Abstract
Moringa oleifera is a tropical plant that is native to India. The extractant from M. oleifera seeds can be used for water treatment, because coagulation-active components are contained in the seeds. M. oleifera coagulant (MOC) is traditionally extracted with water and used for the treatment of turbid water. Recently, many studies have been focused on revealing its practical application and improving its coagulation activity, including those on MOC purification or heavy metal removal by MOC. MOC has the potential for use in drinking water and wastewater treatment, especially in tropical regions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coagulation is a major technology for water treatment, and many kinds of coagulants are used in conventional water treatment processes for tap water production, wastewater treatment, and water recycling. These coagulants can be classified mainly into inorganic coagulants, synthetic organic polymers, and natural coagulants. The inorganic polymer “polyaluminum chloride (PAC)” is widely used in water treatment, especially in Japan, and the inorganic salts “aluminum sulfate (alum)” and ferric chloride, which are other famous coagulants used worldwide (Kumar et al. 2012). Synthetic organic polymers, such as polyacrylamide and its copolymer with other monomers, are widely used, especially in water treatment (Mallevialle et al. 1984).
Natural coagulants have been demanded and studied for use in water treatment because of their low cost and safety. Coagulants containing Al or Fe as the main component remain in water treatment residuals (WTRs). WTRs are reused and recycled for various purposes, of which reuse on farmland is an important one. Al or Fe in the coagulants can combine with phosphoric acid, which is added to the soil as a nutrient, and produce AlPO4 or FePO4, essentially inhibiting the nutrient function in the field. This may lead to some difficulty in the acceptance of WTR recycling on farmland and would be a reason why natural coagulants should be paid greater attention.
Some famous natural coagulants are chitosan (Gassara et al. 2015; Zonoozi et al. 2011) and alginic acid (Maryam et al. 2014). Plant materials, e.g., seeds, also have been studied in order to develop natural coagulants. Among the plant materials that have been studied are Pisum sativum (Rajamohan et al. 2018), common oak acorn (Antov et al. 2018), Durio zibethinus (Suffian et al. 2018), Jatropha curcas (Abidin et al. 2017), Ocimum basilicum (Sorour et al. 2015), and Cassia obtusifolia (Yee and Yeong 2014). Moringa oleifera is a tropical plant that contains a coagulation-active component in its seeds. In a review paper written by Dr. Mustapha Hassan Bichi (Bichi 2013), the use of M. oleifera as a coagulant/flocculant in water treatment is well summarized. He wrote that the use of M. oleifera seeds for domestic household water treatment has been traditionally known in certain rural areas in Sudan and that one of the best-known uses in West Asia for M. oleifera is using its powdered seeds to flocculate contaminants and purify drinking water.
The M. oleifera tree, seedpod, and seeds before and after being shelled are shown in Fig. 4.1. M. oleifera belongs to the family of Moringaceae, which contains only a single genus of shrubs. The occurrence of M. oleifera is summarized in the review paper by Bichi (2013). As M. oleifera is found in nature and does not receive harmful synthetic or other treatments, even when it is not used correctly in coagulation, leading to an overdose of the natural substance, no adverse effects on public health are expected in the case of slight overdosage. In addition, given its organic nature, coagulant sludge generated through M. oleifera coagulation is likely safer for use as animal feed or plant fertilizer. M. oleifera is a fast-growth tree, commonly found in semiarid, tropical, and subtropical areas including India, South and Central America, Africa, and Southeast Asia (Olsen 1987; Jahn 1988). Thus, M. oleifera coagulation is a pragmatic option for providing access to safe drinking water to rural communities in developing countries worldwide, which is the Sustainable Development Goals (SDGs: “sustainable water management” in it).
M. oleifera seeds contain dimeric cationic proteins, which can be extracted from the seeds using water or a salt solution. This M. oleifera extract can then be used for coagulation applications. Coagulation with the M. oleifera extract is effective for turbidity removal, especially for highly turbid water, meaning M. oleifera extract can be used as a coagulant (M. oleifera coagulant: MOC). For example, previous studies demonstrated that water-extracted MOC achieved more than 60% removal of turbidity for highly turbid surface waters, i.e., up to 750 NTU (Bichi 2013). As described in the literature, attempts have been made to enhance the coagulation activity of MOCs, including via solvent changes and different purification processes in order to remove unnecessary organics. Some of this research, including that carried out by the authors, is introduced and summarized in this review paper.
