Abstract
Greenhouse gas accumulation and climate change impact reduction requires widespread utilization of green technology. However, escalating demand for crops as a food source coupled with the finite availability of arable land makes cultivation of biofuel crops unsustainable. Algal biomass can be grown using non-arable areas such as lakes, oceans, or deserts, thus avoiding the current problem of land use competition with the food supply chain. Third-generation biofuels mainly consist of algal biofuels. However recently, hybrid use of algae for production of biofuels and also treating the wastewater for greenhouse gas reduction is gaining ground. Algae have the potential to produce valuable substances for the food, feed, cosmetic, pharmaceutical, and waste treatment industries. Microalgae mass cultures using solar energy and concentrated CO2 sources can be used to produce renewable fuels such as methane, ethanol, biodiesel, oils and hydrogen and for other fossil fuel sparing products and processes. Recently developed hybrid technologies include biomass production, wastewater treatment, and GHG mitigation for production of prime products as biofuels. This also helps in atmospheric pollution control such as the reduction of GHG (CO2 fixation) and bioremediation of wastewater microalgae growth. However, the selection of efficient strain, cultivation systems, microbial metabolism, and biomass production are important steps of viable technology for microalgae-based biodiesel production and phytoremediation. This chapter will discuss the latest developments in area of selection, production, and accumulation of target bioenergy carrier’s strains, as well as the third-generation biofuels and hybrid technology for development of oil, biodiesel, ethanol, methanol, biogas production, and GHG mitigation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Anthropogenic carbon dioxide emissions largely from fossil fuels in energy and transportation sectors have raised the concentration of CO2 from pre-industrial levels of 280 ppm to about 415 ppm today, and they will continue to raise to about 570 ppm by the twenty-second century (Aaron and Tsouris 2005). This will have disastrous consequences on human health, climate, and agriculture (Kumar 2013, 2018; Kumar et al. 2018, 2020). In general, the use of photosynthetic organisms as a feedstock mitigates ever-increasing anthropogenic CO2 emissions. High photosynthetic carbon sequestration efficiencies and carbon capture percentages of 90% make algae very well suited as a source for biofuels and bioproducts (Demirel 2018b). Algae are capable of eliminating 513 tons of CO2 and producing up to 100 tons of dry biomass per hectare per year (Bilanovic et al. 2009).
The first generation of biofuels from food crops or their derivatives require farmland or wasteland but irrigation and fertilizer use improved their growth and productivity. Thus, the debate is focussed on food vs. fuel. It also posed a severe threat to food security triggering food versus fuel dilemma (Kumar 2018; Kumar et al. 2018, 2020; Nazneen and Kumar 2014).
The second-generation biofuels derived from lignocellulosic biomass were largely extracted from agricultural waste, lignocellulosic materials, food waste, etc. Although they addressed the above-mentioned problems, their processing technology and biofuel production has not been perfected for large-scale utilization (Anindita and Kumar 2013; Kumar and Gupta 2018).
2 Third-Generation Biofuels
Photosynthetic organisms include filamentous and unicellular macro- and microalgae, and cyanobacterial species. Microalgae have the potential to produce valuable substances for the food, feed, cosmetic, pharmaceutical, and waste treatment industries. Commercial culture of microalgae has >40 years history with some of the main species grown being Spirulina for health food, Dunaliella salina and Haematococcus pluvialis for carotenoid production, and several species for aquaculture (Lee 1997; Borowitzka 1999; Carvalho et al. 2006; see review Behera et al. 2019). Biorefinery approach with integrated biology, ecology, and engineering could lead to a feasible algal-based technology for a variety of biofuels and bioproducts (Allen et al. 2018).
The environmental consequence of global warming has led to a paradigm shift towards renewable fuels (Maity et al. 2014). Algal biofuel is an example of third-generation biomass which has the potential to replace fossil fuels and animal feeds (John et al. 2011; Sharma and Singh 2017; Gajraj et al. 2018; Kumar et al. 2018, 2020). Macro and microalgae transform solar energy into the carbon storage products, leading to lipid accumulation, including those that can be transformed into biodiesel, bioethanol, and biomethanol (Kraan 2013; Maity et al. 2014). Maity et al. (2014) reported that microalgae provide mainly TAG (triacylglycerols), a potential source of biofuel at large scale.
Although wastewater treatment and CO2 removal by microalgae have been studied separately for a long time, there is no detailed information available on combining both processes (Craggs et al. 2011; Li et al. 2017; Kumar 2018; Kumar et al. 2019, 2020).
3 Wastewater Treatment
Discharge of untreated/partially treated brewery wastewater leads to environmental problems, such as water scarcity, excessive growth of undesirable microbes that cause loss of aquatic lifeforms (Okolo et al. 2018), and health-related problems in communities around the discharge areas (Norman 1997). Wastewater is a complex mixture of natural organic and inorganic materials, as well as man-made compounds. Different sources of pollutants include “Discharge of either raw or treated sewage from towns and villages; dis-charge from manufacturing or industrial plants; run-off from agricultural land; and leachates from solid waste disposal sites” these sites of pollution have problems so that a solution is sought (Gray 1989; Horan 1990; Showkat and Najar 2019). Three quarters of organic carbon in sewage are present as carbohydrates, fats, proteins, amino acids, and volatile acids. The inorganic constituents include large concentrations of sodium, calcium, potassium, magnesium, chlorine, sulphur, phosphate, ammonium salts bicarbonate, and heavy metals (Talbot et al. 1990; Horan 1990; Lim et al. 2010). Scarcity of water, the need for energy and food are forcing us to explore the feasibility of wastewater recycling and resource recovery (De la Noue and De Pauw 1988).
Microbiological composition of sewage wastewater environment is an ideal media for a wide range of microorganisms, especially bacteria, viruses, and protozoa. The majority is harmless and can be used in biological sewage treatment, but sewage also contains pathogenic microorganisms. However algae have been used in hybrid technology which produces biofuels along with removing the heavy metals and pollutants from wastewater. In conventional wastewater treatment system, the removal of biochemical oxygen demands (BOD ) suspended solids, nutrients (NO3−-N, NO2−-N, NH4+-N and PO43−-P), coliform bacteria, and toxicity are the main goal for getting purified wastewater (Abdel-Raouf et al. 2012; Lim et al. 2010; Amenorfenyo et al. 2019; Kumar et al. 2020).
3.1 Hybrid Technologies
Molazadeh et al. (2019) reviewed microalgae-based CO2 biofixation, various microalgae cultivation systems, as well as concept of integration of CO2 biofixation process and wastewater treatment. This helps in photosynthetic biomass production, wastewater treatment, and GHG mitigation. Thus, hybrid technology offers atmospheric pollution control such as the reduction of GHG (CO2 fixation) coupling wastewater treatment with microalgae growth (Weissman and Goebel 1988; Abdel-Raouf et al. 2012; Maity et al. 2014) (Figs. 10.1 and 10.2). Sharma and Singh (2017) made systematic analyses of energy demand and GHG emission statistics of various nations, as well as all the steps involved in overall process from algal strain selection to biodiesel production. Microalgae including eukaryotic algae and cyanobacteria, as well as macro-algae, have demonstrated to be an environmental-friendly and sustainable alternative to energy-intensive and conventional biological treatment processes (Mohsenpour et al. 2021).
Hybrid technology: Microalgae and wastewater treatment. (Source: Abdel-Raouf, N., Al-Homaidan, A. A., and Ibraheem, I. B. (2012). Microalgae and wastewater treatment. Saudi J. Biol. Sci. 19, 257–275. doi: https://doi.org/10.1016/j.sjbs.2012.04.005. Open access: Reprinted with Licence no. 4880801372566 dated 2nd Aug 2020 RightsLink)
Microalgae for third-generation biofuel production, mitigation of greenhouse gas emissions, and wastewater treatment. (Source: Maity, J. P., Bundschuh, J., Chen, C.-Y., & Bhattacharya, P. (2014). Microalgae for third-generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives – A mini review. Energy, 78, 104–113. https://doi.org/10.1016/j.energy.2014.04.003. Reproduced with licence no 5064180534847 dated 8th May 2021)
Currently, algae wastewater treatment and its biomass use are attracting attention worldwide (Simate et al. 2011). Liu et al. (2013) highlighted the use of microalgae to address the problems of wastewater treatment. The current progress of hybrid technologies (biomass production, wastewater treatment, GHG mitigation) for production of prime products as biofuels offer atmospheric pollution control, such as the reduction of GHG (CO2 fixation) coupling wastewater treatment with microalgae growth (Maity et al. 2014).
Wang et al. (2017) reported reuse of secondary municipal effluent from wastewater treatment plants to alleviate freshwater resource shortage. Wastewater treatment (WWT) for biofuel production provides additional incentives (Milano et al. 2016). This integrated approach (as illustrated in Fig. 10.5) is postulated to provide resource recovery-based monetary benefits, as well as 800–1400 GJ/ha/year energy which can be a source of energy at the community level (Mehrabadi et al. 2015). Furthermore, algae-based strategies for the removal of toxic minerals, such as As, Br, Cd, Hg, Pb, Sc, and Sn ions, have also been reported individually or in combination (Abdel-Raouf et al. 2012; Amenorfenyo et al. 2019). Olajire (2020) reviewed some of these challenges with a focus on key issues: water consumption and waste generation, energy efficiency, emission management, environmental impact of brewing process, and best environmental management for breweries. The potential for remediation of inorganic nitrogen and phosphorus from wastewater by microalgae is well documented (Shi et al. 2007; Abhinandan et al. 2015; Whitton et al. 2015). Abhinandan et al. (2015) studied cultivation of microalgae species namely Chlorella pyrenoidosa and Scenedesmus abundans in rice mill effluent (i.e., paddy soaked water) for nutrient removal.
