Abstract
Abiotic stress factors such as drought, flooding, cold, heat waves, ultra violet radiations, oxidizing agents and salinity in the current era of climate change is jeopardizing the plant growth and development leading to crop failure worldwide. Engineered plants with improved tolerance to abiotic stresses would provide opportunities to adapt crops to future climates coupled with enhanced food productivity and sustainable agricultural development. Growth and development of plants involve a wide range of sophisticated genetic, hormonal, metabolic and environmental events which are tightly regulated by internal and external cues, such as phytohormones (including various biostimulants and different organic and inorganic elicitors), temperature, light irradiation, etc. Out of these, phytohormones such as jasmonates, gibberellins, abscisic acid, brassinosteroids, nitric oxide, salicylic acid, etc. have evolved to control vital functions in regulating various plant physiological and developmental processes, ranging from seed germination, photosynthesis, leaf senescence, pollen growth, to plant defense responses, and ameliorating various abiotic stresses. The role of such phytohormones in conferring plant adaptation under dynamic climate changes is still in infancy stage. Few reports are available on the current topic. In this chapter, we attempt to summarize recent studies that have provided insights of the plant environmental adaptability and the multidimensional role of different phytohormones viz. salicylic acid (SA), nitric oxide (NO) and hydrogen sulphite (H2S) in regulating various developmental processes and stress tolerance, taken together with the molecular mechanisms of phytohormone signalling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
28.1 Introduction
According to an estimate of Food and Agricultural Organization [FAO] (2009), the aim of world agriculture for increasing the 70% food production by the year 2050 for approximately 2.3 billion newborn populations is facing a great hurdle in the light of hunger, poverty coupled with the dynamic environmental factors and over-exploitation of natural endowments. A global emerging problem in current times is climate change and global warming which can be considered as ‘global warning’ to plant stress physiologists as well. A variation in global climate is further supposed to accelerate as global climatic projections indicate a significant increase of 2–4 °C in mean temperature over the next half of the present century (IPCC Intergovernmental Panel on Climate Change 2007). A global level, agriculture is widely considered as one of the prime prone sectors affected by a change in climatic conditions (Abbas 2013). Climate change and agriculture are correlated, as a change in climate of a particular region is the main reason of biotic and abiotic stresses, which pose severe impact on the agriculture. Changing environmental pattern and variability in climate accelerate the impacts of abiotic stresses in crop plants (Thornton et al. 2014; Raza et al. 2019). The crop productivity improvement under abiotic stress is one of the biggest and prime challenges faced by the agricultural scientific community worldwide. Despite extensive research being done in the field, the research for yielding abiotic stress-resistant crops remains very scanty. This can be attributed to the complex nature of environment × genotype interactions and particularly, the capacity to quantify the dynamic physiological response profile of crop plants to a dynamic environment (Moshelion and Altman 2015; Dalal et al. 2019).
Abiotic stress factors such as salinity, drought, heavy metals, UV-B radiation, air pollution and heat stress will further intensity these effects (Khan et al. 2015; Pereira 2016; Wani et al. 2018; Ali et al. 2019) and may strongly impact yield of crop plants and quality of agricultural products (Knutti et al. 2016; Pereira 2016; Feller et al. 2017). Efforts have been made to know potential tolerance mechanisms and plant response under such stressed conditions. As plants are sessile organisms, they continuously encounter different environmental pressures at morphological, physiological, biochemical and molecular levels. Plants have to adapt to these abiotic stresses induced adverse impacts at cellular and subcellular levels to optimally perform the growth and development. Under the danger of climate change, the adaptive underlying mechanisms of plants should perform optimally to counter these challenges. Nevertheless, these adaptive mechanisms can be engineered by exogenous application of phytohormones. In the present chapter, an attempt is made to exploit the tendency of external application of some phytohormones in different crop plants to boost their tolerance against environmental pressures. In the following section, we are discussing the potentiality of hydrogen sulphide, nitric oxide, and salicylic acid mediated mechanisms in countering salinity, drought, heat and metal stress in different species of plants.
28.2 Hydrogen Sulphide (H2S)
Hydrogen sulphide (also known as dihydrogen sulphide or sulfane), is a chemical compound with the chemical formula H2S. It is a colourless gas, with a characteristic foul odour of rotten eggs. Production of H2S involves microbial (usually by sulphate reducing microorganisms) breakdown of organic matter in an anaerobic condition, such as sewers and sewages. H2S is a new gaseous signalling molecule that have different sources and subcellular compartments generally associated with cysteine metabolism in a plant body (Gotor et al. 2013; Scuffi et al. 2014; Birke et al. 2015; Corpas et al. 2019a) that tends to regulate physiological process in plants (Li et al. 2016a; Mostofa et al. 2015; Banerjee et al. 2018; Corpas et al. 2019b) and have tendency to show adaptation to various environmental stresses which includes heavy metal exposure, temperature, drought and salt stresses (Lisjak et al. 2013; Li et al. 2016b; He et al. 2018). H2S can likely exert anti-inflammatory, antioxidant, cyto-protective, antiapoptotic and organ protective effects, further improving environmental stress tolerance of cells (Yuan et al. 2017). It was believed to be a toxic gas and tend to have hazardous effects on environment but by recent several researches it has emerged as an important molecule that helps in signalling with numerous effects on various physiological processes both related to plants as well as animal systems. After nitric oxide (NO) and carbon monoxide (CO), H2S has been recognized as the third endogenous gaso-transmitter in plants. Hydrogen sulphide plays a vital role in cell signalling pathways by being an important component. In plants, H2S generation can be related to cysteine (cys) metabolism (Papenbrock et al. 2007). D-Cys desulfhydrase (D-CDES) and L-Cys desulfhydrase (LSD) derogate cysteine to hydrogen sulphide, ammonia and pyruvate and hence are responsible for the release of H2S into cell (Kopriva 2006). Moreover, participation of H2S in ethylene-induced stomatal closure has also been proven. Increase in glutathione level, alterations of enzyme activities and influences on both NO and H2O2 metabolisms are some of the intracellular effects of H2S. Recent studies have also showed that in regulation of proharvest senescence of horticulture H2S plays an important role. In the accompanying section, the role of H2S under various abiotic stresses is discussed briefly.
28.2.1 Role of H2S in Salt Stress
Salt stress is included in abiotic kind of stress that usually limits the agricultural productivity mostly seen in arid and semiarid regions of the world. It tends to be one of the major environmental stresses which results in ionic toxicity and induces an osmotic stress in plants to a major extent that leads to different nutritional disorders. H2S is known to enhance the salt tolerance, as is seen in barley seedling roots where it acts as a messenger molecule resulting in the modulation of different physiological processes in plant by decreasing the net K+ efflux (Chen et al. 2015). Moreover, the effect of H2S was seen in poplar species as well. When poplar was subjected to salt stress, both long term as well as short term, it results in increased Na+ efflux as well as an increased H+ influx for its roots caused by NaHS. Depolarization-activated K+ channels resulted in K+ loss induced by NaCl. Other researches revealed about the role of H2S in salt stress in rice plants. Results have shown a decreased growth with NaHS. Salt stressed rice plants have a decreased content of chlorophyll, carotenoid and soluble proteins. Under salt stress, a vital role of H2S was witnessed in ion homeostasis, where the ability to decrease the uptake of Na+ and Na+/K+ ratio and to balance mineral contents. Furthermore, it was revealed how implication of this gaso-transmitter, H2S, can manage the salt stress that makes the rice plants to adapt the adversities of environment (Mostofa et al. 2015). In a study performed on the roots of Arabidopsis thaliana, Li et al. (2014) exposed the plants to 100 mM NaCl stress which caused electrolyte leakage and disturbed the Na+/K+ ratio. However, the follow-up treatment with NaHS enhanced the salt tolerance by maintaining the homeostasis of ions via regulating the plasma membrane Na+/H+ antiporter system. It has also been documented that salt stress also initiate biosynthesis of endogenous H2S. In a study carried out in seedlings of Medicago sativa, Lai et al. (2014) found that there was subsequent increase in the biosynthesis of H2S from 30 to 70 nmol g−1 FW when the concentration of NaCl increased from 50 to 300 mM. Integrity of photosynthetic apparatus can be deteriorated by salt stress which eventually has an immense effect on photosynthetic efficiency of plant. In Nicotiana tabacum, Da Silva et al. (2017b) observed that salinity stress induced the accumulation of endogenous H2S was responsible for redox defense systems to prevent the inhibitory effects of salinity stress. Thus, it is clear that H2S is an important molecule in plants implicated to confer salt stress tolerance.