2 Historical Coagulation/Flocculation Via Moringa oleifera
2.1 Coagulation/Flocculation Via Moringa oleifera Seed
Several studies have been done on the performance of M. oleifera seeds as an alternative coagulant or coagulant aid. Earlier studies recommended the use of M. oleifera seed extracts as coagulants for water treatment in African and South Asian countries where this plant is considered indigenous (Bichi 2013; Olsen 1987). If MOCs become widely used in drinking water and wastewater treatment in other countries as well, M. oleifera may become a cash product, bringing more economic benefits for the producing countries.
Ndacigengesere and Narasiah (Ndacigengesere and Narasiah 1996) have studied turbidity removal by water-extracted MOC as the primary coagulant in the first stage of MOC research, and the result was up to 80–99% removal for both raw waters and synthetic turbid waters. For the application of MOC, coagulation of target materials by MOC has been investigated. Unlike inorganic turbid materials, organic suspended solids such as bacteria, algae, and organic matter were difficult targets for coagulation. Sengupta et al. (2012) reported the removal of helminth eggs from irrigation water, turbid water, wastewater, and tap water by water (tap water)-extracted MOC. Margarida et al. (2017) aimed to develop a water treatment sequence using MOC combined with an activated carbon in order to remove Microcystis aeruginosa and natural organic matter through an integrated process. They demonstrated higher than 80% removal of M. aeruginosa cells from water with 150–200 μg/L of chlorophyll a (indicates the algae amount), with water-extracted MOC removing them by the combination of coagulation/flocculation and dissolved air flotation (DAF) processes. MOCs has been applied in the removal of dyes, surfactants, perfluorooctane sulfonate (PFOS), and perfluorooctanoate (PFOA), too (Beltran-Heredia et al. 2009; Beltran-Heredia and Sanchez-Martin 2009; Kumar et al. 2015). For dye removal, the influences of coagulation pH, temperature, and initial dye concentration have also been tested by using the Langmuir isotherm model fitting. The ability of water-extracted MOC to remove an anionic surfactant, sodium lauryl sulfate, has been evaluated, with up to 80% removal observed via the coagulation/flocculation process. pH and temperature were found to be not very important factors in this removal efficiency, unlike the dye removal. PFOS and PFOA are persistent organic pollutants, and their occurrence in the environment can cause toxicological effects to humans. The removal efficiency of these compounds by water-extracted MOC was investigated and compared with those of alum and ferric chloride. Kumar et al. (2015) reported that water-extracted MOC was more effective in reducing PFOS and PFOA than the conventional inorganic coagulants, with reduction efficiencies up to 65 and 72%, respectively. Kumar et al. also tried a combination of activated carbon and MOC for removal as like Margarida et al. (2017). This significantly increased removal efficiency, with the maximum removal efficiencies reported as 98 and 94% for PFOS and PFOA, respectively, with low-dose MOC (with powder-activated carbon).
Regarding practical application of MOCs, some researchers have checked its coagulation activity, including direct usage of the seed itself, for real surface water and wastewater. Poumaye et al. (2012) dried and transformed into a powder the seeds of M. oleifera to clarify the surface water, i.e., the river M’Poko. By using sand/coal filtration with coagulation treatment with MOC seed powder, the turbidity and a quantity of organic matter could be treated to the required standards. Vieira et al. evaluated M. oleifera seed with a mixed culture that was used for the biodegradation of hydrocarbons present in the effluent from fuel distribution terminals contaminated with diesel oil and gasoline under different coagulation conditions (Vieira et al. 2012). The biodegradation was evaluated by varying the M. oleifera seed concentration, drying temperatures, and seed drying times. They compared M. oleifera seed with chitosan, and the results indicated that Chitosan is a superior coagulant compared with M. oleifera for the sedimentation.
However, MOCs does not always lead to better coagulation activity. Muyibi and Evison (1995) found that the residual turbidity of samples increased with the decrease in initial turbidity at the optimum dosage of water-extracted MOC. This indicates that water-extracted MOC may not be an efficient coagulant for low turbidity water. It is a problem for the usage of water-extracted MOC for drinking water treatment, because the turbidity of its raw water is usually low, especially in Japan. This is also mentioned by other researchers (Pereira et al. 2017). Pereira et al. mentioned another problem—an increase in the organic matter after coagulation/flocculation with MOCs. Organic matter, especially the dissolved organic carbon (DOC) component of total organic carbon (TOC), is related to biochemical oxidation demand (BOD) and chemical oxidation demand (COD) and can cause undesired colors and odors of the water. This increase is a classical problem with treated water, which is likely caused by unnecessary components eluted during extraction of the coagulation active components from the seeds, as Beltrán-Heredia et al. mentioned (Beltrán-Heredia et al. 2012).
There is a possibility that these problems can be solved by improving the method for extracting the coagulation-active components from the M. oleifera seeds or purification method for them. These developments are necessary for the wide use of MOCs, not only for drinking water treatment but also for other water treatment processes.