3.2 Steps of Wastewater Treatment
3.2.1 Cultivation
Cultivating microalgae under photosynthetic conditions on wastewater with low cost resources and easy separation of the biomass from the treated wastewater and high value products can be obtained in an eco-friendly manner. It can also be used to decontaminate pesticides and heavy metals. Singh et al. (2016) presented a hypothetical model of cyanobacteria in sustainable agriculture and environmental management (Fig. 10.3). Cyanobacteria could play a potential role in the enhancement of agriculture productivity and mitigation of GHG emissions (Singh 2011). Cyanobacteria are also useful for wastewater treatment and are an effective bio-fertilizer source and have the ability to degrade the various toxic compounds, even the pesticides (Cohen 2006). They also help in ecological restoration of degraded lands (Singh 2014, 2015a, b) (Fig. 10.4). Wastewater treatment, however, can also be organized or categorized by the nature of the treatment process operation being used, for example, physical, chemical, or biological. Li et al. (2020) reported that coupling algal growth on wastewater with hydrothermal liquefaction (HTL) is regarded as an environment-enhancing pathway for biomass amplification, wastewater management, sustainable energy generation, and value-added products generation. They proposed a paradigm shift involving enhanced algal wastewater treatment and bioenergy production for field application (Fig. 10.3).
Algal growth on wastewater with hydrothermal liquefaction (HTL). (Source: Li H, Watson J, Zhang Y, Lu H, Liu Z. Environment-enhancing process for algal wastewater treatment, heavy metal control, and hydrothermal biofuel production: A critical review. Bioresour Technol. 2020 Feb; 298: 122421. doi: https://doi.org/10.1016/j.biortech.2019.122421. Epub 2019 Nov 14. PMID: 31767428. Licence no 5065350239998 dated 10th May)
A hypothetical model exhibiting the potential roles of cyanobacteria in sustainable agriculture and environmental management. (Singh, J. S., Kumar, A., Rai, A. N., & Singh, D. P. (2016). Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Frontiers in microbiology, 7, 529. https://doi.org/10.3389/fmicb.2016.00529. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY))
Cyano bioremediation using cyanobacteria is a green clean tool for decontamination of synthetic pesticides from agro- and aquatic ecosystems (Kumar and Singh 2017) (Figs. 10.4 and 10.5).
A model showing the technologies used for production of biofuels from the mass cultivation of cyanobacteria. (Source: Singh, J. S., Kumar, A., Rai, A. N., & Singh, D. P. (2016). Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Frontiers in Microbiology, 7, 529. https://doi.org/10.3389/fmicb.2016.00529. Reproduced under CC-BY license which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and the source are credited)
Microalgal production inside industrial premises using effluent rich flue gas and wastewater would produce biomass for meeting the ever increasing energy demands, with the added benefits of wastewater treatment (WWT) and emission control (Milano et al. 2016). The algal biomass can be converted into various types of renewable biofuels including bioethanol, biodiesel, biogas, photo biologically produced biohydrogen, and further processing for bio-oil and syngas production through liquefaction and gasification, respectively (Kraan 2013). This integrated approach of microalgal wastewater treatment with resource recovery for maximizing the derivable products is illustrated in Fig. 10.6 (Behera et al. 2019).
Integration of microalgal wastewater treatment with resource recovery for maximizing the derivable products. (Source: Behera, B., Acharya, A., Gargey, I. A., Aly, N., & P, B. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. https://doi.org/10.1016/j.biteb.2018.08.001. Reproduced under Licence number 4906420744566 dated 12th September)
3.2.2 Physical Treatment
Physical wastewater treatment, a pretreatment stage, has been used generally to reduce suspended solids, as well as grease and oil from wastewater through sedimentation by gravitational force (Jayanti and Narayanan 2004).
3.2.3 Chemical Treatment
Chemical treatment processes involve pH adjustment or coagulation/flocculation by adding different chemicals to the effluent to alter its chemistry (Simate et al. 2011). Flocculation involves stirring/agitation of chemically treated effluent to induce coagulation that improves sedimentation performance by increasing particle size, thereby increasing settling efficiency (Simate et al. 2011).
3.2.4 Use of Consortia of Bacteria Cyanobacteria/Microalgae
From an environmental friendly perspective, bacteria, cyanobacteria, and microalgae, and the consortia have been largely considered for biological treatment of wastewaters (Perera et al. 2019). Perera et al. (2019) highlighted the use of specific molecular techniques of proteomics, genomics, transcriptomics, metabolomics, and genetic engineering to develop more stable consortia of bacteria and cyanobacteria/microalgae with their improved biotechnological capabilities in wastewater treatment.
3.2.5 Overview of Microalgal Nutrient Remediation Mechanisms
Direct remediation is the most commonly discussed mechanism of remediation and is achieved through interconnected biochemical pathways for the uptake of the target nutrients into the biomass for storage (Seco and Ferrer 2015) or assimilation into nucleic acids and proteins for biomass growth (Cai et al. 2013).
Whitton et al. (2015) described that nutrient remediation with microalgae occurs through one of two pathways. A nitrogen source is required for the synthesis of proteins (Powell et al. 2008) or assimilation into nucleic acids and proteins for biomass growth (Cai et al. 2013; see also Whitton et al. 2015). The microalgae can be utilized for total nitrogen (TN) removal (nitrification and denitrification), with NO3− assimilation observed following the near-complete exhaustion of NH4+ (Eixler et al. 2006) from the source wastewater. Phosphate, in the preferred form of (H2PO4)2 and (HPO4)2, is transported across the cell membrane via energized transport and assimilated into nucleotides following phosphorylation for the synthesis of ribosomal RNA (Beuckels et al. 2015; see Whitton et al. 2015).
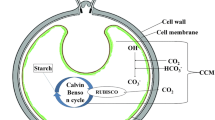
4 Algal CO2 Fixation
According to Benemann and Pedroni (2003), approximately 1.8 tons of CO2 can be fixed through photosynthesis by producing 1 ton algal biomass in high rate algal pond (HRAP)-based wastewater treatment (see Craggs et al. 2014). Raeesossadati and Ahmadzadeh (2015) reported CO2 fixation rates of several microalgae and cyanobacteria species under different CO2 concentrations and culture conditions for biomass production (see also Moheimani et al. 2015). According to Allen et al. (2018), some of the best biofuel microalgae strains are Nannochloropsis spp., Chlorella vulgaris, Chlorella minutissima, Chlorella protothecoides, Botryo-coccus braunii, Spirulina platensis, Chlorella emersonii, Spirulina maxima, Phaeodactylum tricornutum, Scenedesmus obliquus, Chlorococcum spp., Dunaliella tertiolecta, Crypthecodinium cohnii, Dunaliella salina, Schizochytrium spp., Chlamydomonas reinhardtii, and Microcystis aeruginosa in terms of high growth rate and lipid contents. These algae usually require light, nutrients, and carbon dioxide, to produce high levels of polysaccharides such as starch and cellulose. These polysaccharides can be extracted to fermentable sugars through hydrolysis and further fermentation to bioethanol and separated through distillation (Allen et al. 2018; Rodionova et al. 2017).
5 Algal Ccultivation for Biomass Production
High carbon capture percentages of 90% paired with the ability to perform high photosynthetic carbon sequestration efficiencies and harvesting and using the totality of the biomass make algae a suitable source for biofuels and bioproducts (Demirel 2018b).
Microalgae mass cultures can use solar energy for the biofixation of power plant flue gas and other concentrated CO2 sources into biomass Gajraj et al. (2018). Owing to the presence of low lignin and hemicellulose content in algae in comparison to lignocellulosic biomass, the algal biomass has been considered more suitable for the bioethanol production (Chen et al. 2013).
Macro- and microalgae are a promising new source of biomass that may complement agricultural crops. They are found in diverse environments, some species thriving in freshwater, others in saline conditions and sea water (Benemann 1993; Carlsson et al. 2007; Schenk et al. 2008). As demonstrated here, microalgae appear to be the only source of renewable biodiesel that is capable of meeting the global demand for transport fuels (Chisti 2007). There are three types of macroalgae, brown, green, and red algae approximately 40 kg wet biomass/m2 of gulf-weed (Sargassum muticum), compared to 2.3 kg/m2 and 6.6 kg/m2 of green laver (Ulva lactuca) and agar weed (Gelidium amansii), respectively. The brown algae such as sea mustard (Undaria pinnatifida) and kelp (Saccharina japonica) are one of the promising biomass for biofuel production because cultivation productivity based on area size is the highest among three types of macroalgae (Clarens et al. 2010; Eshaq et al. 2011; Slade and Bauen 2013; Rajkumar et al. 2014; Directive 2009/28/EC). Silambarasan et al. (2021) studied use of Chlorella sp., Scenedesmus sp., and their consortium for the biorefinery approach. Moreover, deoiled algal biomass (DAB) waste used as a biofertilizer combined with inorganic fertilizer resulted in the greater improvement of Solanum lycopersicum (Silambarasan et al. 2021).
