Abstract
Plants face different types of biotic and abiotic stresses during their life span. Heavy metal (HM) stress is considered as one of the most challenging and emerging threats to sustainable agricultural development and overall economic yield of various plant species. Increasing levels of HMs in arable soils is a main environmental issue due to their deleterious effects on plant growth and productivity. The exogenous application of different plant growth regulators is a well-known strategy to alleviate the adverse effects of HMs stress on plants. In the present review, the role of 5-aminolevulinic acid (ALA) in the alleviation of HM stress in different plants is elaborated. 5-Aminolevulinic acid is identified as a highly efficient ameliorating agent to sustainably neutralize the harmful effects of abiotic stresses in plants. In particular, the role of ALA has been increasingly recognized in improving plant HM stress-tolerance via ALA-mediated control of principal plant-metabolic processes. However, various underlying mechanisms that unravel ALA-induced plant HM stress-tolerance remain unexplored. The application of ALA on HM-stressed plants improves plant height, root length, chlorophyll pigments, antioxidant enzyme activities, nutrient uptake and soluble protein contents and minimizes ultra-structural damage, oxidative stress and HM uptake. Furthermore, it triggers modification of glutathione reductase, ascorbic acid and GSH contents in HM-stressed plants. The lower concentration of ALA proved to be more beneficial in stress amelioration. The cost-effectiveness and efficiency of ALA in improving growth and production of plants under varying growth conditions is still not clear. Nevertheless, over-accumulation of ALA through genetic manipulation can enhance stress-tolerance in plants which is the key area to be investigated. This review article elaborates the potential role of ALA in HM tolerance and highlights the future research dimensions in the related ambits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The group of elements in the periodic table having densities greater than 5 g/cm3 are regarded as heavy metals (HMs) (Alloway 1995; Singh et al. 2016). Different anthropogenic activities are the major source of HM contamination in soil and water bodies thus causing serious problems for living organisms (Rouphael et al. 2008; Rizwan et al. 2016; Qayyum et al. 2017). The most widespread anthropogenic sources of HMs include industries, indiscriminate utilization of agricultural inputs, metalliferous mining and smelting, military warfare and trainings, sewage sludge disposal, automobile emissions, and open waste dumping (Alloway 1995; Shi et al. 2005; Rehman et al. 2015; Rizwan et al. 2017). Plants take up and accumulate HM ions from the surrounding environment. The accumulation of HMs induces toxic effects on plant growth attributes as well as on consumer health (Stobrawa and Lorenc-Plucinska 2008; Jaishankar et al. 2014; Afshan et al. 2015; Abbas et al. 2017). According to the U.S. Environmental Action Group, the toxicity caused by various HMs is one of the major reasons affecting health of more than 10 million people and posing serious threats to sustainable agricultural development in eight countries such as China, Russia, Ukraine, India, Dominican Republic, Kyrgyzstan, Peru, and Zambia (Khan et al. 2015). The exposure to low doses of HMs for a long period of time disturbs plant functions and human health (Fu et al. 2008; Adrees et al. 2015a; Jabeen et al. 2016; Rehman et al. 2017). A number of reviews have been surveyed to observe the effects of HMs on plant growth and development (Babula et al. 2009; Adrees et al. 2015b; Singh et al. 2016; Farid et al. 2018; Rizwan et al. 2018). HMs may exist in dissolved or suspended form. The suspended form is more harmful to plants as compared to the dissolved form (Alloway 1995; Keller et al. 2015). Metals such as cadmium (Cd), copper (Cu), molybdenum (Mo), nickel (Ni), lead (Pb), zinc (Zn), and beryllium (Be) are more detrimental to crops as compared to manganese (Mn), iron (Fe), arsenic (As), and mercury (Hg) (Ali et al. 2018). Some HMs (Cu, Mo, Mn, and Zn) at lower concentrations serve as vital nutrients to plants and animals but are toxic at higher levels (Jarup 2003; Azevedo and Lea 2005; Rehman et al. 2016).
Plant growth regulators (PGRs) play important regulatory roles in signaling networks, developmental processes and engineering tolerance in plants either directly or indirectly to an array of biotic and abiotic environmental stresses (Ahmad et al. 2018; Bali et al. 2018; Jan et al. 2018; Wani et al. 2016; Handa et al. 2018). 5-Aminolevulinicacid (ALA) is a white to off white, odorless and water soluble crystalline solid PGR, having a molecular weight of 167.59 g/mol. The chemical name for ALA is 5-amino-4 oxypentanoic acid hydrochloride with structural formula C5H9NO3HCl (Fig. 1). ALA is the main predecessor in the biosynthesis of porphyrins such as, chlorophyll and hemeA glutamyltRNA intermediates, in plants. ATP and NADPH as cofactor react to synthesize ALA from glutamate which, at lower concentration helps in chlorophyll biosynthesis (Castelfranco et al. 1974; Beale 1990; Hotta et al. 1997b). ALA has both growth enhancing and stress-tolerance abilities. It has been observed that ALA at lower concentrations improves plant growth and yield (Hotta et al. 1997a, b; Roy and Vivekanandan 1998; Watanabe et al. 2000). An increase in chlorophyll content and photosynthetic ability was also noted at lower concentrations of ALA. ALA strengthens plants against abiotic stress by increased enzymatic activities or by improving the antioxidant defense system that protects the plant membrane system against reactive oxygen species (Korkmaz 2012). It also promotes vegetative expansion (Tanaka and Tanaka 2011; Naeem et al. 2010; Zavgorodnyaya et al. 1997) and helps the plants against harmful effects of different abiotic stresses including HMs. It is observed that enzymes involved in the production and breakdown of ALA play an important role in chlorophyll synthesis (Tanaka et al. 1992; Bogorad 1967). ALA not only stimulates plant development but also enhances yield through improvement in carbon dioxide fixation and nitrogen assimilation (Tanaka et al. 1992). Other notable changes include vegetation greenness (Maruyama-Nakashita et al. 2010), improved rate of photosynthesis (Akram and Ashraf 2011b; Hotta et al. 1997b), enhanced chlorophyll activity (Tanaka and Tanaka 2007, 2011; Naeem et al. 2010), and better functioning of nitrite reductase (Tanaka and Tanaka 2007; Mishra and Srivastava 1983). Moreover, ALA was effective in terms of promoting plant growth and development at lower concentrations (Chakraborty and Tripathy 1990; Balestrasse et al. 2010). On the contrary, ALA at higher concentrations promotes increased production of reactive oxygen species (ROS), which cause oxidative stress in plants (Pattanayak and Tripathy 2011; Balestrasse et al. 2010). However, the underlying mechanisms of stress-tolerance and the extent of crop yield improvement by ALA is not fully exposed yet. Considering the importance of ALA, the present review was accomplished to understand the cost-effectiveness and efficacy of ALA-mediated improvement in plant growth and development under different HM stress conditions (Fig. 2).