2.2 Isolation and Improvement in Extraction of Coagulation Active Components
The coagulation active components of water-extracted MOC were found to be soluble cationic proteins with molecular weight of about 13 kDa isoelectric with a pH value of 10–11 (Ndacigengesere et al. 1995). The amino acid sequences of this protein were revealed by Gassenschmidt et al. (1991, 1995). These studies were conducted in 1990; however, the coagulation proteins present in M. oleifera seeds and their characterization have been investigated recently as well. As an example, Alves et al. (2017) reported that globulin and albumin were the highest protein fractions in M. oleifera seeds with 53 and 44%, respectively. By using protein profile analysis, Bodlund I. et al. discovered that the major protein bands had molecular weights of around 6.5 and 9.0 kDa, respectively (Bodlund et al. 2013).
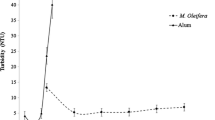
Based on the scientific information about coagulation-active components in M. oleifera seeds, trials to improve extraction of these components would be a reasonable development in order to improve the coagulation activity of MOCs. As an example, our research group found a better solvent for the extraction. To improve the coagulation-active components, we used a salt solution, because Olsen reported that ethanol, hexane, methanol, and acetone could not improve the extraction of the coagulation active component (Olsen 1987). It was well known that the solubility of proteins increases with salt concentration at low salt ionic strength owing to the decrease in mutual association of protein molecules by shielding with the salt molecules (White et al. 1968; Voet and Voet 1990). Because the active component for coagulation in water-extracted MOC is a protein (Gassenschmidt et al. 1991, 1995), it was possible that its solubility could be enhanced by the salt solution, thus improving the coagulation activity of MOCs. Therefore, we tried some salt solutions as a solvent to improve the extraction of the active component. The results led us to the following conclusions, which were reported in Okuda et al. (1999). First, extraction with 1.0 mol/L NaCl solution enhanced the coagulation activity of MOC. Second, the MOC extracted with NaCl solution (salt-extracted MOC) achieved seven times lower turbidity of a kaolin solution than conventional MOC extracted with distilled water could, as shown in Fig. 4.2. Third, salt-extracted MOC was an effective coagulant, with a greater than 95% decrease in the 50 NTU initial turbidity of kaolin using only 4 mL/L, whereas 32 mL/L of water-extracted MOC was necessary for only a 78% turbidity decrease. In Japan, turbidity standard is 2 mg/L for drinking water, so only salt-extracted MOC could archived this level in this experiment. The active component extracted with the salt solution was not the same as that extracted with water; its molecular weight was found to be about 3 kDa by Okuda et al. (2001a). Other research groups confirmed these results. For example, Madrona et al. revealed the coagulation properties and potential for the MOC extracted by a KCl solution (Madrona et al. 2010). Following our research Okuda et al. (2001a), other extractants, including other salt solutions and tap water, were used as the extraction solvent for the improvement of MOCs activity in some studies, in which better results compared to those with distilled water were found. Here, alum could reduce residual turbidity less than 1 mg/L at around 2 mg-Al/L without the increase of residual DOC.
Even in salt-extracted MOC, the problem of increasing residual organic matter still exists. As shown in Fig. 4.2, salt-extracted MOC could decrease the residual DOC from 20 mg-C/L (water-extracted MOC) to 2 mg-C/L at the dosage of MOC for around 7 mg/L of residual turbidity. For example, the standard of TOC (total organic carbon), similar to DOC in treated water, is 3 mg/L in Japan, salt-extracted MOC could meet this standard with turbidity removal. In addition to the research on improving the extraction solvents, the coagulation activity between species of Moringa (Moringa stenopetala and M. oleifera) was compared in order to find coagulation-active components with higher performance. Dalvand et al. compared the efficiency of M. stenopetala seed coagulant (MSC) with that of alum, and they found that a salt-extracted MSC-alum hybrid coagulant could remove a dye (Direct Red 23 azo dye) from textile wastewater. Moreover, a ninhydrin test was used to determine the quantity of primary amines in the salt-extracted MSC and salt-extracted MOC, which showed that salt-extracted MSC was much more effective than salt-extracted MOC for the dye removal (Dalvand et al. 2016).
