Abstract
Algae biofuel has the potential to replace fossil fuels. However, cultivation and productivity of target algae need improvement, while controlling undesired organisms that can lower the efficiency of production systems. A central composite design and response surface model were utilized to predict cultivation optima of marine microalga, Nannochloropsis salina, under a suite of environmental parameters. The effects of salinity, pH, and temperature and their interactions were studied on maximum sustainable yield (MSY, a measure for biomass productivity), lipid content of N. salina, and invading organisms. Five different levels of each environmental predictor variable were tested. The environmental factors were kept within ranges that had previously been determined to allow positive N. salina growth (14.5–45.5 PSU; pH 6.3–9.7; 11–29 °C). The models created for this experiment showed that N. salina’s MSY and lipid content are not strongly affected over the broad range of salinity and temperature values. Calculated optima levels were 28 PSU/20 °C for MSY and 14.5 PSU/20 °C for lipid accumulation, but neither value significantly influenced the model. However, pH was the most important factor to influence algae productivity, and pH optimum was estimated around 8. Both MSY and lipid content were strongly reduced when pH deviated from the optimum. Occurrence of invading organisms seemed stochastic, and none of the environmental factors studied significantly influenced abundance. In conclusion, pH should be kept around 8 for maximum productivity of N. salina. Temperature and salinity should be kept around 20 °C and 28 PSU; however, moderate variations are not too much of a concern and might enhance lipid content of N. salina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An important limitation within algae biofuel production is the lack of optimized cultivation systems (Chisti 2007; Brennan and Owende 2010; Mata et al. 2010). Current production systems include inexpensive, open ponds where algae cultures are under risk of contamination by invading organisms. Such organisms that are contamination risks to these systems include unwanted predators (rotifers and ciliates), competitors (other algae species), yeast, molds, fungi, bacteria and viruses (Becker 1994; Borowitzka 1998). Surprisingly, the ecology of algae production systems (how the desired algal strain and invading organisms, competitors or predators, interact with each other and their environment) has been neglected. A decisive plan with a novel ecological approach has the ability to provide unique solutions to address the current limitations of algal biofuel growth systems. By manipulating environmental factors within cultures, we should be able to simultaneously promote algal growth while restricting competitor or predator growth.
It is well known that microalgae growth can be manipulated by environmental parameters, such as salinity (Renaud and Parry 1994; Doan et al. 2011; Moazami et al. 2012; Bartley et al. 2013), pH (Rocha et al. 2003; Moheimani and Borowitzka 2011; Bartley et al. 2014), and temperature (Sukenik et al. 1993; Rocha et al. 2003; Van Wagenen et al. 2012). Additionally, it has been shown that growth can be affected by controlling oxygen levels (Chi et al. 2009), nitrogen sources (Chen et al. 2011; Sforza et al. 2012; Campos et al. 2014), and the use of insecticides (Ma et al. 2006). Previous research often applies a focused approach when addressing the effects of a single parameter on algae species. A new strategy is necessary to estimate interactions among variables, while keeping the number of required experimental units manageable. The response surface method (RSM) allows researchers to explore optimum conditions of several predictor variables in a single experiment (Inouye 2001). This method may be completed with a central composite design (CCD), which helps to optimize the estimation of a second-order model, allowing for reduced costs and time required during experimentation. In this study, we aim to utilize this method to identify a suite of optimum conditions for algae biomass production and lipid accumulation while limiting invading organisms for a variety of environmental parameters.
Our study species, Nannochloropsis salina (and its close relatives) has been identified as a suitable biofuel microalga (Chisti 2007; Mata et al. 2010). Members of this genus have been explored for their promise for biofuel development, human supplements (Roessler 1990; Cheng-Wu et al. 2001; Moheimani and Borowitzka 2011), and a feed source in hatcheries (Zittelli et al. 2000; Rebolloso-Fuentes et al. 2001; Zittelli et al. 2003). N. salina (Class: Eustigmatophyceae) exhibits high growth rates, lipid productivity, and a wide tolerance range for different environmental parameters (Richmond and Cheng-Wu 2001). Previous research within our lab has led to proposed optimum conditions for growth and lipid accumulation of 22-34 PSU for salinity (Bartley et al. 2013), pH level of 8–9 (Bartley et al. 2014), and temperature around 20–25 °C (Macanowicz and Boeing, unpublished data).
