Abstract
The present day global environmental pollution is resultant of modernization, industrialization, urbanization, and several other anthropogenic activities, which involve the huge application of trace metals. Among the trace metals, Arsenic (As) is known as the leading toxicant to the environment worldwide and having the various toxic effects on human and animal health. Exposure of As causes various types of health effects like dermal and neurological problems, reproductive and pregnancy effects, cardiovascular effects, diabetes mellitus, diseases of the respiratory system, multiorgan cancers, etc. The persistence of As in the environment may pollute or contaminate soils and aqueous system as both natural components or as the result of human activity. In recent years, the development of efficient green chemistry methods for detoxification of trace metal poisoning has become a major focus of researchers. It has been investigated in order to find an eco-friendly and recyclable technique for the removal of trace elements contamination from the natural resources. Bioremediation process in this regards is an option that offers the possibility to reduce or render trace and toxic elements such as As using plants and microbes. Among the various bioremediation processes, phytoremediation and bioremediation using microbes are quite effective. Phytoremediation includes the removal of contaminants with the help of green plants, while the microbial bioremediation includes the removal of trace and toxic elements by microorganisms (bacteria, fungi, yeast and algae) as sorbents. The aim of this chapter is to give an overview of the As contamination in the environment and also the mechanism of removal of the As from the contaminated resources by the potent application of plants and microbes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Industrial wastewaters is mainly responsible for the heavy metal contamination in the environment (Goutam et al. 2018; Bharagava et al. 2017a; Gautam et al. 2017; Saxena et al. 2016; Saxena and Bharagava 2015). However, Arsenic (As) is both a geogenic and anthropogenic contaminant, which poses a significant threat to human life, health, and social well-being in the current scenario. It is widely spread in a number of areas worldwide, including countries in Asia, North and Latin America, parts of Europe and Africa. Exposure to As in concentrations exceeded from the World Health Organization provisional guide value (WHO 2011) through the consumption of As-enriched groundwater is having adverse impacts on human health (Bhattacharya et al. 2014). The As calamity is severe among Asian countries, especially the Ganga-Meghna-Brahmaputra plain of India and Bangladesh (Chakraborti et al. 2013, 2016).
Identification of the safe aquifers in naturally occurring As-contaminated areas is the main challenge for the scientific community worldwide. There are some chemical and geological processes which are responsible for the mobilization of As; hence the proper knowledge of these processes will be very useful to overcome the situation. Studies have been documented that more than 6 million people are at the risk from drinking As-contaminated water >50 μg/L in West Bengal, while this number is 30–35 million in Bangladesh (Chakraborti et al. 2002; Mandal and Suzuki 2002; Srivastava and Sharma 2013). It has been reported that 0.9 million people from 15 districts of Bihar are at health risk, a state of India which is located in the eastern part of the Gangetic plain (Saha 2009). The situation is also the same in other states of India like Uttar Pradesh, Jharkhand, Chhattisgarh, Assam, West Bengal, and Manipur; these data show that As is posing a serious risk to the population in mass.
Arsenic has been classified as a class (I) human carcinogen due to its sensitivity and mobilization at normal pH range of 6.5–8.5 (range of natural groundwater). The toxicity of the As varies with the species, inorganic and organic. The inorganic species (arsenite and arsenate) are more toxic than the organic (monomethylarsonic acid, dimethylarsinic acid, arsenobetaine etc.). Furthermore, As(III) is much time toxic than the As(V) (Hughes et al. 2011). Inorganic forms of As are dominant in natural water, while the organic species dominate in surface water bodies where the bacterial activities are more prominent like industrial and agricultural runoff (Smedley and Kinniburgh 2002). Thus it is suggested that estimation of the total As would not be very useful to evaluate its human health hazard.
The persistence of As in the environment may pollute or contaminate soils and aqueous stream as both natural components or as the result of human activity. In recent years, the development of efficient green chemistry methods for detoxification or cleaning of trace metal poisoning has become a major focus of researchers. Several investigations have been performed globally in order to find an eco-friendly and recyclable technique for the removal of traces elements contamination from the natural resources. Plants and microbe have the tendency to uptake trace elements from the media termed as process bioremediation. Bioremediation process offers the possibility to reduce or render trace and toxic elements. Among the various bioremediation processes, phytoremediation and bioremediation using microbes are quite effective. Phytoremediation includes the removal of contaminants with the help of green plants, while the microbial bioremediation includes the removal of trace and toxic elements by microorganisms (bacteria, fungi, yeast and algae) as sorbents (Saxena et al. 2018; Bharagava et al. 2017b, c; Saxena and Bharagava 2017; Chandra et al. 2015). The aim of this chapter is to give an overview of the As contamination in the environment and also the mechanism of removal of the As from the contaminated resources by the potent application of plants and microbes.
12.2 Arsenic in the Environment: Occurrence and Sources
Arsenic is a class I carcinogen, also termed as “king of poison.” It is found in the earth crust in a trace amount including in air, water, and soils. The wide extent of the As has caused a menace to the lives of several hundred million people in different regions worldwide and has resulted into the world’s largest environmental calamity (Smedley and Kinniburgh 2002; Ravenscroft et al. 2009). Arsenic is not newly known but also has been used in ancient time 300 BC in hardening the bronze and use of As compounds as an ulcer treatment.
An average concentration of As (1800 μg/kg) has been reported in the earth crust (Mason 1966). The concentration of As may occur up to five times in shales and alluvium. Arsenic has been reported as a major component in more than 200 minerals, such as arsenides, sulfides, oxides, arsenates, and arsenites including elemental As. Arsenopyrite (FeAsS) is known as the most abundant As ore mineral. High As concentration also has been observed in many oxide minerals and hydrous metal oxide either as part of the mineral structure or as sorbed species which includes a coating on the edges of clay minerals and on the surface of the calcite (Goldberg and Glaubig 1988; Smedley and Kinniburgh 2002). Muds and clay tend to have higher As concentration rather than sand and sandstones (Ravenscroft et al. 2009). An average order of 5000–10,000 μg/kgAs concentration in soils has been reported. Boyle and Jonasson (1973) and Shacklette et al. (1974) quoted an average value of 7200 and 7400 μg/kg, respectively, for American soils. Average As concentration in the stream sediment was found in the range of 5000–8000 μg/kg in England and Wales (AGRG 1978). A considerable concentration (range 1200–2600 μg/kg) has also been reported in sediments of the river Ganges, from Brahmaputra river with a range 1400–5900 μg/kg and from Meghna river with a range 1300–5600 μg/kg (Datta and Subramanian 1997). Arsenic enters into groundwater through weathering processes of As mineral bearing rocks followed by runoff, deposition, and leaching (NIH 2010).