2.3 Purification of Active Components from Crude Extractant
MOCs are problematic for real application, as mentioned at Sect. 4.2.1, because of the increase of organic matter in treated water, which likely is caused by unnecessary components in MOC. To remove these components from salt-extracted MOC, Ghebremichael et al. tried purification using ion exchange. They discussed the chemical characteristics, coagulation, and antimicrobial properties of purified MOC (p-MOC) (Ghebremichael et al. 2005). Dezfooli et al. reported that coagulant proteins from M. oleifera seeds could be purified by removing the seed oil followed by a protein salting out method with 40% (NH4)2SO4 combined with subsequent dialysis and heat treatment (Dezfooli et al. 2016). In another study, Baptista et al. reported the efficiency of ultrafiltration for MOC (Baptista et al. 2015). They used surface water for the evaluation of salt-extracted MOC performance, and better performance in the removal of color (89%), turbidity (89%), compounds with UV254 nm absorbance (76%), and SUVA (62%) was achieved by using this p-MOC purified by membrane filtration. This same research group developed another method for active component purification, i.e., the fractionation of proteins. With it, 87% removal of color, 90% removal of turbidity, and 79% removal of UV254 nm were achieved using 13 mg/L of the globulin coagulant from M. oleifera seeds for treatment of low turbidity water (50 NTU) without leading to an excessive increase in the DOC of the treated water (Alves et al. 2017).
Regarding purification, our research group also tried to isolate the active coagulation component in the salt-extracted MOC and established a method for such (Okuda et al. 2001a, b). In that study, the active component was isolated (purified) from salt-extracted MOC through a sequence of steps that included salting out via dialysis, removal of lipids and carbohydrates via homogenization with acetone, and fractionation via anion exchange (Fig. 4.3) by using DOC concentration and coagulation activity as indicators. After the ion exchange fractionation based on carbon concentration, one fraction eluted at 0.3 M NaCl gradient had high coagulation activity (peak 2 in Fig. 4.3, coagulation activity was calculated as like Eq. 4.1). Specific coagulation activity of the active components (ratio of DOC to MOC dosage) in this fraction was much higher than the crude extract (salt-extracted MOC). The active component was not the same as that of the water-extracted MOC; its molecular weight was only about 3 kDa.
Anion exchange chromatogram of pre-purified salt-extracted MOC. Open circles show coagulation activities, closed circles show DOC, and the dashed line shows the NaCl gradient (Okuda et al. 2001a)
In our related research, the coagulation mechanism of the p-MOC was also studied, which seemed to involve an enmeshment by the insoluble matters formed from the coagulation-active component. Other coagulation mechanisms such as double-layer compression (Fig. 4.4), interparticle bridging, and charge neutralization were not responsible for the coagulation by the salt-extracted p-MOC based on zeta potential and molecular weight analysis. The formation of insoluble matter was affected by bivalent cations, such as Ca2+, which may connect each active component molecule in the salt-extracted p-MOC to form the net-like structure shown in Fig. 4.5.
Model for the coagulation mechanism by salt-extracted p-MOC (Okuda et al. 2001b)
Based on the isolation and identification of the active component of salt-extracted MOC, a simple purification method was developed over a series of studies. The two-step purification consisting of (1) acetone washing of powdered active components insolubilized by sedimentation via dialysis and (2) their re-dissolution in NaCl solution was established as a practical purification method for salt-extracted MOC. The coagulation activity and residual DOC of this practical p-MOC are shown in Fig. 4.6. It can be seen that no residual DOC increases by keeping high coagulation activity was obtained at low carbon dosage to compare with salt-extracted MOC before purification, as shown in Fig. 4.2. This indicates that the method consisting of dialysis and re-dissolution was enough to prevent DOC from increasing in water treated via coagulation. We believe that this research was a stepping stone for other researches on the purification of MOCs.
To decrease the contamination by unnecessary components of MOC, not only the purification of the extracted solution was evaluated in our recent study, but also the pretreatment of the seed powder. Figure 4.7 shows the effect of prewashing time (washed with 100 mL distilled water per 1 g of seed powder at 5 °C) on the coagulation activity of salt-extracted MOC (extracted with 100 mL 0.3 M NaCl solution per 1 g of prewashed seeds), with oil-extracted (cold press method) seed powder used in this experiment. The coagulation activity (ratio of turbidity decreases against it in coagulation experiment without MOC) and residual DOC of the treated water are shown in Fig. 4.7. The DOC of the initial turbid water (kaolin solution with tap water) was 0.9 mg/L, and 50–60% of residual DOC could be reduced with two rounds of distilled water prewashing of the seed powder while maintaining high coagulation activity. Two rounds of prewashing seemed to be the best, as some decrease in coagulation activity was observed after three rounds of prewashing. This was due to the extraction of some coagulation-active components via prewashing. In that study, the optimum temperature and volume of distilled water for prewashing were also investigated, but no significant difference was observed in these conditions (data not shown).
