Abstract
Several coagulants/flocculants have been studied in order to remove the color and turbidity of raw water, employing natural ones demonstrated advantages in relation to chemicals. Moringa oleifera Lam is a natural polymer that has been gaining prominence in water treatment. It acts as a clarifying agent, providing a cationic protein that destabilizes the particles contained in a liquid medium. The main objective of the present work is to study the efficiency in terms of removing color and turbidity of raw water in order to obtain drinking water. For this purpose, different coagulant solutions were obtained utilizing three solutions of KCl in different concentrations (0.01, 0.1, and 1 M) and pure water combined with M. oleifera Lam seed. Each coagulant solution obtained was studied with concentrations ranging from 50 to 600 ppm of Moringa in solution. The pH was varied (4.0, 6.0, and 8.0) with 25% and 50% sodium hydroxide solution (NaOH) and hydrochloric acid (HCl), respectively. The tests were conducted with the “Jar Test Device” and the efficiency of the process was evaluated regarding the reduction of color and turbidity. The best results were found employing the coagulant solutions extracted with 1 M salt solution, pH 8.0, and different concentrations of coagulant solution. It is important to explain that the best results were in various concentration ranges, as the concentration of protein in solution becomes higher, the greater is its power as a coagulant. The lowest content of protein was found in the solution extracted with water, which consequently had the lowest values of color and turbidity removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In order to control the quality of water, it is necessary to track various indicators. Nowadays, the concern about contamination of aquatic environments has increased, especially when water is used for human consumption. Thus, there is great importance in either the development of more sophisticated treatments or the improvement of the current ones. Turbidity and color removal is one of the most important steps in a water treatment process, which is generally achieved using coagulants. Many coagulants are widely used in conventional water treatment processes, based on their chemical characteristics. These coagulants are classified into inorganic, synthetic organic polymers, and natural coagulants.
Alum has been the most widely used coagulant because of its proven performance, cost effectiveness, relative easy handling, and availability. Recently, much attention has been drawn on the extensive use of alum. According to Driscoll and Letterman (1995), the utilization of alum has raised a public health concern because of the large amount of sludge produced during the treatment and the high level of aluminum that remains in the treated water. McLachlan (1995) discovered that the intake of large quantity of alum salt may cause Alzheimer disease.
For these reasons, and also due to others advantages of natural coagulants/flocculants over chemicals, some countries such as Japan, China, India, and the United States have adopted the use of natural polymers in the treatment of surface water for the production of drinking water (Kawamura 1991).
The application of natural materials for clarifying turbid waters of rivers is an ancient and home-based practice in tropical developing countries where these natural materials act as primary coagulants. One example of these coagulants is the seed of the tropical tree Moringa oleifera (MO), which contains active agents with excellent activity and coagulating properties. The extract of the seeds has been mentioned for drastically reducing the amount of sludge and bacteria in sewage (Muyibi and Evison 1995).
M. oleifera is a tropical plant belonging to the family Moringaceae (Katayon et al. 2006), a single family of shrubs with 14 known species. MO is native of India but is now found throughout the tropics (Bhatia et al. 2007). It is a non-toxic natural organic polymer. The MO tree is generally known in the developing world as a vegetable, a medicinal plant, and a source of vegetable oil (Katayon et al. 2006). MO is drought-tolerant and has nutritional, medicinal, and water-cleaning attributes. Its leaves, flowers, fruits, and roots are used locally as food ingredients. The medicinal and therapeutic properties of MO have led to its utilization as a cure for different ailments and diseases, physiological disorders, and in Eastern allopathic medicine (Akhtar et al. 2007). Additionally, the coagulant is obtained at extremely low or zero net cost (Ghebremichael et al. 2005).
If MO is proven to be active, safe, and inexpensive, it is possible to use it widely with drinking water and wastewater treatment. Besides, MO may yet have financial advantages bringing more economic benefits for the developing countries (Okuda et al. 1999).
