Abstract
In the present investigation, we studied the possible potentiating effect of salicylic acid (SA) under Cd toxicity in Oryza sativa L. leaves. Cd treatments for 24 h reduced the shoot length, dry biomass and total chlorophyll content followed by high Cd accumulation in shoots. About 16 h presoaking with SA resulted in partial protection against Cd, as observed by minor changes in length, biomass and total chlorophyll. SA priming resulted in low Cd accumulation. Enhanced thiobarbituric acid reactive substances (TBARS), hydrogen peroxide (H2O2) and superoxide anion (O2 −) content were seen when Cd was applied alone, while under SA priming the extent of TBARS, H2O2 and O2 − were significantly low, suggesting SA-regulated protection against oxidative stress. The antioxidant enzymes like Catalase (CAT), guaiacol peroxidase (GPx), glutathione reductase (GR) and superoxide dismutase (SOD) showed varied activities under Cd alone. CAT activity increased after Cd treatment, followed by a decline in GPX and GR activity. SOD also declined at the highest concentrations with an initial increase. Under SA-priming conditions, the efficiency of the antioxidant enzymes was significantly elevated. GPx and SOD activity showed significant increase in activity. The ascorbate activity increased after Cd treatment, followed by a decline in glutathione under SA-free condition. SA priming showed gradual increase in these non-enzymic antioxidants. Our results indicate that Cd-induced oxidative stress can be regulated by SA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of oxygen in the cellular environment poses a constant oxidative threat to cellular structure and function. The univalent reduction of oxygen, which occurs as a result of spin restriction, results in the formation of toxic reactive oxygen species (ROS) such as superoxide radicals (O2 −), hydrogen peroxide (H2O2), hydroxyl radicals (OH−), singlet oxygen (1O2), etc. (Elstner 1982; Halliwell and Gutteridge 1988; Asada 1994; Gille and Singler 1995). These ROS cause oxidative damage to biomolecules such as lipids, proteins and nucleic acids altering cellular functions (Alia et al. 1995; Gille and Singler 1995). Oxidative stress is a major factor in plants subjected to various environmental stresses (Monk et al. 1989; Greger et al. 1995; Harnendez et al. 1999; Prasad et al. 1999; Panda et al. 2003a, b). The occurrence of activated oxygen and symptoms of oxidative injury have been reported in plants subjected to heavy metals like Cd, Cu, Zn, Pb, Al and Cr (Gallego et al. 1996; Chaoui et al. 1997; Prasad et al. 1999; Dietz et al. 1999; Panda et al. 2003a, b; Verma and Dubey 2003; Choudhury and Panda 2004; Chen et al. 2004). However, in order to overcome the deleterious effects of ROS, plants have developed complex antioxidant defence systems that scavenge these ROS. Exposure of heavy metals can provoke pronounced antioxidant responses, which varies with the species and the type of tissue analysed.
Cadmium is a toxic heavy metal with a long biological half life (Himly et al. 1985). Cd toxicity causes leaf rolls, chlorosis and reduced growth of plants, partly due to the suppression of cell growth by the inhibition of proton pump responsible for the process (Aidid and Okamoto 1992; Khale 1993). It can interfere with water balance (Barcelo and Poschenrieder 1990) and damage the photosynthetic apparatus. Cd toxicity is also attributed to the generation of ROS leading to oxidative injury (Hendry et al. 1992; Gallego et al. 1996; Chaoui et al. 1997). Cd can either stimulate or inhibit the activities of antioxidant enzymes. In sunflower, Cd has been reported to decrease the activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidases (APX) and glutathione reductase (GR) initiating the process of lipid peroxidation (Gallego et al. 1996). Induction of lipid peroxidation has also been reported in Phaseolus vulgaris and Phaseolus aureus under Cd toxicity (Lozano-Rodriguez et al. 1997). Heavy metals like Cu and Fe being redox can participate directly in the formation of free radical via Fenton type or Haber–Weiss reactions. Cd being a non-redox metal is unable to participate in such Fenton type reactions. Since it is well established that Cd induces antioxidant responses, it is assumed that Cd is directly or indirectly involved in the formation of free radicals thereby causing oxidative stress (Schutzendubel et al. 2001; Sandalio et al. 2001). Due to the formation of ROS by Cd, the basic photochemical processes are affected resulting in the high sensitivity of photosynthesis to Cd due to metabolic dysfunction (Atal et al. 1991; Malik et al. 1992; Mendelssohn et al. 2001).