28.2.2 Role of H2S in Heat Stress
Heat stress can be defined as excessive increase in soil and air temperatures above a threshold level for ample time that cause usually an irreversible damage to plant physiological functions as well as its development which includes the rate of reproductive development or the photosynthetic rate which can be seen in decreased seed or fruit production. As the plants are sessile, they have to tolerate the temperatures provided by their environment which can never be always optimal (Bita and Gerats 2013). Heat stress can affect the plant in different manners either by high day or high night temperatures or by either high air or high soil temperatures. Several research groups have suggested the implication of H2S to tolerate both high and low temperatures. Heat stress can be attributed as a direct effect of global warming (Banerjee and Roychoudhury 2018). A synchronized antioxidant network is activated by H2S to achieve heat shock protection. It was seen that electrolyte leakage and the production of MDA due to membrane lipid peroxidation was significantly reduced by H2S released from NaHS (Li et al. 2012). H2S interacts with various physiological regulators like Ca2+ and proline. It has been seen that maize seedling when treated with NaHS have elevated the activity of pro-biosynthetic enzyme that facilitated viability of seedling under heat stress due to high endogenous accumulation of proline (Li et al. 2013). Under the heat stress the rate of germination of maize seeds can be increased by NaHS, a derivative of H2S and also enhance the tissue viability and the accumulation of malondialdehyde (MDA) caused by heat treatment has also been lowered (Li et al. 2013). In maize the heat tolerance induced by salicylic acid (SA) is enhanced by NaHS that acts as H2S donor (Li et al. 2015b). Recently, an increase in heat tolerance was achieved by the application of NaHS on wheat seedling through foliage. High temperature in plants also triggers the biosynthesis of the H2S (Fu et al. 2013). In Nicotiana tabacum, when seedlings are under 35 °C, it was found that the expression of LCD was increased which was responsible for the biosynthesis of H2S (Chen et al. 2016). H2S donor also induces the thermotolerance in strawberry plants which were exposed to 42 °C (Christou et al. 2014). They observed that H2S root pretreatment activates systemic thermotolerance via the coordinated network of transcriptional regulation of heat shock proteins and aquaporin.
28.2.3 Role of H2S in Drought Stress
The availability of water content in soil is reduced and various environmental factors that cause a continuous loss of water either by transpiration (loss of water from aerial parts of plant in the form of moisture or water vapour) or by evaporation (process of vaporization) lead to a condition known as drought stress (also known as water stress). It is one of the most important abiotic environmental stresses and can be regarded as a multidimensional stress because it can cause alteration in various physiological as well as morphological functions of a plant. Drought stress connects plant development and yield. Primarily it can affect the crop productivity and hence can weaken the global food security and can increase other related adverse consequences like desiccation, osmotic imbalance, drooping and much more. Different plant species have different tolerance to drought stress. Drought stress tolerance and protection can be regulated by H2S (Jin et al. 2011, 2013, 2017). Mitigation of drought susceptibility can be achieved by H2S (Zhang et al. 2010b). Reduced activity of lipoxygenase (LOX) and malondialdehyde (MDA) content along with increased activities of ascorbate peroxidase (APX) and catalase (CAT) was reported when wheat seedlings were treated with NaHS, a derivative of H2S (Zhang et al. 2010a). In seedlings of Arabidopsis thaliana, Shen et al. (2013) conducted an experiment and found that wild type seeds treated with polyethylene glycol (PEG-8000) mimicked drought stress, which trigger the biosynthesis of H2S. They concluded their study by observing that H2S improved drought resistance through regulating the expression of drought associated miRNAs in Arabidopsis. When wheat seeds were exposed to PEG 6000 induced drought stress for 2 days, a rapid increase from 1.5 to 3.5 mmol g−1 dry weight of H2S production was measured (Zhang et al. 2010a). They further observed that H2S treatment slowed down the activity of lipogenase. Some researchers showed that prolonged exposure to H2S results in increased stomatal aperture whereas short-term exposure to H2S can result in closure of stomata (García-Mata and Lamattina 2010; Lisjak et al. 2010, 2011). In case of wheat (Triticum aestivum L.), drought responsive genes regulated by H2S were also studied.
28.2.4 Role of H2S in Metal Stress
Increased anthropogenic and industrial activities lead to the contamination of water and soil by heavy metals growing as one of the most concerned global environmental problems (Fang et al. 2016; Ye et al. 2017; Wani et al. 2018). Metal stress is responsible for the retarded plant growth as well as development. Moreover, they have a deleterious effect on plant’s physiological processes. The major heavy metal toxicants may include lead (Pb), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni) and zinc (Zn) (Macomber and Hausinger 2011; Wang et al. 2013; Ye et al. 2017; Qin et al. 2018; Zhou et al. 2018; Wani et al. 2018). Various effects like chromosomal aberration, membrane damage, colonization inhibition, oxidative stress, antioxidant enzyme upregulation and even cell death can be caused by the presence of the heavy metals even if these are present in trace amounts (Gong et al. 2009; Ali et al. 2014, 2018; Huang et al. 2015). Blockage of the casparian strips or due to tapering by cell wall of roots can result in accumulation of heavy metals in root cells. The efficiency of toxicants removal and alleviation of heavy metals can be done by the application of H2S. Lead, a heavy metal effects the germination, growth (root elongation and cell division) and various biochemical and physiological attributes (photosynthesis, nutrients uptake, respiration and hormonal equilibrium) in cauliflower, which are however mitigated by the application of NaHS, a donor of H2S. Accumulation of higher amounts of zinc in soil can result from excessive use of zinc enriched fertilizers and the industrial activities. This accumulation can affect the quality of both vegetables and fruit crop. Both the shoot as well as root dry weight of pepper (Capsicum annuum L.) plant was reduced due to zinc stress, whereas, proline content and leaf electrolyte leakage (EL) were enhanced. Pertinence of NaHS increased both the shoot and root growth in zinc stressed plant, but also reduced the leaf EL and proline content in pepper plant. NaHS promoted the plant growth and photosynthesis in chromium stressed plant (Ali et al. 2013). While in wheat plants NaHS can help in relieving the reduction in germination of seeds caused by copper stress (Zhang et al. 2008). Moreover, oilseed rape (Brassica napus L.) stressed by heavy metal lead, alleviated by NaHS, results in growth, photosynthesis and cell structure improvement (Ali et al. 2014). Chen et al. (2018) studied the effect of exogenous H2S on Brassica oleracea L. seed germination and seedling growth under Pb (0.25 and 0.5 mM) stress. They found that Pb markedly inhibited seed germination and seedling growth, root length, shoot length and fresh weight. In addition, they observed that NaHS elevated endogenous contents and reduced the Pb induced malonyldialdehyde, superoxide anion and hydrogen peroxide production. Zanganeh et al. (2018) studied the impacts of seed priming with H2S along with salicylic acid on possible metabolic pathway of amino acids in maize plant under Pb stress. They observed that H2S along with salicylic acid play a significant role in regulating the metabolism of methionine and arginine in maize under Pb stress condition. Mostofa et al. (2015) studied the alleviative role of H2S in rice plants under Cd stress. Their results revealed Cd-induced growth inhibition and biomass reduction. Cd-mediated oxidative stress in rice plants was evidenced by observing increased levels of superoxide, hydrogen peroxide, methylglyoxal and malondialdehyde. H2S reduced the extent of Cd-induced oxidative stress via enhancing redox status and the activities of enzymes related to reactive oxygen species metabolism and methylglyoxal detoxification system. They confirmed the beneficial effect of H2S under Cd stress by using H2S-scavenger hypotaurine that abolished the beneficial effect of H2S in rice plants.
28.3 Nitric Oxide (NO)
Nitric oxide with the formula NO is a colourless gas and is considered as one of the principal oxides of nitrogen. Nitric oxide is a free radical, i.e. it is having an unpaired electron. It is a highly diffusible gas having the ability to diffuse through biological membranes readily. NO has an immense role in various physiological and biochemical (hence is a bioactive molecule) processes of plants. It shows dual character at different concentrations. At low concentrations, it acts as a signalling molecule whereas at higher concentrations, it can damage the cell resulting in nitro-oxidative stress. Hence it has been proven to be useful as well as harmful for plant. Beside this, NO is associated with signal transduction. NO, like H2O2 is considered as an important signalling molecule which makes plants to have adaptive responses to various biotic as well as abiotic environmental stresses (Qiao et al. 2014; Hossain et al. 2015; Santisree et al. 2015; Simontacchi et al. 2015; Sahay and Gupta 2017; Asgher et al. 2017; Sami et al. 2018; Nabi et al. 2019; Sharma et al. 2019). It has been seen that application of NO to seeds or seedlings of different plants resulted in making the plants tolerant to various stresses which includes heat, drought, and salt stresses mainly through two different ways; either by decreasing oxidative toxicity or by increasing antioxidative potential in numerous plants like citrus plants (Tanou et al. 2009), wheat (Wahid et al. 2007), Capsicum annuum (Kaya et al. 2019b), soybean (Ishibashi et al. 2011), maize (De Azevedo Neto et al. 2005), rice (Uchida et al. 2002; Yang et al. 2016), orange seedlings (Fan and Liu 2012), tomato (Siddiqui et al. 2017), strawberry (Kaya et al. 2019a) and other crop species (Hossain et al. 2015; Santisree et al. 2015; Simontacchi et al. 2015). During plant pathogen interactions, NO has an important additional defensive role to play (Durner et al. 1998; Gaupels et al. 2011; Mur et al. 2013). Various researches have revealed the association of NO with plant growth, seed germination, photosynthesis, leaf senescence, pollen growth, and orientation and it is rightly given a name of “plant growth regulator” (Beligni and Lamattina 2001). Moreover, NO has a significant interaction with other plant hormones such as auxins, gibberellins, abscisic acid, cytokinins, ethylene, salicylic acid, jasmonic acid, etc. under diverse stresses. The production of NO is suggested to take place through different pathways which are classified as oxidative or reductive steps.