This present work explores the use and manipulation of three environmental parameters (salinity, pH, and temperature and their interactions) to enhance growth rates and lipid accumulation of N. salina while minimizing undesirable organisms. Thereby, we stay within the tolerance range for N. salina determined in our previous experiments. Here, we emphasize that we are not looking for the effect of a single environmental parameter as in our previous work. Rather, we are optimizing environmental parameters based on what we already know to be tolerance ranges and are looking more specifically for interaction between various environmental parameters. The RSM/CCD method used in this study will allow for insight into the potential relationships between the parameters and reveal their respective optimized conditions, while also limiting the number of experiments required. This research is novel because of its focus on multiple predictor variables (salinity, pH, and temperature), various response factors (growth rate, carrying capacity, maximum sustainable yield, lipid content, and invading organisms), and its unique attempt to combine these parameters. The implications of this work may provide cost-effective, viable, and sustainable cultivation developments for algal biofuel industry.
Methods
Nannochloropsis salina 1776 was obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton via the Los Alamos National Laboratory. The culture medium used during experiments was the F/2 medium for marine algae (Guillard and Rhyther 1962). The experiment was conducted in February/March 2012 for 31 days in a greenhouse located at New Mexico State University in Las Cruces, NM, USA, where open-faced aquaria were subjected to natural light conditions, as well as invading organisms. Algae culture circulation was achieved by means of air pumps with stone aerators using natural air. The airflow for each pump lies at 2500 cm3 min−1, and we used a separate air pump for each aquarium. A preliminary experiment did not yield a difference in growth rate or maximum cell density reached when one or two pumps and stone aerators were used, indicating that our method was sufficient to supply CO2 and mixing. Nannochloropsis salina was initially in an outdoor raceway at Fabian Garcia Agricultural Science Center grown in standard F/2 medium. The experiment was conducted in aquaria with 15-L working volumes and a culture depth of 12 cm in batch culture mode. Salinity, pH, and temperature readings were monitored mid-day three times a week with a Hach Hydrolab (Hach Hydromet). Salinity, pH, and temperature were also monitored at night to ensure they remained consistent. Absorbance readings (Thermo Spectronic) and samples for cell counts were also collected. Samples for algae counts were preserved with Lugol’s solution and stored in dark, cool locations. Absorbance and cell counts resulted in similar growth curves, and we used cell counts for further analysis.
Optimization of conditions
The effects of three factors (salinity, pH, and temperature) on cultures of N. salina were studied. Eighteen aquaria, having 15 unique experimental conditions designated by a central composite design, were monitored. This design consists of a full factorial design incorporating the three variables: four replications are conducted at the center point (optimum of all three environmental variables), eight points compose the corners of a three-dimensional cube with all three environmental variables deviating from the optimum at intermediate levels, and six star points that are centered above the cube faces at a distance of α from the center point test the more extreme values of each of our environmental variables (Tables 1 and 2). Our star points and center points (estimated to be the optimum prior to conducting the current experiment) were based on previous research (Bartley et al. 2013, 2014; Macanowicz and Boeing, unpublished data) that revealed ranges of the three factors that N. salina could exhibit positive growth under given environmental condition. From those values, the intermediate levels were calculated. In order for the response surface to be rotatable, α must be calculated as follows: α = n√(2n − 1), where n is number of factors.
Desired salinity levels were achieved with Instant Ocean in nonsterile deionized water. To maintain desired pH levels within the media, aquaria contained 25 mM of either (1) trisodium citrate for pH 6.3, (2) 4-(2-hydroxyethyl)4piperazine ethanesulfonic acid (Hepes) for pH 7, (3) N-tris[hydroxymethyl]-methylglycine (Tricine) for pH 8, or (4) N-tris[hydroxymethyl]methyl-3-amino-propanesulfonic acid (TAPS) for pH 9 and 9.7. All aquaria contained buffer and during previous work (Bartley et al. 2014), buffers were shown to have no effect on algae growth. Biofuel production industries often utilize CO2 injections to lower pH; however, for this study, this method was not applicable as CO2 also increases carbon availability and probably would have destabilized the pH treatments. Temperatures were maintained through a series of water baths surrounding each aquarium. Three chilling pools were used to maintain temperatures of 11, 15, and 20 °C, while heaters were employed to maintain temperatures of 25 and 29 °C. A series of pumps and interconnected water baths allowed for constant circulation of water between aquaria and chilling pools.