Salicylic acid (SA) is an important signalling element and an endogenous growth regulator involved in local and endemic disease resistance in plants in response to a pathogenic attack (Enyedi et al. 1992; Alverez 2000). SA can modulate plant responses to a wide range of oxidative stress (Shirasu et al. 1997). Treatment of SA for 24 h in Cd-treated barley seedlings showed an increased level of Cd tolerance (Matewally et al. 2003). Application of SA exogenously induced thermo tolerance in mustard and increased the antioxidant efficiency in maize subjected to chilling stress (Raskin et al. 1987; Dat et al. 1998; Janda et al. 1999). In wheat, application of SA can trigger tolerance to salinity and also reduce the toxic effects of various heavy metals (Shakriova and Bezrukova 1997; Mishra and Choudhuri 1999; Berukova et al. 2001). In the present investigation, we have analysed the potentiating role of SA in reducing the toxic effects of Cd in developing leaves.
Materials and methods
Plant material
Dry graded seeds of Oryza sativa L. (cv: Longai) were collected from the Regional Agricultural Research Station, Karimganj, India. Seeds were surface sterilized with 0.1% HgCl2 followed by three rinses in sterile distilled water and germinated in Whatman No. 1. filter paper for 3 days at 30 + 2°C. The germinated seeds were then transferred to plastic pots containing balanced Yoshida nutrient solution. Seedlings were then grown for 3 days inside a growth chamber under continuous white light (20 W Philips TLD, Mumbai, India) with a photon flux density of 52 μ moles m−2 s−1. Cd treatment in the form of CdCl2 was given with nutrient solution at various concentrations after 3 days (0, 100 and 1,000 μM). After 24 h of treatment, the plants were harvested for various biochemical and enzymic estimations. SA treatment was given by presoaking the seeds in 100 μM of SA for 16 h prior to germination. The seeds were then washed with sterile distilled water and then germinated and grown as stated above.
Growth, pigments, lipid peroxidation and ROS analysis
Growth was studied by measuring the shoot length and dry biomass. Plants were dried in an oven at 70°C for 48 h and then weighed to measure the dry biomass. The amount of Cd accumulation in shoots was measured using an atomic absorption spectrometer (PERKIN-ELMNER, Foster City, CA, USA, 3110). The total chlorophyll content was estimated spectrophotometrically by homogenizing the plant material in 80% cold acetone as suggested by Arnon (1949). The possible generation of ROS in terms of total peroxide (H2O2) content and superoxide radical (O2 −) production were also measured. H2O2 content was measured by homogenizing the leaves in 5% trichloroacetic acid (TCA) as suggested by Sagisaka (1976). The O2 − production in leaf tissue was estimated as per the method of Elstner and Heupel (1976) by monitoring the nitrate formation from hydroxylamine. The plant material was homogenized in 3 ml of 65 mM phosphate buffer (pH 7.8) and centrifuged at 5,000g for 10 min. The incubation mixture contained 0.9 ml of 65 mM phosphate buffer, 0.1 ml of 10 mM hydroxylamine hydrochloride and 1 ml of the supernatant plant extract. After incubation at room temperature (25°C) for 20 min, 1 ml of 17 mM sulphanilamide and 1 ml of 7 mM ∝-naphthyl were added. After reaction at 25°C, 1 ml of diethyl ether was added and centrifuged at 1,500g for 5 min. The absorbancy was read at 530 nm. A standard curve with NO2 − was established to calculate the production rate of O2 − from the chemical reaction of O2 − and hydroxylamine. Lipid peroxidation was measured as the amount of thiobarbituric acid reactive substances (TBARS) determined by the thiobarbituric acid (TBA) reaction, as described by Heath and Packer (1968). The leaf tissues (0.2 g) were homogenized in 2 ml of 0.1% (m/v) TCA. The homogenate was centrifuged at 10,000g for about 20 min and the supernatant was collected. To 1 ml of the supernatant, 1 ml of TCA (20% containing 0.5% TBA) was added followed by 0.01 ml of 4% solution of butylated hydroxytoulene prepared in ethanol. The mixture was incubated at 95°C for 30 min and then cooled in ice. It was then centrifuged at 10,000g for 15 min. and the absorbance was measured at 532 nm and corrected for 600 nm.
