosic fraction is fermented to xylitol. Unrean and Ketsub [33] used sugarcane bagasse as substrate for the efficient coproduction of ethanol and xylitol with 0.44 gg−1 and 0.5 gg−1 yield respectively, thus demonstrating the integrated biorefinery approach. The utilization of corncob as a carbon source to produce xylitol has been reported in many studies [59, 61, 79]. Kumar et al. [57] used corncob to produce xylitol by Candida tropicalis MTCC 6192. With the maximum xylose recovery (56.4 gL−1) obtained after subjecting the acid pretreated hydrolysate to combined detoxification methods (activated charcoal, membrane process, and ion-exchange resin process), 0.62 gg−1 yield of xylitol was achieved. Recently, use of some novel carbon sources have also been reported. For instance, Abdul Manaf et al. [80] have used oil palm frund (OPF) as the potential carbon source for xylitol production. Upon subjecting it to mild nitric acid treatment, 18.4 g of xylose per 100 g of OPF was recovered. The obtained hydrolysate was then fermented by K. marxianus ATCC 36,907 which yielded 0.35 gg−1 xylitol. Xylitol and ethanol were simultaneously produced from cashew bagasse hydrolysate using Kluyveromyces marxianus CCA510, and xylitol concentration of 4.17 gL−1 was achieved [73]. Apart from lignocellulosic materials, artificial media containing xylose have also been used as main carbon source in many literatures. Srivani and Pydi Setty [81] used C. parapsilosis NCIM-3323 to ferment synthetic xylose to produce xylitol. After optimization of different process parameters (pH 3.5, temperature 30 °C, and initial xylose concentration 60 gL−1), 28.14 gL−1 of xylitol production was achieved.
9.3 Co-substrate
The utilization of co-substrate is a promising strategy for overcoming the NADPH regeneration constraint and subsequently its adverse impact on xylose conversion to xylitol. For production of xylitol, xylose is consumed under limited oxygen availability, which eventually has a negative impact on supply of NADPH and carbon intermediates. To improve the production of xylitol, use of supplementary carbon source is potential strategy. Apart from the carbon sources, the agro-industry byproducts can also be utilized as vitamins, nitrogen, and mineral sources which may bring down the cost of process. However, major studies on co-substrate utilization have been done using defined and semi-defined media. Tamburini et al. [82] used glucose, galactose, and maltose to study their influence on specific activity level of XR and XDH. It was found that glucose inhibited the reduction of xylose, whereas galactose induced utilization of xylitol for cell growth. Low concentration of maltose enhanced the biomass growth and xylitol accumulation significantly. In the co-substrate studies by Arruda and Felipe [83], glycerol was used as an additional substrate to produce xylitol by Candida guillermondii. Upon the addition of glycerol (0.7 gL−1) to semi-defined media consisting xylose, the xylitol productivity of 1.13 gL−1 h−1 with 0.78 gg−1yield was achieved. It also promoted the cellular growth.Hernández-Pérez et al. [84] utilized different sugars like sucrose, glycerol, and maltose as co-substrates to enhance the bioconversion of sugarcane straw hydrolysate into xylitol via Candida guilliermondii FTI20037. Xylose uptake rate was improved by 8.8% and 6.8% upon addition of 10 gL−1 sucrose and 0.7 gL−1 glycerol respectively. It was also observed that only sucrose addition increased the xylitol concentration to 36 gL−1. It was concluded that the process of xylitol production could be effective and economical by employing sucrose derived from lignocellulosic substrates. Recently, Pappu and Gummadi [85] developed a cybernetic model for estimation of xylitol production by Debaryomyces nepalensis NCYC 3413. The combination of 10 gL−1 of glucose and 90 gL−1 of xylose produced the maximum xylitol of 56.42 gL−1 with productivity 0.6 gL−1 h−1.
9.4 Aeration
Aeration is an important factor in the production of xylitol. Oxygen availability determines whether xylose metabolic pathway will be shifted to respiration or fermentation, thus controlling the consumption of carbon for growth and bioconversion. Recently, there has been a shift towards two-stage aeration from one-stage aeration for improving the xylitol production. In two-stage aeration, the first stage involves high aeration rate to attain high biomass concentration and second stage involves low aeration rate to produce high concentration of xylitol. Raj and Krishnan [86] recently optimized the two-stage aeration process for xylitol production using synthetic media by Candida tropicalis. The aeration rate for initial 12-h fermentation was 3.33 vvm. It was subsequently reduced to 0.33 vvm that resulted in 0.85 gL−1 h−1 xylitol productivity with 0.85 g/g yield. Misra et al. 2013 [78] also studied two-stage aeration on corncob hydrolysate using Candida tropicalis. At initial aeration rate of 0.7 vvm for 24 h followed by 0.3 vvm for 36 h, 0.58 gg−1 yield and 0.283 gL−1 h−1 productivity were observed. In another study for producing xylitol from corncob hydrolysate via Debaryomyces hansenii, two-stage aeration with 0.29 vvm at the initial time of fermentation and subsequently 0.096 vvm resulted in 0.39 gg−1 yield and 0.236 gL−1 h−1 productivity of xylitol [87]. Influence of agitation and aeration upon xylitol production by Kluyveromyces marxianus using tamarind seed was evaluated. At 0.1 vvm aeration rate in semi-anaerobic condition and agitation at 100 rpm, the xylitol yield of 0.71 gg−1 was reported [88].
9.5 Nitrogen source
Yeast extract, peptone, and urea are some of the most common organic nitrogenous sources whereas sources like ammonium nitrate, ammonium chloride, and ammonium sulfate are most common inorganic ones.
Among the two, organic sources are better as they have positive influence on cellular growth of xylitol-producing microorganisms. de Albuquerque et al. [73] studied the impact of various nitrogen sources on production of xylitol via K. marxianus CCA510 from cashew apple bagasse. Out of the three selected nitrogen sources, i.e., ammonium sulfate, urea, and yeast extract, addition of urea resulted in higher xylitol production (12.27 gL−1, which was around 18% higher than that of control) whereas higher biomass production was obtained with yeast extract (12.93 gL−1) followed by ammonium sulfate (10.58 gL−1) and urea (10.43 gL−1). Zhang et al. [89] also evaluated the influence of nitrogen sources on xylitol production by Candida athensensis SB18 from horticultural waste. The medium was added with various nitrogenous sources like ammonium nitrate, casamino acid, ammonium sulfate, and urea. It was observed that addition of urea improved the xylose conversion with higher yield of 0.75 gg−1.