Nkurunziza et al. (2009) reported that the use of MO as a coagulant in water treatment could be applied both at household and industrial scale. As MO is more effective at high turbidity, its use at large scale could be particularly more beneficial during rainy season when water is at its highest turbidity level and the water treatment plants are often forced to close.
Vieira et al. (2009) reported the potential use of MO seeds as a natural adsorbent for the treatment of dairy industry wastewater (DIW) with low cost. The efficiencies of removal obtained were of up to 98%, for both color and turbidity, these results were reached using 0.2 g MO and 0.2 L of 1.0 g/L sorbate solution (DIW). The results showed that MO seed keeps its adsorption power under a pH range between 5.0 and 8.0.
Okuda et al. (1999) compared the coagulation efficiency of two different methods. In the first one, salt solution (1 M NaCl) extraction was employed while water extraction was used in the second, which is the conventional method. The former was found to be more efficient. They observed yet no difference in the coagulation for extracts using any one of the four 1:1 salts (NaCl, KNO3, KCl, and NaNO3).
In the aforementioned context, the present work aims at studying how efficient the removal of color and turbidity is by means of using different seed extracts of M. oleifera Lam as a natural coagulant in the process of producing drinking water.
2 Materials and Methods
The superficial water used in the tests was collected at the same point of the capitation of the water-supplying company of the city of Maringá, Paraná, Brazil, in the basin of the river Pirapó.
The raw water was characterized in terms of chemical and physical parameters after its collection and before the coagulation/flocculation/sedimentation tests. The parameters evaluated were: color and turbidity (HACH DR/2010 spectrophotometer), compounds with absorption in UV-254 nm (LS Logen Scientific, Alpax spectrophotometer), iron concentration, total dissolved solids, and total suspended solids (APHA 1995).
Although the water samples were stored at 4°C, they were brought to room temperature (23–25°C) prior to jar testing. The water temperature was kept constant in all tests because otherwise it could significantly influence the water viscosity.
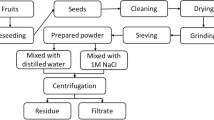
The coagulant solution was prepared using four different extractions as coagulant, which were obtained through extraction with water and KCl solution in three molar concentrations, 0.01, 0.1, and 1 M. The salt and molarity were obtained from data found in literature (Okuda et al. 1999; Nkurunziza et al. 2009).
The coagulant solution of M. oleifera Lam was made ready in the day of testing by grinding 1 g of seed pulp and 100 mL of water or saline solution in the blender. After that, the solution is agitated for 30 min and vacuum filtered.
We used concentrations of the extract of M. oleifera Lam 50, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, and 600 ppm in the coagulation/flocculation, and the pH of the raw water ranged from 4.0, 6.0, and 8.0, thereby trying to avoid inefficiency in the process.
The tests of coagulation/flocculation/sedimentation were performed in the “jar test device” Simple, Milan, Model JT101/6 of six evidence-controlled rotation of the mixing shafts. The working conditions for the process of coagulation/flocculation/sedimentation were rapid mixing speed (100 rpm), time of coagulation (3 min), speed of slow mixing (15 rpm), time of flocculation (15 min), and settling time (60 min).
The efficiency of the process was evaluated by removing color and turbidity measured by spectrophotometer HACH DR/2010, according to the procedure recommended by Standard Methods (APHA 1995)
The method of Lowry et al. (1951) was adopted in order to determine the amount of total proteins extracted in the preparation of the standard solution of M. oleifera Lam and verify the relationship between the dosage of protein and efficiency of the coagulant.
3 Results and Discussion
Although many studies have used synthetic water in the experiments, this work chose to use raw water collected directly from the Pirapó River. Therefore, it is important to consider that the natural compounds may cause variations in their composition, which interfere in the treatment process. All those factors are taken into account when evaluating the obtained results.