28.3.1 Role of NO in Drought Stress
In drought stress, i.e. unavailability of water, there is an interaction between NO and abscisic acid (ABA) (Hancock et al. 2012; Sahay et al. 2019). An exogenous application of NO increased the drought tolerance in cut leaves and seedlings of Triticum aestivum by enhancing ABA synthesis with the help of a NO donor (Zhao et al. 2001). Many researchers through their researches in diverse crop plants have experimentally shown that there is an enhancement of plant tolerance to drought stress with the external application of NO (Lei et al. 2007, Hao et al. 2008, Shao et al. 2010, Tian and Lei 2006, Xing et al. 2004; Cechin et al. 2015; reviewed by Santisree et al. 2015; Montilla-Bascón et al. 2017). Moreover, researches have showed that NO is produced as a signal molecule in response to abscisic acid (Mioto and Mercier 2013). In case of Zea mays, it was seen that by the application of NO, it interact with CK’s and results in regulation of photosynthesis and also an increase in adaptation towards drought stress (Shao et al. 2010). Similar results were also reported in case of Arabidopsis thaliana for the induction of senescence by interaction between CK’s and NO (Mishina et al. 2007). Nevertheless, in Arabidopsis thaliana, it was found that over-expression of two ABA receptors (AtPYL4 and AtPYL5) enhanced drought resistance, antioxidant enzyme activity and osmolyte levels. These observations also bring out the role of NO in drought stress response in Arabidopsis (Shi et al. 2014).
28.3.2 Role of NO in Salt Stress
A worldwide increase in salinity threatens the plant growth, development and production of agriculture (Zhu 2003; Ahmad et al. 2018a; Romero-Munar et al. 2019). According to an estimate there is more than 20% (~45 million ha) of arable land worldwide and approximately 50% of irrigated land is occupied by high salt concentration (Ahmad et al. 2015; Fatma et al. 2016). The high salt conditions are injurious to plant health, as it causes ionic, osmotic and oxidative stress, thereby inhibiting various biochemical and physiological processes responsible for optimal growth and development (Munns and Tester 2008; Khan et al. 2010; Ahanger et al. 2018; Khanam and Mohammad 2018; Jorge et al. 2019). Due to salinity-induced intracellular ion imbalance, the soil nutrient uptake is altered which leads to nutrition deficiencies in plants. While, salinity provokes disintegration of membranes, loss of metabolic functions, leakage of ions, DNA defragmentation, and consequently programmed cell death (Hasegawa et al. 2000; Ahanger and Agarwal 2017; Tang et al. 2019). Salt stress has a relevant effect on a plant as that of the drought stress do. Many experiments have shown the increase in resistance to salt stress (also known as salinity) on the application of NO to the affected plants (Khan et al. 2003; Fatma et al. 2016; Sehar et al. 2019). There are ample evidences suggesting the involvement of NO in alleviation of ill effects of salt stress on various plants. Under salt stress, DELLA proteins play an important role where it restrict the growth and regulate development. Root meristems inhibition under salt stress was achieved by NO generation by auxin concentration reduction (Liu et al. 2015). Furthermore, experiments showed a synergetic interaction between NO and SA resulting in the alleviation of salt stress (Liu et al. 2014). By the application of NO, there was an increase in antioxidant enzyme activity, witnessed in mitochondria of wheat seeds under salt stress, enhancing the germination rate and weight of radicals and coleoptiles. Further experiments were done with wheat plant in which it was seen that the starch content can be reduced and soluble sugar content can be increased on the pretreatment of wheat seeds with NO for 3 days (Almansouri et al. 2001). Association of NO with increased ATP content in wheat seeds was also observed. Enhancement of Na+/K+ ratio was provided by NO application in the callus of Phragmites communis (reed) and Populus euphratica (populus) providing tolerance to salt stress (Zhao et al. 2004). In a recent study, Sehar et al. (2019) studied the involvement of nitric oxide donor (sodium nitroprusside) in the reversal of glucose-inhibited photosynthetic responses in the presence or absence of salt stress (100 mM NaCl) in wheat plants. They found that NO improves photosynthesis of wheat plants in the absence of salt stress, and also reduces glucose-mediated repression of photosynthesis under salt stress. NO accomplished this effect through increase in antioxidant system and concomitant decrease in glucose and ethylene sensitivity under salt stress. In yet another recent experiment, Campos et al. (2019) studied the potential of NO and phytohormone crosstalk in Lactuca sativa plants to salinity stress conditions. Plant exposure to salt stress triggered ionic, osmotic and oxidative stress, which causes imbalance in hormone content, cell death and consequently decreased growth of plants. These changes were correlated with salt ions in tissues. However, NO caused a decrease in Na+ accumulation and stabilized the mineral nutrient status. This resulted in maintenance of photosynthesis rate and growth re-establishment. The NO-signalling also balances the phytohormones content and caused an increase in antioxidant potential and osmotic regulation, with culminates tolerance to the salt stress. Hence it was proved that NO has an immense importance in physiological response alteration which enhances the growth of plant (Liu et al. 2007).
28.3.3 Role of NO in Heavy Metal Stress
There are alterations in root structures due to change in hormonal imbalance under heavy metal stress. One of the common aspects of stress caused by various heavy metals is ROS production (He et al. 2012; Wani et al. 2018). Various researches have showed that NO tends to have an important role in the protection against heavy metal stress like the toxic effects exerted by As (Singh et al. 2009), Cd (Hsu and Kao 2004), Al (Wang and Yang 2005), Cu (Yu et al. 2005) and B (Farag et al. 2017). Application of NO to the plants having toxicities of such heavy metals alleviates the effects under different conditions. NO content sometimes increases and sometimes decreases in response to heavy metal stress making a controversy. Researchers have shown that in case of few plants like pea (Pisum sativum) and Arabidopsis thaliana, Cd tend to reduce the NO production hence resulting in stress (Barroso et al. 2006; Rodríguez-Serrano et al. 2009; Zhu et al. 2012a, b; Xu et al. 2011). In Arabidopsis thaliana, NO accumulation induced by auxin increased the cadmium tolerance (Xu et al. 2011). Application of NO successfully shows an improvement in the antioxidative capacity of Medicago truncatula and reduction of auxin degradation in roots were also seen in the plant under Cd stress (Xu et al. 2010).
28.3.4 Role of NO in Temperature Stress
The adverse effects that results from heat stress can be enlisted as membrane damage, lipid peroxidation, enzyme inactivation, oxidative stress and ultimately disruption of DNA strands (Suzuki and Mittler 2006; Ali et al. 2019). Protein denaturation, oxidative and osmotic stresses and membrane rigidity that can cause ion leakage are some other effects of low temperature (Ruelland et al. 2009; Khan et al. 2017). To deal with such adversities, application of NO turned to be useful to the plants. It was seen that NO also helps in inducing various activities in cold stress like nitric oxide synthase (NOS) and S-nitrosoglutathione reductase (GSNOR) activities. Some residues of other compounds like S-nitrosothiols and nitrates were also observed in case of pea plant (Corpas et al. 2008). Nitrate reductase dependent-NO generation was detected in Arabidopsis thaliana plant when the plant was subjected to 4 °C (Zhao et al. 2009). At low temperatures, there is the production of ABA which leads to the closure of stomata or osmolyte production having deleterious effects on the physiology of plants (Ruelland et al. 2009; Kosová et al. 2012).
28.4 Salicylic Acid (SA)
Salicylic acid (ortho-hydroxy benzoic acid; SA), a ubiquitously distributed signalling molecule, phenolic in nature, plays a pivotal role in tolerance of various kinds of stresses in diverse crop plants; both biotic and abiotic. Additionally, it plays a role in plants growth and development along with defense responses. Furthermore, various physiological as well as molecular processes like uptake of nutrients, fruit ripening, stomatal movements, photosynthesis, biosynthesis of chlorophyll, pathogenesis-associated protein expression, etc. are regulated by the SA mainly in concentration dependent manner (Khan et al. 2015; Nazar et al. 2017; Zaid et al. 2019). Also, biosynthesis of various secondary metabolites like sinapyl alcohol dehydrogenase, cinnamyl alcohol dehydrogenase and cytochrome P450 are encoded by the SA genes along with the production of different chaperones, heat shock proteins and antioxidants (Jumali et al. 2011). SA is synthesized mainly via two different pathways: isochorismate synthase (ICS) and the phenylalanine ammonia lyase (PAL) in plastids. However, ICS pathway is the major route of biosynthesis of SA in most of the plants where, ICS is biosynthesized from chorismic acid, an end product of shikimic acid pathway (Uppalapati et al. 2007; Catinot et al. 2008; Jayakannan et al. 2015). Various researchers have reported a potential role of SA in amelioration of different abiotic stresses like drought (Miura et al. 2013; Nazar et al. 2015; Lee et al. 2019), salinity (Fahad and Bano 2012; Khodary 2004; Amirinejad et al. 2017; Tahjib-ul-Arif et al. 2018; Alsahli et al. 2019), chilling (Yang et al. 2012; Chen et al. 2016; Wang et al. 2018), metal (Zhao et al. 1995; Zaid et al. 2019) and heat (Fayez and Bazaid 2014; Munir and Shabbir 2018). Other effects of SA includes: ethylene biosynthesis is retarded by the exogenous supplementation of SA, enhancement in the production of photosynthetic pigments and photosynthetic machinery and alleviation of deleterious effects in plants, resulting from exposure to heavy metal (Zhao et al. 1995; Zhang and Chen 2011). PR1 and PR2 (pathogenesis-related genes) are reported to be the SA-inducible genes mainly involved in the modulation of some abiotic stresses in plants (Miura et al. 2013). Also, there are reports suggesting the SA receptors to belong to the family of receptor-like protein kinases (RLKs) as SA is capable of modulating the expression of various RLKs in plants (Ohtake et al. 2000). Despite considerable investigations, the role of SA in stress tolerance at molecular level remains largely unknown.