Currently, cultivating microalgae has gained large momentum among researchers due to their photosynthetic rate of CO2 fixation and its versatile nature to grow in various wastewater systems (Abhinandan and Shanthakumar 2015). However, the efficiency of wastewater treatment differs species to species. Abhinandan and Shanthakumar (2015) reviewed the use of microalgae group Chlorophyta for industrial wastewater treatment, domestic wastewater treatment, and nutrient removal.
Several physicochemical factors govern algal growth such as carbon concentration, medium pH, and bubbling depth, on absorption and utilization of supplied CO2 (Yin et al. 2019). They reported that increasing CO2 bubbling depth and keeping higher carbon concentration and higher pH in microalgae culture can improve CO2 absorption ratio, which will optimize the bio fixation of CO2.
Applications of aquatic ecology to algal cultivation systems, in optimizing nutrients designing and constructing biotic communities, can help to maximize algal biomass yields (Shurin et al. 2013; Bartley et al. 2016; Smith and Mcbride 2015). Algal biofilms in natural ecosystems represent three-dimensional, multispecies, and multi-layered structures which involve consortia of heterotrophic and photoautotrophic prokaryotic and eukaryotic organisms (Berner et al. 2015; Gross et al. 2015).
Some microalgae can be grow under saline conditions in desert zones near the ocean when freshwater supply is not feasible (Mussgnug et al. 2010). According to Demirel (2018a), microalgae can fix CO2 10–50 times more efficient than other energy plants making them a very well-suited resource for biofuels and bio products.
Kargupta et al. (2015) studied growth of Chlorella pyrenoidosa and Scenedesmus abundans in a tubular batch photo bioreactor with provision for continuous flow of 10% CO2 enriched air through the headspace. They reported that CO2 sequestration and growth rate were comparable at height/diameter ratio of 8 and 16.
6 Bioreactors
Whitton et al. (2015) summarized different types of bioreactors with sub-categories, either open to the environment or enclosed, which include suspended and non-suspended systems (see also Christenson and Sims 2011) (Fig. 10.7).
Categories of microalgal bioreactors for wastewater remediation. (Source: Whitton, R Francesco Ometto, Marc Pidou, Peter Jarvis, Raffaella Villa & Bruce Jefferson (2015) Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment, Environmental Technology Reviews, 4: 133–148, DOI: https://doi.org/10.1080/21622515.2015.1105308. This is an open access article distributed under the terms of the Creative Commons CC BY license)
6.1 Suspended Type Bioreactors
6.1.1 Suspended Open High Rate Algal Pond (HRAP)
High rate algal ponds (HRAPs) are open shallow raceway ponds about 30–40 cm deep, with a paddle wheel that are used for low energy wastewater treatment (Mehrabadi et al. 2017a, b). It is a raceway-configured open pond mixed via a paddle wheel to circulate the algal culture and prevent settlement where sunlight is the primary method of irradiation, and as such, culture depths of 20–60 cm are typical (García et al. 2017). High rate algal ponds (HRAP) also provide more efficient natural disinfection. HRAP performance can be further enhanced by bubbling CO2 into the pond during the day to promote algal growth when it is often carbon-limited. Craggs et al. (2014) presented the design and operation and performance of HRAP systems and their application for economical, low-energy upgrade of conventional wastewater treatment ponds combined with energy recovery and biofuel production. Currently, the HRAP-based wastewater treatment is the most economical and environmental approach to produce algal biomass for conversion to biofuels. Kumar et al. (2010) developed technology for efficient use of microalgal CO2 fixation integrated with wastewater treatment (Kumar et al. 2018; Gajraj et al. 2018). Currently, by producing 1 ton algal biomass in HRAP-based wastewater treatment approximately 1.8 tons of CO2 can be fixed through photosynthesis (Benemann and Pedroni 2003). However, there is a critical need for a cost-effective alternative upgrade option for pond systems. This can include:
-
1.
Solids are removed and digested in covered anaerobic digester ponds (CADP) anaerobically.
-
2.
Aerobic treatment by sunlight-powered algal growth on the supernatant;
-
3.
Removal of algal growth and subsequent conversion to biofuel; and
-
4.
Further polishing of the treated effluent as required.
The HRAP supports a symbiotic community of microalgae and bacteria for the assimilation of nutrients and organic matter (Park and Craggs 2014). It can be found operational at full scale with a demonstration plant located in New Zealand with individual pond footprints of 1.25 ha (Sutherland et al. 2014). The major drawback in integrating waste water treatment (WWT) with algal cultivation among others (as shown in Fig. 10.8) (Behera et al. 2019) is the low lipid content of the biomass due to the presence of bacteria (lipid content <10%) available in HRAPs that reduces the biomass energy (Mehrabadi et al. 2015).
Pros and cons of utilizing microalgae in WWT HRAPs. (Source: Behera, B., Acharya, A., Gargey, I. A., Aly, N., & P, B. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. https://doi.org/10.1016/j.biteb.2018.08.001. Reproduced under Licencenumber 4906420744566 dated 12th September)
The concept of industrial symbiosis, with synergistic effects of achieving WWT and biofuel production, is well-known (see Behera et al. 2019). The algal growth rate is dependent on the complex interaction of various environmental, operational, and biological factors. Different parameters influence microalgal growth and associated energy production in WWT HRAPs (Behera et al. 2011) (Fig. 10.9).
Parameters influencing microalgal growth and associated energy production in WWT HRAPs. (Source: Behera, B., Acharya, A., Gargey, I. A., Aly, N., & P, B. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. https://doi.org/10.1016/j.biteb.2018.08.001. Reproduced under licence number 4906420744566 dated 12th September)
6.1.2 Suspended Closed Photobioreactor (PBR)
Bioprocess engineers have developed photobioreactors (PBRs) aiming for mass culture of microalgae. Photobioreactors are devices that are optimized to convert light energy into biomass energy and offer the highest levels of experimental control for developing optimal microalgae production systems (Chisti 2007). Photobioreactors (Hulatt et al. 2017) obtained light conversion efficiency up to 0.70 g biomass per mol of PAR for Nannochloropsis sp. (Davis et al. 2011; Ehimen et al. 2011).
Allen et al. (2018) reported some possible algal-based processes for biofuel and by-product productions: OP open pond, PBR photobioreactor (Fig. 10.10).
Some possible algal-based processes for biofuel and by-product productions. OP open pond, PBR photobioreactor. (Source: Allen, J., Unlu, S., Demirel, Y. et al. Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour. Bioprocess. 5, 47 (2018). https://doi.org/10.1186/s40643-018-0233-5. This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited)
Behera et al. (2019) extensively reviewed the design considerations, mass transfer characteristics, economic and energy consideration for increasing the performance of closed PBRs.
A PBR is an example of a closed, suspended system available in various configurations, including horizontal or vertical tubular photo bioreactor (TPBR) (Fig. 10.11) (Abdel-Raouf et al. 2012; Molina et al. 2001; Hulatt and Thomas 2011; Bechet et al. 2013) or flat panel reactors (Hu et al. 1998; Ugwu et al. 2008; Sierra et al. 2009) and bio-film (Blanken et al. 2014).
Schematic photobioreactor design that follows a horizontal tube. (Source: Abdel-Raouf N., Al-Homaidan, A. A., and Ibraheem, I. B. (2012). Microalgae and wastewater treatment. Saudi J. Biol. Sci. 19, 257–275. doi: https://doi.org/10.1016/j.sjbs.2012.04.005. Open access Reprinted with Licence no. 4880801372566 dated 2nd Aug 2020 RightsLink)
Flat-plate photo bioreactors with short light path lengths as they have a high surface area to volume ratio and consume less energy than tubular systems (Zou et al. 2000; Jorquera et al. 2010; Vejrazka et al. 2012).
Some species of microalgae synthesize very long chain fatty acids (carbon chains 20+ in length), including eicosapentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6n-3) (Guiheneuf and Stengel 2013). Nannochloropsis is a genus of robust, oleaginous microalgae that synthesizes EPA during balanced growth and is a promising candidate for commercial applications (Sharma and Schenk 2015). Hulatt et al. (2017) examined changes in the biochemical composition of Nannochloropsis sp. cultivated in optimized flat-plate photo bioreactors as a potential feedstock for aqua feeds (Fig. 10.12) (Yustinadiar et al. 2020).
Configuration of the flat-plate photobioreactor systems. Photobioreactors used a 14 mm light path length with illumination by warm white LED lights. Photobioreactors were set up, monitored, and data logged using a custom program running on a Linux single board computer. (This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hulatt CJ, Wijffels RH, Bolla S, Kiron V (2017) Production of Fatty Acids and Protein by Nannochloropsis in Flat-Plate Photobioreactors. PLoS ONE 12(1): e0170440. https://doi.org/10.1371/journal.pone.0170440)
6.2 Non-suspended Open Algal Biofilms
Microalgae may grow in suspension, but also in biofilms. Gross et al. (2015) summarizes the state of the art of different algal biofilm systems in terms of their design and operation. Microalgae biofilms represent an alternative to the suspension-based systems and could be used as a production platform for microalgae biomass (Blanken et al. 2014). The advantage of algal biofilm systems is that algae can be simply harvested through scraping and thus avoid the expensive harvesting procedures used in suspension-based harvesting, such as flocculation and centrifugation. A biofilm looks as a slimy, green layer and consists of large numbers of microalgae entrapped in a gel-like matrix (Miranda et al. 2017) (Fig. 10.13).