The characteristics of the superficial water used in this study are presented in Table 1.
It can be observed that the water used in the experiments has apparent color, turbidity, solids, and amount of compounds with absorption in UV-254 nm relatively high.
It is noticeable that the water has high turbidity and color. Of note, Nkurunziza et al. (2009) concluded that MO is not a good coagulant in water with low turbidity and color.
3.1 Indicative Value of Protein
The indicative values of protein present in the solutions of Moringa with water and with KCl at different molarities (0.01, 0.1 and 1 M) are presented in Table 2.
Table 2 shows the value of protein as a function of salt solution molarity. An increase in the amount of protein is observed due to the elevation of KCl concentration. This is an important fact because it is known that the MO seed protein is the most important compound in the process of clarifying the water. MO is reported to have a cationic dimeric protein of high molecular weight, which destabilizes the particles in the water through a process of neutralization and adsorption, flocculate colloids followed by sedimentation (Vieira et al. 2009). It is important to notice that the lower value found for the concentration of protein was extracted with water.
3.2 Influence of KCl Molarity and pH
Figures 1 and 2 show the relationship between turbidity and color removals (%), respectively, as a function of MO concentration (ppm) for the four prepared coagulant solutions (with water and KCl at different molarity). These coagulation/flocculation tests were carried out using raw water at pH equal to 8.0.
In order to remove turbidity of raw water with pH 8.0, as shown in Fig. 1, the best results were obtained with the highest saline concentration, or 1 M KCl, presenting a removal degree of approximately 99.7%. For 0.1 M KCl, the value was 80%, 60% for 0.01 M KCl, and only 45% to extraction with water. Therefore, the decline in the saline concentration causes decrease in percentages of turbidity removal. This may be related to the amount of protein found in the three different salt solutions (Table 2). As the level of protein rises, the higher is the coagulation activity; consequently, the better is the efficiency of removal for both color and turbidity.
Okuda et al. (1999) studied the coagulation process in water solutions using coagulants obtained from M. oleifera and extracting the active component with 1 M NaCl, which allowed the authors to obtain a reduction of turbidity of 95%. In that case, the water sample had an initial turbidity of 50 µT and a 4-ppm solution of coagulant obtained with 1 M NaCl was used. For the extraction with water, 32 ppm was necessary to reach a turbidity removal rate of 78%. The pH was measured to be 7.0.
Nkurunziza et al. (2009) employed a solution of 3% MO, extracted with a 1-M NaCl solution, finding turbidity removal rates of 83.2% (given an initial water turbidity of 50 µT) and 99.8% (initial water turbidity of 450 µT). The conclusion is that the seed of MO is most effective for the treatment of water with higher turbidity.
The color removal rate exhibited the same trend as the turbidity removal. By means of Figs. 1 and 2, it can be observed that the concentration chosen seems not to influence the percentage of removal of both turbidity and color.
For 1 M KCl, 450 ppm of the coagulant significantly increased removal of color. As concentration of the coagulant increased, color removal was also increased from approximately 80% to almost 100%.
The color removal was smaller than turbidity removal, ranging from 30%, 70%, and 72% to 83% for water and KCl solutions at 0.01, 0.1, and 1 M, respectively.
The results presented for the extraction of water are not the most representative, but may present an alternative affordable, simple, and low cost for water treatment in small rural communities, thus improving the quality of water consumed.
Figures 3 and 4 show turbidity and color removals (%), respectively, function of MO concentration (ppm) for the four prepared coagulation solutions at pH equal to 6.0.
Figures 3 and 4, for both turbidity and color, show that for pH 6.0, the best results were obtained performing the extraction with 1 M KCl; this behavior was similar to that observed with pH 8.0. However, the decrease in pH caused the percentage of removal to drop from 99.7% to 70% for turbidity, and from 98.5% to 80% for color.