28.4.1 Role of SA in Heat Stress
Over the past years, different researchers have identified a putative role of SA in regulation of heat stress in plants through the modulation of various physiological and metabolic processes. Heat stress tolerance in different plants under the influence of SA is mainly explained by the amendment of the antioxidant defence mechanism in plants. Activity of various antioxidant enzymes is modulated mainly through the endogenous H2O2 levels under the influence of SA in plants resulting in improvement of plant growth and development. Various researchers have reported the amelioration in growth, biochemical and physiological attributes of different plants viz. corn and soybean (Khan et al. 2003), wheat (Shakirova 2007), Brassica juncea (Fariduddin et al. 2003), wheat seedlings (Hayat et al. 2005), barley seedlings (Pancheva et al. 1996), maize (Khodary 2004), cucumber, grape and many other important plants (Shi et al. 2006; Wang et al. 2010) under heat stress, by the exogenous application of SA to the plant. Furthermore, survival percentage of maize seedlings has been reported to increase by the pretreatment of seeds under heat stress with SA. Also, an enhanced accumulation of osmolytes leading to an improvement of antioxidant system of the plants under heat stress has been reported (Li et al. 2015a, 2015b; Li 2015). After a pretreatment with SA and heat stress, Agrostis stolonifera showed an improvement in growth (increase in green leaf index), membrane stability, lipid peroxidation and photosynthesis. Similarly, the activities of different antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT) and contents of proline, phenolics and flavonoids in Digitalis trojana were enhanced by the pretreatment of SA in callus cultures under heat stress (Cingoz and Gurel 2016). A reduction in the oxidative damage by heat stress was recorded in Arabidopsis plants after the foliar-application of SA treatment (Larkindale and Knight 2002). Moreover, the leaf-applied SA induced long-term heat tolerance in the grape plant, which might be due to the Ca2+ homeostasis and antioxidant systems (Wang and Li 2006). Many other derivatives of SA (sulphosalicylic acid, methyl salicylate) have been reported to influence the heat tolerance in plants like cucumber and holm oak, respectively (Shi et al. 2006; Llusia et al. 2005). An increase in the contents of protein as well as proline was observed by both SA treatment and heat acclimation leading to the induction of POD and APX activities and a reduction in CAT activity (Chakraborty and Tongden 2005). Kaur et al. (2009) have observed that SA pretreatment leads to heat tolerance at seedling stage in Brassica species by increasing total soluble sugar and growth of plants as well as by enhancing the activities of some enzymes (invertase, CAT and POD). Moreover, the expression of some noteworthy proteins (heat shock proteins; HSPs) along with some new proteins was stimulated after pretreatment with SA. In yet another study, it has been reported that the expression of HSP21 was modulated by the application of SA to the leaves of the plant (grapevine) under study. The levels of HSP21 remained high throughout the experiment in the SA-treated plants as compared to the control, helping the plant to recover thus conferring heat tolerance to the plant (Wang et al. 2011). There are other reports related to heat tolerance by foliar application of SA, involving HSPs in tomato, mung bean and hyacinth bean. The genes involved in the ascorbate-glutathione cycle and activities of several reactive oxygen species (ROS) scavenging antioxidant enzymes are regulated by the SA treatment in these plants (Rai et al. 2018a, 2018b). In a study carried out by Liu et al. (2006), an interrelationship has been reported between SA and ABA. It was observed that the levels of SA and ABA was modulated during heat stress and the presence of SA (conjugated as well as free form) is much imported to the plant as compared to ABA in conferring heat tolerance to the plants. Clarke et al. (2004) correlated the level of SA and basal heat tolerance in Arabidopsis genotypes with modified SA signalling. Thus, from the above discussion, it is clear that SA modulated different mechanisms to confer heat stress tolerance in various crop plants.
28.4.2 Role of SA in Metal Stress
Heavy metals present in the agricultural lands due to various anthropogenic activities lead to perturbation of various physiological, biochemical and metabolic processes in different plants leading to a disturbed growth and reduced productivity (Weast 1984; Boussama et al. 1999; Aftab et al. 2011; Ahmad et al. 2018b; Wani et al. 2018). Various researchers have reported an important role of SA in alleviating the stress caused by the heavy metals present in the environment. Lead- and mercury-induced stress in rice has been studied by Mishra and Choudhuri (1999) where pretreatment with SA protected rice from the heavy metals induced membrane disruption. Also, Cd-induced toxicity in barley (Metwally et al. 2003), maize (Pál et al. 2002; Krantev et al. 2008), mustard (Zaid et al. 2019), peppermint (Ahmad et al. 2018b), soybean (Drazic and Mihailovi 2005), alfalfa (Drazic et al. 2006) and rice (Panda and Patra 2007; Chao et al. 2010) was positively alleviated by SA application (foliar applied or pretreatment) to these plants. Yang et al. (2003) studied the effect of Al on Cassia tora where it was found that SA application conferred tolerance to the plant to withstand the deleterious effects of the heavy metal stress. It was reported that the Cassia tora under Al stress accumulated citrate in their roots as result of SA application. Ill effects caused by lead and mercury in rice were also mitigated by the exogenous application of salicylic acid. It was reported that the enhancement in the activity of lipoxygenase was regulated by the application of SA (exogenous) (Mishra and Choudhuri 1999). Gill et al. (2016) studied the protective effects of exogenously applied SA on Cr toxicity in the black and yellow-seeded Brassica napus L. The findings of these authors demonstrated the enhanced activity of enzymatic antioxidants and gene expression, secondary metabolism and the transcript level of specific stress associated proteins in root and leaf of these plants under Cr toxicity. Cd tolerance due to the application of SA in different plants is attributed to the improvement of antioxidant system of the plant as SA enhances the activity of SOD, peroxidase, dehydroascorbate reductase, GR which help in scavenging the ROS produced in these plants (Ahmad et al. 2018b; Khanam and Mohammad 2016; Kazemi et al. 2011; Zhang et al. 2011; Bai et al. 2014). Furthermore, in a report by Krantev et al. (2008) similar results were recorded where the growth of the plant, activities of RUBP carboxylase and PEP, carboxylase and antioxidant enzymes (APX, SOD) were improved whereas, activity of CAT, rate of lipid peroxidation and leakage of electrolytes was reduced in maize leaves, thus conferring Cd tolerance to the plant. Along with the above mentioned physiological and biochemical processes, SA also modulates the expression of certain genes and proteins that lead to lower H2O2 content in the plants resulting in membrane stability under heavy metal stress (Chao et al. 2010). Kumari and Pandey-Rai (2018) studied the effect of SA on Artemisia annua under arsenic (As) stress. It was reported that SA helps in sequestration of As into the vacuoles and the regulation of artemisinin biosynthesis by the synthesis of oxylipins, which modulate the expression of MAPKs. Moreover, different transcription factors (MYC, WRKY) are upregulated by these MAPKs resulting into a positive effect on the biosynthesis of secondary metabolites (artemisinin) along with the mitigation of heavy metal stress.
28.4.3 Role of SA in Drought Stress
Drought stress (water deficit) that affects plants, is a multidimensional stress which leads to various dysfunctions (physiological and biochemical) at different organizational levels of the plant like turgor reduction, growth, photosynthetic rate, stomatal conductance and damages of cellular components, decrease in water potential, production of radical scavenging compounds, regulation and alteration of gene expression, etc. (Janda et al. 2007; Yordanov et al. 2000; Farooq et al. 2009; Aimar et al. 2011; Kunert et al. 2016; Joshi et al. 2016). In a study by Da Silva et al. (2017a), SA application enhanced the growth of sesame plant under drought stress resulting in mitigating the ill effects of the stress. Also, there are reports where SA application delimits the synthesis of ROS in plants under water stress. Furthermore, application of SA increases the expression of mitochondrial alternative oxidase (AOX; an important enzyme for stress tolerance) in stressed plants (Tang et al. 2017; Zhang and Chen 2011). Responses of tomato plant under drought stress showing negative growth, reduction in photosynthetic and biochemical parameters (membrane stability index, leaf water potential, NR and CA activity, contents of chlorophyll and relative water) and an upregulation of proline content and antioxidant enzymes (CAT , POX and SOD) were enhanced by the application of exogenous SA at lower concentration (Hayat et al. 2008). From the survey of scientific literature, various reports indicate a stress-tolerance role of SA in different plants like tomato and bean (Senaratna et al. 2000), Salvia officinalis (Abreu and Munné-Bosch 2008), wheat (Hamada 1998; Hamada and Al-Hakimi 2001; Singh and Usha 2003), barley (Bandurska and Stroinski 2005), sunflower (Hussain et al. 2008), etc. Soaking of seeds (wheat) in SA (an aqueous degraded product of acetyl SA), confer drought resistance to the seedlings, improving the shoot and root dry weights, transpiration rate by protecting the photosynthetic apparatus of the plant from oxidation (Hamada 1998; Hamada and Al-Hakimi 2001). Singh and Usha (2003) also confirmed these results, where the plants showed a marked enrichment in growth (moisture content, dry mass), physiology (activity of Rubisco and superoxide dismutase (SOD) and content of total chlorophyll) and yield of the plants irrespective of the concentration of SA or levels of drought stress applied when compared to the control plants. Similar reports where seeds of tomato and bean have been soaked in SA/acetyl SA to study the response of the plants to drought stress. It was recorded that the seedling survival rate increased by the applied concentration (0.1 Mm and 0.5 mM) of SA/acetyl SA (Senaratna et al. 2003). Bandurska and Stroinski (2005) interestingly reported that the application of SA to the barley plants improved ABA content, suggesting a concomitant role of ABA for the development of drought tolerance by the application of SA. However, some of the reports suggest that by the application of SA (to maize) did not improve drought tolerance, however rendering plants more susceptible to drought (Németh et al. 2002). Horváth et al. (2007) also reported negative results for the application of SA to Chinese spring wheat however, application of SA to winter wheat Cheyenne gave no effect to the plant. Contrary to the previous two plants application of an SA analogue (4-hydroxybenzoic acid) increased the drought tolerance in Cheyenne plants. Thus, suggesting the role of SA in imparting drought tolerance depends upon the type and concentration of SA applied and the genotype of the plant and severity of the stress (Yuan and Lin 2008).