Bio-flocculation of Isochrysis sp. cells by BAPS-52-2 filaments. (a–f) Attachment of Isochrysis sp. cells to BAPS-52-2 filaments. Secreted EPS shown by red arrows. (g) Isochrysis cells (left wells, controls) and Isochrysis cells mixed with BAPS-52-2 filaments (right wells) at day 0 and day 10 (h). Green pigmentation produced by biofilm produced by monocultured BAPS-52-2 filaments at day 10 ((i) upper well) and Biofilm #102 at day 10 ((i) bottom well). Scale bars (a–f) 20 μm; (g–i) 1 cm. (Source: Miranda, A.F., Ramkumar, N., Andriotis, C. et al. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol Biofuels 10, 120 (2017). https://doi.org/10.1186/s13068-017-0798-9 (http://creativecommons.org/licenses/by/4.0/))
Miranda et al. (2017) isolated and characterized a number of natural microalgal biofilms from freshwater, saline lakes, and marine habitats (Fig. 10.13). Structurally, these biofilms represent complex consortia of unicellular and multicellular, photosynthetic and heterotrophic inhabitants, such as microalgae, bacteria, cyanobacteria, diatoms, and fungi. Symbiotic microalgal–bacterial biofilms can be very attractive for municipal wastewater treatment (Boelee et al. 2014). Boelee et al. (2014) described that microalgae remove nitrogen and phosphorus and simultaneously produce the oxygen that is required for the aerobic, heterotrophic degradation of organic pollutants. However, attempts are on to obtain a balanced system where no additional oxygen is required.
6.3 Non-suspended Enclosed Immobilized Cell System
Abdel-Raouf et al. (2012) reported that one of the major problems in the utilization of microalgae for the biological tertiary treatment of wastewater is their recovery from the treated effluent. However, cell immobilization allows high cell density of immobilized cells, improves the product yield and the volumetric productivity of bioreactors. Immobilization appears to offer several advantages in comparison with batch or continuous fermentation where free microorganisms are used.
It has been reported that Phormidium laminosum immobilized on polymer foam has the potential to remove nitrate in a continuous flow system with uptake efficiencies above 90% (Sawayama et al. 1998).
Matrix immobilization is a variant of the attachment theme of reactors through the entrapment of living microalgae cells within a natural or artificial resin, e.g., alginate or carrageenan beads (Mallick 2002; Mehta and Gaur 2005). Shi et al. (2007) reported removal of nitrogen and phosphorus from wastewater by two green microalgae (Chlorella vulgaris and Scenedesmus rubescens) using a novel method of algal cell immobilization, the twin-layer system. In the twin-layer system, microalgae are immobilized by self-adhesion on a wet, microporous, ultrathin substrate (the substrate layer) (Fig. 10.14).
Configuration of twin-layer wastewater treatment system. (Source: Shi, J., Podola, B. & Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19, 417–423 (2007). https://doi.org/10.1007/s10811-006-9148-1. Reproduced with Licence no 4881430070033 dated 3rd August 2020)
7 Harvesting
Harvesting of the microalgae requires the separation of a low amount of biomass consisting of small individual cells from a large volume of culture medium. The microalgal cell surface is negatively charged, and thus harvesting typically involves dosing a positively charged metal coagulant to neutralize the surface charge, allowing the cells to aggregate together, creating flocs (Henderson et al. 2008a). These flocs are removed through filtration, sedimentation, centrifugation, or dissolved in air flotation (DAF) (Henderson et al. 2008b). DAF utilizes the natural tendency of algae to float by raising the biomass to the surface, where it is skimmed off and recovered.
7.1 Extraction Methods
Various methods, including autoclaving, bead-beating, microwaves, sonication, and a 10% NaCl solution, have been tested to identify the most effective cell disruption method. The total lipids from Botryococcus sp., Chlorella vulgaris, and Scenedesmus sp. were extracted using a mixture of chloroform and methanol (1:1) (Lee et al. 2010; Mercer and Armenta 2011) Harun et al. (2013) presented approximate mass balance for biomass to biodiesel and fertilizer/animal feed supply chain (Fig. 10.15).
An approximate mass balance for biomass to biodiesel and fertilizer/animal feed supply chain. (Source: Harun R, Doyle M, Gopiraj R, Davidson M, Forde GM, Danquah MK (2013) Process economics and greenhouse gas audit for microalgal biodiesel production, in: advanced biofuels and bioproducts. Springer, New York, pp 709–744): Source: Allen, J., Unlu, S., Demirel, Y. et al. Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour. Bioprocess. 5, 47 (2018). https://doi.org/10.1186/s40643-018-0233-5. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
However, unicellular algae are difficult to harvest, resulting in recent research into non-planktonic algae, especially filamentous species such as Oedogonium sp. and Tribonema sp. (Roberts et al. 2013). For instance, Wang et al. (2013) reported that for the filamentous algae trialled, Cladophora sp. was most efficient under low N:P ratio wastewater whilst Pseudanabaena sp. was better for removing nitrogen from high N:P ratio wastewater.
Harun et al. (2013) presented approximate mass balance for biomass to biodiesel and fertilizer/animal feed supply chain (Fig. 10.15).
8 Algal Biofuel Production
Demirbas (2006) investigated microalgal biomass for bio-oil production and found the quality superior to the wood. Microalgal biomass with the diversified biochemical content of lipids, carbohydrates, and proteins can be processed via a variety of thermal and biochemical conversion routes into biodiesel, biohydrogen, biomethane, bio-oil, bio-crude oil, etc. (Behera et al. 2019). Behera et al. (2015) discussed the importance of the algal cell contents, many strategies for product formation through various conversion technologies.
Recently, attempts have been made for bioethanol production through fermentation process using algae as the feed stock (Singh 2011; Nguyen and Vu 2012; Chaudhary et al. 2014; Kumar et al. 2018). Sunil Kumar and Buddolla (2019) reported that many species of microalgae can be induced to accumulate significant quantities of lipids, thus contributing to an elevated oil yield, which is later converted into biodiesel by a process called transesterification. Rodionova et al. (2017) presented a scheme for production of biofuels from the hybrid technology (Fig. 10.16).
Algal cultivation for biofuel production. (Source. Rodionova, M. V, Poudyal, R. S., Tiwari, I., Voloshin, R. A., Zharmukhamedov, S. K., Nam, H. G., Allakhverdiev, S. I. (2017). Biofuel production: Challenges and opportunities. International Journal of Hydrogen Energy, 42(12), 8450–8461. https://doi.org/10.1016/j.ijhydene.2016.11.125. License Number 4883421498494 dated Aug 07, 2020)
9 Pyrolysis
Pyrolysis is the thermochemical process involving the application of heat (300–700 °C and higher) at atmospheric pressure under anoxic (the total absence of oxygen) conditions (Kazemi et al. 2019). Porphy and Farid (2012) produced bio-oil from pyrolysis of algae (Nannochloropsis sp.) at 300 °C after lipid extraction, which is composed of 50 wt% acetone, 30 wt% methyl ethyl ketone, and 19 wt% aromatics, such as pyrazine and pyrrole. Similarly, Choi et al. (2014) carried out pyrolysis study on a species of brown algae Saccharina japonica at a temperature of 450 °C and obtained about 47% of bio-oil yield. They reviewed thermochemical conversion of microalgae into bio-crude oil through pyrolysis and hydrothermal liquefaction technologies. Kazemi et al. (2019) presented HTL process of microalgae biomass (Fig. 10.17).
Schematic diagram of HTL process of microalgae biomass. (1) Slurry reservoir (2) high-pressure pump (3) reactor (4) gas cylinder (5) heat exchanger (6) condenser (7) separation vessel. (Source: Kazemi Shariat Panahi, H., Tabatabaei, M., Aghbashlo, M., Dehhaghi, M (2019). Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresource Technology Reports, 7, 100216. https://doi.org/10.1016/j.biteb.2019.100216. Licence 4906430857910 dated 12th September 2020)
Allen et al. (2018) biorefinery approach with integrated biology, ecology, and engineering has been presented in Fig. 10.18.
Microalgae biorefinery model. (Koyande AK, Show PL, Guo R, Tang B, Ogino C, Chang JS. Bio-processing of algal bio-refinery: a review on current advances and future perspectives. Bioengineered. 2019 Dec;10(1):574-592. https://doi.org/10.1080/21655979.2019.1679697. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited)
10 Discussion
Anthropogenic emissions of excessive atmospheric CO2 is leading to climate change causing cyclones never heard of, like Tauktae which was responsible for large number of deaths in India (https://economictimes.indiatimes.com/news/india/168-deaths-98-tauktae-hit-hospitals-resume-work/articleshow/82809054.cms). The frequency and fury of cyclones has been increasing to devastating levels due to heating of oceans which results in formation of cyclones. The world’s largest iceberg, dubbed A-76, has calved from Antarctica. The animation in the link shows a giant slab of ice breaking off from the Ronne Ice Shelf, lying in the Weddell Sea (https://www.nbcnews.com/science/science-news/worlds-largest-iceberg-just-broke-antarctic-ice-shelf-rcna974). This is also outcome of global warming.
The Paris Accord commits to reduction of global emissions to zero by 2050 in order to achieve 1.5 °C (Kumar et al. 2020). Biodiesel derived from oil crops is a potential renewable and carbon-neutral alternative to petroleum fuels (Behera et al. 2015; Kumar 2018; Kumar et al. 2019, 2020). According to Chisti (2007), commercial production of biodiesel or fatty acid methyl ester (FAME) usually involves alkaline-catalysed transesterification of triglycerides found in oilseed crops (mainly rapeseed in Europe and soybean in the United States) with methanol. However, escalating demand for these crops as a food source coupled with the finite availability of arable land makes their cultivation for biofuels unsustainable (Chisti 2007).