Algal biomass measurements and quantification of invading organisms
Cell density of N. salina and invading organisms were estimated by processing samples using a Benchtop B3 Series FlowCAM (Fluid Imaging Technologies, USA) to monitor growth rates and densities. A FlowCAM is a flow cytometer that records, counts, and measures the images of individual particles for a given volume of sample flowing at a constant rate. Samples were diluted so that no more than 30 organisms were counted per frame. Each sample was processed by the FlowCAM for 3 min, resulting in >10,000 organisms being counted and measured. Organisms present were grouped into size classes, and subsequently, number of particles per milliliter was calculated. The size classes were based on size parameters of N. salina and invaders (mainly diatoms). The dilution was entered into the FlowCAM, and particle densities for the different organisms were automatically calculated.
FAME analysis
Using optical density and cell counts, we monitored the growth phase of each tank. Growth subsided after 2 weeks and reached carrying capacity (also called steady state or stationary phase). After another 2 weeks (day 30), we gathered 1-L samples and froze them for subsequent lipid analyses.
Lipid content was monitored by transesterification and analysis of fatty acid methyl esters (FAME) by gas chromatography mass spectrometry (GC/MS) for each treatment. In situ transesterification followed by GC/MS quantitation of FAMEs as a measure of lipid productivity has emerged as a superior method to gravimetric lipid determination (Griffiths et al. 2010; Bigelow et al. 2011; Laurens et al. 2012). One liter of each culture was centrifuged, and the pellet was collected and transferred to a 15-mL conical tube. The pellet was then washed with deionized water, shaken, and recentrifuged. This process was repeated five times for each collection to remove salts from the sample prior to analysis. Samples were then lyophilized and tissue placed in a pre-weighed 16-mL glass vial to which 5 mL 0.2 N potassium hydroxide in methanol was added and allowed to stand for 30 min in a 40 °C water bath. An internal standard, glyceryl tritridecanoate, was included in the reaction to monitor conversion efficiency. Each sample vial was vortexed in 10 min intervals. The reaction was quenched after 30 min with 1 mL of 1 M glacial acetic acid. Each sample was back-extracted with 2 mL hexane with methyl tricosanoate C23:0, an internal standard for quantification/internal standard calibration. The hexane fraction was further diluted and analyzed via GC/MS under conditions as described by Patil et al. (2012).
Briefly, all samples were analyzed with a Hewlett Packard 5890 Gas Chromatograph with a 5972 Mass Selective Detector and 7673 Autosampler. Two-microliter injections were loaded onto a 30-m × 0.25-mm diameter (0.25-μm film thickness) Agilent DB-23 capillary column with helium carrier gas. The initial temperature was 80 °C and ramped 20 °C min−1 to 220 °C and held for 6 min for a total run time of 13.3 min. Chromatographic signals were matched to a standard mix (Supelco 37 Comp. FAME mix 10 mg cm−3 in methylene chloride), and internal calibration was achieved using a C23:0 internal standard spiked at 50 μg mL−1.
Calculations and statistical analyses
Nannochloropsis growth rate (r) was calculated from cell counts using SPSS 22.0 software [IBM Corp. 2013]. We used data from the entire duration of the experiment and applied the logistic growth equation, which is appropriate for microalgae growth (Xu and Boeing 2014):
In the above equation, N t is the population size at time t, N 0 is the initial population size at day 1 of our experiment, r is population growth rate, and t are the days of our experiment. K represents carrying capacity and is the maximum cell density, at which a certain parameter has become limiting (e.g., light, nutrients), and no further positive population growth occurs. Details on how to calculate logistic growth in SPSS are described by Campos et al. (2014).
Logistic growth rate largely depends on how quickly carrying capacity is reached and not on the maximum algae density that is achievable under given conditions. Thus, logistic growth rate is not a good estimate for microalgae productivity (Xu and Boeing 2014; Campos et al. 2014). Therefore, we also calculated maximum sustainable yield (MSY) of algae density (Xu and Boeing 2014):
Interestingly, results for MSY are similar than for exponential growth rate calculations:
where d = density, n = day of experiment after which positive growth ceases, and Δt = time in days (Campos et al. 2014).
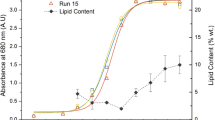
Due to high number of level for three factors, we had 15 different treatments and any graph attempting to present all the information gathered would be difficult to read. In this paper, we present a graph with growth curves (cell density over time) for selected treatments (center points and star points) (see Tables 1 and 2 and Fig. 1). We report, however, our calculated growth rates, carrying capacity, and MSY for all treatments in Table 2.