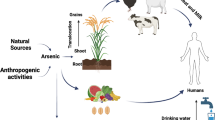
Along with the natural source of As occurrence, there are several anthropogenic ways by which it can enter into the environment which include combustion of coal, application in agricultural practices using As-based pesticides, chromated copper arsenate (CCA) for wood preservation, activities like smelting of base metal ores, and mining activities (Smedley and Kinniburgh 2002).
The concentration of As in freshwater strongly depends on the source of As, local geochemical environment, and the amount available. It can vary by more than 103 orders of magnitude. The main factors for mobilization and accumulation of As are rock-water interactions and availability of favorable physical and geochemical conditions in the aquifers (Smedley and Kinniburgh 2002). The presence of As in the aquifers is controlled by many factors that include redox potential, pH, adsorption/desorption, precipitation/dissolution, As speciation, and biological transformation.
12.3 Aqueous Speciation and Toxicity of Arsenic
Arsenic behaves differently among the heavy metalloids and oxyanion-forming elements (e.g., As, Sb, Mo, Cr, Re, Se, and V) in its sensitivity and mobilization at the typical values of pH (6.5–8.5) found in the natural aquifers. Arsenic can be found in several oxidation states (−3, +3, 0 and +5) and forms organic and inorganic species in the environment (Smedley and Kinniburgh 2002). Inorganic forms are dominant in natural water, while organic forms are found in surface water where biological activities take place or where waters are significantly affected by industrial waters containing organic impurities. Inorganic forms are oxyanions of trivalent arsenite As(III) and or pentavalent arsenate As(V), and major organic species are dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA). Inorganic species are more toxic than the organic (NIH 2010).
12.4 Arsenic in Groundwater and Surface Water
Concentration of As higher than WHO guideline (10 μg/L) has been reported in several areas worldwide. Arsenic is widely spread in groundwaters of many states of India including Uttar Pradesh, Bihar, Jharkhand, Chhattisgarh, Assam, West Bengal, and Manipur. More than 170,000 water samples from tube wells were analyzed from all surveyed states of India, and half of the samples had As >10 μg/L. The maximum As concentration was detected in a tube well from West Bengal as 3700 μg/L, which was 370 times higher than the WHO guideline value (10 μg/L) and 74 times higher than the Indian standard of As (50 μg/L) in drinking water. Elevated concentrations of As in groundwater were also detected in other states such as Bihar and Uttar Pradesh. Altogether 13.85 and 6.96 million people from all surveyed states were exposed to As greater than 10 and 50 μg/L, respectively (Chakraborti et al. 2017).
Higher concentration of As has been also observed in the river water samples, mainly in the seasonal river channels or tributaries of the Ganges. The concentration of As varied between the Ganges and its tributaries as follows: Ganges (2–4 μg/L), Bay (23 μg/L), Burhi Gandak (17–24 μg/L), Kamala (10 μg/L), Bagmati (13 μg/L), Santi (5 μg/L), Blan (12 μg/L), and Punpun (6 μg/L) (unpublished). Higher As concentration has not been observed in most studies on the middle and lower Gangetic plain. However, an elevated concentration of As (20–22 μg/L) has been reported in a small stream (river Gobra) in Murshidabad (Stuben et al. 2003) and river Jalangi and upper Ichamati (range 37–101 μg/L) of West Bengal (Mukherjee et al. 2009). It has been suggested that the reduction of Mn in the Gobra while the groundwater discharge served as a source of As in river Jalangi and upper Ichamati is due to elevated As concentrations. In a study done in middle Gangetic plain, it was observed that the river or channels which are small and locally recharged (Burhi Gandak, Bay, Bagmati, and Blan) contain high levels of As, while the river Ganges and its channels contain much fewer concentrations (unpublished). The probable reason for higher concentrations of As may be lean flow into the channels and recharge from the adjacent aquifers or may be upstream anthropogenic inputs. The river Kamala originates in the Himalayas and contains much less As concentration in its upper reaches near Jainagar city of Madhubani district in Bihar state.
12.5 Arsenic in Sediments and Its Relation with the Geological Settings in Fluvial Regions
The Ganges basin, also known for world’s largest alluvium sedimentation and deposition, is an end result of the India-Asia plate collision that had started in Palaeogene geological period. In Gangetic plain, the sediment deposition is divided into Pleistocene and Holocene deposition (Revenscroft 2001). A flat topography exists with a north to south slope in the Holocene alluvium Gangetic plain. Acharyya and Shah (2007) have discussed geomorphologic and quaternary morphostratigraphy of Gangetic plain on 1:50,000 scale, along with field observations to identify fluvial landforms and soil characteristics. The terrain is divided into two types of deposition: the older alluvium (Pleistocene) is characterized by the presence of yellow-brown clay with profuse calcareous and ferruginous concentrations. The newer alluvium (Holocene) is characterized by unoxidized, organic-rich sand, silt, and clay and restricted to low-lying fluvial and fluviolacustrine settings. The major part of Ganga plain consists of interfluves upland terrace surface (Singh 1996). The plain has been incised by dendritic drainage and channels containing a good amount of organic muds of Holocene age (Ravenscroft et al. 2005). Shah (2008) revealed a mineral assemblage (quartz, muscovite, chlorite, kaolinite feldspar, amphibole, and goethite) with the help of XRD studies on soil samples of As-safe older alluvium and As-contaminated newer alluvium from Gangetic plain. In Gangetic plain tube wells are tapped mostly in shallow aquifers, which hold 30% of total replenishable groundwater. Shallow aquifers are the main source of drinking water to fulfill the daily requirements of the local population and remain as the major input for societal development.
Primarily mineral assemblage of quartz, calcite (CaCO3), muscovite, and chlorite with minor amounts of smectite, feldspar, hematite, siderite, goethite, and magnetite was reported in Gangetic plain (Kumar et al. 2016). The significant presence of altered feldspar, chert, and chlorite indicated the sedimentary and metasedimentary origin of sands (Ahmed et al. 2004). The presence of calcite and muscovite in the upper oxidized sediments suggested a close relationship with chelation of the metals in the zone of oxidation (Hasan et al. 2009). Peaks of hematite (Fe2O3) and goethite (FeO(OH)) were observed throughout the profile, but magnetite (Fe3O4) was reported only in the upper yellowish oxidized silty clay. The presence of this secondary mineral was noted in early studies of As in Southeast Asia by Islam et al. (2004) and Anawar et al. (2006). Jönsson and Sherman (2008) investigated on sorption of As to siderite and find As(V) sorbs strongly, but As(III) sorbs weakly. Mumford et al. (2012) reported 184,000 μg/kg of As in a siderite, which included quartz, so the actual concentration in the siderite is probably higher. Hence, siderite was reported as decidedly a sink for As in Gangetic plain.