Appropriation of the metabolic products of microorganisms as sources of energy and specialized organic compounds represents a growing form of agriculture (Shurin et al. 2013).
Many microalgae species have been adapted to grow efficiently in wastewater. Thus, the cost of production may be decreased due to the simultaneous use of wastewater and cultivation of specific nutrient-rich microalgae. Therefore, microalgae-mediated CO2 bio-mitigation can be more economic, cost-effective, and eco-friendly, when it is incorporated into a wastewater treatment infrastructure (Kuo et al. 2016; Collotta et al. 2018).
According to Molazadeh et al. (2019), three major methods are being taken into action in order to remove excess CO2 which is the largest contributor to the greenhouse effect: (1) transforming CO2 to organic matters through photosynthesis. The use of third-generation biofuels does not require land and water like crops which compete with food crops (Kumar 2018; Kumar et al. 2010, Chap. 3). (2) Using chemical reactions including chemical/physical solvent scrubbing, adsorption, cryogenics, and membranes, (3) The storage of CO2 emitted underground or into the ocean. According to Miranda et al. (2017), next generation of bioenergy feedstocks should meet key selection criteria: (1) low freshwater requirement; (2) high growth rates and biomass production; (3) high harvesting index in short rotation period; (4) high content of bioenergy-producing molecules (5) ability to grow on marginal lands and lack of competition with agricultural crops for arable lands; (6) low costs for growth and harvest; and (7) production of high-value co-products (Henry 2010).
However, the mitigation through biological CO2 fixation is an economically practical and environmentally sustainable technology which has achieved much attention as an alternative method in the long term (Kumar et al. 2010, 2018, 2020; Kumar 2013, 2018).
Microalgae are autotrophic organisms that capture CO2 present in the air for oxygenic photosynthesis. Because of high tolerant capability to elevated levels of CO2 in the atmosphere, microalgae are preferred over other carbon sequestration methods (Behera et al. 2019).
The relative supplies of different mineral nutrients and light can have strong phytoplankton and their value as biofuel feedstocks. Large supplies of P relative to N may favour competitive dominance by heterocystous N2-fixing cyanobacteria (Schindler et al. 2008), which have low cellular lipid contents. Several species of eukaryotic algae accumulate lipid in their cells under conditions of N-starvation (Shurin et al. 2013).
There are three main sources of wastewater, municipal (domestic), agricultural, and industrial wastewater, which contain a variety of ingredients (Chiu et al. 2015). Industrial waste from metal processing industries as well as textile, leather, tannery, and electroplating, have varying amounts of toxic metal ions. Likewise heavy metal ions such as Cd, Cr and Zn, as well as organic chemical toxins such as surfactants, hydrocarbons, and biocides are all present in industrial wastewaters (Ahluwalia and Goyal 2007).
Water from domestic wastewater treatment plants or livestock operations offers a potential source of cheap nutrients that might otherwise be discharged into surface waters (Craggs et al. 2011; Sturm et al. 2012). Recent development of hybrid technologies (biomass production, wastewater treatment, GHG mitigation) for production of prime products as biofuels offer atmospheric pollution control such as the reduction of GHG (CO2 fixation) coupling wastewater treatment with microalgae growth (Maity et al. 2014). Marine microalgae constitute a useful form of biomass to overcome environmental problems as they can be grown on saline water. However, the availability of innovative approaches for maximizing the treatment efficiency, coupled with biomass productivity, remains the major bottleneck for commercialization of microalgal technology (Abinandan et al. 2018). However in addition to algae culture and growth, it is also essential to develop cost-effective technologies for efficient biomass harvesting, lipid extraction, and biofuels production (Safi et al. 2014). As single-celled molecular factories, microalgae can also be cultivated on marginal land unsuitable for agriculture, using waste streams or saline water supplies (Clarens et al. 2010; Marjakangas et al. 2015). Nannochloropsis is a genus of robust, oleaginous microalgae that synthesizes eicosapentaenoic acid (EPA) during balanced growth and is a promising candidate for commercial applications (Davis et al. 2011; Kilian et al. 2011; Radakovits et al. 2012; Sharma and Schenk 2015).
Although some components in the wastewater, such as nitrogen and phosphorus, act as nutrients for microalgae, algal growth rates are lower in different industrial wastewaters, and thus potential for the large-scale treatments of industrial wastewaters containing high levels of heavy metal ions for algal culture are not so promising at the moment. The main challenges are not limited to: industrial-scale capturing of carbon dioxide using microalgal species, the tolerances of specific algal strains grown in a wide variety and concentration of heavy metal ions in industrial wastewaters but optimization of several parameters like simultaneous removal of CO2 and treatment of wastewaters containing heavy metal ions (Molazadeh et al. 2019).
11 Conclusion
Renewable energy plays a critical role in addressing issues of energy security and climate change at global and domestic scales. Biomass offers only means of absorption of global carbon dioxide the major cause of global warming. However, first- and second-generation biofuels compete mostly for land and water with arable lands. Third-generation biofuels derived from algae (or a consortium of microbes) offer triple benefits like algal biomass production, wastewater treatment, and greenhouse gas mitigation. This is receiving increasing attention worldwide as an alternative and renewable source for energy production. Algal biomass can be grown using non-arable areas such as lakes, oceans, or deserts, thus avoiding the current problem of land use competition with the food supply chain. The potential for remediation of inorganic nitrogen and phosphorus from wastewater by microalgae is considered an environmental approach to nutrient polishing. It also has benefits of (a) sequestering of CO2 from the atmosphere during photosynthesis (b) oxygenating the treated effluent and biofuel production. The chapter presented some of these processes in detail.
References
Aaron D, Tsouris C (2005) Separation of CO2 from flue gas: a review. Sep Sci Technol 40:321–348. https://doi.org/10.1081/SS-200042244
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IB (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Abhinandan S, Shanthakumar S (2015) Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: a review. Renew Sust Energ Rev 52:123–132
Abhinandan S, Bhattacharya R, Shanthakumar S (2015) Efficacy of Chlorella pyrenoidosa and Scenedesmus abundans for Nutrient Removal in Rice Mill Effluent (Paddy Soaked Water). Int J Phytoremed 17(1–6):377–381. https://doi.org/10.1080/15226514.2014.910167
Abinandan S, Subashchandrabose SR, Venkateswarlu K, Megharaj M (2018) Nutrient removal and biomass production: advances in microalgal biotechnology for wastewater treatment. Crit Rev Biotechnol 38(8):1244–1260. https://doi.org/10.1080/07388551.2018.1472066
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243. https://doi.org/10.1016/j.biortech.2005.12.006
Allen J, Unlu S, Demirel Y, Black P, Riekhof W (2018) Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour Bioprocess 5:47. https://doi.org/10.1186/s40643-018-0233-5
Amenorfenyo DK, Huang X, Zhang Y, Zeng Q, Zhang N, Ren J, Huang Q (2019) Microalgae brewery wastewater treatment: potentials, benefits and the challenges. Int J Environ Res Public Health 16(11):1910. https://doi.org/10.3390/ijerph16111910
Anindita R, Kumar A (2013) Pretreatment methods of lignocellulosic materials for biofuel production: a review. J Emerg Trends Eng Appl Sci 4(2):181–193
Bartley ML, Boeing WJ, Daniel D, Dungan BN, Schaub T (2016) Optimization of environmental parameters for Nannochloropsis salina growth and lipid content using the response surface method and invading organisms. J Appl Phycol 28:15–24
Bechet Q, Muñoz R, Shilton A, Guieysse B (2013) Outdoor cultivation of temperature-tolerant chlorella sorokiniana in a column photobioreactor under low power-input. Biotechnol Bioeng 110:118–126. https://doi.org/10.1002/bit.24603. PMID: 2276710
Behera S, Mohanty RC, Ray RC (2011) Ethanol production from mahula (Madhuca latifolia L.) flowers using free and immobilized (in Luffa cylindrical L. sponge discs) cells of Zymomonas mobilis MTCC 92. Ann Microbiol 61:469–474. https://doi.org/10.1007/s13213-010-0160-y
Behera S, Singh R, Arora R, Sharma NK, Shukla M (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2:1–13. https://doi.org/10.3389/fbioe.2014.00090
Behera B, Acharya A, Gargey IA, Aly N, Balasubramanian P (2019) Bioprocess engineering principles of Microalgal cultivation for sustainable biofuel production. Bioresour Technol Rep 5:297–316. https://doi.org/10.1016/j.biteb.2018.08.001