Reprinted with permission from (Akram and Ashraf 2013)
Chemical structure of ALA. (
Plant Response to Environmental Stresses
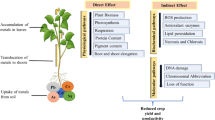
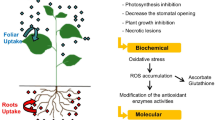
Different biotic and abiotic stresses (HM, drought, salinity, temperature, pathogen and pest) lead to physiological, morphological, biochemical, and molecular changes in plants. HMs, temperature, salinity, and drought stimulate cellular damage by orchestrating the rate of reactive oxygen species (ROS) production. ROS are produced in the apoplast of plant cells via NADPH oxidases and accumulate in the mitochondria, chloroplasts and even in the nucleus (Van Breusegem et al. 2008; Czarnocka and Karpiński 2018) as demonstrated in Fig. 3. HM stress, like drought and salinity, leads to distraction of ion circulation, the photosynthetic mechanism, and growth of the cells (Rucinska-Sobkowiak 2016). Oxidative stresses which normally harmonize with drought, salinity, and temperature can cause protein denaturation resulting in altered configurations and functions. Consequently, these adverse environmental stresses stimulate identical cell signaling corridors and cellular reactions. The biochemistry of the cells is changed during biochemical alteration in plants. These adaptations give rise to metabolic pathways in the form of lower molecular weight metabolites, generation of particular proteins, a mechanism of toxicity removal, and variations in phytohormone levels. The aptitude of living organisms to cope with varying environmental conditions enhances their endurance and reproduction capacity (Fujita et al. 2006; Smirnoff 1995).
HMs are taken up by the plants from the external environment via specific transporters (Fig. 3) like zinc–iron permease (ZIP), heavy metal transport ATPase (CPxandP1B-ATPase), natural resistant associated macrophage protein (NRAMP), cation diffusion facilitator (CDF), and ATP-binding cassette (ABC) transporters which are either present at the plasma membrane or vacuolar membranes (Park et al. 2012). In addition to transporters, there are cysteine-rich metal binding peptides like phytochelatins (PCs) or metallothionines (MTs) and glutathione (GSH) which are also important players in the metal transport system in plants (Jalmi et al. 2018) (Fig. 3). Plants require both essential metals (micronutrients) and metalloids (B, Cu, Fe, Mn, Mo, Ni, and Zn) for appropriate metabolic and physiological system functioning in optimal and suboptimal environmental conditions. However, HMs that are essential for growth and metabolism of plants cause toxic effects on metabolic pathways at higher concentrations. The HMs exert toxicity by overcrowding of functional groups like enzymes, nutrients and the ion transport system or substitution of important ions on cellular sites, enzyme denaturing, and distraction of cell reliability. HMs also impose toxicity by free radical formation, that is, ROS overproduction (Smirnoff 1995).
Importance of ALA in Plant Growth Constraints
In recent years, the use of plant growth regulators to mitigate HM stress has been regarded as a good strategy for sustainable agriculture production (Sakpirom et al. 2018). 5-Aminolevulinic acid has great importance in many physiological and biochemical processes in plants (Yossouf and Awad 2008; Tanaka et al. 1992; Beale and Castelfranco 1974; Sasaki et al. 2002; Korkmaz et al. 2010; Balestrasse et al. 2010; Li et al. 2011). A number of studies have shown that ALA enhanced plant growth by increasing chlorophyll levels and photosynthesis rates in different plants like rice (Hotta et al. 1997a), barley, garlic, potato (Tanaka et al. 1992), and date palm (Phoenix dactylefera) (Al-Khateeb et al. 2006). ALA has also been observed to improve the carbon to nitrogen ratio (Maruyama-Nakashita et al. 2010). ALA at a higher concentration (≥ 5 mM) also showed herbicidal effect on plants (Kumar et al. 1999; Tanaka and Tanaka 2007). Normally, antioxidants (enzymatic and non-enzymatic) have extensive roles in promoting plant growth by controlling the production of reactive oxygen species (ROS) during stress and non-stress conditions (Shahbaz and Ashraf 2008; Mittler 2002; Ashraf and Akram 2009; Perveen et al. 2010, 2011, 2012; Akram et al. 2012). ALA also plays an important role in the plant antioxidant defense system (Xu et al. 2009; Akram et al. 2012; Memon et al. 2009). It was observed that ALA helps to enhance antioxidant enzyme activities like peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) in pakchoi (Brassica campestris) leaves. Moreover, an increase in chlorophyll content and photosynthetic rate was also recorded in Brassica campestris (Memon et al. 2009). ALA at lower concentrations stimulated the production of antioxidant enzymes such as POD, CAT, ascorbate peroxidase (APX) and SOD in Ginkgo biloba (Xu et al. 2009). The elevated levels of non-enzymatic antioxidants like glutathione (GSH) have also been observed under ALA treatment. However, a decline in dehydroascorbate (DHA) and no alteration in GSSG level were also evident in Ginkgo biloba as a result of ALA treatment. According to these observations, ALA also behaves as a light capturing pigment (Senge 1993; Burnham and Lascelles 1963; Sano and Granick 1961). Reports indicate that ALA has an important role in mineral nutrient uptake and transformation in kudzu (Pueraria phaseoloides). Substantial improvements in kudzu dry biomass, chlorophyll content, photosynthetic rate, stomatal conductance, starch, nutrients, and sugar levels were observed as a result of ALA application (Xu et al. 2010). Awad (2008) reported that ALA appreciably enhanced photosynthetic pigments and the chlorophyll a/b ratio in date palm.