12.6 Arsenic in Agricultural Soils: A Close Relation with Groundwater Extraction
The total concentration of As observed in agricultural soils ranged from 3527 to 14,690 μg/kg in Gangetic plain (Kumar et al. 2016), and this finding is approximately two to seven times higher than the world’s typical average value of 2000 μg/kg for igneous and sedimentary rocks (Mandal and Suzuki 2002). A high variability was observed in the concentration of As in agricultural soils in the Gangetic plain (Kumar et al. 2016). It is known that presence of higher concentration of As in soils may be attributed to geogenic contribution (Kumar et al. 2016; Meharg and Rahman 2003), although few studies indicate higher As concentration in soils than sediments (Chowdhury et al. 1999). This suggests that other sources may contribute to the anomalous high concentration of As in soils. The situation of an elevated As in agricultural soils has been documented in previous studies conducted from As prone areas of Bangladesh, a country in which it has been confirmed that As levels were elevated in zones where As in groundwater used for irrigation was high (Meharg and Rahman 2003). Mean As concentration in agricultural soils has been reported as 101,000 μg/kg in West Bengal, India (Norra et al. 2005). Another study conducted in West Bengal in an As-contaminated area revealed a range of 10,000–35,000 μg/kg in agricultural soils (Sanz et al. 2007). Arsenic concentration of surface soils having a range of 1090–2480 μg/kg and any specific trend was not observed in a concentration with the depth of 2 mbgl (Vicky-Singh et al. 2010). The As concentration in agricultural soils varied from 880 to 4960 μg/kg in Feni district of Bangladesh, while it ranged from 3110 to 8900 for Dhamrai, Bangladesh, and 17,600–65,000 μg/kg for Faridpur, Bangladesh (Ahsan et al. 2009). A range of 8500–10,300 μg/kg in Japanese paddy soils, 2000–4600 μg/kg in South Korean soils, and 6700–9100 μg/kg in soils from Thailand (Mandal and Suzuki 2002) has been observed.
12.7 Arsenic in Dietary Components
In study from central Gangetic basin it was reported that the vegetables with highest mean As concentration were luffa, brinjal, cucumber, ladyfinger, gourd, and green gram with mean values of 800, 492, 399, 375, 268, and 174 μg/kg, respectively (Kumar et al. 2016). In other dietary components, rice, wheat, and maize have the highest mean As concentrations (Kumar et al. 2016). Variation in As and other elements’ accumulation in plants depends on many factors, viz., availability of As in water and soils, accumulation capacity by plant, lifespan of plants grown, etc. (Roychowdhury et al. 2003). Among all food categories such as vegetables, rice, wheat, maize, and green gram, vegetables contained elevated concentrations of As. In another study, it was reported that As concentrations (dry wt.) in wheat and maize were 24 μg/kg and 11 μg/kg, respectively, from Maner district of Bihar, India (Singh and Ghosh 2011).
12.8 Ecotoxicological and Health Effects of Arsenic
Arsenic is a toxic element, known as class (I) human carcinogen and widely distributed in the environment as both inorganic and organic forms (Hughes et al. 2011). In general, the inorganic forms (arsenite and arsenate) of As are much more toxic than the organic forms (monomethylarsonic acid, dimethylarsinic acid, arsenobetaine, etc.) of As. Arsenite is generally more toxic than arsenate, and humans are exposed to both forms of inorganic As from water and food. There are many pathways by which As can enter the human body via food chain (ingestion by water and food sources), and occupational exposure is the most common (Rahman et al. 2009). Various inorganic species (arsenite and arsenate) and organic species (methylated anionic species, volatile As hydride, and organo As) in food materials have been reported as the main pathways to human exposure (Momplaisir et al. 2001).
12.9 Bioremediation Strategies for Arsenic Detoxification
Several conventional remediation methods such as solidification and stabilization, soil flushing/washing, electrokinetics, chemical reduction/oxidation, in situ oxidation, thermal desorption, surfactant-enhanced aquifer remediation, vitrification, pneumatic fracturing, excavation/retrieval, landfill, etc. are available (Saxena et al. 1999; Wenzel et al. 1999). Most of these traditional remediation methods used for in situ and ex situ remediation of contaminated sites are uneconomical, unsustainable, and destructive. Bioremediation is the use of plants or microorganisms to decontaminate an environment by transforming or degrading pollutants. In situ bioremediation is a well-established technology for the treatment of contaminated sites, especially when the pollutants are diffused in large areas. Such managed areas are available for safe uses at an economically acceptable price.
Generally, metal contaminants are found in various matrix of the environment such as soils, sediments, and water. Metal contaminants may be present naturally or produced anthropogenically through various industrial processes. The main reason for the remediation of the metal-contaminated site is that metals are nonbiodegradable in nature; however, they can be transmuted through various processes such as sorption, methylation, complexation, and changes in valence state. These conversions affect the movement and bioavailability of metal pollutants in a different matrix.
Arsenic bioremediation is an economical and environment friendly method. Arsenic is a chemical analog to phosphorus in several plants, and it is easily taken up by plants (Tu and Ma 2003). There are several ways and methods of As bioremediation that are discussed in the next sections of this chapter.
12.9.1 Arsenic Remediation Using Plants (Phytoremediation)
The generic term “phytoremediation” contains two words: the Greek prefix phyto means “plant,” attached to the Latin root medium which means “to correct or remove an evil.” In general, phytoremediation is a set of mechanisms that use different plants to absorb, extract, contain, or immobilize contaminants (organic and inorganic) from different components of the environment (such as air, water, and soil) (Salt et al. 1998). Arsenic is an unnecessary constituent for plants, and inorganic As species are usually extremely toxic for plants. Usually, the As concentrations found in most of the terrestrial plants are below 10 mg kg−1 under normal conditions (Matschullat 2000).
Arsenate present in the soil act as phosphate analog in plants by replacing phosphate in several biochemical and also interrupt phosphate metabolism. For example, in some plants arsenate can interrupt mitochondrial oxidative phosphorylation and thus, the production of the nucleotide adenosine triphosphate (ATP), which is the main energy source for cells (Fayiga and Saha 2016). After absorption As can find its way into the fruit/grains of plants and accumulate there. There are several factors which can affect the phytoremediation of As-polluted soil and water, such as bioavailability of As in the soil, redox potential, speciation of As in soil, phosphate concentrations, the presence of co-contaminants, plant age, plant nutrition, rhizosphere characteristics, and biological associations with microbes (Fayiga and Saha 2016). During phytoremediation, plant removes or uptakes metals by using one or more of these mechanisms, i.e., phytoextraction, phytostabilisation, rhizofiltration, phytodegradation, and phytovolatilization.
Arsenic inhibits plant growth, delays seed germination, and causes foliage chlorosis and necrosis (Odutayo et al. 2015). Presence of As in plants may lead to a decrease in crop yield, as As can disturb the uptake and transport of nutrients in plants (Paivoke and Simola 2001). Phytoremediation has a benefit over traditional remediation technology of As-contaminated soils such as burial and chemical stabilization, which may cause long-term health intimidations due to leakage or chemical uncertainty (Allen 2001; Förstner and Haase 1998; Bhattacharya et al. 2007). Thus phytoremediation technology has the possibility to become an environment friendly and economical unconventional remediation technique for As-contaminated sites (Bhattacharya et al. 2007).