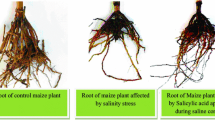
28.4.4 Role of SA in Salinity Stress
Salinity is one of the important abiotic stresses, affecting the growth and physiology of plants. Salt stress may result into responses like, osmotic conductance, toxicity and imbalance of specific ion, and oxidative stress, leading to the production of reactive oxygen species (Tester and Davenport 2003) and inversely resulting in a decrease of plant weight and thus reduction in plant productivity (Ashraf and Harris 2004). SA is a phytohormone that modulates plant physiological responses conferring stress tolerance to the plant (Ahmad et al. 2011; Janda et al. 2012; Khan et al. 2014). In a report by Azooz et al. (2011), treatment with SA to Vicia faba under salt stress (sea water treatment) ameliorated the ill effects of salinity leading to an increase of osmolyte and free amino acids (proline) accumulation. Also an increase in biomass accumulation, growth and antioxidant system has been registered in the plant under study. Khan et al. (2014) have also confirmed the beneficial effects of SA application to salt-stressed Vigna radiata L. leading to a decrease in the levels of ethylene and better partitioning of salt ions. Also, some reports confirm an increase in the levels of endogenous ABA and proline along with an improvement of growth and yield of wheat plant under salt stress in response to SA application (Shakirova et al. 2003). Improvement of growth, accumulation of biomass, cell division, and activity improvement of photosynthetic apparatus as well as different antioxidant enzymes have been reported after the application of SA (Da Silva et al. 2017a). Janda et al. (2012) reported that salt stress have deleterious effects on the photosynthesis and membrane stability of barley plant; which were improved by the exogenous application of SA. Similar results were reported in maize, where the application of SA to salt-stressed plant leads to reduction in accumulation of Na and improvement of growth and development of the plant under salt stress (Gunes et al. 2007). Furthermore, various researchers have reported the amelioration of salt stress induced changes in different plants viz. barley (El-Tayeb 2005), wheat (Hamada and Al-Hakimi 2001; Sakhabutdinova et al. 2004), tomato (Tari 2002; Tari et al. 2004, 2010; Szepesi 2005; Szepesi et al. 2008a, b; Gémes et al. 2008), Salvia officinalis L. (Sahar et al. 2011), Arabidopsis (Poór et al. 2012) Iris hexagona (Wang et al. 2001). Moreover, application of SA to salt-stressed barley plants by improving the contents of photosynthetic pigment and maintenance of membrane integrity resulted in salt tolerance (El-Tayeb 2005). Application of SA to salt-stressed plants also result in a decrease in lipid peroxidation due to a decrease in Na content and a significant increase in the contents of K and Mg. The activities of various antioxidant enzymes (SOD, CAT, GPX) was also increased by the application of SA with a decrease in contents of dehydroascorbate reductase and in the ascorbate and glutathione contents (He and Zhu 2009). The activity of these antioxidant enzymes was also modulated by the application of SA in tomato plants along with an increase in the accumulation of various osmolytes (sugars, sugar alcohol, proline) in the plant (Tari 2002; Tari et al. 2004, 2010; Szepesi 2005; Szepesi et al. 2008a, b; Gémes et al. 2008). Sahar et al. (2011) also reported similar results in Salvia officinalis L. plant. In contrast to the earlier reports, Hao et al. (2012) studied a line of mutant Arabidopsis (snc1, NahG, npr1–1, snc1/NahG and wild type plant) where high SA accumulation lead to an increase in salt-induced damage and reduced SA proved to be favourable for the plant. Similarly, reports for soybean under salt stress show a decrease in the levels of endogenous free SA content (Hamayun et al. 2010). Thus, from the above literature it is clear that SA is a potent phytohormone which confers salt stress resistance in different crop plants.
References
Abbas F (2013) Analysis of a historical (1981–2010) temperature record of the Punjab province of Pakistan. Earth Interact 17:1–23
Abreu ME, Munné-Bosch S (2008) Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: a case study in field-grown Salvia officinalis L. plants. Environ Exp Bot 64:105–112
Aftab T, Khan MMA, da Silva JAT, Idrees M, Naeem M (2011) Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J Plant Growth Regul 30:425–435
Ahanger MA, Agarwal RM (2017) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460
Ahanger MA, Alyemeni MN, Wijaya L, Alamri SA, Alam P, Ashraf M, Ahmad P (2018) Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS One 13:e0202175
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard Brassica juncea (L.) Czern. & Coss. Plants can be alleviated by salicylic acid. S Afr J Bot 77:36–44
Ahmad P, Hashem A, Abd-Allah EF, Alqarawi AA, John R, Egamberdieva D, Gucel S (2015) Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front Plant Sci 6:868
Ahmad P, Abd_Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KH (2018a) Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci Rep 8:13515
Ahmad B, Jaleel H, Sadiq Y, Khan MMA, Shabbir A (2018b) Response of exogenous salicylic acid on cadmium induced photosynthetic damage, antioxidant metabolism and essential oil production in peppermint. Plant Growth Regul 86:273–286
Aimar D, Calafat M, Andrade AM, Carassay L, Abdala GI, Molas ML (2011) Drought tolerance and stress hormones: from model organisms to forage crops. In: Vasanthaiah H, Kambiranda D (eds) Plants and environment. InTech, Rijeka, Croatia, pp 137–164
Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang G (2013) Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem 32:2234–2239
Ali B, Song WJ, Hu WZ, Luo XN, Gill RA, Wang J, Zhou WJ (2014) Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastructural changes in oilseed rape. Ecotoxicol Environ Saf 102:25–33
Ali S, Rizwan M, Zaid A, Arif MS, Yasmeen T, Hussain A, Abbasi GH (2018) 5-Aminolevulinic acid-induced heavy metal stress tolerance and underlying mechanisms in plants. J Plant Growth Regul 37:1423–1436
Ali S, Rizwan M, Arif MS, Ahmad R, Hasanuzzaman M, Ali B, Hussain A (2019) Approaches in enhancing thermotolerance in plants: an updated review. J Plant Growth Regul 12:1–25. https://doi.org/10.1007/s00344-019-09994-x
Almansouri M, Kinet JM, Lutts S (2001) Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 231:243–254
Alsahli A, Mohamed AK, Alaraidh I, Al-Ghamdi A, Al-Watban A, El-Zaidy M, Alzahrani SM (2019) Salicylic acid alleviates salinity stress through the modulation of biochemical attributes and some key antioxidants in wheat seedlings. Pak J Bot 51:1551–1559
Amirinejad AA, Sayyari M, Ghanbari F, Kordi S (2017) Salicylic acid improves salinity-alkalinity tolerance in pepper (Capsicum annuum L.). Adv Hort Sci 31:157–163
Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res 24:2273–2285
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Azooz MM, Youssef AM, Ahmad P (2011) Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int J Plant Physiol Biochem 3:253–264
Bai X, Dong Y, Kong J, Xu L, Liu S (2014) Effects of application of salicylic acid alleviates cadmium toxicity in perennial ryegrass. Plant Growth Regul 75:695–706
Bandurska H, Stroinski A (2005) The effect of salicylic acid on barley response to water deficit. Acta Physiol Plant 27:379–386
Banerjee A, Roychoudhury A (2018) Small heat shock proteins: structural assembly and functional responses against heat stress in plants. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN (eds) Plant metabolites and regulation under abiotic stress. Academic Press, Elsevier, London, pp 367–374
Banerjee A, Tripathi DK, Roychoudhury A (2018) Hydrogen sulphide trapeze: Environmental stress amelioration and phytohormone crosstalk. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2018.08.028
Barroso JB, Corpas FJ, Carreras A, Rodríguez-Serrano M, Esteban FJ, Fernández-Ocana A, Del Río LA (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793
Beligni MV, Lamattina L (2001) Nitric oxide in plants: the history is just beginning. Plant Cell Environ 24:267–278
Birke H, De Kok LJ, Wirtz M, Hell R (2015) The role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant Cell Physiol 56:358–367
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Boussama N, Quariti O, Ghorbal MH (1999) Changes in growth and nitrogen assimilation in barley seedlings under cadmium stress. J Plant Nutr 22:731–752
Campos FV, Oliveira JA, Pereira MG, Farnese FS (2019) Nitric oxide and phytohormone interactions in the response of Lactuca sativa to salinity stress. Planta:1–15
Catinot J, Buchala A, Abou-Mansour E, Métraux JP (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett 582:473–478
Cechin I, Cardoso GS, Fumis TDF, Corniani N (2015) Nitric oxide reduces oxidative damage induced by water stress in sunflower plants. Bragantia 74:200–206
Chakraborty U, Tongden C (2005) Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. Curr Sci 89:384–389
Chao YY, Chen CY, Huang WD, Kao CH (2010) Salicylic acid mediated hydrogen peroxide accumulation and protection against cd toxicity in rice leaves. Plant Soil 329:327–337
Chen Z, Zhang L, Zhu C (2015) Exogenous nitric oxide mediates alleviation of mercury toxicity by promoting auxin transport in roots or preventing oxidative stress in leaves of rice seedlings. Acta Physiol Plant 37:194
Chen X, Chen Q, Zhang X, Li R, Jia Y, Jia A, Hu X (2016) Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiol Biochem 104:174–179
Chen Z, Yang B, Hao Z, Zhu J, Zhang Y, Xu T (2018) Exogenous hydrogen sulfide ameliorates seed germination and seedling growth of cauliflower under lead stress and its antioxidant role. J Plant Growth Regul 37:5–15