Significance of ALA against Abiotic Stress
ALA application is also used to mitigate the effects of abiotic stresses on plants. Induction of temperature stress-tolerance has also been reported in rice, potato and soybean with the application of ALA (Balestrasse et al. 2010; Hotta et al. 1998). ALA treatment under reduced light conditions enhanced chilling resistance by controlling constraints in chlorophyll biosynthesis, photosynthesis and respiration (Wang et al. 2004). ALA applications improved grain yield of wheat (Al-Thabet 2006) and barley under drought stress (Al-Khateeb et al. 2006). Its applications also reduced salt stress in crops like date palm (Youssef and Awad 2008), oilseed rape (Naeem et al. 2010), potato (Zhang et al. 2006), pakchoi (Wang et al. 2005), and spinach (Nishihara et al. 2003). The ALA treatment controls many physiological processes related to plant growth under salinity stress like seed germination, photosynthesis, and absorption of nutrients (Hotta et al. 1997a, b; Youssef and Awad 2008). ALA application also enhanced photosynthetic activity in cucumber under salt stress (Wu et al. 2018). The application of ALA not only enhanced nutrient uptake in plants under stressed and non-stressed conditions (Akram et al. 2018) but also improved the antioxidant defense system under chilling stress (Liu et al. 2018).
ALA as Stress-Tolerance Agent
ALA has the ability to mitigate harmful effects of stress on plant growth and production (Akram et al. 2012). However, the physiological mechanism of stress alleviation by ALA still remains unclear. Different abiotic stresses such as salinity, temperature, drought and heavy metals, and so on, modify the ALA status in plants (Hodgins and van Huystee 1986; Cornah et al. 2003; Genisel et al. 2017). However, it is not totally obvious which metabolic process is disturbed in plants with modified ALA status.
ALA Against Heavy Metal Stress
Plants under HM stress experience different harmful effects. ALA acts as a plant growth regulator and resists the toxic effects of heavy metals. The ameliorative role of ALA under HM stress in plants is discussed below (Table 1).
Plant Growth, Biomass, and Chlorophyll Contents
Usually, HMs at higher concentrations disturb plant growth attributes, biomass and chlorophyll contents. A considerable decline has been reported in plant height, root length, number of leaves, dry and fresh weight of roots, stem and leaves, and root morphological parameters like root diameter, root tips, root surface area, and root volume of Brassica napus at higher Cd concentrations. However, ALA application at the 25 mg/L concentration significantly mitigated the adverse effects of Cd and appreciably improved all the above-mentioned plant growth attributes (Ali et al. 2013a, b; Xu et al. 2015). ALA application under Cd stress extensively enhanced chlorophyll a, b and total chlorophyll and considerably reduced metal concentrations in shoots and roots of B. napus (Ali et al. 2013a). In a recently reported study, sunflower plants under Cr stress had significantly reduced plant growth attributes and photosynthetic pigments. The application of ALA markedly enhanced plant growth under Cr stress (Farid et al. 2018). The application of ALA in cauliflower enhanced plant growth, photosynthetic pigments, and antioxidant enzymes activities under Cr stress (Ahmad et al. 2017). Higher concentrations of Pb also extensively reduced the fresh and dry weight of Brassica napus leaf, stem and roots. However, ALA application alleviated the toxic effects of Pb and enhanced plant biomass under Pb stress. ALA application without Pb stress showed no alteration in plant biomass (Ali et al. 2014). Tian et al. (2014) reported that combined application of ALA and Pb promoted root surface area, root diameter, root volume, number of root tips and total chlorophyll content as compared to Pb treatment only. Lead considerably reduced chlorophyll contents in B. napus. However, ALA application under Pb stress showed no changes in chlorophyll contents (Ali et al. 2014). By increasing the concentration of ALA Xiaomenget al. (2018) reported an increase in chlorophyll a/b in Matricaria recutita plants. The possible reason for enhancement in plant growth might be due to the potential of ALA to resist HM-induced stress and reduce lipid peroxidation (Youssef and Awad 2008) by triggering the heme-based antioxidant system to scavenge overproduced ROS (Nishihara et al. 2003). The application of ALA confers tolerance against metal stress by reducing the uptake of toxic metals by the plant, improving the nutrient level, photosynthetic pigments and upholding of the photosynthetic mechanism. Generally, ALA has a promotive role in regulating a number of metabolic processes and improves plant growth and yield under HM toxicity.
Variations in Photosynthetic Traits and Carotenoid Contents
It is well documented that photosynthetic traits like stomatal conductance, net photosynthesis and transpiration rate decline under Cd stress. However, application of ALA, with or without Cd stress, shows improvement in photosynthetic traits. The ameliorative effect of ALA under Cd stress has been observed at the 25 mg/L concentration (Ali et al. 2013a). The combination of ALA and Pb also enhanced stomatal conductance, net photosynthetic rate and transpiration rate in oilseed rape (Tian et al. 2014). The above-mentioned results indicate the positive impact of ALA on photosynthetic traits under HM stress.
Effects on Malondialdehyde and Reactive Oxygen Species
Malondialdehyde (MDA) is a signal molecule of free radicals in plant tissues. Its concentration may vary under varying environmental stresses. The MDA contents in roots were higher under Cd stress whereas ALA application with Cd stress extensively reduced MDA production. Minimum production of MDA was observed at 25 mg/L of ALA (Ali et al. 2013b, 2015). Cadmium stress enhanced the production of ROS in oilseed rape (Brassica napus L.). ALA at 25 mg/L reduced H2O2, O2 and OH contents under Cd stress (Ali et al. 2013b). However, ALA application without Cd stress showed no decrease in MDA and ROS production in the leaves and roots of B. napus. Like Cd, Pb stress also enhanced MDA contents in oilseed rape. However, ALA application with Pb stress considerably declined MDA contents in the leaves and roots of B. napus. Lead at higher concentrations alone increased ROS production. ALA application with 100 and 400 µM concentrations of Pb reduced ROS contents in the leaves and roots of B. napus (Ali et al. 2014). The above reports showed that MDA and ROS generation was increased under HM stress. However, ALA application reduced MDA and ROS generation and conferred resistance against HM stress by improving antioxidant enzyme activity.