Higher plants can bear high levels of As by two basic approaches, which are (a) exclusion, whereby carriage of As is limited, and low, moderately constant As concentrations are retained in the shoot or grain over a wide range of soil concentrations, and (b) accumulation, whereby As is accumulated in less/nontoxic form(s) in upper parts of plant at both high and low soil concentrations (Baker et al. 2000). Some studies conducted on As remediation using various plant species are given in Table 12.1.
Most of the plants do not accumulate As, and its concentrations in leaves or seeds are often below 1 mg/kg. Several authors reported that some tropical and subtropical plant species can tolerate and uptake various inorganic and organic forms of As (Meharg and Hartley-Whitaker 2002). Ma et al. (2001a, b) conducted a screening study on several plant species growing at an As-contaminated site in Florida and reported that Pteris vittata, as an As hyperaccumulator fern. The results showed that its leaves can contain an excess of 1% As (dry weight basis). This was due to the capability of the fern to translocate As from the lower parts of plants (roots) to the upper parts (leaves) and accumulate it, due to its ability to uphold high phosphate in its roots (Tu and Ma 2003; Luongo and Ma 2005). When As enters in plants through the roots of plants and through P transporters (Pit and Pst proteins), As(V) is reduced to As(III) before being ejected to cell vacuoles.
In another study, Wang et al. (2007) examined the discrepancy of As accumulation by ferns collected at various sites in south China (Guangxi Province) and observed genotypic variations within P. vittata that could be useful in breeding improved cultivars. Similarly, Aldrich et al. (2007) studied the accumulation of As(III) and As(V) by mesquite a desert plant species and its possible use for decontamination of As-contaminated soils. During experiment, mesquite seedlings were grown in agar-based medium containing 5 mg/L of either As(III) or As(V). The results of the study revealed that the As(V) concentration was significantly higher than the As(III) concentration in all parts of the plant. It happened as As(V) was reduced to As(III) inside the mesquite plant revealed by X-ray absorption spectroscopy (XAS). Therefore the mesquite plants could be a potential candidate for the phytoremediation of As-contaminated soils in arid regions.
A greenhouse experiment with P. vittata showed that frond As concentration increased from 29.4 to 15,861 mg kg−1 in soil spiked with 1500 mg kg−1 As in 2 weeks showing the high translocation of As in the fern (Ma et al. 2001a, b). In another study, Tu and Ma (2002) reported that the fern (P. vittata) was also able to remove As from soils containing different As species at different As concentrations. A very large percentage of the As (75–93%) accumulated in the fronds showing that the fern was able to translocate As within the plant (Ma et al. 2001a, b; Zhang et al. 2002; Lombi et al. 2002; Tu et al. 2002). Many plants have been reported to tolerate As in As-contaminated soils but are not hyperaccumulators because they accumulate As only in their roots (Bondada and Ma 2003; Srivastava et al. 2006).
Alvarado et al. (2008) observed the elimination of As (0.15 mg/L) from polluted water by two aquatic plants, i.e., water hyacinth (Eichhornia crassipes) and lesser duckweed (Lemna minor). Two plant densities were used: 1 kg/m2 and 4 kg/m2 (on a wet basis) for lesser duckweed and water hyacinth, respectively. The results showed that water hyacinth removed 18% of As and the removal rate was 600 mg As/ha/d. The results showed that L. minor removed 140 mg As/ha/d (5%) from the As-polluted water.
Salido et al. (2003) performed field and greenhouse trials to assess the performance of phytoremediation of two metals (As and lead) from polluted soil at an EPA Superfund site (Barber Orchard). To remove As Chinese brake ferns (Pteris vittata) were used in the study. Results showed that the concentration of As in shoots of ferns was about 20 times higher than the soil As concentration under field conditions. According to the study, to bring the As concentration to safe levels (40 mg/kg), it was estimated that 8 years would be required. Results also indicated that reduction in the acid-extractable quantity of soil As and increase in the pH of soil may improve As removal from the contaminated site.
12.9.2 Arsenic Remediation Using Bacteria
Plants often exist in mutual relationships with other microorganisms, especially bacteria and fungi (Fayiga and Saha 2016). It is important to know the effects and role of As accumulation on these microbes. It is also important to know what role these microbes play in As uptake by the fern. Arsenic-resistant bacteria have been isolated in the fronds and rhizosphere of P. vittata (Rathinasabapathi et al. 2006; Huang et al. 2010).
Arsenic bioremediation depends on microbial action to detoxify, mobilize, or immobilize As through oxidation-reduction, biomethylation, sorption, and complexation processes (Wang and Zhao 2009). In prokaryotes and unicellular eukaryotes, arsenate (AsV) and arsenite (AsIII) enter the cell through phosphate (Pi) transporters and aquaglyceroporins, respectively. In bacteria, genes localized in the ars operon regulate As resistance. It typically comprises three genes, arsR, arsB, and arsC, encoding three proteins, which convert arsenate to arsenite and extrudes arsenite from the cells (Rosen 2002; Tripathi et al. 2007).
Takeuchi et al. (2007) conducted a study on nine bacterial strains of marine and nonmarine origins for As resistance and removal. The As-resistant and accumulating bacteria are extensively present in the marine and nonmarine aquatic environments. Further, they reported that As-accumulating bacterial species, such as Marinomonas communis, were possible candidates for remediation of As-polluted aquatic mediums. During the experiment, M. communis indicated the high As tolerance with median effective concentration (EC50) value of 510 mg Asl−1 and was capable of removing As from culture medium amended with arsenate. Salmassi et al. (2002) isolated a heterotrophic bacterial strain (Agrobacterium albertimagni strain AOL15) which can oxidize As(III) from the surface of aquatic macrophytes collected in a Hot Creek. The isolated bacterial strain can oxidize 585 μM As(III) within 24 h in mannitol medium in laboratory conditions.
Similarly, Liu et al. (2011) piloted a laboratory study for As removal from contaminated soil by using genetically modified bacteria. The study showed that those bacteria which have expressed arsM gene efficiently removed As through volatilization from the As-contaminated soil. Further, it was reported that use of genetically engineered microorganisms is a cost-effective and capable approach for As bioremediation from contaminated sites.
In past years, many microorganisms were isolated from As-contaminated sites, and these were potentially involved in As removal (Routh et al. 2007). Results of the study showed that A. bolidensis (a novel gram-positive, facultatively anaerobic, coccus-shaped actinomycete) actively reduced As(V) to As(III) in aqueous media in laboratory conditions.