10 Various fermentation configurations
For efficient xylitol production, many different modes of fermentation like batch, continuous, fed-batch, and recycle have been reported in recent years. There are different types of bioreactors like stirred tank, fluidized bed, airlift, and basket-type stirred tank reactor which utilize free, immobilized, and adapted cells for desired product formation. Table 4 summarizes the studies on xylitol production in different types of bioreactors. Studies on bioreactors and their mode of operations play a significant role in scaling up the process for xylitol production. Bioreactors provide a conducive environment for controlling fermentation parameters like dissolved oxygen concentration, temperature, and pH which regulates the biomass growth and subsequently enhances the production of xylitol. Table 5 summarizes the recent studies on xylitol production by wild strains of yeast with different configurations. In the following section, different modes of operations involved during the fermentation by xylitol-producing organisms are reviewed.
10.1 Batch configuration
This configuration is widely used to produce xylitol as it is simple to operate and contamination can be controlled easily. Most of the studies of batch configurations are done at the flasks level. Xu et al. [106] conducted the shake flask xylose fermentation via C. tropicalis 31949 from sugarcane bagasse to evaluate the effect of inoculum amount, initial xylose concentration, and inoculum quantity. The optimum conditions were determined as follows: initial xylose concentration 100 g/l inoculum age 26 h and inoculum amount 10% which produced 62.98 gL−1 xylitol. Morais Junior et al. [72] also did shake flask study to optimize the different growth parameters for producing xylitol via C. tropicalis JA2 from sugarcane bagasse. Fermentation under the optimal conditions resulted in xylitol concentration, yield, and productivity of 109.5 gL−1, 0.86 gg−1, and 2.81gL−1 h−1 respectively.Jia et al. [79] also reported the xylitol yield as 0.77 gg−1and productivity 2.45 gL−1 h−1 after carrying out the shake flask fermentation of pretreated (combined tetrabutylammonium hydroxide extraction and acid hydrolysis) corncob hydrolysate by Candida troplicalis. Apart from shake flask studies, batch configuration has been applied in bioreactors for efficient production of xylitol. Vaz de Arruda et al. [100] studied the fermentation using bench and pilot scale bioreactors to produce xylitol via Candida guillermondii from sugarcane baggasse. kLa (16 h−1) was chosen as the scale-up parameter and was applied in 2.4 L, 8 L, and 125 L reactors. All the experiments from bench to pilot scale level demonstrated a minimum yield of 0.6 gg−1. In all the fermentations, similar productivity values were observed (about 0.31gl−1 h−1), thus indicated that it was independent of reactor capacity. However, xylitol yield in 125 L reactor was 0.55 gg−1, almost 17% lesser than the yield for bench reactor (0.66 gg−1). Kumar et al. [59] fermented detoxified (ion-exchange resin) corncob hydrolysate via Candida tropicalis MTCC 6192 to produce xylitol. Xylose fermentation was conducted 14 L stirred tank bioreactor. After 90 h of batch fermentation, the highest xylitol yield and concentration obtained were 0.6 gg−1 and 29.6 g/L respectively. Zhang et al. [89] utilized hemicellulosic hydrolysate from horticultural waste to produce xylitol via Candida athensensis SB18. The fermentation process was done in 2 L stirred tank reactor by setting air flow rate, agitation speed, and temperature to 0.7 Lmin−1, 200 rpm, and 30 °C respectively. The maximum xylitol concentration obtained was 100 gL−1 with yield 0.89 gg−1 and 0.98 g−1 h−1 productivity.
10.2 Fed-batch configuration
In this configuration, substrate and nutrients are fed to reactor while no product leaves out of it. Problems related to substrate inhibition and cell growth can be resolved due to the controlled feeding rate. This strategy can be adopted for enhancing the productivity. Unrean and Ketsub [33] compared the batch and fed-batch configuration in shake flask for coproduction of xylitol and ethanol using sugarcane bagasse using Candida tropicalis and Saccharomyces cerevisiae respectively. The ethanol and xylitol concentration obtained in batch configuration were 41gL−1 and 6.8 gL−1. To enhance the yield of ethanol and xylitol, fed-batch with pulse feeding of pretreated bagasse and hemicellulose hydrolysate was applied which resulted in the maximum ethanol and xylitol concentration as 56.1 gL−1 and 24 gL−1 respectively. Ping et al. [34] fermented untreated corncob hydrolysate to produce xylitol via Candida tropicalis CCTCC M2012462. Fermentation experiments were conducted in 5L bioreactor using fed-batch configuration at 35 °C and 200 rpm. After 84 h, the maximum xylitol concentration, yield and productivity were 38.8gL−1, 0.7 gg−1, and 0.46 gL−1 h−1 respectively. Li et al. [103] proposed a double-stage fed-batch configuration to suppress the negative effects of inhibitory compounds during fermentation of corncob via Candida tropicalis to produce xylitol. The fermentation process was done in 3.7 L bioreactor. The two-stage fed-batch configuration resulted in xylitol concentration of 96.5 gL−1 with yield and productivity as 0.83 gg−1, 1.01 gL−1 h−1 respectively, 12.6% and 65.57% higher than that of batch configuration. Himabindu and Gummadi [107] produced xylitol by Debaromyces nepalensis using fed-batch mode. Xylose and nitrogenous source were fed intermittently and productivity of 0.43 gL−1 h−1 with 0.64 gg−1 yield. Sirisansaneeyakul et al. [108] employed high-cell density culture of Candida magnoliae TISTR 5663 for xylitol production by repeated fed-batch configuration in a 2 L stirred tank bioreactor under limited oxygen condition (aeration rate 1.0 vvm and agitation rate 300 rpm). The xylose and nitrogen were fed which resulted in xylitol yield of 0.727, 0.719, and 0.720 gg−1, respectively. The final concentration of xylitol ranged between 235 and 284 gL−1 and an average productivity was 1.149 gL−1 h−1 in the total duration of 750 h.
10.3 Continuous configuration
Continuous configuration helps in maintaining high productivities for extended periods of time as it substantially eliminates the idle times for discharge, charge, sterilization, and cleaning of vessel. Several growth-related parameters of microorganism could be easily regulated and monitored. For instance, the specific growth rate of organism can be controlled easily by changing the dilution rate at the steady state. The estimation of process parameters that affects the fermentation process like aeration rate is also possible with ease. It also offers extra stability in synthesis of products, henceforth many bioreactors have been operated at continuous mode to produce xylitol. Martínez et al. [109] fermented sugarcane baggase hydrolysate in a 1.25 L stirred tank reactor by Candida guilliermondii FTI 20,037. The maximum xylitol productivity of 0.7 gL−1 h−1 and yield of 0.58 gg−1 was achieved by employing lower kLa values of 10 and 20 h−1. Martínez et al. [110] also studied the influence of dilution rate and pH upon xylitol production by Candida guilliermondii FTI 20,037 in continuous configuration using sugarcane baggase hydrolysate. The maximum xylitol concentration of 28.7 gL−1 with 0.63 gg−1 yield was obtained by adjusting dilution rate, kLa, and pH to 0.01 h−1, 30 h−1, and 4 respectively. Zahed et al. [38] studied combined xylitol and ethanol production in a membrane bioreactor by fermenting rice straw hydrolysate using Candida tropicalis NCIM 3119 and Saccharomyces cerevisiae NCIM 3090 respectively. The continuous mode produced 55 gL−1 of ethanol and 31gL−1 of xylitol in comparison to the batch mode, where the concentration of ethanol and xylitol were 33.4 gL−1 and 25.1 gL−1 respectively. For sequential production of lactic acid and xylitol from vine shoot trimmings Salgado et al. [111] combined 2L and 10L CSTRs. After adjusting the dilution rate to 0.043 h−1, maximum xylitol yield and productivity of 0.55gg−1 and 0.218gL−1 h−1 respectively were achieved.