The tests of coagulation/flocculation performed using coagulant solution obtained by extracting the protein with water and with KCl showed that the ability to coagulate in terms of reduction in residual color and turbidity of water is closely related to the content of proteins released in the process of extraction. Extraction with water was not an efficient method to remove color and turbidity.
By means of Figs. 3 and 4, it is shown that increasing the concentration of salt solution of extraction provides a release of larger proteins, which reflects an empowerment of coagulation.
Figures 5 and 6 show the turbidity and color removals (%), respectively, for raw water treatment at pH 4.0 using the four proposed coagulation solutions at different concentrations.
The lowest values of removal of both turbidity and color were found when pH was equal to 4.0, as shown in Figs. 5 and 6. This fact was expected since Okuda et al. (2001) stated that the coagulant activity of MO seed is low in pH levels smaller than 7.0.
The result is effective because it is near the pH of the raw water. So if the process would be implemented in a water treatment plant, it would not be necessary to correct the pH, making the process simple and easy to use.
In the work undertaken by Okuda et al. (2001) and Ndabigengesere and Narasiah (1998), we used a simulation of raw water; thus, the purpose of this study was to evaluate the different levels of pH and evaluate if the behavior was similar when raw water was capitated directly into the river.
Analyzing the pH, the best percentages of removal were observed at pH 8.0. This was also detected by Okuda et al. (2001). These authors studied the removal of turbidity in pH 0 to 14 and observed high coagulation activity at pH 8 or higher and little coagulation activity was noted with pH below 7. Ndabigengesere and Narasiah (1998) studied the removal of turbidity in pH 0 at 8.0 and observed the same behavior.
Based on the results obtained, it is observed that the seeds of MO are more effective if extracted using 1 M KCl in varying concentrations as well as with pH 8.0. Besides, it is possible to say that the mentioned process can be used to treat water at a low cost. For low pH (below 7.0) Amagloh and Benang (2009) recommend the combination of MO coagulant with another that has better results in removal.
3.3 Relationship Between the Mass of Protein, Salt Concentration, and Turbidity Removal
For pH 8.0, which showed the best results, we evaluated the relationship between the mass of protein, salt concentration, and the percentage of turbidity and color removals.
The indicative values of mass of protein present in the solutions of Moringa with water and with KCl at different molarities (0.01, 0.1, and 1 M) and the corresponding percentage of turbidity and color removals are presented in Table 3.
Table 3 shows that in the extraction with water, 9 g of protein led to 52% of turbidity and 33% of color removals; in the extraction with 0.01 M KCl, 9 g protein, the same mass of protein, led to a 65% of turbidity and 69% for color removal.
In the extraction with 0.01 M KCl, 48 g of protein led to 66% of turbidity and 68% of color removals; in the extraction with 0.1 M KCl, 48 g protein, the same mass of protein, led to a 79% of turbidity and 72% for color removal; and for extraction with 1 M KCl, 48 g protein led to a 96% of turbidity and 82% for color removal.
These results indicate that the process of coagulation and removal of compounds that give color and turbidity of water can be influenced by the protein amount by the amount of salt in the middle. Through Table 3, we can see clearly that these two factors contribute to the increase in the efficiency of removing color and turbidity of water. The highest density of charges, due to the dissociation of salt, can contribute to this increase in clotting.
Okuda et al. (1999) found no difference in the efficiency of coagulation in extracts using different salts (NaCl, KNO3, KCl, NaNO3), but emphasize the increase in the efficiency of coagulation when the coagulant extract is obtained with a higher concentration of saline, i.e., 1 M salt.
With the results presented in Table 3, we can say that MO is an effective coagulant in water treatment, for high turbidity and color, and adding salt boosted its effect.
4 Conclusions
The following conclusions can be draw from this ongoing study:
-
In general, the extraction of the active compound was efficient and can be applied to the coagulation/flocculation in varying concentrations for water obtaining treatment.