Dey et al. (2016) isolated two rod-shaped gram-positive bacteria, from As-contaminated groundwater of West Bengal, India. These isolated bacteria (Bacillus sp. and Aneurinibacillus aneurinilyticus) can tolerate arsenate and arsenite concentration up to 4500 ppm and 550 ppm, respectively. The results showed that the isolates can remove 51.4–51.9% of arsenite and 50.3–53.37% of arsenate, respectively, from a As amended culture media. The isolated bacterial species were As resistant and can be used asa potential candidate for the bioremediation of As.
12.9.3 Arsenic Remediation Using Algae
Phytoremediation is the use of algae (micro or macro) for the biotransformation of contaminants, comprising metals, nutrients, and xenobiotics from industrial liquid effluents and from terrestrial polluted sites. The alga is commonly present on earth and has adapted to a diversity of habitats, with wide tolerance to environmental conditions. Microalgae have the capability of removing environmental toxicants such as heavy metals, hydrocarbons, and pesticides through various mechanisms, such as biosorption, bioconcentration, biotransformation, and biovolatisation.
Becker (1983) observed that some planktonic algae have a great capability for the remediation of metal pollutants from wastewater. These algae absorb or remove heavy metal residues from wastewaters and the separation of the metal-saturated algae from the medium is also done at low cost, resulting in good-quality reusable effluent water and valuable biomass which could be used for various uses. The efficiency of the phytoremediation depends on several parameters, i.e., growth rate of algae, transfer factor achieved by the algae, the quantity of heavy metal in the medium, preferred extent of metal removal from the medium, and metal recovery in relation to investment and operating expenses (Becker 1983).
Microalgae can potentially be used in remediation of As from contaminated media, due to their capability to bioaccumulate and biotransform As (Bahar et al. 2013). Recently, Wang et al. (2013) examined two freshwater green algae species (Chlamydomonas reinhardtii and Scenedesmus obliquus) to assess the As bioaccumulation kinetics under different conditions. They found that the As bioaccumulation was significantly greater in phosphate-limited conditions as compared to phosphate-enriched conditions. This may be due to the competition between As(V) and phosphate for the identical transporter to enter the cells due to their structural resemblance.
12.9.4 Arsenic Remediation Using Fungi and Yeast
Mycorrhizal fungi and plant association help plants to reduce metal toxicity, by binding metals present in the contaminated soil to fungal hyphae (Koslowsky and Boerner 1989; Gadd 1993). So the plants grown in metal-contaminated soil and infected by fungi show better resistance than normal plants. As a result, plants infected by these fungi show a higher degree of resistance to metals, because the metals are accumulated in fungal hyphae and are not translocated to plant parts (Bradley et al. 1981; Brown and Wilkins 1985; Dehn and Schüepp 1989).
Several other As accumulator fungal species have been reported by different authors, such as Scopulariopsis brevicaulis (Gosio 1892), Phaeolus schweinitzii (Pearce et al. 1998), Fusarium oxysporum (Granchinho et al. 2002), Sinorhizobium meliloti (Carrasco et al. 2005), Neosartorya fischeri, Aspergillus clavatus (Cernansky et al. 2007, 2009), Aspergillus candidus (Vala 2010), Aspergillus niger (Mukherjee et al. 2010), Trichoderma sp., Neocosmospora sp., and Rhizopus sp. (Srivastava et al. 2011).
In a study, Chen et al. (2007) studied the effect plant phosphorus on arbuscular mycorrhizal fungi Glomus mosseae and As accumulation by Medicago sativa. The phosphorous and As interactions were also studied in plants. The results of the study indicate that due to fungal colonization, plant dry weight increased more than sixfolds and also considerably increased in total uptake (phosphorus and As contents). Notwithstanding of phosphorus and As addition levels, phosphorous content was two times higher in shoot and root of both the arbuscular mycorrhizal fungi and plants. The results also showed that an As concentration was significantly lesser than corresponding uninoculated controls. The decrease in As concentration in the shoot was due to the “dilution effects” caused by reduced As partitioning to upper parts of the plant and stimulated the growth of arbuscular mycorrhizal fungi.
In another study, the arsenate tolerance level in a fungus (Aspergillus niger) was observed by Mukherjee et al. (2010). The results point out that A. niger had a great arsenate uptake capacity and tolerance. Further, they reported that the A. niger can tolerate oxidative stress by influencing its antioxidative defense mechanism and may be used for the removal of arsenate from contaminated water.
In yeasts, As tolerance is provided by three connecting genes in the cluster ACR1, ACR2, and ACR3: ACR1 encrypts a putative transcription factor; ACR2 encrypts an arsenate reductase, and ACR3 encrypts a plasma membrane AsIII-efflux transporter. This approach confirms the reduction and removal of As(V) from the cytosol to the exterior medium. The additional mechanism that functions in yeast for the removal of cytosolic As is an ABC-type transporter, yeast cadmium factor, which is positioned at the vacuolar membrane and sequesters glutathione conjugates of AsIII (AsIII–GS3) in the vacuole (Ghosh et al. 1999).
12.10 Conclusion
Among all the metalloids, Arsenic (As) is recognized as the leading toxicant worldwide and having various toxic effects on human and animal health as well as on the environment. Exposure of As may be by various routes including direct inhalation from the atmosphere, ingestion through contaminated food and water and dermal absorption. It causes various types of health effects like dermal and neurological involvement, reproductive and pregnancy effects, cardiovascular effects, diabetes mellitus, diseases of the respiratory system, multiorgan cancers etc. The source and occurrence and accumulation of As in the environment could be natural and anthropogenic causing environmental contamination. Due to its harmful effects, it has been investigated in order to find an eco-friendly technique for the removal of As contamination from the natural resources. Bioremediation process in this regards is an option that offers the possibility to reduce or render trace and toxic elements such as As using plants and microbes. Among the various bioremediation processes, phytoremediation and bioremediation using microbes are quite effective. Phytoremediation includes the removal of contaminants with the help of green plants, while the microbial bioremediation includes the removal of trace and toxic elements by microorganisms (bacteria, fungi, yeast and algae) as sorbents.