Continuous fermentation process also utilizes the immobilized cells as they offer good stability with high productivity, high cell density, and reusability. Ding [112] produced xylitol using immobilized cells of Candida sp. ZU04 in fluidized bed bioreactor from corn cob hydrolysate. Initially higher aeration rate (1.00 vvm) was set to accelerate the glucose consumption that resulted in rapid increase of cells in alginate within 24 h. The biomass in alginate increases rapidly within first 24 h due to the fast consumption of glucose at high aeration rate of 1.00 vvm. In the second phase, when aeration was lowered down to 0.3 vvm, xylitol productivity and yield were enhanced to 0.84 gL−1 h−1and 0.73 gg−1 respectively. Soleimani and Tabil [113] compared the bioconversion productivities of immobilized and free cells of C. guilliermondii FTI 20,037 for producing xylitol using oat hulls. When the continuous fermentation was done with free cells at 1.25 vvm, xylitol yield, productivity, and concentration obtained were 0.87 gg−1, 0.57 gL−1 h−1, and 55 gL−1. These results were almost comparable to the continuous fermentation employing immobilized cells.
10.4 Cell recycle
For the improvement of yield and productivity of a bioprocess, there is a requirement of running the bioreactors with high cell concentration than in traditional batch, fed batch, and continuous modes. Cell immobilization, recycle with centrifugation, and membrane-based recycle system have been efficiently employed to maintain the high cell density in bioreactors. Choi et al. [114] carried out cell recycle using hollow fiber membrane in the batch fermentation by Candida tropicalis for xylitol production. The optimized cell-recycle fermentation produced xylitol with yield, productivity, and concentration of 0.82 gg−1, 4.94 gL−1 h−1, and 189 gl−1 respectively. These results were 2.2 times higher in final concentration of product and 1.3 times higher in xylitol productivity compared with the batch culture.Kwon et al. [95] stated that the isolated strain Candida tropicalis KCTC 10,457 which was able to produce the xylitol in the fed-batch configuration with the productivity of 4.88 gL−1 h−1. In order to enhance the productivity of xylitol, the cells were recycled using submerged membrane bioreactors. After 10 rounds of recycle, very high yield and productivity of 0.85 and 12 gL−1 h−1 respectively were achieved. Cunha et al. [115] carried out repeated batch with cell recycle using entrapped cells of Candida guillermondii for xylitol production. After the third cycle, the maximum xylitol yield, concentration, and productivity were 0.77 gg−1, 39.7gL−1, and 0.53 gl−1 h−1 respectively were obtained. Figure 3 illustrates the different fermentation configurations for xylitol production.
11 Prospect of metabolic engineering for improving xylitol production
Xylitol-producing wild strains have exhibited good productivity from different lignocellulosic biomass. However, interventions targeting the modification of strains by metabolic engineering can potentially improve xylitol production. Although there has been progress made in metabolic engineering techniques, developing an unchanging and tolerant recombinant strain is one of the primary challenges that needs to be resolved. Some factors governing gene modification include XDH gene suppression, XR enzyme expression, co-factors availability, and heterologous xylose transporter expression. Partial or complete suppression of XDH gene can stop the xylitol to get oxidized into xylulose henceforth increase its accumulation [116]. This technique not only enhances the productivity but also diminishes the requirement of regulating the oxygen supply. However, for maintaining the cell growth, there is need of additional substrate along with xylose. Ko et al. [117] designed a mutant of Candida tropicalis by disrupting XDH gene. Addition of glycerol to xylose during the fermentation resulted in xylitol yield and productivity of 0.97 gg−1 and 3.2 gL−1 h−1 respectively. In another study by Pal et al. [118], mutant Debaryomyces hansenii was constructed after removal of XDH gene. In case of mutant, xylitol concentration was 2.5 times higher than that of native strain. XR is the main enzyme that is involved in the conversion of xylose to xylitol. Its activity can be increased by either improving the expression of innate enzyme or by inserting the heterologous gene [119]. Inclusion of robust constitutive promoter like the glyceraldehyde-3-phosphate dehydrogenase can improve XR expression [30]. Jeon et al. [120] constructed mutant Candida tropicalis by incorporating highly efficient XR codon from Neurospora crassa using pGAPDH promoter. Xylitol yield and productivity were increased by 62% and 73% respectively in comparison to the parent strain. Saccharomyces cerevisiae by itself cannot assimilate xylose; however, inclusion of genes of XR enzyme from Pichia and Candida has considerably improved the xylitol productivity of this strain [119, 121, 122]. Xylitol production may also be improved by increasing the availability of cofactor NADPH that indirectly enhances activity of XR enzyme. Enzymes 6PGDH and G6PDH are aimed for genetic alterations as they promote the production of NAPDH [30]. Overexpression of enzyme G6PD in modified Saccharomyces BJ3505 strain enhanced the amount of NADPH and improved the productivity of xylitol by 20% [123]. Ahmad et al. [124] disrupted glycerol kinase and coexpressed gcy1, 2, 3 genes from Scheffersomyces stipites in BSXDH-3 strain to increase production of NADPH. Due to sumptuous amount of cofactor, the obtained xylitol productivity was 30% more than parent strain. The transportation of xylose into the cell may limit its assimilation and xylitol productivity. Both xylose and glucose strive for identical transport systems, which prefer hexoses over pentoses. Subsequently, assimilation of xylose is restricted due to the prior consumption of glucose. Jeon et al. [125] modified the recombinant Candida tropicalis by expressing xylose transporter gene (AT5G59250) derived from Arabidopsis thaliana. Fermentation of xylose glucose mixture by this recombinant strain exhibited 29% and 25% higher xylose consumption rate and xylitol productivity respectively than control strain. In another study, Kluyveromyces marxianus YZJ074 was modified by over expressing three transporter genes (CiGXF1), (KmFPS1), (CiGXS1) which converted almost 100% xylose to xylitol [126].