Enzyme extraction and assay
Extraction of the antioxidant enzymes were determined by homogenizing the leaves in 0.1 M phosphate buffer (pH 6.8) in a prechilled mortar and pestle under cold conditions. The extract was then centrifuged at 4°C for 15 min at 14,000g in a cooling centrifuge. The supernatant was used for the assay of CAT and guaiacol peroxidase (GPx), as suggested by Chance and Maehly (1955). The 5 ml of the assay mixture of CAT comprised of 3 ml of 0.1 M phosphate buffer (pH 6.8), 1 ml 30 mM H2O2 and 1 ml of the enzyme extract. The reaction was stopped by adding 10 ml of 2% H2SO4 after 1 min. incubation at 20°C. The acidified reaction mixture was titrated against 0.01 M KMnO4 to determine the quantity of H2O2 utilized by the enzyme. The 3 ml of the assay mixture of GPx comprised 0.1 M phosphate buffer (pH 6.8), 30 mM guaiacol, 30 mM H2O2 and 0.3 ml enzyme extract. The rate of change in absorbance at 420 nm was measured using a UV-visible spectrophotometer (Systronics, Mumbai, India). SOD was recorded as suggested by Giannopolitis and Ries (1977). About 3 ml of assay mixture of SOD contained 79.2 mM Tris–HCl buffer (pH 8.9) containing 0.12 mM EDTA and 10.8 mM tetramethyldiamine, bovine serum albumin (0.0033%), 6 mM nitroblue tetrazolium (NBT), 600 μM riboflavin in 5 mM KOH and 0.2 ml enzyme extract. The reaction mixture was illuminated by placing the glass tubes between two fluorescent tubes (Philips, 20 W, India), and by switching the light off the reaction was terminated. The increase in absorbance due to Formazen formation was recorded at 560 nm. The activity of GR was measured as suggested by Smith et al. (1988). The reaction mixture for GR comprised 1 ml of 0.2 M potassium phosphate buffer (pH 7.5) containing 1 mM EDTA, 0.5 ml of 3 mM DTNB (5,5′-dithiobis-2-nitrobenzoic acid) in 0.01 M potassium phosphate buffer (pH 7.5), 0.1 ml of 2 mM NADPH, and 1 ml enzyme extract and distilled water to make up the final volume to 2.9 ml. The reaction was initiated by adding 0.1 ml of 2 mM oxidized glutathione (GSSG). The increase in absorbance at 412 nm was recorded at 25°C for 5 min. The activity was expressed as absorbance change (ΔA412) g fresh weight−1 s−1.
Determination of non-enzymic antioxidants
Non-enzymic antioxidants, ascorbate and glutathione were estimated as suggested by Oser (1979) and Griffith (1980), respectively. The leaf tissues were homogenized in 5% (w/v) sulphosalicylic acid and the homogenate was centrifuged at 10,000g for 10 min. The reaction mixture for ascorbate consisted of 2 ml 2% Na-molybdate, 2 ml 0.15 N H2SO4, 1 ml 1.5 mM Na2HPO4 and 1 ml tissue extract. It was then incubated at 60°C in a water bath for 40 min, cooled and centrifuged at 3,000g for 10 min and the absorbance was measured at 660 nm. For estimation of glutathione, the tissue extract was neutralized in 0.5 ml of 0.5 M potassium phosphate buffer (pH 7.5). The reaction mixture contained 0.5 ml of 0.1 M Na phosphate buffer (pH 7.5) containing EDTA, 0.2 ml of 6 mM DTNB, 0.1 ml of 2 mM NADPH and 1 ml of 1-U yeast-GR Type III (Sigma, St. Louis, MO, USA). The change in absorbance at 412 nm was followed at 25°C until it reached 5 U. Each experiment was repeated thrice and the data represented are means of three separate experiments +SE.