Benemann JR (1993) Utilization of carbon dioxide from fossil fuel-burning power plants with biological systems. Energy Conserv Manag 34:999–1004
Benemann MA, Pedroni PM (2003) Biofixation of CO2 and greenhouse gas abatement with microalgae. Final report prepared for US DOE. US DOE, Washington, DC
Berner F, Heimann K, Sheehan M (2015) Microalgal biofilms for biomass production. J Appl Phycol 27(5):1793–1804
Beuckels A, Smolders E, Muylaert K (2015) Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res 77:98–106
Bilanovic D, Andargatchew A, Kroeger T, Shelef G (2009) Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations-response surface methodology analysis. Energy Convers Manag 50:262–267. https://doi.org/10.1016/j.enconman.2008.09.024
Blanken W, Janssen M, Cuaresma M, Libor Z, Bhaiji T, Wijffels RH (2014) Biofilm growth of Chlorella sorokiniana in a rotating biological contactor based photobioreactor. Biotechnol Bioeng 111:2436–2445. https://doi.org/10.1002/bit.25301
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2014) Balancing the organic load and light supply in symbiotic microalgal-bacterial biofilm reactors treating synthetic municipal wastewater. Ecol Eng 64:213–221. https://doi.org/10.1016/j.ecoleng.2013.12.035
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev 19:360–369
Carlsson AS, van Beilen JB, Moeller R, Clayton D (2007) In: Bowles D (ed) Micro- and macro-algae: utility for industrial applications, outputs from the EPOBIO project. University of York, CPL Press, Newbury, p 86
Carvalho AP, Meireleles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Chaudhary L, Pradhan P, Soni N, Singh P, Tiwari A (2014) Algae as a feedstock for bioethanol production: new entrance in biofuel world. Int J Chem Technol Res 6:1381–1389
Chen CY, Zhao XQ, Yen HW, Ho SH, Cheng CL, Bai F et al (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10. https://doi.org/10.1016/j.bej.2013.03.006
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001. PMID: 17350212
Chiu SY, Kao CY, Chen TY, Chang YB, Kuo CM, Lin CS (2015) Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour Technol 184:179–189. https://doi.org/10.1016/j.biortech.2014.11.080
Choi JH, Woo HC, Suh DJ (2014) Pyrolysis of seaweeds for bio-oil and bio-char production. Chem Eng Trans 37:121–126. https://doi.org/10.1016/j.biortech.2014.09.068
Christenson L, Sims R (2011) Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv 29(6):686–702
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819. PMID: 20085253
Cohen RRH (2006) Use of microbes for cost reduction of metal removal from metals and mining industry waste streams. J Clean Prod 14:1146–1157. https://doi.org/10.1016/j.jclepro.2004.10.009
Collotta MP, Champagne WM, Tomasoni G (2018) Wastewater and waste CO2 for sustainable biofuels from microalgae. Algal Res 29:12–21. https://doi.org/10.1016/j.algal.2017.11.013
Craggs RJ, Heubeck S, Lundquist TJ, Benemann JR (2011) Algae biofuel from wastewater treatment high rate algal ponds. Water Sci Technol 63:660–665
Craggs R, Park J, Heubeck S, Sutherland D (2014) High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N Z J Bot 52(1):60–73. https://doi.org/10.1080/0028825X.2013.861855
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
De la Noue J, De Pauw N (1988) The potential of microalgal biotechnology. A review of production and uses of microalgae. Biotechnol Adv 6:725–770. https://doi.org/10.1016/0734-9750(88)91921-0
Demirbas A (2006) Oily products from mosses and algae via pyrolysis. Energy Sources 28:933–940. https://doi.org/10.1080/009083190910389
Demirel Y (2018a) Sugar versus lipid for sustainable biofuels. Int J Energy Res 42:881–884. https://doi.org/10.1002/er.3914
Demirel Y (2018b) Biofuels. In: Comprehensive energy systems. Elsevier, New York, pp 875–908. https://doi.org/10.1016/B978-0-12-809597-3.00125-5
EC Directive (2009) On the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC.2009.04.23. Off J EU L 140:16e62
Ehimen EA, Sun ZF, Carrington CG, Birch EJ, Eaton-Rye JJ (2011) Anaerobic digestion of microalgae residues resulting from the biodiesel production process. Appl Energy 88:3454–3463. https://doi.org/10.1016/j.apenergy.2010.10.020
Eixler S, Karsten U, Selig U (2006) Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 45(1):53–60
Eshaq FS, Ali MN, Mohd MK (2011) Production of bioethanol from next generation feed-stock alga Spirogyra species. Int J Eng Sci Technol 3:1749–1755
Gajraj RS, Singh GP, Kumar A (2018) Third-generation biofuel: algal biofuels as a sustainable energy source. In: Kumar A, Ogita S, Yau Y-Y (eds) Biofuels: greenhouse gas mitigation and global warming next generation biofuels and role of biotechnology. Springer, Heidelberg, pp 307–326
García D, Alcántara C, Blanco S, Pérez R, Bolado S, Muñoz R (2017) Enhanced carbon, nitrogen and phosphorus removal from domestic wastewater in a novel anoxic-aerobic photobioreactor coupled with biogas upgrading. Chem Eng J 313:424–434
Gray NF (1989) Biology of wastewater treatment. Oxford University Press, Oxford
Gross M, Jarboe D, Wen Z (2015) Biofilm-based algal cultivation systems. Appl Microbiol Biotechnol 99:5781–5789. https://doi.org/10.1007/s00253-015-6736-5
Guiheneuf F, Stengel DB (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11:4246–4266. https://doi.org/10.3390/md11114246. PMID: 24177672
Harun R, Doyle M, Gopiraj R, Davidson M, Forde GM, Danquah MK (2013) Process economics and greenhouse gas audit for microalgal biodiesel production, in: advanced biofuels and bioproducts. Springer, New York, pp 709–744
Henderson RK, Parsons SA, Jefferson B (2008a) Successful removal of algae through the control of zeta potential. Sep Sci Technol 43(7):1653–1666
Henderson R, Parsons SA, Jefferson B (2008b) The impact of algal properties and pre-oxidation on solid-liquid separation of algae. Water Res 42(8–9):1827–1845
Henry RJ (2010) Evaluation of plant biomass resources available for replacement of fossil oil. Plant Biotechnol J 8(3):288–293
Horan NJ (1990) Biological wastewater treatment systems. Theory and operation. John Wiley and Sons Ltd, Chichester
Hu Q, Kurano N, Kawachi M, Iwasaki I, Miyachi S (1998) Ultrahigh-cell-density culture of a marine green alga Chlorococcum littorale in a flat-plate photobioreactor. Appl Microbiol Biotechnol 49:655–662
Hulatt CJ, Thomas DN (2011) Energy efficiency of an outdoor microalgal photobioreactor sited at mid-temper- ate latitude. Bioresour Technol 102:6687–6695. https://doi.org/10.1016/j.biortech.2011.03.098. PMID: 21511466
Hulatt CJ, Wijffels RH, Bolla S, Kiron V (2017) Production of fatty acids and protein by nannochloropsis in flat-plate photobioreactors. PLoS One 12(1):e0170440. https://doi.org/10.1371/journal.pone.0170440
Jayanti S, Narayanan S (2004) Computational study of particle-eddy interaction in sedimentation tanks. J Environ Eng 130:37. https://doi.org/10.1061/(ASCE)0733-9372130:1(37)
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186e193
Jorquera O, Kiperstock A, Sales E, Embirucu M, Ghirardi M (2010) Comparative energy life-cycle analysis of microalgal biomass production in open ponds and photobioreactors. Bioresour Technol 101:1406–1413. https://doi.org/10.1016/j.biortech.2009.09.038. PMID: 19800784
Kargupta W, Ganesh A, Mukherji S (2015) Estimation of carbon dioxide sequestration potential of microalgae grown in a batch photobioreactor. Bioresour Technol 180:370–375. https://doi.org/10.1016/j.biortech.2015.01.017
Kazemi SPH, Tabatabaei M, Aghbashlo M, Dehhaghi M (2019) Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresour Technol Rep 7:100216. https://doi.org/10.1016/j.biteb.2019.100216
Kilian O, Benemann CS, Niyogi KK, Vick B (2011) High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci U S A 108:21265–21269
Kraan S (2013) Mass cultivation of carbohydrate rich microalgae, a possible solution for sustainable biofuel production. Mitig Adapt Strateg Glob Chang 18:27–46. https://doi.org/10.1007/s11027-010-9275-5
Kumar A (2013) Biofuels utilisation: an attempt to reduce GHG’s and mitigate climate change. In: Nautiyal S, Rao K, Kaechele H, Raju K, Schaldach R (eds) Knowledge systems of societies for adaptation and mitigation of impacts of climate change. Environmental science and engineering. Springer, Berlin, pp 199–224
Kumar A (2018) Global warming, climate change and greenhouse gas mitigation. In: Kumar A, Ogita S, Yau Y-Y (eds) Biofuels: greenhouse gas mitigation and global warming next generation biofuels and role of biotechnology. Springer, Heidelberg, pp 1–16
Kumar SM, Buddolla V (2019) Chapter 12 - Future prospects of biodiesel production by microalgae: a short review. In: Buddolla V (ed) Recent developments in applied microbiology and biochemistry. Academic Press, London, pp 161–166. https://doi.org/10.1016/B978-0-12-816328-3.00012-X
Kumar A, Gupta N (2018) Potential of lignocellulosic materials for production of ethanol. In: Kumar A, Ogita S, Yau Y-Y (eds) Biofuels: greenhouse gas mitigation and global warming next generation biofuels and role of biotechnology. Springer, Heidelberg, pp 271–290