Christou A, Filippou P, Manganaris GA, Fotopoulos V (2014) Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol 14:42
Cingoz GS, Gurel E (2016) Effects of salicylic acid on thermotolerance and cardenolide accumulation under high temperature stress in Digitalis trojana Ivanina. Plant Physiol Biochem 105:145–149
Clarke SM, Mur LAJ, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermo tolerance but is not essential for acquired thermo tolerance in Arabidopsis thaliana. The Plant J 38:432–447
Corpas FJ, Chaki M, Fernandez-Ocana A, Valderrama R, Palma JM, Carreras A, Barroso JB (2008) Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol 49:1711–1722
Corpas FJ, González-Gordo S, Cañas A, Palma JM (2019a) Nitric oxide and hydrogen sulfide in plants: which comes first? J Exp Bot. https://doi.org/10.1093/jxb/erz031
Corpas FJ, Barroso JB, González-Gordo S, Muñoz-Vargas MA, Palma JM (2019b) Hydrogen sulfide: a novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J Integ Plant Biol Doi. https://doi.org/10.1111/jipb.12779
Da Silva AS, Suassuna JF, de Melo AS, Costa RR, de Andrade WL, da Silva DC (2017a) Salicylic acid as attenuator of drought stress on germination and initial development of sesame. Rev Bras Eng Agríc Ambient 21:156–162
Da Silva CJ, Fontes EPB, Modolo LV (2017b) Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci 256:148–159
Dalal A, Bourstein R, Haish N, Shenhar I, Wallach R, Moshelion M (2019) Dynamic physiological Phenotyping of drought-stressed pepper plants treated with “productivity-enhancing” and “survivability-enhancing” biostimulants. Front Plant Sci 10:905
De Azevedo Neto AD, Prisco JT, Eneàs-Filho J, Medeiros JV, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Drazic G, Mihailovi CN (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517
Drazic G, Mihailovi_ CN, Lojic M (2006) Cadmium accumulation in Medicago sativa seedlings. Biol Plant 50:239–244
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Nat Acad Sci U S A 95:10328–10333
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Fahad S, Bano A (2012) Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak J Bot 44:1433–1438
Fan QJ, Liu JH (2012) Nitric oxide is involved in dehydration/drought tolerance in Poncirus trifoliata seedlings through regulation of antioxidant systems and stomatal response. Plant Cell Rep 31:145–154
Fang H, Liu Z, Jin Z, Zhang L, Liu D, Pei Y (2016) An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ Poll 213:870–877
Farag M, Najeeb U, Yang J, Hu Z, Fang ZM (2017) Nitric oxide protects carbon assimilation process of watermelon from boron-induced oxidative injury. Plant Physiol Biochem 111:166–173
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41:281–284
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. In Sustainable agriculture (pp. 153–188). Springer, Dordrecht.
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521
Fayez KA, Bazaid SA (2014) Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J Saudi Soc Agr Sci 13:45–55
Feller U, Kingston-Smith AH, Centritto M (2017) Abiotic stresses in agroecology: a challenge for whole plant physiology. Front Environ Sci 5:13
Food and Agricultural Organization [FAO] (2009) How to feed the world in 2050. Food and Agriculture Organization, Rome, Italy. http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf
Fu P, Wang W, Hou L, Liu X (2013) Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc Bot Pol 82:295–302
García-Mata C, Lamattina L (2010) Hydrogen sulfide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188:977–984
Gaupels F, Kuruthukulangarakoola GT, Durner J (2011) Upstream and downstream signals of nitric oxide in pathogen defense. Curr Opin Plant Biol 14:707–714
Gémes K, Poór P, Sulyok Z, Szepesi Á, Szabó M, Tari I (2008) Role of salicylic acid pre-treatment on the photosynthetic performance of tomato plants (Lycopersicon esculentum mill. L. Cvar. Rio Fuego) under salt stress. Acta Biol Szeg 52:161–162
Gill RA, Zhang N, Ali B, Farooq MA, Xu J, Gill MB, Bizeng Mao Zhou W (2016) Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ Sci Pollut Res 23:20483–20496
Gong J, Wang B, Zeng G, Yang C, Niu C, Niu Q, Zhou W, Liang Y (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164:1517–1522
Gotor C, García I, Crespo JL, Romero LC (2013) Sulfide as a signaling molecule in autophagy. Autophagy 9:609–611
Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, Cicek N (2007) Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol 164:728–736
Hamada AM (1998) Effects of exogenously added ascorbic acid, thiamin or aspirin on photosynthesis and some related activities of drought-stressed wheat plants. In: Garab G (ed) Photosynthesis: mechanisms and effects, vol 4. Kluwer Academic Publisher, Dordrecht, pp 2581–2584
Hamada AM, Al-Hakimi AMA (2001) Salicylic acid versus salinity-drought-induced stress on wheat seedlings. Rostlinna Vyroba 47:444–450
Hamayun M, Khan SA, Khan AL, Shinwari ZK, Hussain J, Sohn EY, Lee IJ (2010) Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Pak J Bot 42:3103–3112
Hancock JT, Neill SJ, Wilson ID (2012) Nitric oxide and ABA in the control of plant function. Plant Sci 181:555–559
Hao GP, Xing Y, Zhang JH (2008) Role of nitric oxide dependence on nitric oxide synthase-like activity in the water stress signaling of maize seedling. J Integ Plant Biol 50:435–442
Hao L, Zhao Y, Jin D, Zhang L, Bi X, Chen H, Li G (2012) Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant Soil 354:81–95
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Biol 51:463–499
Hayat S, Fariduddin Q, Ali B, Ahmad A (2005) Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agr Hung 53:433–437
Hayat S, Hasan SA, Fariduddin Q, Ahmad A (2008) Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Inter 3:297–304
He Y, Zhu ZJ (2009) Exogenous salicylic acid alleviates NaCl toxicity and increases antioxidant enzyme activities in Lycopersicon esculentum. Biol Plant 52:792–795
He H, Zhan J, He L, Minghua G (2012) Nitric oxide signaling in aluminum stress in plants. Protoplasma 249:483–492
He H, Li Y, He LF (2018) The central role of hydrogen sulfide in plant responses to toxic metal stress. Ecotoxicol Environ Saf 157:403–408
Horváth E, Pál M, Szalai G, Páldi E, Janda T (2007) Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol Plant 51:480–487
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from H2O2 detoxification and scavenging. Front Plant Sci 6:420
Hsu YT, Kao CH (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Huang Z, Chen G, Zeng G, Chen A, Zuo Y, Guo Z, Tan Q, Song Z, Niu Q (2015) Polyvinyl alcohol-immobilized Phanerochaete chrysosporium and its application in the bioremediation of composite-polluted wastewater. J Hazard Mater 289:174–183
Hussain M, Malik MA, Farooq M, Ashraf MY, Cheema MA (2008) Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J Agr Crop Sci 194:193–199
IPCC Intergovernmental Panel on Climate Change (2007) Climate change 2007: impacts, adaptation and vulnerability. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) Contribution of working group II to fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, p 1000
Ishibashi Y, Yamaguchi H, Yuasa T, Iwaya-Inoue M, Arima S, Zheng SH (2011) Hydrogen peroxide spraying alleviates drought stress in soybean plants. J Plant Physiol 168:1562–1567
Janda T, Szalai G, Leskó K, Yordanova R, Apostol S, Popova LP (2007) Factors contributing to enhanced freezing tolerance in wheat during frost hardening in the light. Phytochemistry 68:1674–1682
Janda K, Hideg E, Szalai G, Kovács L, Janda T (2012) Salicylic acid may indirectly influence the photosynthetic electron transport. J Plant Physiol 169:971–978
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2015) Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul 76:25–40
Jin ZP, Shen JJ, Qiao ZJ, Yang GD, Wang R, Pei YX (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun 414:481–486
Jin ZP, Xue SW, Luo YN, Tian BH, Fang HH, Li H, Pei Y (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46
Jin Z, Wang Z, Ma Q, Sun L, Zhang L, Liu Z, Pei Y (2017) Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil 419:141–152
Jorge TF, Tohge T, Wendenburg R, Ramalho JC, Lidon FC, Ribeiro-Barros AI, António C (2019) Salt-stress secondary metabolite signatures involved in the ability of Casuarina glauca to mitigate oxidative stress. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2019.103808
Joshi R, Wani SH, Singh B, Bohra A, Dar ZA, Lone AA, Singla-Pareek SL (2016) Transcription factors and plants response to drought stress: current understanding and future directions. Front Plant Sci 7:1029
Jumali SS, Said IM, Ismail I, Zainal Z (2011) Genes induced by high concentration of salicylic acid in ‘Mitragyna speciosa’. Aus J Crop Sci 5:296
Kaur P, Ghai N, Sangha MK (2009) Induction of thermotolerance through heat acclimation and salicylic acid in brassica species. Afr J Biotechnol 8:619–625
Kaya C, Akram NA, Ashraf M (2019a) Influence of exogenously applied nitric oxide on strawberry (Fragaria× ananassa) plants grown under iron deficiency and/or saline stress. Physiol Plant 165:247–263
Kaya C, Akram NA, Sürücü A, Ashraf M (2019b) Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci Horticul 255:52–60
Kazemi M, Hadavi E, Hekmati J (2011) Role of salicylic acid in decreases of membrane senescence in cut carnation flowers. Am J Plant Physiol 6:106–112
Khan W, Prithiviraj B, Smith DL (2003) Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol 160:485–492
Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MMA (2010) Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant 32:121
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462