References
Acharyya SK, Shah BA (2007) Groundwater arsenic contamination affecting different geologic domains in India–a review: influence of geological setting, fluvial geomorphology and quaternary stratigraphy. J Environ Sci Health A 42:1795–1805
AGRG (1978) The Wolfson geochemical atlas of England and Wales. Clarendon Press, Oxford
Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Alam SM, Bhuyian MH (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl Geochem 19:181–200
Ahsan DA, DelValls TA, Blasco J (2009) Distribution of arsenic and trace metals in the floodplain agricultural soil of Bangladesh. Bull Environ Contam Toxicol 82:11–15
Aldrich MV, Peralta-Videa JR, Parsons JG, Gardea-Torresdey JL (2007) Examination of arsenic(III) and (V) uptake by the desert plant species mesquite (Prosopis spp.) using X-ray absorption spectroscopy. Sci Total Environ 379(2–3):249–255
Allen A (2001) Containment landfills: the myth of sustainability. Eng Geol 60:3–19
Alvarado S, Guedez M, Lue-Meru MP, Nelson G, Alvaro A, Jesus AC, Gyula Z (2008) Arsenic removal from waters by bioremediation with the aquatic plants water hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour Technol 99:8436–8440
Anawar HM, Akai J, Yoshioka T, Konohira E, Lee JY, Fukuhara H, TariKulAlam M, Garcia-Sanchez A (2006) Mobilization of arsenic in groundwater of Bangladesh: evidence from an incubation study. Environ Geochem Health 28:553–565
Bahar MM, Megharaj M, Naidu R (2013) Bioremediation of arsenic-contaminated water: recent advances and future prospects. Water Air Soil Pollut 224:1722
Baker AJ, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton, pp 85–107
Becker EW (1983) Limitations of heavy metal removal from waste water by means of algae. Water Res 17(4):459–466
Bharagava RN, Saxena G, Mulla SI, Patel DK (2017a) Characterization and identification of recalcitrant organic pollutants (ROPs) in tannery wastewater and its phytotoxicity evaluation for environmental safety. Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-017-0490-x
Bharagava RN, Chowdhary P, Saxena G (2017b) Bioremediation: an ecosustainable green technology: its applications and limitations. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 1–22. https://doi.org/10.1201/9781315173351-2
Bharagava RN, Saxena G, Chowdhary P (2017c) Constructed wetlands: an emerging phytotechnology for degradation and detoxification of industrial wastewaters. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 397–426. https://doi.org/10.1201/9781315173351-15
Bhattacharya P, Mukherjee AB, Bundschuh J, Zevenhoven R, Loeppert RH (2007) Arsenic in soil and groundwater environment: biogeochemical interactions, health effects and remediation, Trace metals and other contaminants in the environment (Series Editor Nriagu JO), vol 9. Elsevier, Amsterdam, p 645
Bhattacharya P, Naidu R, Polya DA, Mukherjee A, Bundschuh J, Charlet L (2014) Arsenic in hydrological processes-sources, speciation, bioavailability and management. J Hydrol 518:279–283
Bondada BR, Ma LQ (2003) Tolerance of heavy metals in vascular plants: arsenic hyperaccumulation by Chinese brake fern (Pteris vittata L.). In: Chandra S, Srivastava M (eds) Pteridology in the new millenium. Kluwer Academic Publishers, Dordrecht, pp 397–420
Boyle RW, Jonasson IR (1973) The geochemistry of as and its use as an indicator element in geochemical prospecting. J Geochem Explor 2:251–296
Bradley R, Burt AJ, Read DJ (1981) Mycorrhizal infection and resistance to heavy metal toxicity in Calluna vulgaris. Nature 292:335–337
Brown MT, Wilkins DA (1985) Zinc tolerance of mycorrhizal Betula. New Phytol 99:101–106
Carrasco JA, Armario P, Pajuelo E, Burgos A, Caviedes MA, Lόpez R et al (2005) Isolation and characterization of symbiotically effective rhizobium resistant to arsenic and heavy metals after the toxic spill at the Aznalcóllar pyrite mine. Soil Biol Biochem 37:1131–1140
Cernansky S, Urik M, Sevc J, Khun K (2007) Biosorption and biovolatilization of arsenic by heat-resistant fungi. Environ Sci Pollut Res 14:31–35
Cernansky S, Kolencik M, Sevc J, Urik M, Hiller E (2009) Fungal volatilization of trivalent and pentavalent arsenic under laboratory conditions. Bioresour Technol 100:1037–1040
Chakraborti D, Rahman MM, Paul K, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Mukherjee SC (2002) Arsenic calamity in the Indian sub-continent-what lessons have been learned? Talanta 58:3–22
Chakraborti D, Rahman MM, Murrill M, Das R, Patil SG, Sarkar A, Dadapeer HJ, Yendigeri S, Ahmed R, Das KK (2013) Environmental arsenic contamination and its health effects in a historic gold mining area of the Mangalur greenstone belt of Northeastern Karnataka, India. J Hazard Mater 262:1048–1055
Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, Mukherjee SC (2016) Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 152:520–529
Chakraborti D, Rahman MM, Das B, Chatterjee A, Das D, Nayak B, Pal A, Chowdhury UK, Ahmed S, Biswas BK, Sengupta MK, Hossain MA, Samanta Roy MM, Dutta RN, Saha KC, Mukherjee SC, Pati S, Kar PB, Mukherjee A, Kumar M (2017) Hydrogeol J 25(4):1165–1181
Chandra R, Saxena G, Kumar V (2015) Phytoremediation of environmental pollutants: an eco-sustainable green technology to environmental management. In: Chandra R (ed) Advances in biodegradation and bioremediation of industrial waste, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 1–30. https://doi.org/10.1201/b18218-2
Chen B, Xiao X, Zhu Y-G, Smith FA, Xie ZM, Smith SE (2007) The arbuscular mycorrhizal fungus Glomus mosseae gives contradictory effects on phosphorus and arsenic acquisition by Medicago sativa Linn. Sci Total Environ 379:226–234
Chowdhury TR, Basu GK, Mandal BK, Biswas BK, Samanta G, Chowdhury UK, Chanda CR, Lodh D, Roy SL, Saha KC, Roy S (1999) Arsenic poisoning in the Ganges delta. Nature 401:545–546
Datta DK, Subramanian V (1997) Texture and mineralogy of sediments from the Ganges-Brahmaputra-Meghna river system in the Bengal basin, Bangladesh and their environmental implications. Environ Geol 30:181–188
Dehn B, Schüepp H (1989) Influence of VA mycorrhizae on the uptake and distribution of heavy metals in plants. Agricult Ecosyst Environ 29:79–83
Dey U, Chatterjee S, Mondal NK (2016) Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol Rep 10:1–7
Dominguez MT, Maranon T, Murillo JM, Schulin R, Robinson BH (2008) Trace element accumulation in woody plants of the Guadiamar Valley, SW Spain: a large-scale phytomanagement case study. Environ Pollut 152:50–59
Fayiga AO, Saha UK (2016) Arsenic hyperaccumulating fern: implications for remediation of arsenic contaminated soils. Geoderma 284:132–143