R software (R Core Team 2014) was used to estimate model coefficients (using the stats::lm() method), estimate optimum levels of the parameters (using the stats::optim() method), and to create surface plots (using the lattice::wireframe() method).
Each of five different response variables was modeled via linear regression using second-order polynomials, including second- and third-order interactions. The five response variables explored were as follows: growth rate (r), carrying capacity (K), MSY, invading organism density, and lipid content. All models were analyzed using all 18 aquaria with their respective experimental conditions specified by the CCD. The coefficients of each second-order polynomial model describe the estimated effects of their respective parameter on the response variable in the model and can be written as follows:
where Y ijkl is the response variable being studied and e ijkl is the random error term. Hence, this full model has ten variable terms and an intercept. Determination of a final model for each response variable started with the above full model and iterated through removing the nonsignificant term having the highest p value until all remaining terms were significant at a 0.05 significance level (Neter et al. 2004).
Results
Algae growth model
At the center points (all environmental parameters at the predicted optima), algae showed positive growth for the first 3 weeks of the experiment, and then reached the stationary phase/carrying capacity, where no additional population growth was observed (Fig. 1). The logistic growth equation was the appropriate way to calculate growth rate, as indicated by high coefficients of determination (R 2, Table 2). However, none of the environmental parameters significantly entered the model to explain differences in growth rate or carrying capacity. However, our results show that the MSY (which is calculated from logistic growth rate and carrying capacity) model can explain the effects of the three environmental factors on MSY of N. salina (Table 2). The simplified model (reduced to just significant variables) contains only two variable terms. The equation for MSY as a function of significant parameter estimates is as follows:
Nannochloropsis salina growth curves under optimized environmental conditions (center points, top graph) and star points (one environmental parameter is above or below optimum). sal salinity (PSU), temp temperature (°C). The center points were replicated four times, while no replication was done for the star points
Due to the model limitations imposed by the limited number of significant parameters, pH (p < 0.0001) and pH2 (p < 0.0001), only optimum pH levels could be estimated (pH 7.730). Figure 2 illustrates the estimating function and shows the optimizing pH value. This reduced model has an R 2 of 0.760. Salinity (p = 0.0567) and a pH/salinity interaction (p = 0.0665) were marginally nonsignificant (Fig. 3). Temperature was also not a significant parameter in the model. However, the highest observed MSY was achieved at experimental treatment levels of 28 PSU and 20 °C, respectively.
Lipid content model
Our resulting lipid content model was able to sufficiently explain the effects of the chosen factors on the lipid content (% by cell dry weight) of N. salina (Table 2). Once again, only two variables remained in the simplified model, pH (p < 0.0002) and pH2 (p < 0.0002). The estimating equation for lipid content as a function of these significant parameters is as follows:
Here, the optimum pH level was estimated to be 8.056. Figure 1 illustrates the estimating function and shows the optimizing pH value. This model has an R 2 of 0.618. While salinity and temperature could not be directly estimated, lipid accumulation was greatest at 14.5 PSU and 20 °C, respectively.
Invading density model
Invading organisms consisted almost exclusively of diatoms. The invading density model was unable to sufficiently explain the effects of salinity, temperature, or pH on invading organisms (p = 0.5653 for the null hypothesis that no terms in the full model are important in predicting invading organisms; the lowest p value for any single variable model was p = 0.2918).
Discussion
Estimating effects of environmental variables and their interactions is challenging due to bias created by keeping other conditions constant and partial correlations among the variables. The models created for this experiment show that they may be used to locate optimum levels of given parameters and identified pH to be an important parameter when maximizing algae biomass production and lipid accumulation.
Algae cultures exhibited typical logistic population growth, where they rapidly increased in numbers for the first couple of weeks before reaching the stationary phase, due to a limiting factor, most likely nutrients or light. However, neither logistic growth rate nor carrying capacity were adequate response variables to measure impacts of environmental variations. This supports earlier findings (Campos et al. 2014) that the calculation of logistic growth rate is strongly influenced by how soon it reaches maximum cell density. For example, the logistic growth rate is higher when the carrying capacity is reached early, even if achievable maximum cell density (carrying capacity) is low. If carrying capacity is twice as high but reached a few days later, logistic growth rate is lower. Thus, logistic growth rate is not a good indicator for biomass productivity. MSY is a product of logistic growth rate and carrying capacity and has also been previously shown to be a reliable response variable for algae biomass production (Xu and Boeing 2014; Campos et al. 2014).