Khan TA, Fariduddin Q, Yusuf M (2017) Low-temperature stress: is phytohormones application a remedy? Environ Sci Pollut Res 24:21574–21590
Khanam D, Mohammad F (2016) Effect of structurally different plant growth regulators (PGRS) on the concentration, yield, and constituents of peppermint essential oil. Int J Geogr Inf Syst 23:1–10
Khanam D, Mohammad F (2018) Plant growth regulators ameliorate the ill effect of salt stress through improved growth, photosynthesis, antioxidant system, yield and quality attributes in Mentha piperita L. Acta Physiol Plant 40:188
Khodary SFA (2004) Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Inter J Agr Biol 6:5–8
Knutti R, Rogelj J, Sedláček J, Fischer EM (2016) A scientific critique of the two-degree climate change target. Nat Geosci 9:13
Kopriva S (2006) Regulation of sulfate assimilation in Arabidopsis and beyond. Ann Bot 97:479–495
Kosová K, Prášil IT, Vítámvás P, Dobrev P, Motyka V, Floková K, Trávničková A (2012) Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J Plant Physiol 169:567–576
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
Kumari A, Pandey-Rai S (2018) Enhanced arsenic tolerance and secondary metabolism by modulation of gene expression and proteome profile in Artemisia annua L. after application of exogenous salicylic acid. Plant Physiol Biochem 132:590–602
Kunert KJ, Vorster BJ, Fenta BA, Kibido T, Dionisio G, Foyer CH (2016) Drought stress responses in soybean roots and nodules. Front Plant Sci 7:1015
Lai D, Mao Y, Zhou H, Li F, Wu M, Zhang J, Xie Y (2014) Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci 225:117–129
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Lee BR, Zhang Q, Park SH, Islam MT, Kim TH (2019) Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Horticul Environ Biotechnol 60:31–40
Lei Y, Yin C, Li C (2007) Adaptive responses of Populus przewalskii to drought stress and SNP application. Acta Physiol Plant 29:519–526
Li Z (2015) Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal Behav 10:e1051278
Li ZG, Gong M, Xie H, Yang L, Li J (2012) Hydrogen sulphide donor sodium hydrosulfide induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2C and calmodulin. Plant Sci 185–186:185–189
Li ZG, Ding XJ, Du PF (2013) Hydrogen sulphide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li J, Jia H, Wang J, Cao Q, Wen Z (2014) Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251:899–912
Li X, Lawas LMF, Malo R, Glaubitz U, Erban A, Mauleon R, Heuer S, Zuther E, Kopka J, Hincha DK, Jagadish KSV (2015a) Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ 38:2171–2192
Li Z, Xie L, Li X (2015b) Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J Plant Physiol 177:121–127
Li ZG, Min X, Zhou ZH (2016a) Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci 7:1621
Li Z, Zhang Y, Zhang X, Peng Y, Merewitz E, Ma X, Huang L, Yan Y (2016b) The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ Exp Bot 124:22–38
Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48:931–935
Lisjak M, Teklic T, Wilson ID, Wood M, Whiteman M, Hancock JT (2011) Hydrogen sulfide effects on stomatal apertures. Plant Signal Behav 6:1444–1446
Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ 36:1607–1616
Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD (2006) Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot 57:3337–3347
Liu Y, Wu R, Wan Q, Xie G, Bi Y (2007) Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol 48:511–522
Liu S, Dong Y, Xu L, Kong J (2014) Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul 73:67–78
Liu S, Yang R, Pan Y, Ma M, Pan J, Zhao Y, Cheng Q, Wu M, Wang M, Zhang L (2015) Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicol Environ Safety 119:35–46
Llusia J, Penuelas J, Munne-Bosch S (2005) Sustained accumulation of methyl salicylate alters antioxidant protection and reduces tolerance of holm oak to heat stress. Physiol Plant 124:353–361
Macomber L, Hausinger RP (2011) Mechanisms of nickel toxicity in microorganisms. Metallomics 3:1153–1162
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mioto PT, Mercier H (2013) Abscisic acid and nitric oxide signaling in two different portions of detached leaves of Guzmania monostachia with CAM up-regulated by drought. J Plant Physiol 170:996–1002
Mishina TE, Lamb C, Zeier J (2007) Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant Cell Environ 30:39–52
Mishra A, Choudhuri MA (1999) Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant 42:409–415
Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. The Plant J 73:91–104
Montilla-Bascón G, Rubiales D, Hebelstrup KH, Mandon J, Harren FJ, Cristescu SM, Prats E (2017) Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci Rep 7:13311
Moshelion M, Altman A (2015) Current challenges and future perspectives of plant and agricultural biotechnology. Trends Biotechnol 33:337–342
Mostofa MG, Rahman A, Ansary MM, Watanabe A, Fujita M, Tran LS (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:14078
Munir M, Shabbir G (2018) Salicylic acid mediated heat stress tolerance in selected bread wheat genotypes of Pakistan. Pak J Bot 50:2141–2146
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Mur LAJ, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJM, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 5:pls052
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133
Nazar R, Umar S, Khan NA, Sareer O (2015) Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. South Afr J Bot 98:84–94
Nazar R, Iqbal N, Umar S (2017) Heat stress tolerance in plants: action of salicylic acid. In: Salicylic acid: a multifaceted hormone. Springer, Singapore, pp 145–161
Németh M, Janda T, Horváth E, Páldi E, Szalai G (2002) Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci 162:569–574
Ohtake Y, Takahashi T, Komed Y (2000) Salicylic acid induces the expression of a number of receptor-like kinase genes in Arabidopsis thaliana. Plant Cell Physiol 41:1038–1044
Pál M, Szalai G, Horváth E, Janda T, Páldi E (2002) Effect of salicylic acid during heavy metal stress. Acta Biol Szeged 46:119–120
Pancheva TV, Popova LP, Uzunova AM (1996) Effect of salicylic acid on growth and photosynthesis in barley plants. J Plant Physiol 149:57–63
Panda, SK, Patra HK (2007). Effect of salicylic acid potentiates cadmium-induced oxidative damage in Oryza sativa L. leaves. Acta Physiologiae Plantarum, 29(6): 567–575
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants - from the field to the test tube and back. Plant Biol 9:582–588
Pereira A (2016) Plant abiotic stress challenges from the changing environment. Front Plant Sci 7:1123. https://doi.org/10.3389/fpls.2016.01123
Poór P, Szopkó D, Tari I (2012) Ionic homeostasis disturbance is involved in tomato cell death induced by NaCl and salicylic acid. In Vitro Cell Dev Biol Plant 48:377–382
Qiao W, Li C, Fan LM (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93
Qin L, Zeng G, Lai C, Huang D, Xu P, Zhang C, Cheng M, Liu X, Liu S, Li B, Yi H (2018) “Gold rush” in modern science: fabrication strategies and typical advanced applications of gold nanoparticles in sensing. Coord Chem Rev 359:1–31
Rai KK, Rai N, Rai SP (2018a) Response of Lablab purpureus L. to high temperature stress and role of exogenous protectants in mitigating high temperature induced oxidative damages. Mol Biol Rep 45:1375–1395
Rai KK, Rai N, Rai SP (2018b) Salicylic acid and nitric oxide alleviate high temperature induced oxidative damage in Lablab purpureus L plants by regulating bio-physical processes and DNA methylation. Plant Physiol Biochem 128:72–88
Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plan Theory 8:34
Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueño MC, Luis A, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Romero-Munar A, Baraza E, Gulías J, Cabot C (2019) Arbuscular mycorrhizal fungi confer salt tolerance in giant reed (Arundo donax L.) plants grown under low phosphorus by reducing leaf Na+ concentration and improving phosphorus use efficiency. Front Plant Sci 10:843
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49:35–146
Sahar K, Amin B, Taher NM (2011) The salicylic acid effect on the Salvia officianlis L. sugar, protein and proline contents under salinity (NaCl) stress. J Stress Physiol Biochem 7:80–87
Sahay S, Gupta M (2017) An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 67:39–52
Sahay S, Khan E, Gupta M (2019) Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in brassica genotypes by combining the role of stress modulators, markers and antioxidants. Nitric Oxide 89:81–92
Sakhabutdinova AR, Fatkhutdinova DR, Shakirova FM (2004) Effect of salicylic acid on the activity of antioxidant enzymes in wheat under conditions of salination. App Biochem Microbiol 40:501–505
Sami F, Faizan M, Faraz A, Siddiqui H, Yusuf M, Hayat S (2018) Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 73:22–38
Santisree P, Bhatnagar-Mathur P, Sharma KK (2015) NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci 239:44–55
Scuffi D, Alvarez C, Laspina N, Gotor C, Lamattina L, Garcíamata C (2014) Hydrogen sulfide generated by l-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166:2065–2076
Sehar Z, Masood A, Khan NA (2019) Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ Exp Bot 161:277–289
Senaratna T, Touchell D, Bunn T, Dixon K (2000) Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul 30:157–161
Senaratna T, Merritt D, Dixon K, Bunn E, Touchell D, Sivasithamparam K (2003) Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul 39:77–81
Shakirova FM (2007) Role of hormonal system in the manisfestation of growth promoting and anti-stress action of salicylic acid. In: Hayat S, Ahmad A (eds) A plant hormone. Springer, Dordrecht
Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR (2003) Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant science, 164(3):317–322
Shao R, Wang K, Shangguan Z (2010) Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: probed by ESR spectroscopy and fast OJIP fluorescence rise. J Plant Physiol 167:472–479
Sharma A, Soares C, Sousa B, Martins M, Kumar V, Shahzad B, Thukral AK (2019) Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: a review on molecular and biochemical aspects. Physiol Plant. doi:https://doi.org/10.1111/ppl.13004
Shen J, Xing T, Yuan H, Liu Z, Jin Z, Zhang L, Pei Y (2013) Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by microRNA expressions. PLoS One 8:e77047
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135
Shi H, Ye T, Zhu JK, Chan Z (2014) Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J Exp Bot 65:4119–4131
Siddiqui MH, Alamri SA, Al-Khaishany MY, Al-Qutami MA, Ali HM, Hala AR, Kalaji HM (2017) Exogenous application of nitric oxide and spermidine reduces the negative effects of salt stress on tomato. Hort Environ Biotechnol 58:537–547
Simontacchi M, Galatro A, Ramos-Artuso F, Santa-Maria GE (2015) Plant survival in a changing environment: the role of nitric oxide in plant responses to abiotic stress. Front Plant Sci 6:977
Singh B, Usha K (2003) Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul 39:137–141
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Szepesi Á (2005) Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biol Szeg 49:123–125
Szepesi Á, Csiszár J, Gallé Á, Gémes K, Poór P, Tari I (2008a) Effect of long-term salicylic acid pre-treatment on tomato (Lycopersicon esculentum mill. L.) salt stress tolerance: changes in glutathione S-transferase activities and antocyanin contents. Acta Agron Hung 56:129–138
Szepesi Á, Poór P, Gémes K, Horváth E, Tari I (2008b) Influence of exogenous salicylic acid on antioxidant enzyme activities in the roots of salt stressed tomato plants. Acta Biol Szeg 52:199–200
Tahjib-Ul-Arif M, Siddiqui MN, Sohag AAM, Sakil MA, Rahman MM, Polash MAS, Tran LSP (2018) Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J Plant Growth Regul 37:1318–1330
Tang Y, Sun X, Wen T, Liu M, Yang M, Chen X (2017) Implications of terminal oxidase function in regulation of salicylic acid on soybean seedling photosynthetic performance under water stress. Plant Physiol Biochem 112:19–28
Tang H, Niu L, Wei J, Chen X, Chen Y (2019) Phosphorus limitation improved salt tolerance in maize through tissue mass density increase, Osmolytes accumulation, and Na+ uptake inhibition. Front Plant Sci 10:856. https://doi.org/10.3389/fpls.2019.00856
Tanou G, Molassiotis A, Diamantidis G (2009) Hydrogen peroxide- and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J Plant Physiol 166:1904–1913
Tari I (2002) Acclimation of tomato plants to salinity stress after a salicylic acid pretreatment. Acta Biol Szeg 46:55–56
Tari I, Simon LM, Deér KA, Csiszár J, Bajkán SZ, Kis GY, Szepesi A (2004) Influence of salicylic acid on salt stress acclimation of tomato plants: oxidative stress responses and osmotic adaptation. Acta Physiol Plant 26:237
Tari I, Kiss G, Deer AK, Csiszár J, Erdei L, Gallé Á, Simon LM (2010) Salicylic acid increased aldose reductase activity and sorbitol accumulation in tomato plants under salt stress. Biol Plant 54:677–683
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–507
Thornton PK, Ericksen PJ, Herrero M, Challinor AJ (2014) Climate variability and vulnerability to climate change: a review. Glob Chang Biol 20:3313–3328
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant 50:775–778
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL (2007) The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with pseudomonas syringae pv. Tomato DC3000. Mol Plant-Mic Interac 20:955–965
Wahid A, Perveen M, Gelani S, Basra SM (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Wang LJ, Li SL (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170:685–694
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Wang Y, Mopper S, Hasenstein KH (2001) Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J Chem Ecol 27:327–342
Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, Cheng JS (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:34–40
Wang GF, Seabolt S, Hamdoun S, Ng G, Park J, Lu H (2011) Multiple roles of WIN3 in regulating disease resistance, cell death, and flowering time in Arabidopsis. Plant Physiol 156:1508–1519
Wang H, Yuan X, Wu Y, Huang H, Zeng G, Liu Y, Wang X, Lin N, Qi Y (2013) Adsorption characteristics and behaviors of graphene oxide for Zn (II) removal from aqueous solution. Appl Surf Sci 279:432–440
Wang Y, Wen T, Huang Y, Guan Y, Hu J (2018) Salicylic acid biosynthesis inhibitors increase chilling injury to maize (Zea mays L.) seedlings. Plant Growth Regul 86:11–21
Wani W, Masoodi KZ, Zaid A, Wani SH, Shah F, Meena VS, Mosa KA (2018) Engineering plants for heavy metal stress tolerance. Rend Lin Sci Fis e Nat 29:709–723
Weast RC (1984) CRC handbook of chemistry and physics, 64th edn. CRC Press, Boca Raton
Xing H, Tan L, An L, Zhao Z, Wang S, Zhang C (2004) Evidence for the involvement of nitric oxide and reactive oxygen species in osmotic stress tolerance of wheat seedlings: inverse correlation between leaf abscisic acid accumulation and leaf water loss. Plant Growth Regul 42:61–68
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Xu J, Wang W, Sun J, Zhang Y, Ge Q, Du L, Liu X (2011) Involvement of auxin and nitric oxide in plant cd-stress responses. Plant Soil 346:107
Yang ZM, Wang J, Wang SH, Xu LL (2003) Salicylic acid-induced aluminium tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 217:168–174
Yang Z, Cao S, Zheng Y, Jiang Y (2012) Combined salicyclic acid and ultrasound treatments for reducing the chilling injury on peach fruit. J Agr food Chem 60:1209–1212
Yang L, Ji J, Harris-Shultz KR, Wang H, Wang H, Abd-Allah EF, Luo Y, Hu X (2016) The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in Rice subjected to heavy metal cadmium stress. Front Plant Sci 7:190. https://doi.org/10.3389/fpls.2016.00190
Ye S, Zeng G, Wu H, Zhang C, Dai J, Liang J, Yu J, Ren X, Yi H, Cheng M, Zhang C (2017) Biological technologies for the remediation of co-contaminated soil. Crit Rev Biotechnol 37:1062–1076
Yordanov I, Velikova V, Tsone V (2000) Plant response to drought, acclimation and stress tolerance. Photosynthetica 30:171–186
Yu CC, Hung KT, Kao CH (2005) Nitric oxide reduces cu toxicity and cu-induced NH4+ accumulation in rice leaves. J Plant Physiol 162:1319–1330
Yuan S, Lin HH (2008) Role of salicylic acid in plant abiotic stress. Zeits für Nat 63:313–320
Yuan S, Shen X, Kevil CG (2017) Beyond a Gasotransmitter: hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antiox Redox Signal 27:634–653
Zaid A, Mohammad F, Wani SH, Siddique KM (2019) Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol Environ Saf 180:575–587
Zanganeh R, Jamei R, Rahmani F (2018) Impacts of seed priming with salicylic acid and sodium hydrosulfide on possible metabolic pathway of two amino acids in maize plant under lead stress. Mol Biol Res Comm 7:83
Zhang WN, Chen WL (2011) Role of salicylic acid in alleviating photochemical damage and autophagic cell death induction of cadmium stress in Arabidopsis thaliana. Photochem Photobiol Sci 10:947–955
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529
Zhang H, Wang MJ, Hu LY, Wang SH, Hu KD, Bao LJ, Luo JP (2010a) Hydrogen sulfide promotes wheat seed germination under osmotic stress. Russ J Plant Physiol 57(4):532–539
Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL (2010b) Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integ Plant Biol 52:556–567
Zhang F, Zhang H, Xia Y, Wang G, Xu L, Shen Z (2011) Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep 30:1475–1483
Zhao HJ, Lin XW, Shi HZ, Chang SM (1995) The regulating effect of phenolic compounds on the physiological characteristics and yield of soybeans. Acta Agron Sin 21:351–355
Zhao Z, Chen G, Zhang C (2001) Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Func Plant Biol 28:1055–1061
Zhao L, Zhang F, Guo J, Yang Y, Li B, Zhang L (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134:849–857
Zhao MG, Chen L, Zhang LL, Zhang WH (2009) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151:755–767
Zhou C, Lai C, Zhang C, Zeng G, Huang D, Cheng M, Hu L, Xiong W, Chen M, Wang J, Yang Y, Jiang L (2018) Semiconductor/boron nitride composites: synthesis, properties, and photocatalysis applications. App Cata B 238:6–18
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhu M, Dai S, Chen S (2012a) The stomata frontline of plant interaction with the environment perspectives from hormone regulation. Front Biol 7:96–112
Zhu XF, Jiang T, Wang ZW et al (2012b) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater 239:302–307
Acknowledgements
We are thankful to the host institution Aligarh Muslim University, Aligarh 202002, India and our workplace at Plant Physiology and Biochemistry Section, Department of Botany, Aligarh Muslim University, Aligarh for providing us seat to work. We apologize to those authors whose work could not be cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sadiq, Y., Zaid, A., Khan, M.M.A. (2020). Adaptive Physiological Responses of Plants under Abiotic Stresses: Role of Phytohormones. In: Hasanuzzaman, M. (eds) Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I. Springer, Singapore. https://doi.org/10.1007/978-981-15-2156-0_28
Download citation
DOI: https://doi.org/10.1007/978-981-15-2156-0_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2155-3
Online ISBN: 978-981-15-2156-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)