Results
Growth parameters and chlorophyll content
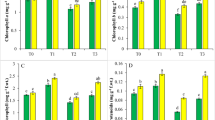
Treatment with Cd reduced the shoot length and dry biomass with an initial increase at 100 μM concentration (Fig. 1a, b). About 18% inhibition of shoot length was observed at 1,000 μM of Cd alone. Under SA treatment, lesser inhibition was seen with only 10% decrease at 1,000 μM (Fig. 1a). Dry biomass also showed a similar trend. Initial increase was observed under both the conditions with concomitant decrease at 1,000 μM for both Cd alone and under SA treatment (Fig. 1b). The decrease in growth was accompanied by a decrease in the total chlorophyll content (Fig. 1c). Under Cd alone, the total chlorophyll content decreased by 31 and 40%, respectively, at 100 and 1,000 μM of Cd alone as compared to the control. Under SA pretreatment, the total chlorophyll decreased by 21% at 100 μM and 36% at 1,000 μM of Cd. Under control conditions, the total chlorophyll content was higher under SA pretreatment as compared to control without SA.
Cd uptake
The accumulation of Cd was significantly high under SA-free conditions as compared to SA-primed ones. Cd accumulation significantly increased with increase in Cd concentration in the nutrient solution, where the highest accumulation was recorded at 1,000 μM of Cd alone. SA priming reduced the extent of Cd accumulation in leaves as compared to Cd alone. Under both the conditions, plants treated with Cd under SA-free conditions showed higher accumulation of the metal (Fig. 2).
ROS and lipid peroxidation
The production of ROS like H2O2 and O2 − in rice leaves was enhanced under Cd treatment (Fig. 3a, b). In the case of Cd alone, the H2O2 content increased significantly with increase in the concentration of Cd. At 10 μM, the H2O2 content increased by 57% at 100 μM and 63% at 1,000 μM, as compared to controls. On priming with SA, there was a reduction in the extent of H2O2 production as compared to SA-free plants. Under SA-primed conditions, the H2O2 content increased only by 30 and 34% at 100 and 1,000 μM, respectively (Fig. 3a). The superoxide anion production also showed significant enhancement after Cd treatment. When Cd was applied alone, the production of O2 − was significantly high as compared to SA-primed plants. At the highest concentration of Cd, highly significant enhancement in O2 − was observed, about 620% under Cd alone, which was significantly high as compared to SA-primed plants (Fig. 3b). The increase in production of ROS was accompanied by an increase in TBARS content, indicating lipid peroxidation in the shoots of rice plants under Cd treatment (Fig. 4a, b). Application of Cd alone increased the TBARS content by 489 and 754% at 100 and 1,000 μM, respectively. Increase in TBARS content was significantly low under SA primed with only 92 and 237% at 100 and 1,000 μM, respectively. The increase in TBARS content under SA-free conditions suggested toxic effects of Cd, which was reduced after SA priming.
Enzyme activities
Under Cd treatment, significant enhancement in the activities of various antioxidant enzymes could be observed (Fig. 5a–d). Application of Cd in nutrient solution increased the CAT activity in leaves. The increase was consistent with the increase in Cd concentration. Application of SA increased the antioxidant efficiency, where significant increase in CAT activity was observed. At 1,000 μM of both SA-free and SA-primed Cd-treated plants, there was about a 147 and 160%, respective, increase in CAT active to their controls (Fig. 5a). The GPx activity decreased with increase in Cd concentration. At 100 and 1,000 μM, it decreased by 11 and 15%, respectively. SA pre-treatment, however, increased the GPx activity (Fig. 5b). Significant reduction in GR activity was observed under both the conditions (Fig. 5c), while SOD showed an initial increase with simultaneous decrease in activity at 1,000 μM of Cd under Cd alone. Under SA-primed conditions, significant enhancement in SOD activity was seen in leaves as compared to SA-free plants (Fig. 5d).