Surprisingly, both the MSY and the lipid content models contained only pH and pH2 as the significant variables, and salinity was marginally nonsignificant. We had predicted that temperature would be the main influence on MSY. The ranges of environmental parameters used were all within a previously determined range of tolerance for N. salina. For example, from previous experiments (Macanowicz and Boeing, unpublished data), we already knew that N. salina cultures would die and crash in cell densities at temperatures above 30 °C. We did not push the limits of this experiment past the known tolerance ranges. We were able to keep our temperature treatments within ± 2 °C, except for a couple of times, where the 11 °C went up to 15 °C for a few hours. Some compensatory growth might have occurred during those time periods. We conducted similar previous experiments with N. salina (in aquaria in a greenhouse, using F/2 medium, natural light, sparging ambient CO2 concentration to keep cultures suspended and aerated) (Bartley et al. 2013, 2014; Campos et al. 2014). The growth rates and maximum densities achieved in this experiment were comparable to previous experiments. In previous experiments, algae responses to various environmental conditions were strong and obvious. Thus, we do not believe that our results were masked by another factor limiting growth. It is also important to note that pH is on a logarithmic scale, and so, the difference between the pH star and center points would be more pronounced than the differences between the salinity and temperature star points and the center points. It is conceivable, however, that in our design, we surpassed the tolerance levels for pH but not temperature. For example, we previously found that N. salina grew well at pH 9, but died at pH 10 (Bartley et al. 2014). In this experiment, the upper star point was pH 9.7, which had drastic negative effects on MSY and lipid content. In contrast, in our previous experiment, we found high growth rate at 25 °C and N. salina dying at 30 °C. Our upper star point for temperature was 29 °C, which had obviously not surpassed the tolerance level for N. salina. However, we are surprised by the limited influence temperature has on MSY over a broad range. Replication of all our treatments (which would have added 15 additional aquaria as experimental units) would have been useful for higher confidence in this particular result. Variability among replicates in algae cultures is commonly high in outdoor algae cultures. Nevertheless, we conclude that within the ranges used in this experiment, pH is more influential on N. salina productivity than salinity or temperature. This also becomes obvious when comparing the center points (all environmental parameters are at the predicted optimum) with the star points (one parameter is significantly above or below the predicted optimum) in Table 2. MSY values, when salinity and temperature are not optimal (2.023–2.866 cells × 106 mL−1 day−1), are similar to MSY values for the center points (2.47 cells × 106 mL−1 day−1). However, when pH is suboptimal, MSY is sharply decreased (0.099–0.591). Similarly, lipid content is even increased when salinity and temperature are suboptimal (star points) compared to the center points. While when pH is suboptimal, lipid content is reduced by orders of magnitude. We would like to emphasize that our goal was not to maximize production per se, and certain environmental parameters like light and CO2 concentration were not optimized. We merely wanted to evaluate the impact of three selected environmental parameters and increasing CO2 concentration (through CO2 sparging) would have likely destabilized our pH treatments as well as changed the concentration of an essential nutrient for algae photosynthesis in the various treatments.
pH was estimated to be optimum at approximately a level of pH 8. Salinity and temperature optimum levels were not estimable; however, maximum sustainable yield and lipid content were maximized at 28 PSU/20 °C and 14.5 PSU/20 °C, respectively. Lipid accumulation is known to occur when Nannochloropsis cells are stressed through nitrogen starvation and other suboptimal environmental factors (Rodolfi et al. 2009; Bartley et al. 2014). Our research provides novel findings, due to its unique approach that explores three parameters and multiple response variables in a single experiment. Sporalore et al. (2006) used a similar approach with Nannochloropsis oculata, employing the response surface method utilizing a CCD to study the effects of four variables (temperature, incident light intensity, pH, and aeration rate) on growth rate. Similar to our findings, they estimated optimum temperature to be around 21 °C and pH at 8.4 for maximum growth. They also estimated light intensity to be optimized around 52 μmol photons m−2 s−1 and a 14.7 VVH aeration rate. However, they did not incorporate lipid content or invading organisms into their research.