Changes in Antioxidant Enzymes and Total Soluble Protein Content
The HMs like Cd without ALA application reduced SOD and POD activities in B. napus. However, combined application of ALA and Cd significantly enhanced SOD and POD activities. Application of ALA (25 mg/L) alone showed momentous alteration in APX activity. But combined application of ALA and Cd treatments enhanced APX and CAT activities in B. napus (Ali et al. 2013b). Contrary to that, increasing Pb concentrations without ALA application enhanced POD and declined SOD activity. ALA applications mitigated the negative effect of Pb. The ALA application under Pb stress promoted SOD activity in the leaves and roots of B. napus. ALA at higher Pb concentrations showed no change in POD activity. It was observed that Pb at 100 and 400 µM concentrations declined APX activity in the leaves and roots of plants. However, ALA application under Pb stress enhanced APX activity in the leaves and roots of B. napus. Increasing Pb concentrations enhanced CAT activity in the roots and declined in the leaves of B. napus. Reports showed that ALA application had no momentous effect on CAT activity under Pb stress in the leaves and roots of B. napus. ALA application without metal stress showed no appreciable effects on antioxidant enzyme activities (Ali et al. 2014). Higher Pb concentrations reduced total soluble protein extensively in the leaves and roots of B. napus. The ALA application under Pb stress enhanced total soluble proteins in the roots, whereas no change was observed in the leaves of B. napus (Ali et al. 2014). Ali et al. (2013b) investigated that ALA and Cd applications, alone or in combination, showed no impact on total soluble protein in B. napus roots. From the above reports, it was found that ALA application in combination with HMs enhanced the activity of antioxidant enzymes. It is assumed that plants under ALA application might be more efficient at neutralizing excess ROS.
Alteration in Nutrients Uptake
Lead stress enhances nitrogen concentrations in plant leaves as compared with roots. ALA application under Pb stress reduces Na+ uptake in plant roots. ALA application without metal stress considerably increased Ca2+ uptake in B. napus leaves. Combined application of ALA and Pb extensively enhanced potassium and calcium ions in leaves and roots, and magnesium in leaves of B. napus (Ali et al. 2014). Application of Pb considerably decreased micro and macronutrients in the leaves and roots of B. napus but at lower concentrations showed no noticeable change in Cu and Zn in leaves or Cu and S in roots. The ALA application without metal stress showed no alteration in nutrient concentrations. However, ALA application alone also reduced Fe in leaves but Zn and P in roots of B. napus. The ALA along with Pb applications notably enhanced the macronutrients N, P, and S concentrations in the leaves and roots of B. napus. Similarly, Zn concentration was enhanced in the roots of B. napus under ALA and Pb combined application. The ALA application with lower concentrations of Pb improved Fe content in B. napus roots (Ali et al. 2014). The above results elucidate that HM stress disrupts nutrient uptake dynamics in plants. However, ALA application mitigates the toxic effects of HMs and enhances the plant’s ability to accumulate micro and macronutrients to maintain optimum nutrient assimilation.
Modification in Glutathione Reductase (GR), Ascorbic Acid (AsA) and GSH Contents
Reports showed that Pb at lower and higher concentrations without ALA application declined GR activity in plant roots. However ALA application under Pb stress showed no considerable effect on GR activity in the leaves of B. napus. Higher Pb concentration alone notably decreased AsA contents in the leaves and roots of B. napus. The ALA application significantly mitigated Pb toxicity and promoted AsA contents in the roots of B. napus (Ali et al. 2014). AsA demonstrated no considerable change under ALA application alone. While at 25 mg/L of ALA dosage, appreciable enhancement in AsA content was noted (Ali et al. 2013b). Higher concentrations of Pb declined GSH and GSSG contents in the leaves. The ALA application with Pb stress enhanced GSH content in the leaves and roots of B. napus. No change in GSH/GSSG ratio of leaves and roots was observed under Pb stress. Nonetheless, ALA application under Pb stress showed a promotive effect on the total glutathione contents (Ali et al. 2014). Cadmium stress showed a steady decline in glutathione contents. ALA at the 25 mg/L concentration slowly enhanced GSH contents under higher Cd concentrations (Ali et al. 2013b). From the above discussion it is clear that ALA application under metal stress enhanced GSH, AsA, and total glutathione content. The ameliorative effect of ALA might be due to enhanced resistive ability of plants against ROS that triggers the production of GSH and total glutathione.
Effect on Metal Uptake
ALA application reduced Pb uptake in shoots and roots of B. napus. ALA application at 25 mg/L decreased Pb uptake in shoots and roots at higher Pb concentration (Tian et al. 2014). Report showed that ALA mitigates the toxic effects of metals by improving plants resistance against HM stress.
Ultra-structural Changes
Higher Pb concentration totally injured mesophyll cells and reduced intercellular spaces. Lead at a higher concentration also showed harmful effects on chloroplast thylakoid membranes. ALA application alleviated the damaging effect of Pb on leaf ultrastructure in rapeseed plant. ALA at 25 mg/L showed appreciable changes in root cell tips under Pb stress (Tian et al. 2014). ALA application under HM stress improves antioxidant enzyme activity and reduces ROS production, avoiding ultra-structural damage in plant roots as documented by Castelfranco and Jones (1975).
ALA Application Methods
Different approaches of ALA application at various concentrations play a considerable part in the plant physio-chemical attributes under stress conditions (Youssef and Awad 2008; Hotta et al. 1997a, b; Akram and Ashraf 2011a, b; Naeem et al. 2010; Tanaka and Tanaka 2011). The following are the different modes of ALA applications as plant growth promoter.
Foliar Method
One of the methods used to enable plants to remain healthy against stressful conditions is the foliar application of ALA, which was found helpful in enhancing growth and related attributes of various plants.
Non-stress Condition
Foliar application of ALA (50–250 mg/L) in pakchoi plants increased photosynthetic rates. Antioxidant enzyme activities and total chlorophyll contents were improved by ALA under normal conditions (Memon et al. 2009). An improvement in plant biomass, photosynthetic rate, chlorophyll contents, nutrient level and stomatal conductance were also observed by foliar application of ALA under a non-stressed environment in kudzu plants (Xu et al. 2010). Foliar ALA application increased antioxidant enzyme activities, GSH and H2O2 under normal conditions in the Ginkgo biloba plant (Xu et al. 2009). ALA foliar application also enhanced chlorophyll content in date palm (Al-Khateeb et al. 2006). The above reports showed that foliar applications of ALA at lower concentrations considerably promoted plant growth attributes in terms of photosynthetic traits, nutrients, antioxidant enzyme activity, and chlorophyll content under no stress conditions.