Finkelman RB, Belkin HE, Zheng B (1999) Health impacts of domestic coal use in China. Proc Natl Acad Sci India Sect A Phys Sci USA 96:3427–3431
Förstner U, Haase I (1998) Geochemical demobilization of metallic pollutants in solid waste-implications for arsenic in waterworks sludges. J Geochem Explor 62:29–36
French CJ, Dickinson NM, Putwain PD (2006) Woody biomass phytoremediation of contaminated brownfield land. Environ Pollut 141:387–395
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Gautam S, Kaithwas G, Bharagava RN, Saxena G (2017) Pollutants in tannery wastewater, pharmacological effects and bioremediation approaches for human health protection and environmental safety. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 369–396. https://doi.org/10.1201/9781315173351-14
Ghosh M, Shen J, Rosen BP (1999) Pathway of As(III) detoxification in Sachharomyces cerevisiae. Proc Natl Acad Sci U S A 96:5001–5006
Goldberg S, Glaubig RA (1988) Anion sorption on a calcareous, montmorillonitic soil-arsenic. Soil Sci Soc Am J 52:1297–1300
Gosio B (1892) Action of microphytes on solid compounds of arsenic: a recapitulation. Science 19:104–106
Goutam SP, Saxena G, Singh V, Yadav AK, Bharagava RN (2018) Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem Eng J 336:386–396. https://doi.org/10.1016/j.cej.2017.12.029
Granchinho SCR, Franz CM, Polishchuk E, Cullen WR, Reimer KJ (2002) Transformation of arsenic (V) by the fungus Fusarium oxysporum melonis isolated from the alga Fucus gardneri. Appl Organomet Chem 16:721–726
Hartley W, Dickinson NM, Riby P, Lepp NW (2009) Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut 157:2654–2662
Hasan MA, von Brömssen M, Bhattacharya P, Ahmed KM, Sikder AM, Jacks G, Sracek O (2009) Geochemistry and mineralogy of shallow alluvial aquifers in Daudkandi upazila in the Meghna flood plain, Bangladesh. Environ Geol 57:499–511
Huang A, Teplitski M, Rathinasabapathi B, Ma LQ (2010) Characterization of arsenic-resistant bacteria from the rhizosphere of arsenic hyperaccumulator Pteris vittata L. Can J Microbiol 56:236–246
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Islam MS, Zaman MWU, Rahman MM (2013) Phytoaccumulation of arsenic from arsenic contaminated soils by Eichhornia crassipes L., Echinochloa crusgalli L. and Monochoria hastata L. in Bangladesh. Int J Environ Prot 3(4):17–27
Jönsson J, Sherman DM (2008) Sorption of As (III) and As (V) to siderite, green rust (fougerite) and magnetite: implications for arsenic release in anoxic groundwaters. Chem Geol 255:173–181
Koslowsky SD, Boerner REJ (1989) Interactive effects of aluminium, phosphorus and mycorrhizae on growth and nutrient uptake of Panicum virgatum L (Poaceae). Environ Poll 61:107–125
Kumar M, Rahman MM, Ramanathan AL, Naidua R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Liu S, Zhang F, Chen J, Sun GX (2011) Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J Environ Sci 23(9):1544–1550
Lombi E, Zhao FJ, Fuhrmann M, Ma LQ, McGrath SP (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156:195–203
Luongo T, Ma L (2005) Characteristics of arsenic accumulation by Pteris and non-Pteris ferns. Plant Soil 277:117–126
Ma L, Komar KM, Tu C (2001a) A fern that hyperaccumulates arsenic-A hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils. Nature 409:579
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001b) A fern that hyper-accumulates arsenic. Nature 409:579
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mason B (1966) Principles of geochemistry, 2nd edn. McGraw-Hill, New York
Matschullat J (2000) Arsenic in the geosphere-a review. Sci Total Environ 249:97–312
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Meharg AA, Rahman MM (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37:229–234
Mkandawire M, Dudel G (2005) Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci Total Environ 336(1–3):81–89
Momplaisir GM, Rosal CG, Heithmar EM (2001) Arsenic speciationmethods for studying the environmental fate of organoarsenic animal-feed additives. U.S. EPA, NERL, Las Vegas, pp 01–11
Mukherjee A, Bhattacharya P, Shi F, Fryar AE, Mukherjee AB, Xie ZM, Jacks G, Bundschuh J (2009) Chemical evolution in the high arsenic groundwater of the Huhhot basin (Inner Mongolia, PR China) and its difference from the western Bengal basin (India). Appl Geochem 24(10):1835–1851
Mukherjee A, Das D, Mondal SK, Biswas R, Das TK, Boujedaini N, Khuda-Bukhsh R (2010) Tolerance of arsenate-induced stress in Aspergillus niger, a possible candidate for bioremediation. Ecotoxicol Environ Saf 73:172–182
Mumford AC, Barringer JL, Benzel WM, Reilly PA, Young LO (2012) Microbial transformations of arsenic: mobilization from glauconitic sediments to water. Water Res 46:2859–2868
National Institute of Hydrology (NIH), Roorkee, Central Groundwater Board (CGWB) and Ministry of Water Resources, Government of India (GoI) (2010) Mitigation and remedy of groundwater arsenic menace in India: a vision document by New Delhi. pp 1–7
Norra S, Berner Z, Agarwala P, Wagner F, Chandrasekharam D, Stüben D (2005) Impact of irrigation with As rich groundwater on soil and crops: a geochemical case study in West Bengal Delta Plain, India. Appl Geochem 20:1890–1906
Odutayo OI, Feyisola RT, Godonu KG, Makinde OA (2015) Phytotoxicity level and effects of arsenic phytoextraction using Helianthus annuus L. (sunflower). J Nat Sci Res 5(1):37–41
Paivoke AE, Simola LK (2001) Arsenate toxicity to Pisum sativum: mineral nutrients, chlorophyll content, and phytase activity. Ecotoxicol Environ Saf 49(2):11–21
Pearce RB, Callow ME, Macaskie LE (1998) Fungal volatilization of arsenic and antimony and the sudden infant death syndrome. FEMS Microbiol Lett 158:261–265
Rahman F, Chen Z, Naidu R (2009) A comparative study of the extractability of arsenic species from silverbeet and amaranth vegetables. Environ Geochem Health 31(1):103–113
Rathinasabapathi B, Raman SB, Kertulis G, Ma LQ (2006) Arsenic-resistant proteobacterium from the phyllosphere of arsenic-hyperaccumulating fern (Pteris vittata L.) reduces arsenate to arsenite. Can J Microbiol 52:695–700
Ravenscroft P (2001) Distribution of groundwater arsenic in Bangladesh related to geology. In: Jack G, Bhattacharya P, Khan AA (eds) Groundwater arsenic contamination in the Bengal Delta plain of Bangladesh. KTH Special Publication. TRITA-AMI report 3084. pp 41–56
Ravenscroft P, Burgess WG, Ahmed KM, Burren M, Perrin J (2005) Arsenic in groundwater of the Bengal basin, Bangladesh: distribution, filed relations, and hydrological setting. Hydrogeol J 13:727–751
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. Wiley-Blackwell, Chichester
Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Lett 529:86–92