Our observed optimum pH for N. salina growth (pH 8) was consistent with previous research on Nannochloropsis spp. (e.g., pH fluctuations between 6.46 to 8.28 without CO2 addition (Abu-Rezq et al. 1999); pH 7.8 (Sukenik et al. 1993); pH 8.4 (Sporalore et al. 2006); pH 8–9 (Bartley et al. 2014)) while another study utilized a lower pH for growth of N. oculata (pH 7.4–7.8 (Brown et al. 1993)). Although not significant, a previous study also found a reduction in lipid content at pH values that deviated from pH 8 (Bartley et al. 2014). In our study, we are unable to differentiate between indirect (e.g., carbon speciation and related uptake by our alga) and direct physiological effects of pH. Variations in pH can influence algae growth and lipid accumulation by influencing availability and species of carbon, trace metals, and other nutrients (Chen and Durbin 1994; Hill et al. 2014). Higher pH limits availability of CO2 and HCO3 –, which are essential for algae growth. Direct physiological damage to the algae may occur at extreme pH values. Søgaard et al. (2011) found that direct pH effects on growth of three algae species was more important than indirect effects even at high pH values. Organisms invading algae production systems seem mainly driven by stochastic events, season, and geographic location and are less dependent on environmental parameters of the algae production systems (Bartley et al. 2013; 2014; Campos et al. 2014).
The observed salinity range for N. salina growth (14.5–41.5) in this experiment was very broad and demonstrates the hardiness toward salinity of this particular species. The optimum of 28 PSU for biomass production is consistent with previous research (e.g., 22–34 (Bartley et al. 2013); 25–30 PSU (Renaud and Parry 1994); 20–40 PSU (Abu-Rezq et al. 1999); 22–49 PSU (Hu and Gao 2006)). We expected N. salina to exhibit significantly lower growth rates at the more extreme salinity treatments; however, our results did not confirm those expectations. Other research has found that salinity tolerance can vary widely among species, with some diatom species being able to grow at salinities above 100 PSU (Søgaard et al. 2011). The increased lipid content at more extreme salinity values (star points) was not pronounced enough and obscured by the cube points (simultaneous suboptimal levels of several parameters) to be picked up by the model.
We were unable to narrow in on a temperature optimum with this work. Calculated MSY and lipid content were greatest at 20 °C. However, MSY in the star points treatment (Table 2) was even higher than most of the cube points and similar to the center point values. Nannochloropsis salina does not seem overly sensitive to temperature over a broad range of values, similar to our findings with salinity. The alga was originally isolated from a cold marine environment in Scotland. This might be explaining tolerance toward colder temperatures. Furthermore, in nature, microalgae are adapted to day-night temperature variations. However, this is still a surprising result, since temperature typically has a large impact on metabolic rate of organisms and we do not have a good explanation for this finding. A follow-up experiment using additional replicates could shed additional light on this phenomenon. Although some work has also found N. salina having a wide temperature tolerance range (Richmond and Cheng-Wu 2001), other research was able to identify optimum temperature levels for Nannochloropsis spp. (e.g., 24–26 °C for Nannochloropsis sp. (Abu-Rezq et al. 1999); 25 ± 5 °C for N. gaditana (Rocha et al. 2003); and 21 °C for Nannochloropsis oculata (Sporalore et al. 2006)). Van Wagenen et al. (2012) found that N. salina exhibited maximum specific growth rate at 26 °C and that lipid content was increased after exposing cultures to extreme temperature. Similarly, we found increased lipid content at the temperature star points (Table 2). Temperature is a more difficult and costly factor to influence. High temperatures also lead to high water evaporation losses in open systems. Thus, a confirmation of a broad temperature tolerance, in which high biomass production of our N. salina strain could be achieved, would economically be a positive result.
Densities of invading organisms stayed over an order of magnitude below the densities of N. salina and did not dominate in any of the aquaria. Thus, contamination issues in a saline inland algae production facility seems to be of minor concern. Diatoms are the most common invader in Las Cruces, NM, USA (Bartley et al. 2013, 2014; Campos et al. 2014). They are algae with silicate cell walls and a potential for rapid growth in spring, when nutrients are abundant and temperatures are still cool (Sommer et al. 1986). They are also commonly found in highly saline systems (Clavero et al. 2008). Although their invasion into our aquaria was too stochastic for the response surface model to find a relationship, we notice the following: maximum diatom densities were reached in the cube point aquaria, where all parameters (pH, salinity, and temperature) were below the predicted optimum. Further high diatom densities were found in star point aquaria, where salinity was very high as well as the one with the coldest temperature. Minimum diatom densities were two orders of magnitude lower and observed in the cube point aquaria with elevated pH and lower salinity and temperature. Low diatom densities also occurred in the two star point aquaria, when pH and salinity were at their lowest values. Clear relationships between diatom density and environmental parameters might be obscured by stochastic events that determine timing of invasion. Purposeful addition to experimental treatments might better demonstrate the relationship between invaders and environmental factors.