Non-enzymic antioxidants
Both ascorbate and glutathione showed an increasing trend under Cd alone and also under SA-primed conditions. Ascorbate significantly increased at 100 and 1,000 μM of Cd (Fig. 6a, b). At 100 and 1,000 μM, the ascorbate increased by 278 and 326% as compared to the controls. Under SA-primed conditions, the increase was about 179 and 289% at 100 and 1,000 μM (Fig. 6a). Glutathione showed an initial increase at 100 μM under SA-primed conditions. Under SA-free conditions, glutathione decreased by 28 and 44%, respectively, at 100 and 1,000 μM of Cd. At 100 μM of Cd under SA-primed conditions, glutathione increased by 18%, which again decreased at 1,000 μM of Cd. At 1,000 μM, the decrease was about 10% as compared to the control (Fig. 6b).
Discussion
In the present investigation, we have shown that Cd-induced oxidative stress can be regulated by SA in O. sativa L. leaves. Cd is a toxic heavy metal and is known to inhibit growth (Aidid and Okamoto 1992; Veselov et al. 2003). In the present investigation, a decrease in shoot growth was seen under Cd alone with an initial increase at 100 μM. SA priming resulted in potentiating the toxic effects of Cd on growth, which tallies with the findings of Matewally et al. (2003), where the toxic effect of Cd on the growth of barley was reduced after 24 h of SA priming. Significant reduction in the total chlorophyll content was observed under Cd treatment. The inhibition of growth seen by the decrease in dry biomass and shoot elongation may be due to reduction in photosynthesis evidenced by the reduction in total chlorophyll content (Somashekaraiah et al. 1992; Bazzaz et al. 1992; Sandalio et al. 2001). SA priming also resulted in lesser inhibition of total chlorophyll content, thereby reducing the inhibitory effect of Cd on photosynthesis and growth. Accumulation of Cd in leaves was significantly high under SA-free conditions as compared to SA-primed ones. SA priming resulted in reduction in Cd uptake and accumulation as seen earlier in the case of barley and Cassia tora where SA treatment reduced the Cd and Al uptake in these plants, respectively (Matewally et al. 2003; Yang et al. 2003). As a consequence of a general stress response, cytotoxic H2O2 gets accumulated in cells (Levine et al. 1994) and can act as a secondary messenger (Dietz et al. 1999). Under both SA-free and SA-primed conditions, accumulation of H2O2 was observed in leaves. The production of O2 − was enhanced under Cd treatment. Increased production of O2 − under SA-free conditions indicated the normal reduction of O2 −–H2O2 and other electron carriers during mitochondrial electron transport chain. O2 − being less cannot cross the cytoplasmic membrane and dismutate readily to H2O2 (Vranova et al. 2002). High H2O2 and O2 − had been reported earlier in the case of various other plants under Cr, Zn, Pb, etc. (Dietz et al. 1999; Panda 2003; Panda et al. 2003a, b; Skorzynska-Polit et al. 2004; Choudhury and Panda 2004). As a result of the production of ROS, such as H2O2, O2 − and OH−, lipid peroxidation can be initiated, which can disrupt the functionality and integrity of biological membranes (Halliwell and Gutteridge 1988). Under SA-free conditions, Cd significantly prompted TBARS production in leaf tissues as reported for various other heavy metals like Cr, Zn, Pb, As, etc., in the case of higher plants (Mishra and Choudhuri 1996; Panda et al. 2003a, b; Panda and Khan 2003). Under SA-primed condition, the extent of TBARS production in leaves was significantly low as compared to SA-free condition as for SA-treated barley under Cd stress (Matewally et al. 2003). In order to repair the damage initiated by ROS, plants have evolved a complex antioxidant system by which it can scavenge the ROS (Alscher et al. 1997). In this present investigation, significant enhancement of CAT activity was observed in leaves under Cd, which further showed high efficiency under SA priming. GPx activity, however, showed loss