Stress Condition
Foliar application of ALA under drought stress conditions improved plant growth traits and yield in wheat (Al-Thabet 2006). Under cold stress, foliar application of ALA enhanced plant biomass, proline, and chlorophyll contents in pepper. Low temperature stress also enhanced SOD activity in pepper (Korkmaz et al. 2010). ALA also induced tolerance against chilling stress by improving photosynthetic attributes, chlorophyll b content and stomatal opening in melon (Cucumis melo) plants (Wang et al. 2004). Foliar application of ALA under heat stress enhanced plant growth traits by reducing MDA content, superoxide radicals (O2−), and H2O2 in cucumber plants (Zhen et al. 2012). Salt stress in spinach enhanced antioxidant enzyme activities and photosynthetic traits under ALA foliar application (Nishihara et al. 2001, 2003). Foliar application of ALA at 20–80 mg/L enhanced plant growth attributes in sunflower (Akram and Ashraf 2011a).
The above reports showed the prospective advantages of foliar applied ALA in mitigating the harmful effects of unfavorable environments in different plants. Reports also suggested that foliar application of ALA might have a great effect at the early growing stages like germination and seedling establishment.
Seed Treatment Before Sowing
Seed treatment with ALA before sowing enhances plant development in addition to physiological attributes under stressed and non-stressed conditions (Korkmaz and Korkmaz 2009; Wang et al. 2005; Korkmaz et al. 2010).
Non-stressed Condition
An improvement in plant growth traits, chlorophyll content, photosynthetic attributes and yield was noticed without stress conditions in seed treatment of Vigna species (Roy and Vivekanandan 1998). Pre-sowing treatment of rice and horseradish seeds with ALA, enhanced plant growth traits, total chlorophyll content, and yield under normal conditions (Hotta et al. 1997a).
Stress Condition
Reports showed that pre-sowing seed treatment with ALA application under cold stress improved the germination rate in pepper (Korkmaz and Korkmaz 2009). Pre-sowing seed treatment with ALA enhanced seed germination under salinity stress in pakchoi (Wang et al. 2005). The ALA seed treatment promoted photosynthetic ability, antioxidant enzyme activity, and chlorophyll contents under low light conditions in watermelon (Sun et al. 2009). The above reports suggested that seed priming with ALA appreciably stimulated seed germination, plant growth parameters, the antioxidant defense system, and improved yield in different plant species under stress and normal conditions. Practically, seed priming with ALA could be used as a valuable tool to promote plant growth and production under different stresses.
Root Medium
Soil application of ALA has been reported to promote plant growth attributes and production under stressed and normal conditions.
Non-stressed Condition
An enhancement in chlorophyll a and total chlorophyll content in date palm was noted under normal growth conditions and ALA as a Pentakeep fertilizer (Awad 2008). Yoshida et al. (2005) noted improvement in tulip bulbs under normal conditions at 2000–5000 times dilution of Pentakeep.
Stress Condition
Under cold stress, ALA applied in the root medium of soybean enhanced chlorophyll and antioxidant enzyme activity (Balestrasse et al. 2010). The ALA treatment enhanced photosynthetic traits, stomatal conductance and chlorophyll content in Cucumis melo (Wang et al. 2004). Under salinity stress, ALA treatment promotes tuber formation in potato (Zhang et al. 2006). ALA application under cold stress enhances rice dry weight and seedling growth (Balestrasse et al. 2010). Similarly, ALA application enhanced photosynthetic traits, chlorophyll contents, stomatal conductance, and respiration rate under cold stress in melon (Wang et al. 2004). ALA treatments through the roots neutralize salinity stress and enhance antioxidant enzymes activities in cucumber (Zhen et al. 2012). The above reports suggest that ALA application in the plant growth medium under different stresses could mitigate the toxic effects by improving plant physio-chemical traits. ALA addition in the soil medium is not economical as a large amount of soil is required for this purpose. Hence, further study is needed to modify the effectiveness of ALA in the soil medium to suppress undesirable effects of environmental stresses.
Assessment of ALA Application Methods
All the above-mentioned methods were reported to be beneficial in improving plant growth attributes and physiological processes (Fig. 4). ALA is an expensive compound and present in plants in minute quantities. So application methods that require large quantities of ALA are not cost-effective. Foliar application usually depends on the leaf and plant growth stage (Akram et al. 2012; Ashraf and Foolad 2007; Ashraf et al. 2010a). Chlorophyll production or its use is more dynamic at the early developmental stages in plants (Xu et al. 2010; Memon et al. 2009; Naeem et al. 2010; Tanaka and Tanaka 2011). Therefore, the application of ALA as a foliar spray appreciably affects early growth stages like germination and seedling establishment. ALA at a low dosage (1–100 mg L−1) promotes plant growth and yield while at higher concentration (≥ 150 mg L−1) causes inhibitory effects on plants (Tanaka and Tanaka 2007; Sasaki et al. 2002).
Genetic Treatment
Crop resistance against environmental stresses by genetic manipulation is one of the concerned issues being discussed nowadays. Numerous plants have been developed for improvement in stress resistance by enhancing hormone production, organic osmolytes, antioxidants and nutrients (Mittler 2002; Yamaguchi and Blumwald 2005; Ashraf and Akram 2009; Ashraf et al. 2010a; Munns and Tester 2008). ALA application also enhanced the plant’s ability to resist environmental stress. The application of ALA synthase in fungi enhanced the ALA level in this organism as documented by Elrod et al. (2000). Fewer reports are available regarding stress resistance in plants and their ALA accumulation level. Jung et al. (2004) reported the addition of the Bradyrhizobium japonicum aminolevulinic acid synthase (ALA-S) gene in rice. Synthesis of ALA was enhanced significantly causing improvement in chlorophyll content. The authors conclude that ALA is the main precursor in chlorophyll biosynthesis. Increase in ALA-mediated chlorophyll biosynthesis can lead to enhanced net photosynthesis. Endogenous ALA biosynthesis can also optimize different growth and developmental processes in plants. The above reports showed enhancement in ALA production by genetic manipulation of plants which could be exploited as a tool to impart ALA-mediated HM stress-tolerance in plants (Fig. 5).