12 Xylitol recovery and downstreaming
Recovery of the product is of utmost importance for determining the feasibility and viability of any process. There is a requirement of minimum but efficient steps of downstream process so as to produce cost-effective product. There have been several literatures aiming at recovery of highly purified xylitol [127]. The technique of crystallization is widely adapted for downstreaming of xylitol after chemical catalysis because pure xylitol is obtained in a single step with lower energy consumption in comparison to distillation and other purification routes [128]. Before crystallization, there may be the requirement of different purification steps, like clarification using activated charcoal, liquid–liquid extraction, ion-exchange chromatography, pH adjustment, membrane separation, and precipitation, which is well reported in the literature. The recovery of xylitol is expensive and complicated because of its low concentration and fermentation broth which consists of several impurities like cell debris, unreacted sugars (glucose, xylose and arabinose), and sugar alcohols like sorbitol and arabitol. Sampaio et al. [129] employed activated charcoal (20 g/l) for 1 h at 25 C for clarification of fermented broth which removed around 69% of total proteins and 94% of the impurities. Xylitol was almost completely recovered in the fermentation medium. Kresnowati et al. [130] carried out the xylitol purification using combined ultrafiltration and electrodeionization method that was able to remove 99% of biomass and microorganisms, > 46% of xylose, 99% pigment, > 90% acetic acid, and > 99% ionic impurities with 30–50% xylitol loss. Wei et al. [26] studied xylitol crystallization and purification from hemicellulosic hydrolysate of corn cob. The fermentation broth was blanched and desalted via activated carbon and ion-exchange resins. The addition of crystal seeds of xylitol to the supersaturated solution resulted in 60.2% crystallization yield and 95% purity.
13 Future recommendations and conclusion
This review mainly highlights the recent studies of xylitol production by wild strains of yeast. Lately, there has been a noteworthy boost in demand for xylitol due to its wide commercial application in various industrial sectors like food, pharmaceuticals, and dental-related products. Currently, the commercial production of xylitol is carried out by chemical route and will continue to grow and expand in the future. Due to issues of carbon foot print and high energy requirements, biotechnological production of xylitol can be a game changer, as it is not only eco-friendly and efficient but it can also be easily incorporated into biorefineries for meeting out the demand and supply of xylitol. However, the xylitol production through biotechnological route has been restricted to the lab scale. Although a large number of xylitol-producing wild strains are documented in the literatures, but their potential for efficient xylitol production with higher productivities has not been explored fully. Most of the recent studies have been at shake flask level in batch mode. Comparatively, there are very few literatures available on continuous and high-cell density culture, which have the potential to enhance the yield and productivity of xylitol-producing organisms and subsequently can be employed for the scale up studies at pilot level. Development of stable, inhibitor tolerant, and robust recombinant strain is another challenge that needs to be addressed. Unlike chemical plant, separate plant for xylitol production by biotechnological route is not cost-effective. Apart from improvement in fermentation strategies, there are different challenges that need to be addressed in the other main steps like pretreatment, detoxification, and xylitol purification for cost-competitive production. However, inclusion of xylitol in a biorefinery may add to the techno-economic feasibility of this process and subsequently can increase the profitability of biorefinery.
Data availability
Not applicable.
Code availability
Not applicable.
References
Maguire A, Rugg-Gunn AJ (2003) Xylitol and caries prevention - is it a magic bullet? Br Dent J 194:429–436. https://doi.org/10.1038/sj.bdj.4810022
Edelstein S, Smith K, Worthington A et al (2008) Comparisons of six new artificial sweetener gradation ratios with sucrose in conventional-method cupcakes resulting in best percentage substitution ratios. J Culin Sci Technol 5:61–74. https://doi.org/10.1300/J385v05n04_05
Elamin K, Sjöström J, Jansson H, Swenson J (2012) Calorimetric and relaxation properties of xylitol-water mixtures. J Chem Phys 136(10):104508. https://doi.org/10.1063/1.3692609
Chen X, Jiang ZH, Chen S, Qin W (2010) Microbial and bioconversion production of D-xylitol and its detection and application. Int J Biol Sci 6:834–844. https://doi.org/10.7150/ijbs.6.834
Ritter AV, Bader JD, Leo MC et al (2013) Tooth-surface-specific effects of xylitol: randomized trial results. J Dent Res 92:512–517. https://doi.org/10.1177/0022034513487211
Uittamo J, Nieminen MT, Kaihovaara P et al (2011) Xylitol inhibits carcinogenic acetaldehyde production by Candida species. Int J Cancer 129:2038–2041. https://doi.org/10.1002/ijc.25844
Werpy T, Petersen G (2004) Top value added chemicals from biomass Volume I. Us Nrel Medium: ED; Size: 76 pp. pages. https://doi.org/10.2172/15008859
Ravella SR, Gallagher J, Fish S, Prakasham RS (2012) Overview on commercial production of xylitol, economic analysis and market trends BT - D-Xylitol: fermentative production, application and commercialization. In: da Silva SS, Chandel AK (eds) Springer. Berlin Heidelberg, Berlin, Heidelberg, pp 291–306
Salgado JM, Converti A, Domínguez JM (2012) Fermentation strategies explored for xylitol production. D-Xylitol 161–191
Venkateswar Rao L, Goli JK, Gentela J, Koti S (2016) Bioconversion of lignocellulosic biomass to xylitol: an overview. Bioresour Technol 213:299–310. https://doi.org/10.1016/j.biortech.2016.04.092
Dasgupta D, Bandhu S, Adhikari DK, Ghosh D (2017) Challenges and prospects of xylitol production with whole cell bio-catalysis: a review. Microbiol Res 197:9–21. https://doi.org/10.1016/j.micres.2016.12.012