of activity under SA-free conditions, while under SA, the activity showed an increasing trend. Both CAT and GPx were involved in the detoxification of H2O2 in leaves. However, in the absence of GPx, CAT played a provital role in H2O2 detoxification. Similar results were reported for Cd and other heavy metals such as Cu, Cr and Pb (De Vos and Schat 1991; Karatglis et al. 1991; Patra and Panda 1998). In the case of Brassica juncea under Zn and Vigna radiata under Al showed increase in peroxidase activity with increase in concentration of the metals (Prasad et al. 1999; Panda et al. 2003b). Significant decline in GR activity was seen for both SA-free and SA-primed conditions. Although SA treatment increased the GR activity with increase in Cd concentrations, no significant positive effect of SA could be observed in the case of GR. The decline in GR was highly significant for Cd alone. SOD activity showed an initial increase followed by a gradual decrease under SA-free conditions. Priming with SA resulted in an increase in SOD activity. The increase in SOD activity indicated higher H2O2 level seen by the increase in total peroxide content in leaves, which tallies with those observed in the case of B. juncea and V. radiate under Zn and Al treatment (Prasad et al. 1999; Panda et al. 2003b). The high efficiency of CAT under SA-primed condition resulted in the high detoxification of H2O2 as compared to SA-free conditions, imparting protection against ROS and in turn reducing the oxidative threat. The pattern of changes in antioxidant enzymes in leaves indicated oxidative damage to leaves, and Cd-induced oxidative stress was reduced after SA priming, seen by the activities of the antioxidant, as seen in the case of barley under Cd stress (Matewally et al. 2003).
The non-enzymic antioxidants, ascorbate and glutathione showed two different trends in leaf tissues. Ascorbate activity showed an increasing trend on Cd treatment under both SA-free and SA-primed conditions. The enhancement in ascorbate activity indicated the active participation of this antioxidant in detoxifying the toxic ROS directly or through certain enzymes (Rennenberg 1982; Asada and Takahashi 1987), restricting the metal-induced lipid peroxidation and oxidative stress. Glutathione showed a decreasing trend, which again increased after SA priming.
Cd treatment induced oxidative stress in leaves, as evidenced by the decline in growth, increase in lipid peroxidation and changes in antioxidant efficiency in leaves. The potentiating effect of SA can be observed after priming the seeds with 100 μM SA, where the extent of Cd toxicity was highly reduced as observed in the case of lipid peroxidation results and pattern of antioxidants metabolism. Thus, we can conclude that SA can exert Cd tolerance by regulating the antioxidant defence mechanism against toxic concentrations of Cd.
Abbreviations
- SA:
-
Salicylic acid
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
- CAT:
-
Catalase
- GPx:
-
Guaiacol peroxidase
- GR:
-
Glutathione reductase
- SOD:
-
Superoxide dismutase
- NBT:
-
Nitroblue tetrazolium
References
Aidid SB, Okamoto B (1992) Effect of lead, cadmium and zinc on electric membrane potential at xylem symplast interface and cell elongation of Impatiens Balsamina. Environ Exp Bot 32:439–448
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationship in green cells. Physiol Plant 100:224–233
Alverez AL (2000) Salicylic acid in machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44:429–442
Arnon DI (1949) Copper enzyme in the isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense system in plants. CRC, Boca Ration, FL, pp 77–104
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CJ, Artzen CJ (eds) Photoinhibition: topics in photosynthesis. Elsevier, Amsterdam, pp 277–287
Atal N, Saradhi PP, Mohanty P (1991) Inhibition of chloroplast photochemical reaction by treatments of wheat seedlings with low concentration of cadmium: analysis of electron transport activities and changes in fluorescence yield. Plant Cell Physiol 32:943–951