It is difficult to compare results of this study to other studies due to the novel, multi-faceted approached taken in our study. In addition, the densities attained in any experiment depend on algal species, cultivation practices, and other extraneous conditions. The algae cultures were grown under conditions not optimized for production. However, this is one of the few studies attempting to strictly isolate the effect of pH. At large scales, pH is typically controlled by CO2 sparging. Future research should also consider factors like CO2 and light (quality and quantity).
In summary, we find that N. salina is very tolerant to environmental conditions within the range that was tested in this experiment and ultimately responded most sensitively to pH in both biomass productivity and lipid content. It is interesting, however, to note that the four maximum lipid content values were measured at the star points (suboptimal conditions when Nannochloropsis is expected to be stressed, which triggers lipid accumulation) of salinity and temperature, while pH stress led to the opposite effect; the two lowest lipid values were measured at the pH star points. Density of invading organisms (mainly diatoms) was highly variable and no pattern could be detected. This is probably due to stochasticity of invasions into different aquaria.
References
Abu-Rezq TS, Al-Musallam L, Al-Shimmari J, Dias P (1999) Optimum production conditions for different high-quality marine algae. Hydrobiologia 403:97–107
Bartley ML, Boeing WJ, Corcoran AA, Holguin FO, Schaub T (2013) Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54:83–88
Bartley ML, Boeing WJ, Dugan BN, Holguin FO, Schaub T (2014) pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J Appl Phycol 26:1431–1437
Becker EW (1994) Microalgae biotechnology and microbiology. Cambridge University Press, Cambridge, pp 128–142
Bigelow NW, Hardin WR, Barker JP, Ryken SA, MacRae AC, Cattolico RA (2011) A comprehensive GC-MS sub-microscale assay for fatty acids and its applications. J Am Oil Chem Soc 88:1329–1338
Borowitzka MA (1998) Limits to growth. In: Wong YS, Tam NFY (eds) Wastewater treatment with algae. Springer, Berlin, pp 203–218
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Brown MR, Garland CD, Jeffrey SW, Jameson ID, Leroi JM (1993) The gross and amino acid compositions of batch and semi-continuous cultures of Isochrysis sp. (clone T. ISO), Pavlova lutheri and Nannochloropsis oculata. J Appl Phycol 5:285–296
Campos H, Boeing WJ, Dungan BN (2014) Cultivating the marine microalgae Nannochloropsis salina under various nitrogen sources: effect on biovolume yields, lipid content and composition, and invasive organisms. Biomass Bioenergy 66:301–307
Chen CY, Durbin EG (1994) Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar Ecol Prog Ser 109:83–94
Chen M, Tang H, Ma H, Holland TC, Ng KS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresource Technol 102:1649–1655
Cheng-Wu Z, Zmora O, Kopel R, Richmond A (2001) An industrial-size flat plate glass reactor for mass production of Nannochloropsis sp. (Eustigmatophyceae). Aquaculture 195:35–49
Chi Z, Lui Y, Frear C, Shulin C (2009) Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl Microbiol Biotechnol 81:1141–1148
Chisti Y (2007) Biodiesel from microalgae. BiotechnolAdv 25:294–306
Clavero E, Hernández-Mariné M, Grimalt JO, Garcia-Pichel F (2008) Salinity tolerance of diatoms from thalassic hypersaline environments. J Phycol 36:1021–1034
Doan TTY, Sivaloganathan B, Obbard JP (2011) Screening of marine microalgae for biodiesel feedstock. Biomass Bioenergy 35:2534–2544
Griffiths MJ, van Hille RP, Harrison ST (2010) Selection of direct transesterification as the preferred method for assays of fatty acid content of microalgae. Lipids 45:1053–1060
Guillard RRL, Rhyther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Hill PS, Tripati AK, Schauble EA (2014) Theoretical constraints on the effects of pH, salinity, and temperature on clumped isotope signatures of inorganic carbon species and precipitating carbonate minerals. Geochim Cosmochim Acta 125:610–652
Hu H, Gao K (2006) Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol Lett 28:987–992
Inouye BD (2001) Response surface experimental design for investigating interspecific competition. Ecology 82:2696–2706