Winkelhausen E, Jovanovic-Malinovska R, Velickova E, Kuzmanova S (2007) Sensory and microbiological quality of a baked product containing xylitol as an alternative sweetener. Int J Food Prop 10:639–649. https://doi.org/10.1080/10942910601098031
Fisker HO, Nissen V (2006) Effect of gum base and bulk sweetener on release of specific compounds from fruit flavoured chewing gum. Dev Food Sci 43:429–432. https://doi.org/10.1016/S0167-4501(06)80101-9
Sokmen A, Gunes G (2006) Influence of some bulk sweeteners on rheological properties of chocolate. LWT - Food Sci Technol 39:1053–1058. https://doi.org/10.1016/j.lwt.2006.03.002
Lee SH, Choi BK, Kim YJ (2012) The cariogenic characters of xylitol-resistant and xylitol-sensitive Streptococcus mutans in biofilm formation with salivary bacteria. Arch Oral Biol 57:697–703. https://doi.org/10.1016/j.archoralbio.2011.12.001
Tapiainen T, Sormunen R, Kaijalainen T et al (2004) Ultrastructure of Streptococcus pneumoniae after exposure to xylitol. J Antimicrob Chemother 54:225–228. https://doi.org/10.1093/jac/dkh302
Akiyama H, Oono T, Huh WK et al (2002) Actions of farnesol and xylitol against Staphylococcus aureus. Chemotherapy 48:122–128. https://doi.org/10.1159/000064916
Ammons MCB, Ward LS, Dowd S, James GA (2011) Combined treatment of Pseudomonas aeruginosa biofilm with lactoferrin and xylitol inhibits the ability of bacteria to respond to damage resulting from lactoferrin iron chelation. Int J Antimicrob Agents 37:316–323. https://doi.org/10.1016/j.ijantimicag.2010.12.019
Imazato S, Ikebe K, Nokubi T et al (2006) Prevalence of root caries in a selected population of older adults in Japan. J Oral Rehabil 33:137–143. https://doi.org/10.1111/j.1365-2842.2006.01547.x
Wang R, Li L, Zhang B et al (2013) Improved xylose fermentation of Kluyveromyces marxianus at elevated temperature through construction of a xylose isomerase pathway. J Ind Microbiol Biotechnol 40:841–854. https://doi.org/10.1007/s10295-013-1282-6
Islam MS (2011) Effects of xylitol as a sugar substitute on diabetes-related parameters in nondiabetic rats. J Med Food 14:505–511. https://doi.org/10.1089/jmf.2010.0015
Danhauer JL, Kelly A, Johnson CE (2011) Is mother-child transmission a possible vehicle for xylitol prophylaxis in acute otitis media? Int J Audiol 50:661–672. https://doi.org/10.3109/14992027.2011.590824
Mattila PT, Kangasmaa H, Knuuttila MLE (2005) The effect of a simultaneous dietary administration of xylitol and ethanol on bone resorption. Metabolism 54:548–551. https://doi.org/10.1016/j.metabol.2004.11.011
Mohamad NL, Mustapa Kamal SM, Mokhtar MN (2015) Xylitol biological production: a review of recent studies. Food Rev Int 31:74–89. https://doi.org/10.1080/87559129.2014.961077
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. https://doi.org/10.1021/ie801542g
Wei J, Yuan Q, Wang T, Wang L (2010) Purification and crystallization of xylitol from fermentation broth of corncob hydrolysates. Front Chem Eng China 4:57–64. https://doi.org/10.1007/s11705-009-0295-1
Jaffe GM, Szkrybalo W, Weinert PH (1974) Process for producing xylose. Pat US 408
Delgado Arcaño Y, Valmaña García OD, Mandelli D et al (2020) Xylitol: a review on the progress and challenges of its production by chemical route. Catal Today 344:2–14. https://doi.org/10.1016/j.cattod.2018.07.060
Hou-Rui Z (2012) Key drivers influencing the large scale production of xylitol. In: D-Xylitol. Springer, pp 267–289
Felipe Hernández-Pérez A, de Arruda PV, Sene L et al (2019) Xylitol bioproduction: state-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit Rev Biotechnol 39:924–943. https://doi.org/10.1080/07388551.2019.1640658
Rafiqul ISM, Mimi Sakinah AM (2012) A perspective: bioproduction of xylitol by enzyme technology and future prospects. Int Food Res J 19:405–408
Chandel AK, Garlapati VK, Singh AK et al (2018) The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Bioresour Technol 264:370–381. https://doi.org/10.1016/j.biortech.2018.06.004
Unrean P, Ketsub N (2018) Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Ind Crops Prod 123:238–246. https://doi.org/10.1016/j.indcrop.2018.06.071
Ping Y, Ling HZ, Song G, Ge JP (2013) Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis. Biochem Eng J 75:86–91. https://doi.org/10.1016/j.bej.2013.03.022
López-Linares JC, Romero I, Cara C et al (2018) Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour Technol 247:736–743. https://doi.org/10.1016/j.biortech.2017.09.139
Hernández-Pérez AF, de Arruda PV, de Almeida Felipe MdG (2016) Sugarcane straw as a feedstock for xylitol production by Candida guilliermondii FTI 20037. Brazilian J Microbiol 47:489–496. https://doi.org/10.1016/j.bjm.2016.01.019
Sene L, Arruda PV, Oliveira SMM, Felipe MGA (2011) Evaluation of sorghum straw hemicellulosic hydrolysate for biotechnological production of xylitol by Candida guilliermondii. Brazilian J Microbiol 42:1141–1146. https://doi.org/10.1590/S1517-83822011000300036
Zahed O, Jouzani GS, Abbasalizadeh S et al (2016) Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol (Praha) 61:179–189. https://doi.org/10.1007/s12223-015-0420-0
Camargo D, Sene L (2014) Production of ethanol from the hemicellulosic fraction of sunflower meal biomass. Biomass Convers Biorefinery 4:87–93. https://doi.org/10.1007/s13399-013-0096-0
Vallejos ME, Chade M, Mereles EB et al (2016) Strategies of detoxification and fermentation for biotechnological production of xylitol from sugarcane bagasse. Ind Crops Prod 91:161–169. https://doi.org/10.1016/j.indcrop.2016.07.007
Carvalheiro F, Duarte LC, Lopes S et al (2005) Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem 40:1215–1223. https://doi.org/10.1016/j.procbio.2004.04.015
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(1):7. https://doi.org/10.1186/s40643-017-0137-9
Solarte-Toro JC, Romero-García JM, Martínez-Patiño JC et al (2019) Acid pretreatment of lignocellulosic biomass for energy vectors production: a review focused on operational conditions and techno-economic assessment for bioethanol production. Renew Sustain Energy Rev 107:587–601. https://doi.org/10.1016/j.rser.2019.02.024
Gírio FM, Carvalheiro F, Duarte LC, Bogel-Łukasik R (2012) Deconstruction of the hemicellulose fraction from lignocellulosic materials into simple sugars. D-xylitol 3–37