Barceló J, Poschenrieder C (1990) Plant water relation as affected by heavy metal stress: a review. J Plant Nutr 13:1–37
Bazzaz FA, Rolfe GL, Carlson RW (1992) Effect of cadmium on photosynthesis and transpiration of excised leaves of corn and sunflower. Physiol Plant 32:373–377
Berukova MV, Sakhabutdinova R, Fatkhutdinova RA, Kyldiarova I, Shakirova F (2001) The role of hormonal changes in protective action of salicylic acid on growth of wheat seedlings under water deficit. Agrochemiya (Russ.) 2:51–54
Chance B, Maehly AC (1955) Assay of catalase and peroxidase. Method Enzymol 2:746–778
Chaoui A, Mazhoudi SS, Ghorbal MH, Ferjani EEL (1997) Cadmium and zinc induction of lipid peroxidation and effects the antioxidant enzyme activities in bean (Phaseolus vulgaris L). Plant Sci 127:139–147
Chen CT, Chen TH, Lo KF, Chiu CY (2004) Effect of proline on copper transport in rice seedling under excess copper stress. Plant Sci 166:103–111
Choudhury S, Panda SK (2004) Induction of oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. Under lead (Pb) and arsenic (As) phytotoxicity. Curr Sci (in press)
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during the thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1375
De Vos CHR, Schat H (1991) Free radical and heavy metal tolerance. In: Rozema J, Verkleij JAC (eds) Ecological responses to environmental stresses. Kluwer, Dordrecht, pp 1–30
Dietz KJ, Baier M, Kramer U (1999) Free radicals and reactive oxygen species as mediator of heavy metal toxicity in plants. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants: from molecules to ecosystem. Springer, Berlin, pp 73–79
Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant
Elstner EF, Heupel A (1976) Inhibition of nitrate formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Enyedi AJ, Yalpani N, Sliverman P, Raskin I (1992) Signal molecule in systemic plant resistance to pathogens and pests. Cell 70:879–886
Gallego SM, Benavioes MP, Tomaro ML (1996) Effect of heavy metal ions on sunflower leaves-evidence for involvement of oxidative stress. Plant Sci 121:151–159
Giannopolitis CN, Ries SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gille G, Singler K (1995) Oxidative stress in living cells. Folia Microbiol 2:131–152
Greger ML, Kautskey T, Sandberg AC (1995) A tentative model of Cd uptake in Potamogeton petinatus in relation to salinity. Environ Exp Bot 35:215–225
Griffith OW (1980) Determination of glutathione and glutathione disulphide using glutathione reductase and 2- vinyl pyridine. Anal Biochem 106:207–211
Halliwell B, Gutteridge JMC (1988) Free radical in biology and medicine. Clarendon Press, Oxford, p 1
Harnendez JA, Campillo A, Jimenz A, Alarcon TJ, Sevilla F (1999) Response of antioxidant system and leaf water relation to NaCl stress in pea plants. New Phytol 141:241–251
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hendry GAF, Baker AJM, Ewart CF (1992) Cadmium tolerance and toxicity, oxygen radical proCesses and molecular damage in cadmium-tolerant and cadmium-sensitive clones of Holcus lanatus. Acta Bot Neerl 41:271–281
Himly AM, Sabana MB, Oaabees AY (1985) Bioaccumulation of cadmium: toxicity in Megul cephalus. Comp Biochem Physiol 81:139–140
Janda T, Szalai G, Tari I, Paldi E (1999) Hydrophonic treatment with salicylic acid decreases the effect of chilling in maize (Zea mays L.) plants. Planta 208:175–180
Karatglis S, Moustakas M, Symeonidis L (1991) Effect of heavy metals on isoperoxidase of wheat. Biol Plant 33:3–9
Khale H (1993) Response of root of trees to heavy metals. Environ Exp Bot 33:99–119
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative brust orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Light harvesting complex II in radish cotyledons. Physiol Plant 73:518–524
Lozano-Rodriguez E, Hernandez LE, Bonay P, Carpena-Ruiz RO (1997) Distribution of Cd in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128