Kumar A, Singh JS (2017) Cyanoremediation: a green-clean tool for decontamination of synthetic pesticides from agro- and aquatic ecosystems. In: Singh J, Seneviratne G (eds) Agro-environmental sustainability. Springer, Cham. https://doi.org/10.1007/978-3-319-49727-3_4
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J et al (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380. https://doi.org/10.1016/j.tibtech.2010.04.004
Kumar A, Ogita S, Yau Y-Y (eds) (2018) Biofuels: greenhouse gas mitigation and global warming next generation biofuels and role of biotechnology. Springer, Heidelberg, p 432. ISBN 978-81-322-3761-72
Kumar A, Bhansali S, Gupta N, Sharma M (2019) Bioenergy and climate change: greenhouse gas mitigation. In: Rastegari AA, Yadav AN, Gupta A (eds) Prospects of renewable bioprocessing in future energy systems. Biofuel and biorefinery technologies. Springer Nature, Cham, pp 269–290
Kumar A, Yau YY, Ogita S, Scheibe R (eds) (2020) Climate change, photosynthesis and advanced biofuels. Springer, Singapore, p 490. https://doi.org/10.1007/978-981-15-5228-1_1
Kuo CM, Jian JF, Lin TH, Chang YB, Wan XH, Lai JT et al (2016) Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour Technol 221:241–250. https://doi.org/10.1016/j.biortech.2016.09.014
Lee YK (1997) Commercial production of microalgae in the Asia-Pacific rim. J Appl Phycol 9:403–411
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S75. https://doi.org/10.1016/j.biortech.2009.03.058
Li M, Luo N, Lu Y (2017) Biomass energy technological paradigm (BETP): trends in this sector. Sustainability 9(4):567
Li H, Watson J, Zhang Y, Lu H, Liu Z (2020) Environment-enhancing process for algal wastewater treatment, heavy metal control and hydrothermal biofuel production: a critical review. Bioresour Technol 298:122421. https://doi.org/10.1016/j.biortech.2019.122421
Lim S, Chu W, Phang S (2010) Use of Chlorella vulgaris for bioremediation of textile wastewater. J Bioresour Technol 101:7314–7322
Liu X, Saydah B, Eranki P, Colosi LM, Mitchell BG, Rhodes J, Clarens AF (2013) Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour Technol 148:163–171. https://doi.org/10.1016/j.biortech.2013.08.112
Maity JP, Bundschuh J, Chen C-Y, Bhattacharya P (2014) Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: present and future perspectives - a mini review. Energy 78:104–113. https://doi.org/10.1016/j.energy.2014.04.003
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15:377–390
Marjakangas JM, Chen CY, Lakaniemi AM, Puhakka JA, Whang LM, Chang JS (2015) Selecting an indigenous microalgal strain for lipid production in anaerobically treated piggery wastewater. Bioresour Technol 191:369–376
Mehrabadi A, Craggs R, Farid MM (2015) Wastewater treatment high rate algal ponds (WWT HRAP) for low-cost biofuel production. Bioresour Technol 184:202–214
Mehrabadi A, Craggs R, Farid MM (2017a) Wastewater treatment high rate algal pond biomass for bio-crude oil production. Bioresour Technol 224:255–264
Mehrabadi A, Farid MM, Craggs R (2017b) Effect of CO2 addition on biomass energy yield in wastewater treatment high rate algal mesocosms. Algal Res 22:93–103
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. CRC Rev Biotechnol 25:113–152
Mercer P, Armenta RE (2011) Developments in oil extraction from microalgae. Eur J Lipid Sci Technol 113:539–547. https://doi.org/10.1002/ejlt.201000455
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgaebiofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197
Miranda AF, Ramkumar N, Andriotis C et al (2017) Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol Biofuels 10:120. https://doi.org/10.1186/s13068-017-0798-9
Moheimani NR, Parlevliet D, McHenry MP, Bahri PA, de Boer K (2015) Past, present and future of microalgae cultivation developments. In: Moheimani N, McHenry M, de Boer K, Bahri P (eds) Biomass and biofuels from microalgae. Biofuel and biorefinery technologies, vol 2. Springer, Cham. https://doi.org/10.1007/978-3-319-16640-7_1
Mohsenpour SF, Hennige S, Willoughby N et al (2021) Integrating micro-algae into waste water treatment: a review. Sci Total Environ 752:142168. https://doi.org/10.1016/j.scitotenv.2020.142168
Molazadeh M, Ahmadzadeh H, Pourianfar HR, Lyon S (2019) The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front Bioeng Biotechnol 7:42. https://doi.org/10.3389/fbioe.2019.00042
Molina E, Fernandez J, Acien FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131. PMID: 11640983
Mussgnug JH, Klassen V, Schlüter A, Kruse O (2010) Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J Biotechnol 150:51–56
Nazneen S, Kumar A (2014) Energy crops for bio fuel and food security. J Pharmaceut Sci Innov 3(6):507–515
Nguyen THM, Vu VH (2012) Bioethanol production from marine algae biomass: prospect and troubles. J Vietnam Environ 3:25–29. https://doi.org/10.13141/jve.vol3.no1
Norman D (1997) Environmental management systems. Glass Technol 38:146–149
Okolo BI, Nnaji PC, Oke EO, Adekunle KF, Ume CS, Onukwuli OD (2018) Optimizing bio-coagulants for brewery wastewater treatment using response surface methodology. Niger J Technol 36:1104–1113. https://doi.org/10.4314/njt.v36i4.16
Olajire AA (2020) The brewing industry and environmental challenges. J Clean Prod 256:102817
Park JBK, Craggs RJ (2014) Effect of algal recycling rate on the performance of Pediastrum boryanum dominated wastewater treatment high rate algal pond. Water Sci Technol 70(8):1299–1306
Perera IA, Sudharsanam A, Subashchandrabose SR, Venkateswarlu K, Naidu R, Mallavarapu M (2019) Advances in the technologies for studying consortia of bacteria and cyanobacteria/microalgae in wastewaters. Crit Rev Biotechnol 39(5):709–731. https://doi.org/10.1080/07388551.2019.1597828
Porphy SJ, Farid MM (2012) Feasibility study for production of biofuel and chemicals from marine microalgae Nannochloropsis sp. based on basic mass and energy analysis. ISRN Renew Energ 2012:156824. https://doi.org/10.5402/2012/156824
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42(16):5958–5962
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL et al (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropsis gaditana. Nat Commun 3:686
Raeesossadati MJ, Ahmadzadeh H (2015) CO2 environmental bioremediation by microalgae. In: Biomass and biofuels from microalgae, vol 2. Springer, New York, NY, pp 117–136. https://doi.org/10.1007/978-3-319-16640-7
Rajkumar R, Zahira Y, Mohd ST (2014) Algal biofuel production. Bioresources 9(1):1606–1633
Roberts DA, de Nys R, Paul NA (2013) The effect of CO2 on algal growth in industrial waste water for bioenergy and bioremediation applications. PLoS One 8(11):e81631. https://doi.org/10.1371/journal.pone.0081631
Rodionova MV, Poudyal RS, Tiwari I, Voloshin RA, Zharmukhamedov SK, Nam HG, Allakhverdiev SI (2017) Biofuel production: challenges and opportunities. Int J Hydrog Energy 42(12):8450–8461
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278. https://doi.org/10.1016/j.rser.2014.04.007
Sawayama K, Rao K, Hall DO (1998) Nitrate and phosphate ions removal from water by Phormidium laminosum immobilized on hollow fibres in a photobioreactor. Appl Microbiol Biotechnol 49:463–468
Schenk MP, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C et al (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1:20e43
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci U S A 105:11254–11258
Seco A, Ferrer J (2015) Effect of intracellular P content on phosphate removal in Scenedesmus sp. Experimental study and kinetic expression. Bioresour Technol 175:325–332
Sharma K, Schenk PM (2015) Rapid induction of omega-3 fatty acids (EPA) in Nannochloropsis sp. by UV-C radiation. Biotechnol Bioeng 112:1243–1249. https://doi.org/10.1002/bit.25544. PMID: 25708183
Sharma YC, Singh V (2017) Microalgal biodiesel: a possible solution for India’s energy security. Renew Sust Energ Rev 67:72–88. https://doi.org/10.1016/j.rser.2016.08.031
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19(5):417–423
Showkat U, Najar IA (2019) Study on the efficiency of sequential batch reactor (SBR)-based sewage treatment plant. Appl Water Sci 9:2. https://doi.org/10.1007/s13201-018-0882-8
Shurin JB, Abbott RL, Deal MS, Kwan GT, Litchman E, McBride RC, Smith VH (2013) Industrial-strength ecology: trade-offs and opportunities in algal biofuel production. Ecol Lett 16(11):1393–1404. https://doi.org/10.1111/ele.12176
Sierra E, Acién FG, Fernández JM, García JL, González C, Molina E (2009) Characterization of a flat plate photobioreactor for the production of microalgae. Chem Eng J 138(1–3):136–147
Silambarasan S, Logeswari P, Sivaramakrishnan R, Incharoensakdi A, Cornejo P, Kamaraj B, Chi NTL (2021) Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 268:129323. https://doi.org/10.1016/j.chemosphere.2020.129323. PMID: 33359999
Simate GS, Cluett J, Iyuke SE, Musapatika ET, Ndlovu S, Walubita LF, Alvarez AE (2011) The treatment of brewery wastewater for reuse: state of the art. Desalination 273:235–247. https://doi.org/10.1016/j.desal.2011.02.035
Singh JS (2011) Methanotrophs: the potential biological sink to mitigate the global methane load. Curr Sci 100:29–30