Fang C, Thomsen MH, Frankær CG, et al (2019) Factors affecting seawater-based pretreatment of lignocellulosic date palm residues. In: Biorefinery. Springer, pp 695–713
Da Silva SS, Chandel AK (2012) D-Xylitol: Fermentative production, application and commercialization
Santucci BS, Maziero P, Rabelo SC et al (2015) Autohydrolysis of hemicelluloses from sugarcane bagasse during hydrothermal pretreatment: a kinetic assessment. BioEnergy Res 8:1778–1787
Koo B, Park J, Gonzalez R et al (2019) Two-stage autohydrolysis and mechanical treatment to maximize sugar recovery from sweet sorghum bagasse. Bioresour Technol 276:140–145. https://doi.org/10.1016/j.biortech.2018.12.112
Carvalheiro F, Duarte LC, Gírio FM (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res (India) 67:849–864
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 35:489–511
Liu ZH, Chen HZ (2015) Xylose production from corn stover biomass by steam explosion combined with enzymatic digestibility. Bioresour Technol 193:345–356. https://doi.org/10.1016/j.biortech.2015.06.114
Kumar V, Yadav SK, Kumar J, Ahluwalia V (2020) A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour Technol 299:122633. https://doi.org/10.1016/j.biortech.2019.122633
Kumar V, Binod P, Sindhu R et al (2018) Bioconversion of pentose sugars to value added chemicals and fuels: recent trends, challenges and possibilities. Bioresour Technol 269:443–451. https://doi.org/10.1016/j.biortech.2018.08.042
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:1–10
Lima LHA, De Almeida Felipe MDG, Vitolo M, Torres FAG (2004) Effect of acetic acid present in bagasse hydrolysate on the activities of xylose reductase and xylitol dehydrogenase in Candida guilliermondii. Appl Microbiol Biotechnol 65:734–738. https://doi.org/10.1007/s00253-004-1612-8
Kamal SMM, Mohamad NL, Abdullah AGL, Abdullah N (2011) Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Procedia Food Sci 1:908–913. https://doi.org/10.1016/j.profoo.2011.09.137
Kumar V, Sandhu PP, Ahluwalia V et al (2019) Improved upstream processing for detoxification and recovery of xylitol produced from corncob. Bioresour Technol 291:121931. https://doi.org/10.1016/j.biortech.2019.121931
Mussatto SI, Roberto IC (2004) Optimal experimental condition for hemicellulosic hydrolyzate treatment with activated charcoal for xylitol production. Biotechnol Prog 20:134–139. https://doi.org/10.1021/bp034207i
Kumar V, Krishania M, Preet Sandhu P et al (2018) Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour Technol 251:416–419. https://doi.org/10.1016/j.biortech.2017.11.039
Mishra A, Ghosh S (2019) Bioethanol production from various lignocellulosic feedstocks by a novel “fractional hydrolysis” technique with different inorganic acids and co-culture fermentation. Fuel 236:544–553. https://doi.org/10.1016/j.fuel.2018.09.024
Jiang X, He P, Qi X et al (2016) High-efficient xylitol production by evolved Candida maltosa adapted to corncob hemicellulosic hydrolysate. J Chem Technol Biotechnol 91:2994–2999. https://doi.org/10.1002/jctb.4924
Pereira RS, Mussatto SI, Roberto IC (2011) Inhibitory action of toxic compounds present in lignocellulosic hydrolysates on xylose to xylitol bioconversion by Candida guilliermondii. J Ind Microbiol Biotechnol 38:71–78. https://doi.org/10.1007/s10295-010-0830-6
Perna MSC, Bastos RG, Ceccato-Antonini SR (2018) Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii. 3 Biotech 8(2):119. https://doi.org/10.1007/s13205-018-1143-0
Yewale T, Panchwagh S, Rajagopalan S et al (2016) Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. 3 Biotech 6:1–10. https://doi.org/10.1007/s13205-016-0388-8
Rafiqul ISM, Sakinah AMM (2013) Processes for the production of xylitol—a review. Food Rev Int 29:127–156. https://doi.org/10.1080/87559129.2012.714434
Rangaswamy S, Agblevor FA (2002) Screening of facultative anaerobic bacteria utilizing D-xylose for xylitol production. Appl Microbiol Biotechnol 60:88–93. https://doi.org/10.1007/s00253-002-1067-8
Dashtban M, Kepka G, Seiboth B, Qin W (2013) Xylitol production by genetically engineered trichoderma reesei strains using barley straw as feedstock. Appl Biochem Biotechnol 169:554–569. https://doi.org/10.1007/s12010-012-0008-y
Berghäll S, Hilditch S, Penttilä M, Richard P (2007) Identification in the mould Hypocrea jecorina of a gene encoding an NADP+: D-xylose dehydrogenase. FEMS Microbiol Lett 277:249–253. https://doi.org/10.1111/j.1574-6968.2007.00969.x
Sampaio FC, Da Silveira WB, Chaves-Alves VM et al (2003) Screening of filamentous fungi for production of xylitol from d-xylose. Brazilian J Microbiol 34:325–328. https://doi.org/10.1590/s1517-83822003000400007
Yablochkova EN, Bolotnikova OI, Mikhailova NP et al (2003) The activity of xylose reductase and xylitol dehydrogenase in yeasts. Microbiology 72:414–417. https://doi.org/10.1023/A:1025032404238
Misra S, Raghuwanshi S, Gupta P et al (2012) Fermentation behavior of osmophilic yeast Candida tropicalis isolated from the nectar of Hibiscus rosa sinensis flowers for xylitol production. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol 101:393–402. https://doi.org/10.1007/s10482-011-9646-2
Morais Junior WG, Pacheco TF, Trichez D et al (2019) Xylitol production on sugarcane biomass hydrolysate by newly identified Candida tropicalis JA2 strain. Yeast 36:349–361. https://doi.org/10.1002/yea.3394
de Albuquerque TL, Gomes SDL, Marques JE et al (2015) Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal Today 255:33–40. https://doi.org/10.1016/j.cattod.2014.10.054
Rodrussamee N, Lertwattanasakul N, Hirata K et al (2011) Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus. Appl Microbiol Biotechnol 90:1573–1586. https://doi.org/10.1007/s00253-011-3218-2
Wilkins MR, Mueller M, Eichling S, Banat IM (2008) Fermentation of xylose by the thermotolerant yeast strains Kluyveromyces marxianus IMB2, IMB4, and IMB5 under anaerobic conditions. Process Biochem 43:346–350
da Silveira FA, Fernandes TAR, Bragança CRS et al (2020) Isolation of xylose-assimilating yeasts and optimization of xylitol production by a new Meyerozyma guilliermondii strain. Int Microbiol 23:325–334. https://doi.org/10.1007/s10123-019-00105-0
Hedayati Rad F, Sharifan A (2019) Evaluation and statistical optimization of process variables for xylitol production by Candida kefyr. Food Heal 2:31–36