Conclusions and Future Prospects
In this review, we intended to investigate ALA effects on plant growth traits and the mechanisms involved in plant promoting processes under HM stress. A detailed review from the available literature showed that HM stress restricts plant growth and productivity, posing a threat to food security for the ever-increasing population. ALA has the potential to reduce HM stress and enhance plant growth attributes and yield. It also reduces HM toxicity by increasing heme-based antioxidant enzyme activities. Application of ALA enhances plant growth traits under HM stress. Foliar application was found to be the most effective mode of application in promoting plant growth attributes. However, it was not determined what factors could enhance ALA uptake when applied exogenously. The pattern of ALA uptake needs to be investigated. Although ALA application showed an ameliorative effect on plant growth traits, there is still a gap in knowledge regarding the ALA physiological response and its function in signal transduction pathways in plants growing under HM stress. The accurate role of ALA in HM stress alleviation is needed in soil based environmental conditions. A broad range of study is needed at the molecular level to determine how ALA interacts with plant physio-chemical processes and promotes nutrient uptake under HM stress. Genetic exploitation is another area to be investigated with the objective of enhancing ALA production against HM stress. ALA is an expensive compound and naturally occurs in a very low quantity in plants. ALA is extracted from different microorganisms like bacteria and algae, etc. This is the need of the time to develop plants that have the potential to serve as a cheap source of ALA. Furthermore, a deep insight of the ALA-mediated defense networks/plant stress-tolerance mechanism as well as its relationship with other defense signaling pathways in HM-stressed plants can be further investigated by exploring and expanding the knowledge of various approaches, that is, biotechnology, biochemistry, plant stress molecular biology, genomics, proteomics, ionomics, bioinformatics techniques, metabolomics, and computational biology.
References
Abbas T, Rizwan M, Ali S, Rehman MZ, Qayyum MF, Abbas F, Hannan F, Rinklebe J, Ok YS (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Saf 140:37–47
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015a) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Rehman MZ, Irshad MK, Bharwana SA (2015b) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Ahmad R, Ali S, Hannan F, Rizwan M, Iqbal M, Hassan Z, Akram NA, Maqbool S, Abbas F (2017) Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.). Environ Sci Pollut Res 9:8814–8824
Ahmad P, Abd-Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KHM (2018) Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci Rep 8:13515
Akram NA, Ashraf M (2011a) Improvement in growth, chlorophyll pigments and photosynthetic performance in salt-stressed plants of sunflower (Helianthus annuus L.) by foliar application of 5-aminolevulinic acid. Agrochimica 55:94–104
Akram NA, Ashraf MU (2011b) Pattern of accumulation of inorganic elements in sunflower (Helianthus annuus L.) plants subjected to salt stress and exogenous application of 5-aminolevulinic acid. Pak J Bot 43:521–530
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul 32:663–679
Akram NA, Ashraf M, Al-Qurainy F (2012) Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci Hortic 142:143–148
Akram NA, Kausar S, Farid N, Ashraf M, Al-Qurainy F (2018) 5-Aminolevulinic acid induces regulation in growth, yield and physio-biochemical characteristics of wheat under water stress. Sains Malays 47:661–670
Ali B, Huang CR, Qi ZY, Ali S, Daud MK, Geng XX, Liu HB, Zhou WJ (2013a) 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environ Sci Pollut Res 20:7256–7267
Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Xu L, Zhou WJ (2013b) 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul 32:604–614
Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W (2014) Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crops Prod 52:617–626
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 10:0123328
Ali S, Jin R, Gill RA, Mwamba TM, Zhang N, Islam F, Ali S, Zhou W (2018) Beryllium stress-induced modifications in antioxidant machinery and plant ultrastructure in the seedlings of black and yellow seeded oilseed rape. Biomed Res Int 2018:1615968
Al-Khateeb SA, Okawara R, Al-Khateebi AA, Al-Abdoulhady IA (2006) Effects of 5-aminolevulinic acid (5-ALA) on fruit yield and quality of date palm CV, Khalas. Arab Gulf J Sci Res 24:7–11
Al-Thabet SS (2006) Promotive effect of 5-aminolevulinic acid on growth and yield of wheat grown under dry conditions. J Agron 5:45–49
Alloway BJ (1995) Heavy metals in soils, 2nd edn., Blackie, Glasgow, pp 25–34
Ashraf M, Akram NA (2009) Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol Adv 27:744–752
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Akram NA, Arteca RN, Foolad MR (2010a) The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci 29:162–190
Awad MA (2008) Promotive effects of a 5-aminolevulinic acid-based fertilizer on growth of tissue culture-derived date palm plants (Phoenix dactylifera L.) during acclimatization. Sci Hortic 118:48–52
Azevedo RA, Lea PJ (2005) Preface: toxic metals in plants. Braz J Plant Physiol 17:1–1
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2009) Uncommon heavy metals, metalloids and their plant toxicity: a review. In: Organic farming, pest control remediation of soil pollutants, Springer, Dordrecht, pp 275–317
Balestrasse KB, Tomaro ML, Batlle A, Noriega GO (2010) The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71:2038–2045
Bali S, Kaur P, Kohli PK, Ohri P, Thukral AK, Bhardwaj R, Wijaya L, Alyemeni MN, Ahmad P (2018) Jasmonic acid induced changes in physio-biochemical attributes and ascorbate-glutathione pathway in Lycopersicon esculentum under lead stress at different growth stages. Sci Total Environ 645:1344–1360
Beale SI (1990) Biosynthesis of the tetrapyrrole pigment precursor, δ-aminolevulinic acid, from glutamate. Plant Physiol 93:1273–1279
Beale SI, Castelfranco PA (1974) The biosynthesis of δ-Aminolevulinic acid in higher plants: I. accumulation of δ-aminolevulinic acid in greening plant tissues. Plant Physiol 53:291–296