Routh J, Saraswathy A, Collins D (2007) Arsenicicoccus bolidensis a novel arsenic reducing actinomycete in contaminated sediments near the Adak mine (northern Sweden): impact on water chemistry. Sci Total Environ 379:216–225
Roychowdhury T, Tokunaga H, Ando M (2003) Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci Total Environ 308:15–35
Saha D (2009) Arsenic groundwater contamination in parts of middle Ganga plain, Bihar. Curr Sci 97:753–755
Salido AL, Hasty KL, Lim JM, Butcher DJ (2003) Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian mustard (Brassica juncea). Int J Phytoremediation 5(2):89–103
Salmassi TM, Venkateswaren K, Satomi M, Newman DK, Hering JG (2002) Oxidation of arsenite by Agrobacterium albertimagni AOL15, sp. nov., isolated from hot creek, California. Geomicrobiol J 19(1):53–66
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Ann Rev Plant Physiol Plant Mol Biol 49:643–668
Sanz E, Munoz-Olivas R, Camara C, Sengupta MK, Ahamed S (2007) Arsenic speciation in rice, straw, soil, hair and nails samples from the arsenic-affected areas of Middle and Lower Ganga plain. J Environ Sci Health A Tox Hazard Subst Environ Eng 42:1695–1705
Saxena G, Bharagava RN (2015) Persistent organic pollutants and bacterial communities present during the treatment of tannery wastewater. In: Chandra R (ed) Environmental waste management, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 217–247. https://doi.org/10.1201/b19243-10
Saxena G, Bharagava RN (2017) Organic and inorganic pollutants in industrial wastes, their ecotoxicological effects, health hazards and bioremediation approaches. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 23–56. https://doi.org/10.1201/9781315173351-3
Saxena PK, Krishnaraj S, Dan T, Perras MR, Vettakkorumakankav NN (1999) Phytoremediation of metal contaminated and polluted soils. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants-from molecules to ecosystems. Springer, Berlin, pp 305–329
Saxena G, Chandra R, Bharagava RN (2016) Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Rev Environ Contam Toxicol 240:31–69. https://doi.org/10.1007/398_2015_5009
Saxena G, Purchase D, Mulla SI, Saratale GD, Bharagava RN (2018) Phytoremediation of heavy metal-contaminated sites: environmental considerations, field studies, sustainability and future prospects. J Environ Manag
Shacklette HT, Boerngen JG, Keith JR (1974) Selenium, fluorine, and arsenic in superficial materials of the conterminous United States. US Geol. Surv., Circ, vol 692. US Government Printing Office, Washington, DC
Shah BA (2008) Role of quaternary stratigraphy on arsenic-contaminated groundwater from parts of Middle Ganga Plain, UP–Bihar, India. Environ Geol 35:1553–1561
Singh IB (1996) Late Quaternary sedimentation of Ganga plain foreland basin. Geol Surve India Spec Publ 21:161–172
Singh SK, Ghosh AK (2011) Entry of arsenic into food material-a case study. World Appl Sci J 13:385–390
Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Srivastava S, Sharma YK (2013) Arsenic occurrence and accumulation in soil and water of eastern districts of Uttar Pradesh, India. Environ Monit Assess 18:4995–5002
Srivastava S, Singh N (2014) Mitigation approach of arsenic toxicity in chickpea grown in arsenic amended soil with arsenic tolerant plant growth promoting Acinetobacter sp. Ecol Eng 70:146–153
Srivastava M, Ma LQ, Santos J (2006) Three new arsenic hyperaccumulating ferns. Sci Total Environ 364:24–31
Srivastava PK, Vaish A, Dwivedi S, Chakrabarty D, Singh N, Tripathi RD (2011) Biological removal of arsenic pollution by soil fungi. Sci Total Environ 409:2430–2442
Stuben D, Berner Z, Chandrasekharam D, Karmakar J (2003) Arsenic enrichment in groundwater of West Bengal, India: geochemical evidence for mobilization of As under reducing conditions. Appl Geochem 18(9):1417–1434
Takeuchi M, Kawahata H, Gupta LP, Kita N, Morishita Y, Ono Y, Komai T (2007) Arsenic resistance and removal by marine and non-marine bacteria. J Biotechnol 127(3):434–442
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25(4):158–165
Tu C, Ma LQ (2002) Effects of arsenic concentrations and forms on arsenic uptake by the hyperaccumulator ladder brake. J Environ Qual 31:641–647
Tu C, Ma L (2003) Effects of arsenate and phosphate on their accumulation by an arsenic hyperaccumulator Pteris vittata L. Plant Soil 249:373–382
Tu C, Ma LQ, Bondada B (2002) Arsenic accumulation in the hyper-accumulator Chinese brake fern and its utilization potential for phytoremediation. J Environ Qual 31:1671–1675
Vala AK (2010) Tolerance and removal of arsenic by a facultative marine fungus Aspergillus candidus. Bioresour Technol 101:2565–2567
Vamerali T, Bandiera M, Coletto L, Zanetti F, Dickinson NM, Mosca G (2009) Phytoremediation trials on metal-and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ Pollut 157:887–894
Vazquez S, Agha R, Granado A, Sarro MJ, Esteban E, Penalosa JM, Carpena RO (2006) Use of white lupin plant for phytostabilization of Cd and As polluted acid soil. Water Air Soil Poll 177:349–365
Vicky-Singh, Brar MS, Preeti-Sharma, Malhi SS (2010) Arsenic in water, soil, and rice plants in the Indo-Gangetic plains of northwestern India. Commun Soil Sci Plant Anal 41:1350–1360
Wang S, Zhao X (2009) On the potential of biological treatment for arsenic contaminated soils and groundwater. J Environ Manag 90(8):2367–2376
Wang HB, Wong MH, Lan CY et al (2007) Uptake and accumulation of arsenic by 11 Pteris taxa from Southern China. Environ Pollut 145:225–233
Wang Z, Luo Z, Yan C (2013) Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ Sci Pollut Res 20(10):7286–7295
Wenzel WW, Lombi E, Adriano DC (1999) Biogeochemical processes in the rhizosphere: role in phytoremediation of metal-polluted sites. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants – from molecules to ecosystems. Springer, Heidelberg/Berlin, pp 273–303
WHO (2011) Guideline for Drinking-water Quality, fourth edn. World Health Organization, Geneva
Zhang W, Cai Y, Tu C, Ma LQ (2002) Arsenic speciation and distribution in an arsenic hyper-accumulating plant. Sci Total Environ 300:167–177
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumar, M., Yadav, A., Ramanathan, A.L. (2020). Arsenic Contamination in Environment, Ecotoxicological and Health Effects, and Bioremediation Strategies for Its Detoxification. In: Saxena, G., Bharagava, R. (eds) Bioremediation of Industrial Waste for Environmental Safety. Springer, Singapore. https://doi.org/10.1007/978-981-13-1891-7_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-1891-7_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1890-0
Online ISBN: 978-981-13-1891-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)