Misra S, Raghuwanshi S, Saxena RK (2013) Evaluation of corncob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydr Polym 92:1596–1601. https://doi.org/10.1016/j.carbpol.2012.11.033
Jia H, Shao T, Zhong C et al (2016) Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydr Polym 151:676–683. https://doi.org/10.1016/j.carbpol.2016.06.013
Abdul Manaf SF, Md Jahim J, Harun S, Luthfi AAI (2018) Fractionation of oil palm fronds (OPF) hemicellulose using dilute nitric acid for fermentative production of xylitol. Ind Crops Prod 115:6–15. https://doi.org/10.1016/j.indcrop.2018.01.067
Srivani K, Pydi Setty Y (2012) Parametric optimization of xylitol production from xylose by fermentation. Asia-Pacific J Chem Eng 7:S280–S284
Tamburini E, Bianchini E, Bruni A, Forlani G (2010) Cosubstrate effect on xylose reductase and xylitol dehydrogenase activity levels, and its consequence on xylitol production by Candida tropicalis. Enzyme Microb Technol 46:352–359. https://doi.org/10.1016/j.enzmictec.2010.01.001
Arruda PV, Felipe MGA (2009) Role of glycerol addition on xylose-to-xylitol bioconversion by Candida guilliermondii. Curr Microbiol 58:274–278. https://doi.org/10.1007/s00284-008-9321-7
Hernández-Pérez AF, Costa IAL, Silva DDV et al (2016) Biochemical conversion of sugarcane straw hemicellulosic hydrolyzate supplemented with co-substrates for xylitol production. Bioresour Technol 200:1085–1088. https://doi.org/10.1016/j.biortech.2015.11.036
Pappu SMJ, Gummadi SN (2018) Effect of cosubstrate on xylitol production by Debaryomyces nepalensis NCYC 3413: a cybernetic modelling approach. Process Biochem 69:12–21. https://doi.org/10.1016/j.procbio.2018.03.023
Raj K, Krishnan C (2020) Improved co-production of ethanol and xylitol from low-temperature aqueous ammonia pretreated sugarcane bagasse using two-stage high solids enzymatic hydrolysis and Candida tropicalis. Renew Energy 153:392–403. https://doi.org/10.1016/j.renene.2020.02.042
Bustos Vázquez G, Pérez-Rodríguez N, Salgado JM et al (2017) Optimization of salts supplementation on xylitol production by Debaryomyces hansenii using a synthetic medium or corncob hemicellulosic hydrolyzates and further scaled up. Ind Eng Chem Res 56:6579–6589. https://doi.org/10.1021/acs.iecr.7b01120
Martínez-Corona R, Penagos CC, del Carmen Chávez-Parga M et al (2016) Analysis of the effect of agitation and aeration on xylitol production by fermentation in bioreactor with Kluyveromyces marxianus using hydrolized tamarind seed as substrate. Int J Curr Microbiol Appl Sci 5:479–499. https://doi.org/10.20546/ijcmas.2016.506.055
Zhang J, Geng A, Yao C et al (2012) Xylitol production from d-xylose and horticultural waste hemicellulosic hydrolysate by a new isolate of Candida athensensis SB18. Bioresour Technol 105:134–141. https://doi.org/10.1016/j.biortech.2011.11.119
Prabhu AA, Bosakornranut E, Amraoui Y et al (2020) Enhanced xylitol production using non-detoxified xylose rich pre-hydrolysate from sugarcane bagasse by newly isolated Pichia fermentans. Biotechnol Biofuels 13:1–15
Mano JS, Sathyanarayana P (2016) Multi response optimization for enhanced xylitol production by Debaryomyces nepalensis in bioreactor. 3 Biotech 6:1–10. https://doi.org/10.1007/s13205-016-0467-x
Pérez-Bibbins B, De Souza Oliveira RP, Torrado A et al (2014) Study of the potential of the air lift bioreactor for xylitol production in fed-batch cultures by Debaryomyces hansenii immobilized in alginate beads. Appl Microbiol Biotechnol 98:151–161. https://doi.org/10.1007/s00253-013-5280-4
Branco RF, Santos JC, Murakami LY et al (2007) Xylitol production in a bubble column bioreactor: influence of the aeration rate and immobilized system concentration. Process Biochem 42:258–262
Silva CJSM, Mussatto SI, Roberto IC (2006) Study of xylitol production by Candida guilliermondii on a bench bioreactor. J Food Eng 75:115–119
Kwon SG, Park SW, Oh DK (2006) Increase of xylitol productivity by cell-recycle fermentation of Candida tropicalis using submerged membrane bioreactor. J Biosci Bioeng 101:13–18. https://doi.org/10.1263/jbb.101.13
Santos JC, Converti A, de Carvalho W et al (2005) Influence of aeration rate and carrier concentration on xylitol production from sugarcane bagasse hydrolyzate in immobilized-cell fluidized bed reactor. Process Biochem 40:113–118
Mussatto SI, Roberto IC (2003) Xylitol production from high xylose concentration: evaluation of the fermentation in bioreactor under different stirring rates. J Appl Microbiol 95:331–337
Faria LFF, Pereira N, Nobrega R (2002) Xylitol production from D-xylose in a membrane bioreactor. Desalination 149:231–236. https://doi.org/10.1016/S0011-9164(02)00766-X
Dasgupta D, Kurmi AK, Adhikari DK, Ghosh D (2020) Xylitol production from lignocellulosic pentosans using Kluyveromyces marxianus: kinetic modelling of yeast growth and fermentation. Biofuels 11:309–319
Vaz de Arruda P, dos Santos JC, de Cássia Lacerda Brambilla Rodrigues R et al (2017) Scale up of xylitol production from sugarcane bagasse hemicellulosic hydrolysate by Candida guilliermondii FTI 20037. J Ind Eng Chem 47:297–302. https://doi.org/10.1016/j.jiec.2016.11.046
Dalli SS, Patel M, Rakshit SK (2017) Development and evaluation of poplar hemicellulose prehydrolysate upstream processes for the enhanced fermentative production of xylitol. Biomass Bioenerg 105:402–410. https://doi.org/10.1016/j.biombioe.2017.08.001
da Silva DDV, de Arruda PV, Vicente FMCF et al (2015) Evaluation of fermentative potential of Kluyveromyces marxianus ATCC 36907 in cellulosic and hemicellulosic sugarcane bagasse hydrolysates on xylitol and ethanol production. Ann Microbiol 65:687–694. https://doi.org/10.1007/s13213-014-0907-y
Li M, Meng X, Diao E, Du F (2012) Xylitol production by Candida tropicalis from corn cob hemicellulose hydrolysate in a two-stage fed-batch fermentation process. J Chem Technol Biotechnol 87:387–392. https://doi.org/10.1002/jctb.2732
Cheng KK, Zhang JA, Ling HZ et al (2009) Optimization of pH and acetic acid concentration for bioconversion of hemicellulose from corncobs to xylitol by Candida tropicalis. Biochem Eng J 43:203–207. https://doi.org/10.1016/j.bej.2008.09.012