Bogorad L (1967) Aspects of chloroplast assembly. In: Organizational biosynthesis, Elsevier, Amsterdam, pp 395–418
Burnham BF, Lascelles J (1963) Control of porphyrin biosynthesis through a negative-feedback mechanism. Studies with preparations of δ-aminolaevulate synthetase and δ-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J 87:462–472
Castelfranco PA, Jones OT (1975) Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol 55:485–490
Castelfranco PA, Rich PM, Beale SI (1974) The abolition of the lag phase in greening cucumber cotyledons by exogenous δ-aminolevulinic acid. Plant Physiol 53:615–618
Chakraborty N, Tripathy BC (1990) Expression of 5-amino levulinic acid induced photodynamic damage to the thylakoid membranes in dark sensitized by brief pre-illumination. J Biosci 15:199–204
Cornah JE, Terry MJ, Smith AG (2003) Green or red: what stops the traffic in the tetrapyrrole pathway? Trends Plant Sci 8:224–230
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biol Med 122:4–20
Elrod SL, Jones A, Berka RM, Cherry JR (2000) Cloning of the Aspergillus oryzae 5-aminolevulinate synthase gene and its use as a selectable marker. Curr Genet 38:291–298
Farid M, Ali S, Rizwan M, Ali Q, Saeed R, Nasir T, Abbasi GH, Rehmani MI, Ata-Ul-Karim ST, Bukhari SA, Ahmad T (2018) Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol Environ Saf 151:255–265
Fu J, Zhou Q, Liu J, Liu W, Wang T, Zhang Q, Jiang G (2008) High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 71:1269–1275
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Genisel M, Turk H, Dumlupinar R (2017) Exogenous aminolevulinic acid protects wheat seedlings against boron-induced oxidative stress. Rom Biotechnol Lett 22(4):12741–12750
Handa N, Kohli SK, Sharma A, Thukral AK, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018) Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. South Afr J Bot 119:1–10
Hodgins R, Van Huystee RB (1986) Porphyrin metabolism in chill stressed maize (Zea mays L.). J Plant Physiol 125:325–336
Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M (1997a) New physiological effects of 5-aminolevulinic acid in plants: the increase of photosynthesis, chlorophyll content, and plant growth. Biosci Biotech Biochem 61:2025–2028
Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M (1997b) Promotive effects of 5-aminolevulinic acid on the yield of several crops. Plant Growth Regul 22:109–114
Hotta Y, Tanaka T, Luo BS, Takeuchi Y, Konnai M (1998) Improvement of cold resistance in rice seedlings by 5-aminolevulinic acid. J Pesticide Sci 23:29–33
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Ibrahim M, Jabbar A, Farid M, Ali S, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdisc Toxicol 7:60–72
Jalmi SK, Bhagat PK, Verma D, Noryang S, Tayyeba S, Singh K, Shrma D, Sinha AK (2018) Traversing the links between heavy metal stress and plant signaling. Front Plant Sci 9:12. https://doi.org/10.3389/fpls.2018.00012
Jan S, Alyemni MN, Wijaya L, Alam P, Siddique KH, Ahmad P (2018) Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol 18(1):146
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jung S, Yang K, Lee DE, Back K (2004) Expression of Bradyrhizobium japonicum 5-aminolevulinic acid synthase induces severe photodynamic damage in transgenic rice. Plant Sci 167:789–795
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of Silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 241:847–860
Khan MI, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462. https://doi.org/10.3389/fpls.2015.00462
Korkmaz A (2012) Effects of exogenous application of 5-aminolevulinic acid in crop plants. In: Abiotic stress responses plants, Springer, New York, pp 215–234
Korkmaz A, Korkmaz Y (2009) Promotion by 5-aminolevulenic acid of pepper seed germination and seedling emergence under low-temperature stress. Sci Hortic 119:98–102
Korkmaz A, Korkmaz Y, Demirkıran AR (2010) Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ Exp Bot 67:495–501
Kumar MA, Chaturvedi S, Söll D (1999) Selective inhibition of HEMA gene expression by photooxidation in Arabidopsis thaliana. Phytochemisrty 51:847–851
Li DM, Zhang J, Sun WJ, Li Q, Dai AH, Bai JG (2011) 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci Hortic 130:820–828
Liu T, Hu X, Zhang J, Zhang J, Du Q, Li J (2018) H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol 18:34
Maruyama-Nakashita A, Hirai MY, Funada S, Fueki S (2010) Exogenous application of 5-aminolevulinic acid increases the transcript levels of sulfur transport and assimilatory genes, sulfate uptake, and cysteine and glutathione contents in Arabidopsis thaliana. Soil Sci Plant Nutr 56:281–288
Memon SA, Hou X, Wang L, Li Y (2009) Promotive effect of 5-aminolevulinic acid on chlorophyll, antioxidative enzymes and photosynthesis of Pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee). Acta Physiol Plant 31:51–57
Mishra SN, Srivastava HS (1983) Stimulation of nitrate reductase activity by delta amino levulinic acid in excised maize leaves. Experientia 39:1118–1120
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Naeem MS, Jin ZL, Wan GL, Liu D, Liu HB, Yoneyama K, Zhou WJ (2010) 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 332:405–415
Nishihara E, Takahashi K, Nakata N, Tanaka K, Watanabe K (2001) Effect of 5-aminolevulinic acid (ALA) on photosynthetic rate, hydrogen peroxide content, antioxidant level and active oxygen-scavenging enzymes in spinach (Spinacia oleracea L.). J Japan Soc Hortic Sci 70:346–352
Nishihara E, Kondo K, Parvez MM, Takahashi K, Watanabe K, Tanaka K (2003) Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). J Plant Physiol 160:1085–1091
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69:278–288
Pattanayak GK, Tripathy BC (2011) Overexpression of protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS ONE 6:e26532
Perveen SH, Shahbaz M, Ashraf MU (2010) Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol. Pak J Bot 42:3073–3081
Perveen SH, Shahbaz M, Ashraf MU (2011) Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak J Bot 43:2463–2468
Perveen SH, Shahbaz M, Ashraf MU (2012) Changes in mineral composition, uptake and use efficiency of salt stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak J Bot 44:27–35