3 Studies for Practical Use
3.1 Evaluation for Real Water Treatment Systems
For real-use application, it is necessary to compare the capacity and coagulation properties of MOCs to those of traditional coagulants, such as aluminum sulfate (alum). Arnoldsson et al. investigated the optimum coagulant dosage for different levels of turbidity using water-extracted MOC combined with direct rapid sand filtration after coagulation (Arnoldsson et al. 2008). They showed that coagulation with alum led to treatment that was more efficient than that with water-extracted MOC although prolonged sedimentation was necessary for the MOC to produce water of acceptable quality (WHO water guidelines). In contrast, it is mentioned that the treatment with water-extracted MOC did not change the chemistry of the treated water in their experiments. They concluded that water treatment with water-extracted MOC is a sustainable solution for coagulation in drinking water treatment. MOCs were compared with alum in other studies (Okuda et al. 1999; Madrona et al. 2010).
For the cost perspective, the direct use of seed powder for water treatment was studied in further trials for a real application. Our research group conducted one such trial by using a slurry of the cake that remains after oil extraction from M. oleifera seeds (s-MOC), and its results are being introduced. M. oleifera seeds were shelled (dehusked) and grinded into powder for the oil extraction process. The oil in the seed powder was removed via Soxhlet extraction using n-hexane as the solvent for three cycles of extraction and then dried overnight in an oven at 50 °C. Oil extraction is recommended as a method for purifying water-extracted MOC, as well. Samples from the Sungai baluk river, Pahang, Malaysia, of 500 mL were placed in 500 mL beakers, and jar testing was conducted with rapid mixing of 200 rpm for 2 min, 40 rpm for 25 min, and sedimentation for 1 h. Figure 4.8 shows the comparison between the dosage of s-MOC and turbidity. The lowest turbidity value was 4.7 NTU with 1.5 g/L of s-MOC. In this method, coagulation components in seeds were extracted by sample water itself after its addition, then the extracted components would act as a coagulant. There is a possibility that some part of s-MOC remained in the supernatant and contributed residual turbidity; however, the effect was small in this experiment.
3.2 Additional Target Materials
For more understanding, identification, and improvement of MOCs activity for some potential treatment, targets (contaminants in water) are also important for real application of MOCs, meaning “What can MOC treat?” and “What can be removed with a concurrent decrease in turbidity?”
There is a difficulty in coagulation for organic particle removal, as mentioned above. Therefore, instead of its removal ability, Ghebremichael et al. investigated the coagulation and antibacterial activity of p-MOC (Ghebremichael et al. 2005). The p-MOC showed not only enough flocculating ability, but also some antibacterial effects with 1.1–4.0 log reduction of bacteria. Other reports showed that MOC prevents the growth of coliforms and pathogens (Santos et al. 2012) and bacteria (Shan et al. 2017), and that the treatment using MOC could be met for some disinfection requirements (Srivastava 2014; Jabeen et al. 2008). Even treated water rarely is completely free of germs, so it is important to prevent the growth of microorganisms (Amagloh and Benang 2009). Our research group found that MOCs also helped to remove dirt, solid particles, and even some bacteria and fungi (Bina et al. 2010; Eman et al. 2014). In that work, filtration was not conducted during the extraction stage; rather, the suspension (slurry) of oil-free s-MOC (10–30 g/L) was used to treat the Sungai baluk river and wastewater samples. There was coagulation activity (85–94% turbidity removal) in the jar test operated with an initial speed of 150 rpm for 2 min. In addition, s-MOC helped to prevent the growth of microorganisms. The concentration of bacteria was reduced to 7.5 × 104 from 1.7 × 105 CFU/mL in river water and to 1.0 × 104 from 1.1 × 106 CFU/mL in wastewater (Shan et al. 2017) (Table 4.1).
Coagulation of dissolved targets is difficult, but it is possible that target materials are adsorbed by flocs and removed with them via sedimentation or filtration. Santos et al. tried to remove humic acids from water by using salt-extracted MOC (extracted with 0.15 M NaCl) (Santos et al. 2012). Treatment with a low salt-extracted MOC concentration removed humic acids from water, and the extract dosage determined in the study does not impart untoward odor or color to the treated water. It is most difficult to remove soluble inorganic materials such as heavy metals. We reported on heavy metal removal via direct use of M. oleifera seed, s-MOC (Shan et al. 2017). The wastewater samples were collected from the Sungai baluk river. s-MOC was prepared by immersing cake residue, which is seeds residue by removing oil into the distilled water, and different concentrations of s-MOC were used for the jar test. The initial and final heavy metal (Cu, Cd, and Pb) concentrations were measured, and the removal percentages were calculated. The removal of heavy metals increased proportionally with s-MOC dosage until optimum removal was achieved. s-MOC showed high efficiency in the removal of heavy metals from the Sungai baluk river samples, up to 98% Cu and Cd successfully removed. Pb was also reduced by up to 78% (Fig. 4.9). It is possible that heavy metals were removed via coagulation, which includes adsorption onto flocs produced, but it is also possible the seeds themselves absorbed them. This biosorption would become an alternative technique for heavy metals removal from water with combination of coagulation/flocculation.