Malik D, Sheoran IS, Singh R (1992) Carbon metabolism in leaves of cadmium treated wheat seedlings. Plant Physiol Biochem 30:223–229
Matewally A, Finkemeir I, Georgi M, Dietz K-J (2003) Salicylic acid alleviates cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mendelssohn IA, Mckee KL, Kong T (2001) A comparison of physiological indicators of sub lethal cadmium stress in wetland plants. Environ Exp Bot 46:263–275
Mishra A, Choudhuri MA (1999) Effect of salicylic acid on heavy metal induced membrane deterioration mediated by lipooxygenases in rice. Biol Plant 42:409–415
Mishra A, Choudhuri MA (1996) Possible implication of heavy metals (Pb2+ and Hg2+) in the free radical-mediated membrane damage in two rice cultivars. Ind J Plant Physiol 1:43–47
Monk LS, Fagersted KV, Crawford RMM (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol Plant 76:456–459
Oser BL (1979) Hawks physiological chemistry. McGraw-Hills, New York
Panda SK (2003) Heavy metal phytotoxicity induces oxidative stress moss Taxithelium sp. Curr Sci 84:631–633
Panda SK, Chaudhury I, Khan MH (2003a) Heavy metal induced lipid peroxidation and affects antioxidants in wheat leaves. Biol Plant 46:289–294
Panda SK, Singha LB, Khan MH (2003b) Does aluminium phytotoxicity induce oxidative stress in green gram (Vigna radiate)? Bulg J Plant Physiol 29:77–86
Panda SK, Khan MH (2003) Chromium phytotoxicity effects on the germination of green gram (Vigna radiata) seeds. J Phytol Res 15:225–228
Patra JB, Panda B (1998) A comparison of bioChemical responses to oxidative and metal stress in seedlings of barley Hordeum vulgarae L. Environ Pollut 101:99–105; Physiology 33:78–96
Prasad KVSK, Pardha Saradhi P, Sharmila P (1999) Concerted actionantioxidant enzyme and curtailed growth under zinc toxicity in Brassica napus. Environ Exp Bot 42:1–10
Raskin I, Ehmann A, Melander WR, Mecuse BJD (1987) Salicylic acid: a natural inducer of heat production of Arum lilies. Science 237:1601–1602
Rennenberg H (1982) Glutathione metabolism and possible role in higher plants. Phytochemistry 28:2771–2781
Sagisaka S (1976) The occurrence of peroxide in perennial plant Populus glerica. Plant Physiol 57:308–309
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, Del Rio LA (2001) Cadmium induced changes in growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Schutzendubel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Goldbold DL, Polle A (2001) Cadmium induced changes in antioxidant systems, hydrogen peroxide content and differentiation in Scots pine roots. Plant Physiol 127:887–889
Shakriova FM, Bezrukova MV (1997) Induction of wheat resistance against environmental salinization by salicylic acid. Biol Bull 24:109–112
Shirasu K, Nakajima A, Rajshekar K, Dixon RA, Lamb C (1997) Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signal in the activation of defence mechanism. Plant Cell 9:261–270
Skorzynska-Polit E, Darzkiewicz M, Krupa Z (2004) The activity of antioxidant system in cadmium treated Arabidopsis thaliana. Biol Plant 47:71–78
Smith IK, Vierhgeller TL, Throne CA (1988) Assay of glutathione reductase in crude tissue homogenate using 5, 5’, dithiobis (2 – nitrobenzoic acid). Anal Biochem 175:408–413
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung beans (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant 85:85–89
Verma S, Dubey RS (2003) Lead toxicity indices lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Yang MN, Wang J, Wang SH, Xu LL (2003) Salicylic acid induced aluminium tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 217(1):168–174
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Gwozdz.
Rights and permissions
About this article
Cite this article
Panda, S.K., Patra, H.K. Effect of salicylic acid potentiates cadmium-induced oxidative damage in Oryza sativa L. leaves. Acta Physiol Plant 29, 567–575 (2007). https://doi.org/10.1007/s11738-007-0069-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0069-7