Ling H, Cheng K, Ge J, Ping W (2011) Statistical optimization of xylitol production from corncob hemicellulose hydrolysate by Candida tropicalis HDY-02. N Biotechnol 28:673–678. https://doi.org/10.1016/j.nbt.2010.05.004
Xu L, Liu L, Li S et al (2019) Xylitol production by Candida tropicalis 31949 from sugarcane bagasse hydrolysate. Sugar Tech 21:341–347. https://doi.org/10.1007/s12355-018-0650-y
Himabindu K, Gummadi SN (2015) Effect of kLa and fed-batch strategies for enhanced production of xylitol by Debaryomyces nepalensis NCYC 3413. Biotechnol J Int 5:24–36
Sirisansaneeyakul S, Wannawilai S, Chisti Y (2013) Repeated fed-batch production of xylitol by Candida magnoliae TISTR 5663. J Chem Technol Biotechnol 88:1121–1129. https://doi.org/10.1002/jctb.3949
Martínez EA, Silva SS, Felipe MGA (2000) Effect of the oxygen transfer coefficient on xylitol production from sugarcane bagasse hydrolysate by continuous stirred-tank reactor fermentation. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol 84–86:633–641. https://doi.org/10.1385/abab:84-86:1-9:633
Martínez EA, Silva SS, Almeida E, Silva JB et al (2003) The influence of pH and dilution rate on continuous production of xylitol from sugarcane bagasse hemicellulosic hydrolysate by C. guilliermondii. Process Biochem 38:1677–1683. https://doi.org/10.1016/S0032-9592(02)00244-3
Salgado JM, Rodríguez N, Cortés S, Domínguez JM (2012) Coupling two sizes of CSTR-type bioreactors for sequential lactic acid and xylitol production from hemicellulosic hydrolysates of vineshoot trimmings. N Biotechnol 29:421–427. https://doi.org/10.1016/j.nbt.2011.07.003
Ding X (2011) Fermentation of xylitol using immobilized Candida sp.ZU04 cells in three-phase fluidized-bed bioreactor. 2011 Int Conf Remote Sensing, Environ Transp Eng RSETE 2011 - Proc 7591–7593. https://doi.org/10.1109/RSETE.2011.5966129
Soleimani M, Tabil L (2014) Evaluation of biocomposite-based supports for immobilized-cell xylitol production compared with a free-cell system. Biochem Eng J 82:166–173. https://doi.org/10.1016/j.bej.2013.11.011
Choi J-H, Moon K-H, Ryu Y-W, Seo J-H (2000) Production of xylitol in cell recycle fermentations of Candida tropicalis. Biotechnol Lett 22:1625–1628
Cunha MAA, Rodrigues RCB, Santos JC et al (2007) Repeated-batch xylitol bioproduction using yeast cells entrapped in polyvinyl alcohol-hydrogel. Curr Microbiol 54:91–96. https://doi.org/10.1007/s00284-005-0465-4
Jain H, Mulay S (2014) A review on different modes and methods for yielding a pentose sugar: Xylitol. Int J Food Sci Nutr 65:135–143. https://doi.org/10.3109/09637486.2013.845651
Ko BS, Rhee CH, Kim JH (2006) Enhancement of xylitol productivity and yield using a xylitol dehydrogenase gene-disrupted mutant of Candida tropicalis under fully aerobic conditions. Biotechnol Lett 28:1159–1162. https://doi.org/10.1007/s10529-006-9068-9
Pal S, Choudhary V, Kumar A et al (2013) Studies on xylitol production by metabolic pathway engineered Debaryomyces hansenii. Bioresour Technol 147:449–455. https://doi.org/10.1016/j.biortech.2013.08.065
Pal S, Mondal AK, Sahoo DK (2016) Molecular strategies for enhancing microbial production of xylitol. Process Biochem 51:809–819. https://doi.org/10.1016/j.procbio.2016.03.017
Jeon WY, Yoon BH, Ko BS et al (2012) Xylitol production is increased by expression of codon-optimized Neurospora crassa xylose reductase gene in Candida tropicalis. Bioprocess Biosyst Eng 35:191–198. https://doi.org/10.1007/s00449-011-0618-8
Oh EJ, Ha SJ, Rin Kim S et al (2013) Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae. Metab Eng 15:226–234. https://doi.org/10.1016/j.ymben.2012.09.003
Govinden R, Pillay B, Van Zyl WH, Pillay D (2001) Xylitol production by recombinant Saccharomyces cerevisiae expressing the Pichia stipitis and Candida shehatae XYL1 genes. Appl Microbiol Biotechnol 55:76–80. https://doi.org/10.1007/s002530000455
Kwon DH, Kim MD, Lee TH et al (2006) Elevation of glucose 6-phosphate dehydrogenase activity increases xylitol production in recombinant Saccharomyces cerevisiae. J Mol Catal B Enzym 43:86–89. https://doi.org/10.1016/j.molcatb.2006.06.014
Ahmad I, Shim WY, Kim J-H (2013) Enhancement of xylitol production in glycerol kinase disrupted Candida tropicalis by co-expression of three genes involved in glycerol metabolic pathway. Bioprocess Biosyst Eng 36:1279–1284
Jeon WY, Shim WY, Lee SH et al (2013) Effect of heterologous xylose transporter expression in Candida tropicalis on xylitol production rate. Bioprocess Biosyst Eng 36:809–817
Zhang J, Zhang B, Wang D et al (2015) Improving xylitol production at elevated temperature with engineered Kluyveromyces marxianus through over-expressing transporters. Bioresour Technol 175:642–645. https://doi.org/10.1016/j.biortech.2014.10.150
Martínez EA, de Almeida e Silva JB, Giulietti M, Solenzal AIN (2007) Downstream process for xylitol produced from fermented hydrolysate. Enzyme Microb Technol 40:1193–1198. https://doi.org/10.1016/j.enzmictec.2006.09.003
Martínez EA, Canettieri EV, Bispo JAC et al (2015) Strategies for xylitol purification and crystallization: a review. Sep Sci Technol 50:2087–2098. https://doi.org/10.1080/01496395.2015.1009115
Sampaio FC, Passos FML, Passos FJV et al (2006) Xylitol crystallization from culture media fermented by yeasts. Chem Eng Process Process Intensif 45:1041–1046. https://doi.org/10.1016/j.cep.2006.03.012
Kresnowati M, Regina D, Bella C et al (2019) Combined ultrafiltration and electrodeionization techniques for microbial xylitol purification. Food Bioprod Process 114:245–252
Acknowledgements
Authors gratefully acknowledge the constant support provided by Indian Institute of Technology Roorkee (IITR) and Ministry of Human Resource and Development (MHRD).
Funding
The financial assistance for this work was provided by Indian Institute of Technology Roorkee (IITR) and Ministry of Human Resource and Development (MHRD).
Author information
Authors and Affiliations
Contributions
VJ drafted manuscript. VJ and SG revised and modified the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jain, V., Ghosh, S. Biotransformation of lignocellulosic biomass to xylitol: an overview. Biomass Conv. Bioref. 13, 9643–9661 (2023). https://doi.org/10.1007/s13399-021-01904-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01904-0