Effect of different concentrations of s-MOC on heavy metal removal from Sungai baluk river and wastewater samples (Shan et al. 2017)
3.3 Water Softening and Disinfection
Water softening means to decrease the hardness of water, which is caused by ions in water, typically those of calcium and magnesium. Water-extracted MOC shows potential as a softening agent, as evidenced by Muyibi and Evison (1995). They analyzed 17 water samples from hand-dug wells and found that water-extracted MOC can reduce residual hardness, the process of which is influenced by water-extracted MOC dosage and the hardness components, i.e., “calcium only” or “calcium and magnesium”. They also found that the absorption isotherm for softening with water-extracted MOC was linear and of approximately the Langmuir type.
3.4 Novel Studies
Some challenging and progressive approaches based on coagulation/flocculation with MOCs also were tried to exploit those substances. Santos et al. (2016) evaluated the effectiveness of the coagulation/flocculation using M. oleifera functionalized with magnetic iron oxide nanoparticles, which produced flakes that are attracted by an external magnetic field, thereby allowing fast settling and separation of the clarified liquid. The magnetic functionalized MOC could effectively remove 90% of turbidity, 85% of apparent color, and 50% of the compounds with absorption at UV254 nm from surface waters under the influence of an external magnetic field for 30 min. Then, the coagulation/flocculation treatment using magnetic functionalized MOC. Coagulant was able to reduce the values of the physicochemical parameters evaluated with reduced settling time.
The combination with other new technologies is also interesting. Our research group tries to use salt-extracted MOC as a pretreatment of membrane filtration to prevent membrane fouling as an application study for drinking water treatment based on past research (Katayon et al. 2007). In our novel research, coagulation was conducted for the removal of turbidity prior to each microfiltration experiment. Microfiltration treatment of river water without pretreatment resulted in a rapid increase in transmembrane pressure (TMP; an indicator of membrane fouling) from 12 to 24 kPa within three filtration cycles (60 min per cycle), including interval backwashing with clean water without pre-coagulation. Coagulation with salt-extracted MOC performed prior to micro-filtration effectively reduced membrane fouling (1–3 mL-MOC/L), with a significant reduction in membrane fouling observed (based on TMP). The fouling mitigation by salt-extracted MOC pre-coagulation was maximized with a 2 mL/L dosage, which resulted in only an increase in TMP from 12.0 to 12.4 kPa over three filtration cycles. It is possible that unnecessary DOC increased the TMP at dosages higher than 2 mL/L. This indicates that a sufficient level of membrane fouling mitigation in micro-filtration can be achieved via salt-extracted MOC pre-coagulation, even TMP increase could be more prevented by pre-coagulation with alum. It is possible that some increase in TMP (fouling) was caused by MOCs itself (data not shown).
4 Conclusions
The basics of application of M. oleifera seed for water treatment have been studied and increasingly developed in the past 20–30 years, in particular, in the areas of extraction improvement, extract purification, and scientific interest, such as the isolation of active component.
Target materials in raw water, not only “turbidity” but also others, and effect of raw water quality on them in coagulation/flocculation using M. oleifera seed have been energetically revealed in recently.
The most of problem for its real utilization and the usage combined with other novel technologies has been studied.