Laurens LML, Quinn M, Van Wychen S, Templeton DW, Wolfrum EJ (2012) Accurate and reliable quantification of total microalgae fuel potential as fatty acid methyl esters by in situ transesterification. Anal Bioanal Chem 403:167–178
Ma J, Lu N, Qin W, Xu R, Wang Y, Chen X (2006) Differential responses of eight cyanobacterial and green algal species, to carbamate insecticides. Ecotox Environ Safe 62:268–274
Mata TM, Martins A, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Moazami N, Ashori A, Ranjbar R, Tangestani M, Eghtesadi R, Nejad AS (2012) Large-scale biodiesel production using microalgae biomass of Nannochloropsis. Biomass Bioenergy 39:449–453
Moheimani NR, Borowitzka MA (2011) Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl Microbiol Biot 90:1399–1407
Neter J, Kutner M, Nachtsheim C, Wasserman W (2004) Applied linear regression models—4th edition. McGraw-Hill
Patil P, Reddy H, Muppaneni T, Mannarswamy A, Holguin O, Schaub T, Nirmalakhandan N, Cooke P, Deng S (2012) Power dissipation in microwave-enhanced in-situ transesterification of algal biomass to biodiesel. Green Chem 14:809–818
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rebolloso-Fuentes MM, Navarro-Perez A, Garćia-Camacho F, Ramos-Miras JJ, Guil-Guerrero JL (2001) Biomass nutrient profiles of the microalga Nannochloropsis. J Agric Food Chem 49:2966–2972
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Richmond A, Cheng-Wu Z (2001) Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J Biotechnol 85:259–269
Rocha J, Garcia JEC, Henriques MHF (2003) Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Eng 20:237–242
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Roessler PG (1990) Environmental control of glycerolipid metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399
Sforza E, Bertucco A, Morosinotto T, Giacometti GM (2012) Photobioreactors for microalgal growth and oil production with Nannochloropsis salina: from lab-scale experiments to large-scale design. Chem Eng Res Des 90:1151–1158
Søgaard DH, Hansen PJ, Rysgaard S, Glud RN (2011) Growth limitation and three Arctic sea ice algal species: effects of salinity, pH, and inorganic carbon availability. Polar Biol 34:1157–1165
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG model of a seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Sporalore P, Joannis-Cassan C, Duran E, Isambert A (2006) Optimization of Nannochloropsis oculata growth using the response surface method. J Chem Technol Biotechnol 81:1049–1056
Sukenik A, Zmora O, Carmeli Y (1993) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. II Nannochloropsis sp. Aquaculture 117:313–326
Van Wagenen J, Miller TW, Hobbs S, Hook P, Crowe B, Huesemann M (2012) Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 5:731–740
Xu Y, Boeing WJ (2014) Modeling maximum lipid productivity of microalgae: review and next step. Renew Sust Energ Rev 32:29–39
Zittelli GC, Pastorelli R, Tredici MR (2000) A modular flat panel photobioreactor (MFPP) for indoor mass cultivation of Nannochloropsis sp. under artificial illumination. J Appl Phycol 12:521–526
Zittelli GC, Rodolfi L, Tredici MR (2003) Mass cultivation of Nannochloropsis sp. in annular reactors. J Appl Phycol 15:107–114
Acknowledgments
We are grateful for the valuable work from the following undergraduate students: Herman Campos, Levi Chavez, Renee Pardee, Herberto Chaparro, and Zach Brecheisen. Neeshia Macanowicz was vital to the design and construction of the temperature control system. Darren James provided valuable help with statistical analyses for this research. This work is supported by the US Department of Energy under contract DE-EE0003046 awarded to the National Alliance for Advanced Biofuels and Bioproducts and by the Center for Animal Health, Food Safety and Biosecurity at New Mexico State University. This is a New Mexico Agricultural Experiment Station publication, supported by state funds and the US Hatch Act.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartley, M.L., Boeing, W.J., Daniel, D. et al. Optimization of environmental parameters for Nannochloropsis salina growth and lipid content using the response surface method and invading organisms. J Appl Phycol 28, 15–24 (2016). https://doi.org/10.1007/s10811-015-0567-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0567-8