Qayyum MF, Rehman MZ, Ali S, Rizwan M, Naeem A, Maqsood MA, Khalid H, Rinklebe J, Ok YS (2017) Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 174:515–523
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rehman MZ, Rizwan M, Ali S, Fatima N, Yousaf B, Naeem A, Sabir M, Ahmad HR, Ok YS (2016) Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol Environ Saf 133:218–225
Rehman MZ, Khalid H, Akmal F, Ali S, Rizwan M, Qayyum MF, Iqbal M, Khalid MU, Azhar M (2017) Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field. Environ Pollut 227:560–568
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Rizwan M, Ali S, Adrees M, Ibrahim M, Tsang DC, Rehman MZ, Zahir ZA, Rinklebe J, Tack FM, Ok YS (2017) A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 182:90–105
Rizwan M, Ali S, Rehman MZ, Rinklebe J, Tsang DCW, Bashir A, Maqbool A, Tack FMG, Ok YS (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631–632:1175–1191
Rouphael Y, Cardarelli M, Rea E, Colla G (2008) Grafting of cucumber as a means to minimize copper toxicity. Environ Exp Bot 63:49–58
Roy CB, Vivekanandan M (1998) Role of aminolevulinic acid in improving biomass production in Vigna catjung, V. mungo, and V. radiata. Biol Plant 41:211–215
Rucińska-Sobkowiak R (2016) Water relations in plants subjected to heavy metal stresses. Acta Physiol Plant 38:257. https://doi.org/10.1007/s11738-016-2277-2285
Sakpirom J, Kantachote D, Nunkaew T, Khan E (2018) Characterizations of purple non-sulfur bacteria isolated from paddy fields, and identification of strains with potential for plant growth-promotion, greenhouse gas mitigation and heavy metal bioremediation. Res Microbiol 168:266–275
Sano S, Granick S (1961) Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem 236:1173–1180
Sasaki K, Watanabe M, Tanaka T (2002) Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl Microbiol Biotechnol 58:23–29
Senge MO (1993) Recent advances in the biosynthesis and chemistry of the chlorophylls. Photochem Photobiol 57:189–206
Shahbaz M, Ashraf M (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Smirnoff N (1995) Environment and plant metabolism: flexibility and acclimation. BIOS Scientific Publishers, Didcot
Stobrawa K, Lorenc-Plucińska G (2008) Thresholds of heavy-metal toxicity in cuttings of European black poplar (Populus nigra L.) determined according to antioxidant status of fine roots and morphometrical disorders. Sci Total Environ 390:86–96
Sun YP, Zhang ZP, Wang LJ (2009) Promotion of 5-aminolevulinic acid treatment on leaf photosynthesis is related with increase of antioxidant enzyme activity in watermelon seedlings grown under shade condition. Photosynthetica 47:347–354
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346
Tanaka R, Tanaka A (2011) Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim Biophys Acta 1807:968–976
Tanaka Y, Tanaka A, Tsuji H (1992) Stabilization of apoproteins of light-harvesting chlorophyll-a. Plant Physiol Biochem 30:365–370
Tian T, Ali B, Qin Y, Malik Z, Gill RA, Ali S, Zhou W (2014) Alleviation of lead toxicity by 5-aminolevulinic acid is related to elevated growth, photosynthesis, and suppressed ultrastructural damages in oilseed rape. BioMed Res Int 2014:1–11
Van Breusegem F, Bailey-Serres J, Mittler R (2008) Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 147:978–984
Wang LJ, Jiang WB, Huang BJ (2004) Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol Plant 121:258–264
Wang L, Wb J, Liu H, Liu WQ, Kang L, Hou X (2005) Promotion by 5-aminolevulinic acid of germination of pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) seeds under salt stress. J Int Plant Biol 47:1084–1091
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Watanabe K, Tanaka T, Hotta Y, Kuramochi H, Takeuchi Y (2000) Improving salt tolerance of cotton seedlings with 5-aminolevulinic acid. Plant Growth Regul 32:97–101
Wu Y, Jin X, Liao W, Hu L, Dawuda MM, Zhao X, Tang Z, Gong T, Yu J (2018) 5-Aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front Plant Sci 9:123–128
Xiaomeng LI, Li ZH, Qiling SO, CHANG J, Jiabao YE, ZHANG W, Yongling LI, Feng XU (2018) Effects of 5-aminolevulinic acid on the photosynthesis, antioxidant system, and α-bisabolol content of Matricaria recutita. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 46 (2)
Xu F, Chang J, Cheng SY, Zhu J, Li LL, Cheng YW (2009) Promotive effect of 5-aminolevulinic acid on the antioxidant system in Ginkgo biloba leaves. Afr J Biotechnol 8:3769–3776
Xu F, Zhu J, Cheng S, Zhang W, Wang Y (2010) Effect of 5-aminolevulinic acid on photosynthesis, yield, nutrition and medicinal values of kudzu (Pueraria phaseoloides). Trop Grasslands 44:260–265
Xu L, Ali B, Gill RA, Li L, Zhou W (2015) Alleviation of cadmium toxicity by 5-aminolevulinic acid is related to improved nutrients uptake and lowered oxidative stress in Brassica napus. Int J Agric Biol 18:557–564
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10:615–620
Yoshida R, Ohta E, Iwai K, Tanaka T, Okada H (2005) Effects of liquid fertilizer containing 5-aminolevulinic acid on thickening growth in tulip bulbs. In Proceedings of the 32nd Annual Meeting of the Plant Growth Regulation Society of America, Newport Beach, California, USA, 24–27 July pp. 91–94
Youssef T, Awad MA (2008) Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J Plant Growth Regul 27:1–9
Zavgorodnyaya A, Papenbrock J, Grimm (1997) Yeast 5-aminolevulinate synthase provides additional chlorophyll precursor in transgenic tobacco. Plant J 12:169–178
Zhang ZJ, Li HZ, Zhou WJ, Takeuchi Y, Yoneyama K (2006) Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul 49:27–34
Zhen A, Bie ZL, Huang Y, Liu ZX, Fan ML (2012) Effects of 5-aminolevulinic acid on the H2O2-content and antioxidative enzyme gene expression in NaCl-treated cucumber seedlings. Biol Plant 56:566–570
Acknowledgements
The authors want to say thanks to Higher Education Commission (HEC), Pakistan for financial support under HEC Project No.203653/NRPU/R&D/HEC/14/437 and NRPU project No.5634/Punjab/NRPU/R&D/HEC/2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ali, S., Rizwan, M., Zaid, A. et al. 5-Aminolevulinic Acid-Induced Heavy Metal Stress Tolerance and Underlying Mechanisms in Plants. J Plant Growth Regul 37, 1423–1436 (2018). https://doi.org/10.1007/s00344-018-9875-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9875-y