References
Abidin ZZ, Madehi N, Yunus R (2017) Coagulative Behavior of Jatropha curcas and its Performance in Wastewater Treatment. Environ Prog Sustain Energy 36(6):1709–1718
Alves BAT, Oliveira SM, Guttierres GR, Salcedo VAM, Rosangela B, Fernandes VM (2017) Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep Purif Technol 180:114–124
Amagloh FK, Benang A (2009) Effectiveness of Moringa oleifera seed as coagulant for water purification. Afr J Agric Res 4:119–123
Antov MG, Sciban MB, Prodanovic JM, Kukic DV, Vasic VM, Dordevic TR, Milosevic MM (2018) Common oak (Quercus robur) acorn as a source of natural coagulants for water turbidity removal. Ind Crops Prod 117:340–346
Arnoldsson E, Bergman M, Matsinhe N, Persson KM (2008) Assessment of drinking water treatment using Moringa oleifera natural coagulant. Vatten 64:137–150
Baptista ATA, Coldebella PF, Cardines PHF, Gomes RG, Vieira MF, Bergamasco R, Vieira AMS (2015) Coagulation–flocculation process with ultrafiltered saline extract of Moringa oleifera for the treatment of surface water. Chem Eng J 276(1):166–173
Beltran-Heredia J, Sanchez-Martin J (2009) Removal of sodium lauryl sulphate by coagulation/flocculation with Moringa oleifera seed extract. J Hazard Mater 164(2–3):713–719
Beltran-Heredia J, Sanchez-Martin J, Delgado-Regalado A, Jurado-Bustos C (2009) Removal of Alizarin Violet 3R (anthraquinonic dye) from aqueous solutions by natural coagulants. J Hazard Mater 170(1):43–50
Beltrán-Heredia J, Sánchez-Martín J, Muñoz-Serrano A, Peres JA (2012) Towards overcoming TOC increase in wastewater treated with Moringa oleifera seed extract. Chem Eng J 188:40–46
Bichi MH (2013) A review of the applications of Moringa oleifera seeds extract in water treatment. Civil Environ Res 3:1–9
Bina B, Mehdinejad MH, Gunnel D, Guna R, Nikaeen M, Movahedian HA (2010) Effectiveness of Moringa oleifera coagulant protein as natural coagulant aid in removal of turbidity and bacteria from turbid waters. World Acad Sci Eng Technol 4:7–28
Bodlund I, Rajarao GK, Bodlund I, Sabarigrisan K, Chelliah R, Sankaran K (2013) Screening of coagulant proteins from plant material in southern India. Water Sci Technol Water Supply 13(6):1478–1485
Dalvand A, Gholibegloo E, Ganjali MR, Golchinpoo N, Khazaei M, Kamani H, Hosseini SS, Mahvi AH (2016) Comparison of Moringa stenopetala seed extract as a clean coagulant with Alum and Moringa stenopetala-Alum hybrid coagulant to remove direct dye from textile wastewater. Environ Sci Pollut Res Int 23(16):16396–16405
Dezfooli SM, Mussarat S, Salma BF, Hitam SM, Bachmann RT, Uversky VN, Uversky VN (2016) A simplified method for the purification of an intrinsically disordered coagulant protein from defatted Moringa oleifera seeds. Process Biochem 51(8):1085–1091
Eman NA, Tan CS, Makky EA (2014) Impact of Moringa oleifera cake residue application on waste water treatment: a case study. J Water Resour Prot 6:677–687
Gassara F, Antzak C, Ajila CM, Sarma SJ, Brar SK, Verma M (2015) Chitin and chitosan as natural flocculants for beer clarification. J Food Eng. 166:80–85
Gassenschmidt U, Jany KD, Tauscher B (1991) Chemical properties of flocculant—active proteins from Moringa oleifera lam. Biol Chem Hopper-Seyler 372:659
Gassenschmidt U, Jany KD, Tauscher B, Niebergall H (1995) Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochem Biophys Acta 1243:477–481
Ghebremichael KA, Gunaratna KR, Henrikson H, Burmer H, Dalhammar G (2005) A simple purification and activity assay for the coagulant protein from Moringa oleifera seed. Water Res 32(11):2338–2344
Jabeen R, Shahid M, Jamil A, Ashraf MB (2008) Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera. Pak J Bot 40:1349–1358
Jahn SAA (1988) Using Moringa seeds as coagulants in developing countries. J Am Water Works Assoc 80(6):43–50
Katayon S, Noor MM, Tat WK, Halim GA, Thamer AM, Badronnisa Y (2007) Effect of natural coagulant application on microfiltration performance in treatment of secondary oxidation pond effluent. Desalination 204:204–212
Kumar VA, Roshan DR, Puspendu B (2012) A review on chemical coagulation/flocculation technologies for removal of color from textile wastewaters. J Environ Manag 93(1):154–168
Kumar PB, Kumar PS, Fatihah S (2015) A comparative study of coagulation, granular- and powdered-activated carbon for the removal of perfluorooctane sulfonate and perfluorooctanoate in drinking water treatment. Environ Technol 36(17–20):2610–2617
Madrona GS, Serpelloni GB, Salcedo Vieira AM (2010) Study of the effect of saline solution on the extraction of the Moringa oleifera seed’s active component for water treatment. Water Air Soil Pollut 211:409–415