-
The achieved results show that the protein amount is higher for KCl extraction using 1 M.
-
The extract produced through the KCl solution and MO seeds showed good efficiency, being better than the extraction with water. The highest rate of turbidity and color removal were observed when using a high molarity of salt, i.e., 1 M concentration.
-
The experimental results allowed concluding that MO is more effective with pH 8.0.
-
The use of M. oleifera Lam seeds can be considered advantageous and a promising step towards improving the processes of water coagulation/flocculation.
References
Amagloh, F. K., & Benang, A. (2009). Effectiveness of Moringa oleifera seed as coagulant for water purification. African Journal of Agricultural Research, 4(1), 119–123.
Akhtar, M., Moosa Hasany, S., Bhanger, M. I., & Iqbal, S. (2007). Sorption potential of Moringa oleifera pods for the removal of organic pollutants from aqueous solutions. Journal of Hazardous Materials, 141(3), 546–556. doi:10.1016/j.jhazmat.2006.07.016.
APHA—American Public Health Association. (1995). Standard methods for the examination for water and wastewater, 19th ed. Washington, D.C.
Bhatia, S., Othman, Z., & Ahmad, A. L. (2007). Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. Journal of Hazardous Materials, 145(1–2), 120–126. doi:10.1016/j.jhazmat.2006.11.003.
Driscoll, C. T., & Letterman, R. D. (1995). Factors regulating residual aluminium concentrations in treated waters. Environmetrics, 6, 287–309.
Ghebremichael, K. A., Gunaratna, K. R., Henriksson, H., Brumer, H., & Dalhammar, G. (2005). A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Research, 39, 2338–2344. doi:10.1016/j.watres.2005.04.012.
Katayon, S., Noor, M. J. M. M., Asma, M., Ghani, L. A. A., Thamer, A. M., & Azni, I. (2006). Effects of storage conditions of Moringa oleifera seeds on its performance in coagulation. Bioresource Technology, 97(13), 1455–1460. doi:10.1016/j.biortech.2005.07.031.
Kawamura, S. (1991). Effectiveness of natural polyelectrolytes in water treatment. Journal Awa, Japan, 79(6), 88–91.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265.
McLachlan, D. R. C. (1995). Aluminum and the risk for Alzheimer's disease. Environmetrics, 6, 233–275.
Muyibi, S. A., & Evison, L. M. (1995). Moringa oleifera seeds for softening hardwater. Water Research, 29(4), 1099–1105.
Ndabigengesere, A., & Narasiah, K. S. (1998). Quality of water treated by coagulation using Moringa oleifera seeds. Water Research, 32(3), 781–791.
Nkurunziza, T., Nduwayezu, J. B., Banadda, E. N., & Nhapi, I. (2009). The effect of turbidity levels and Moringa oleifera concentration on the effectiveness of coagulation in water treatment. Water Science & Technology, 59, 1551–1558.
Okuda, T., Baes, A. U., Nishijima, W., & Okada, M. (1999). Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Research, 33(15), 3373–3378. doi:10.1016/S0043-1354/(99)00046-9.
Okuda, T., Baes, A. U., Nishijima, W., & Okada, M. (2001). Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Research, 35(3), 830–834. doi:10.1016/S0043-1354(00)00296-7.
Vieira, A. M. S., Vieira, M. F., Silva, G. F., Araújo, A. A., Fagundes-Klen, M. R. Veit, M. T., et al. (2009). Use of Moringa oleifera seed as a natural adsorbent for wastewater treatment. Water, Air, Soil Pollut, published on line. doi: 10.1007/s11270-009-0104-y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madrona, G.S., Serpelloni, G.B., Salcedo Vieira, A.M. et al. Study of the Effect of Saline Solution on the Extraction of the Moringa oleifera Seed’s Active Component for Water Treatment. Water Air Soil Pollut 211, 409–415 (2010). https://doi.org/10.1007/s11270-009-0309-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0309-0