Singh JS (2014) Cyanobacteria: a vital bio-agent in eco-restoration of degraded lands and sustainable agriculture. Climate Change Environ Sustain 2:133–137
Singh JS (2015a) Microbes: the chief ecological engineers in reinstating equilibrium in degraded ecosystems. Agric Ecosyst Environ 203:80–82. https://doi.org/10.1016/j.agee.2015.01.026
Singh JS (2015b) Plant-microbe interactions: a viable tool for agricultural sustainability. Appl Soil Ecol 92:45–46. https://doi.org/10.1016/j.apsoil.2015.03.004
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529. https://doi.org/10.3389/fmicb.2016.00529
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38. https://doi.org/10.1016/j.biombioe.2012.12.019
Smith VH, Mcbride RC (2015) Key ecological challenges in sustainable algal biofuels production. J Plankton Res 37(4):671–682. https://doi.org/10.1093/plankt/fbv053
Sturm BSM, Peltier E, Smith VH, DeNoyelles FJ (2012) Controls of microalgal biomass and lipid production in municipal wastewater-fed bioreactors. Environ Prog Sustain Energy 31:10–16
Sutherland DL, Howard-Williams C, Turnbull MH, Broady PA, Craggs RJ (2014) Seasonal variation in light utilisation, biomass production and nutrient removal by wastewater microalgae in a full-scale high-rate algal pond. J Appl Phycol 26(3):1317–1329
Talbot P, Lencki RW, De la Noüe J (1990) Carbon dioxide absorption characterization of a bioreactor for biomass production of Phormidium bohneri: a comparative study of three types of diffuser. J Appl Phycol 2:341–350
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99(10):4021–4028
Vejrazka C, Janssen M, Streefland M, Wijffels RH (2012) Photosynthetic efficiency of Chlamydomonas reinhardtii in attenuated, flashing light. Biotechnol Bioeng 109:2567–2574
Wang H, Gao L, Chen L, Guo F, Liu T (2013) Integration process of biodiesel production from filamentous oleaginous microalgae Tribonema minus. Bioresour Technol 142:39–44. https://doi.org/10.1016/j.biortech.2013.05.058
Wang JH, Zhang TY, Dao GH et al (2017) Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl Microbiol Biotechnol 101:2659–2675. https://doi.org/10.1007/s00253-017-8184-x
Weissman JC, Goebel RP (1988) Design and analysis of microalgal open pond systems for the purpose of producing fuels. A subcontract report. U.S. Dept. of Energy, Washington, DC. http://www.osti.gov/bridge/product.biblio.jsp?query_id=0&page=0&osti_id=6546458
Whitton R, Ometto F, Pidou M, Jarvis P, Villa R, Jefferson B (2015) Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ Technol Rev 4:133–148. https://doi.org/10.1080/21622515.2015.1105308
Yin D, Wang Z, Wen X et al (2019) Effects of carbon concentration, pH, and bubbling depth on carbon dioxide absorption ratio in microalgae medium. Environ Sci Pollut Res Int 26(32):32902–32910. https://doi.org/10.1007/s11356-019-06287-4
Yustinadiar N, Manurung R, Suantika G (2020) Enhanced biomass productivity of microalgae Nannochloropsis sp. in an airlift photobioreactor using low-frequency flashing light with blue LED. Bioresour Bioprocess 7:43
Zou N, Zhang C, Cohen Z, Richmond A (2000) Production of cell mass and eicosapentaenoic acid (EPA) in ultrahigh cell density cultures of Nannochloropsis sp. (Eustigmatophyceae). Eur J Phycol 35:127–133
Acknowledgements
Authors acknowledge with thanks the authors of the papers and figures used in this chapter with permission: Figure 10.1 Source: Abdel-Raouf, N., Al-Homaidan, A. A., and Ibraheem, I. B. (2012). Microalgae and wastewater treatment. Saudi J. Biol. Sci. 19, 257–275. doi: https://doi.org/10.1016/j.sjbs.2012.04.005. Open access: Reprinted with Licence no. 4880801372566 dated 2nd Aug 2020 RightsLink. Figure 10.2 Maity, J. P., Bundschuh, J., Chen, C.-Y., & Bhattacharya, P. (2014). Microalgae for third-generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives – A mini review. Energy, 78, 104–113. https://doi.org/10.1016/j.energy.2014.04.003. Reproduced with licence no 5064180534847 dated 8th May 2021. Figure 10.3 Li H, Watson J, Zhang Y, Lu H, Liu Z. Environment-enhancing process for algal wastewater treatment, heavy metal control and hydrothermal biofuel production: A critical review. Bioresour Technol. 2020 Feb; 298: 122421. doi: https://doi.org/10.1016/j.biortech.2019.122421. Epub 2019 Nov 14. PMID: 31767428. Licence no 5065350239998 dated 10th May. Figure 10.4 Singh, J. S., Kumar, A., Rai, A. N., & Singh, D. P. (2016). Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Frontiers in microbiology, 7, 529. https://doi.org/10.3389/fmicb.2016.00529. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). Figure 10.5 Kumar A., Singh J.S. (2017) Cyano Remediation: A Green-Clean Tool for Decontamination of Synthetic Pesticides from Agro- and Aquatic Ecosystems. In: Singh J., Seneviratne G. (eds) Agro-Environmental Sustainability. Springer, Cham. https://doi.org/10.1007/978-3-319-49727-3_4. Reproduced with RightsLink licence number 5071420183103 dated 17th May 2021. Figure 10.6 Integration of microalgal wastewater treatment with resource recovery for maximizing the derivable products. (Source: Behera, B., Acharya, A., Gargey, I. A., Aly, N., & P, B. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. https://doi.org/10.1016/j.biteb.2018.08.001. Reproduced under Licence number 4906420744566 dated 12th September). Figure 10.7 Whitton, R Francesco Ometto, Marc Pidou, Peter Jarvis, Raffaella Villa & Bruce Jefferson (2015) Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment, Environmental Technology Reviews, 4: 133–148, DOI: https://doi.org/10.1080/21622515.2015.1105308. This is an open access article distributed under the terms of the Creative Commons CC BY license. Figure 10.8 Source: Behera, B., Acharya, A., Gargey, I. A., Aly, N., & P, B. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. https://doi.org/10.1016/j.biteb.2018.08.001. Reproduced under Licencenumber 4906420744566 dated 12th September. Figure 10.9 Behera, B., Acharya, A., Gargey, I. A., Aly, N., & P, B. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. https://doi.org/10.1016/j.biteb.2018.08.001. Reproduced under Licence number 4906420744566 dated 12th September. Figure 10.10 Allen, J., Unlu, S., Demirel, Y. et al. Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour. Bioprocess. 5, 47 (2018). https://doi.org/10.1186/s40643-018-0233-5. This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Figure 10.11 Abdel-Raouf N., Al-Homaidan, A. A., and Ibraheem, I. B. (2012). Microalgae and wastewater treatment. Saudi J. Biol. Sci. 19, 257–275. doi: https://doi.org/10.1016/j.sjbs.2012.04.005. Open access Reprinted with Licence no. 4880801372566 dated 2nd Aug 2020 RightsLink. Figure 10.12 Yustinadiar, N., Manurung, R. & Suantika, G. Enhanced biomass productivity of microalgae Nannochloropsis sp. in an airlift photobioreactor using low-frequency flashing light with blue LED. Bioresour. Bioprocess. 7, 43 (2020). https://doi.org/10.1186/s40643-020-00331-9. This article is licensed under a Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/. Figure 10.13 Miranda, A.F., Ramkumar, N., Andriotis, C. et al. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol Biofuels 10, 120 (2017). https://doi.org/10.1186/s13068-017-0798-9 (http://creativecommons.org/licenses/by/4.0/). Figure 10.14 Shi, J., Podola, B. & Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19, 417–423 (2007). https://doi.org/10.1007/s10811-006-9148-1. Reproduced with Licence no 4881430070033 dated 3rd August 2020. Figure 10.15 Harun R, Doyle M, Gopiraj R, Davidson M, Forde GM, Danquah MK (2013) Process economics and greenhouse gas audit for microalgal biodiesel production, in: advanced biofuels and bioproducts. Springer, New York, pp 709–744. Allen, J., Unlu, S., Demirel, Y. et al. Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour. Bioprocess. 5, 47 (2018). https://doi.org/10.1186/s40643-018-0233-5. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Figure 10.16 Rodionova, M. V, Poudyal, R. S., Tiwari, I., Voloshin, R. A., Zharmukhamedov, S. K., Nam, H. G., Allakhverdiev, S. I. (2017). Biofuel production: Challenges and opportunities. International Journal of Hydrogen Energy, 42(12), 8450–8461. https://doi.org/10.1016/j.ijhydene.2016.11.125. License Number 4883421498494 dated Aug 07, 2020. Figure 10.17 Kazemi Shariat Panahi, H., Tabatabaei, M., Aghbashlo, M., Dehhaghi, M (2019). Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresource Technology Reports, 7, 100216. https://doi.org/10.1016/j.biteb.2019.100216. Licence 4906430857910 dated 12th September 2020. Figure 10.18 Allen et al. Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour. Bioprocess. 5, 47 (2018). https://doi.org/10.1186/s40643-018-0233-5. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumar, A., Acharya, P., Jaiman, V. (2022). Third-Generation Hybrid Technology for Algal Biomass Production, Wastewater Treatment, and Greenhouse Gas Mitigation. In: Arora, S., Kumar, A., Ogita, S., Yau, Y.Y. (eds) Innovations in Environmental Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-4445-0_10
Download citation
DOI: https://doi.org/10.1007/978-981-16-4445-0_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4444-3
Online ISBN: 978-981-16-4445-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)