Mallevialle J, Bruchet A, Fiessinger F (1984) How safe are organic polymers in water treatment. J Am Water Works Assoc 76(6):87–93
Margarida RT, Serrao SV, Pereira CF, Rosangela B (2017) Green technologies for cyanobacteria and natural organic matter water treatment using natural based products. J Clean Prod 162:484–490
Maryam L, Jing L, Bo M, Jing L (2014) Recovery of struvite via coagulation and flocculation using natural compounds. Environ Technol 35(17–20):2289–2295
Muyibi SA, Evison LM (1995) Optimizing physical parameters affecting coagulation of turbid water with Moringa oleifera seeds. Water Res 29(12):2689–2695
Ndacigengesere A, Narasiah KS (1996) Influence of operating parameters on turbidity removal by coagulation with Moringa oleifera seeds. Environ Technol 17:1103–1112
Ndacigengesere A, Narasiah KS, Talbot BG (1995) Active agents and mechanism of coagulation of turbid water using Moringa oleifera. Water Res 29:703–710
Okuda T, Baes AU, Nishijima W, Okada M (1999) Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res 33(15):3373–3378
Okuda T, Baes AU, Nishijima W, Okada M (2001a) Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res 35(2):405–410
Okuda T, Baes AU, Nishijima W, Okada M (2001b) Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Res 35(3):830–834
Olsen A (1987) Low technology water purification by Bentone Clay and Moringa oleifera seed flocculation as performed in sudanese villages: effects on Schistosoma mansoni cercariae. Water Res 21:517–522
Pereira CF, Rosangela B, Serrao SV, Margarida RT (2017) The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem Eng J 313:226–237
Poumaye N, Mabingui J, Lutgen P, Bigan M (2012) Contribution to the clarification of surface water from the Moringa oleifera: case M’Poko river to Bangui, Central African Republic. Chem Eng Res 90(12):2346–2352
Rajamohan N, Fatma AF, Amal AS (2018) Municipal waste water treatment by natural coagulant assisted electrochemical technique-Parametric effects. Environ Technol Innov 10:71–77
Santos AFS, Paiva PMG, Teixeira JAC, Brito AG, Coelho LCBB, Nogueira R (2012) Coagulant properties of Moringa oleifera protein preparations: application to humic acid removal. Environ Technol 33(1):69–75
Santos TR, Silva MF, Nishi L, Vieira AM, Fagundes-Klen MR, Andrade MB, Vieira MF, Bergamasco R (2016) Development of a magnetic coagulant based on Moringa oleifera seed extract for water treatment. Environ Sci Pollut Res Int 23(8):7692–7700
Sengupta ME, Olsen A, Thamsborg SM, Palsdottir GR, Dalsgaard A, Keraita B, Boateng OK, Keraita B, Boateng OK (2012) Use of Moringa oleifera seed extracts to reduce helminth egg numbers and turbidity in irrigation water. Water Res 46(11):3646–3656
Shan TC, Matar MA, Makky EA, Eman NA (2017) The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl Water Sci 7:1369–1376
Sorour S, Naz C, Reza PA, Sam H (2015) Mucilaginous seed of Ocimum basilicum as a natural coagulant for textile wastewater treatment. Ind Crop Prod 69:40–47
Srivastava M (2014) The health benefits of Moringa oleifera plant. Live Strong Foundation. http://www.livestrong.com/article/431418-the-health-benefits-of-moringa-oleifera-plants/. Accessed at 30 June 2018
Suffian MY, Abdul AH, Suffian YM, Abdul AH, Zuhairi AA, Ahmad ZMFM, Fatihah S, Ahmad BNE, Zuhairi AA (2018) Floc behavior and removal mechanisms of cross-linked Durio zibethinus seed starch as a natural flocculant for landfill leachate coagulation-flocculation treatment. Waste Manag 74:362–372
Vieira RB, Vieira PA, Cardoso SL, Ribeiro EJ, Cardoso VL (2012) Sedimentation of mixed cultures using natural coagulants for the treatment of effluents generated in terrestrial fuel distribution terminals. J Hazard Mater 231–232:98–104
Voet D, Voet JG (1990) Biochemistry. Wiley, New York
White A, Handler P, Smith EL (1968) Principles of biochemistry, 4th edn. Mcgraw-Hill, New York
Yee SKP, Yeong WT (2014) Coagulation-flocculation treatment of high-strength agro-industrial wastewater using natural Cassia obtusifolia seed gum: treatment efficiencies and flocs characterization. Chem Eng 256:293–305
Zonoozi MH, Alavi Moghaddam MR, Arami M (2011) Study on the removal of acid dyes using chitosan as a natural coagulant/coagulant aid. Water Sci Technol 63(3):403–409
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Okuda, T., Ali, E.N. (2019). Application of Moringa oleifera Plant in Water Treatment. In: Bui, XT., Chiemchaisri, C., Fujioka, T., Varjani, S. (eds) Water and Wastewater Treatment Technologies. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3259-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-3259-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3258-6
Online ISBN: 978-981-13-3259-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)