Abstract
Arsenic (As) as a major hazardous metalloid was affected by phytoplankton in many aquatic environments. The toxic dominant algae Microcystis aeruginosa was exposed to different concentrations of inorganic arsenic (arsenate or arsenite) for 15 days in BG11 culture media. Arsenic accumulation, toxicity, and speciation in M. aeruginos as well as the changes of As species in media were examined. M. aeruginosa has a general well tolerance to arsenate and a definite sensitivity to arsenite. Additionally, arsenate actively elevated As methylation by the algae but arsenite definitely inhibited it. Interestingly, the uptake of arsenite was more pronounced than that of arsenate, and it was correlated to the toxicity. Arsenate was the predominant species in both cells and their growth media after 15 days of exposure to arsenate or arsenite. However, the amount of the methylated As species in cells was limited and insignificantly affected by the external As concentrations. Upon uptake of the inorganic arsenic, significant quantities of arsenate as well as small amounts of arsenite, DMA, and MMA were produced by the algae and, in turn, released back into the growth media. Bio-oxidation was the first and primary process and methylation was the minor process for arsenite exposures, while bioreduction and the subsequent methylation were the primary metabolisms for arsenate exposures. Arsenic bioaccumulation and transformation by M. aeruginosa in aquatic environment should be paid more attention during a period of eutrophication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is not only a potential hazardous metalloid but also a natural environmental contaminant to which humans are routinely exposed in food, water, air, and soil (Hughes et al. 2011). In the world, more than 150 million people are exposed to drinking water containing “excessive” arsenic above the World Health Organization's recommended limit of 10 μg L−1 (Nordstrom 2002; Brammer and Ravenscroft 2009). The range of As concentrations found in natural water systems is large, ranging from less than 0.5 μg L−1 to more than 5,000 μg L−1 (Smedley and Kinniburgh 2002). Arsenic concentrations can be up to 20 mg L−1 in some polluted freshwaters (Rahman and Hasegawa 2012). Water contamination with As is a universal and urgent problem that requires immediate action to improve water quality (Rahman 2002).
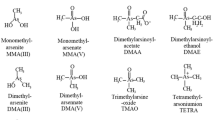
Toxicity and bioaccumulation of As in organisms are dependent not only on its total amount but also on its speciation (Karadjova et al. 2008; Duncan et al. 2010). Although As can be found in the environment in several oxidation states, the trivalent AsIII and pentavalent AsV oxyanions are more prevalent than the organoarsenicals in freshwater (Smedley and Kinniburgh 2002; Sharma and Sohn 2009). It is commonly accepted that, with the exception of the trivalent-methylated metabolites, such as monomethylarsonic acid (MMAIII) and dimethylarsinic acid (DMAIII), and thiolated pentavalent methylated species, such as dimethylthioarsinic acid (DMTAV), the inorganic forms of As are more toxic in mammals than organoarsenicals (Naranmandura and Suzuki 2008; Dopp et al. 2010; Sun et al. 2012; Alava et al. 2012). In aquatic systems, the dominant inorganic As is incorporated into microorganisms such as phytoplankton and is, in turn, converted to methylarsenicals and/or high order organoarsenicals such as arsenosugars (AsS) (Francesconi et al. 1992). Algae plays an important role in As bioaccumulation and biotransformation in the aquatic environment because they show very high As bioaccumulation from surrounding water and thereby determine the amount of As available to higher organisms and their subsequent transformation. Recent studies showed that different algal species have vastly different uptake capacities for inorganic As (Caumette et al. 2011; Duncan et al. 2010; Markley and Herbert 2010).
Cyanobacteria are one of the largest and most important groups of prokaryotic autotrophs with oxygenic photosynthesis in aquatic system. As the most common toxic cyanobacterium in eutrophic freshwater, Microcysis aeruginosa can form harmful algal blooms causing animal poisoning and present risks to human health (Oberholster et al. 2004). Arsenic uptake differs within algal growth phases due to the dominance of different As processes in different stages (e.g., production and excretion of AsIII during log growth phase) (Hellweger et al. 2003; Hellweger and Lall 2004; Hellweger 2005). To date, most of the studies on As uptake, accumulation, and biotransformation by phytoplankton have focused on marine organisms, especially the eukaryotic alga, for the detailed description of AsV detoxification and its metabolism with phosphorus (Maeda et al. 1992a, b; Pawlik-Skowronska et al. 2004; Levy et al. 2005; Qin et al. 2009; Yin et al. 2011b). Little is known about the comparative biotransformation of different inorganic As forms in toxic bloom alga in freshwater, particularly in the speciation transformation and release processes during cyanobacterial blooms. It is requisite to understand these relative aspects due to their potential environmental risks to other organisms and humans if the water is used as drinking water.
Therefore, the main objectives of the present study were to investigate As biotransformation and bioaccumulation in M. aeruginosa as well as their release to the aquatic media at various As-polluted concentrations. Growth studies were undertaken to determine the toxic effects of inorganic As on M. aeruginosa. Changes of As speciation in both alga and media could provide important information to understand the mechanisms of As biotransformation and to predict the possible risks of As-contaminated freshwater during cyanobacterial bloom.
Materials and methods
Organism and culture conditions
The tested bloom cyanobacterium (unicellular M. aeruginosa, FACHB 905) was obtained from the Institute of Hydrobiology, Chinese Academy of Sciences, which was isolated from the eutrophic water of Dianchi Lake (Wu et al. 2007). Batch culture experiments of M. aeruginosa were conducted using BG11 growth media in 250-mL Erlenmeyer flasks with a 16:8 h light:dark cycle under a light intensity of 115 μM photons m−2 s−1 at 25 °C. Test flasks were rotated and shaken three times daily by hand to ensure sufficient gas exchange. The alga culture experiment was run for 15 days.
All stock vessels and culture flasks were soaked in 10 % HNO3 for at least 24 h and then rinsed with Milli-Q water, oven dried, autoclaved, and handled under sterile conditions prior to use. Analytical reagent-grade chemicals and Milli-Q water were used for incubate preparations. The standard stock solutions of arsenate, arsenite, MMA, and DMA were prepared by Na3AsO4·12H2O (Fluka, p.a.), NaAsO2 (Alfa Aesar), NaCH4AsO3 (Fluka), and NaC2H6AsO2 (Sigma-Aldrich), respectively.
Growth rate inhibition bioassays
The 72-h growth rate inhibition bioassays were used to determine the toxicity of inorganic As, AsV as Na3AsO4·12H2O (Fluka, p.a.), and AsIII as NaAsO2 (Alfa Aesar). Cells in exponential growth phase, with the initial cell density of 106 cells mL−1, were added separately into increasing concentrations of AsV (5 μM to 20 mM) and AsIII (5 μM to 500 μM). Fresh algal samples that were transferred to a 10-mm quartz cell were used to measure the turbidity at wavelength of 682 nm using a Thermo ultraviolet visible light spectrophotometer. The number of cells per milliliter was followed every 24 h over a 72-h exposure, which was counted traditionally using a hemocytometer and a microscope. The specific growth rate (μ) of the cells was calculated with the following equation: μ = ln(C t / C 0) / t, where μ is the cell-specific growth rate (per day), C t and C 0 represent the cell density at time t and the initial of the experiment, respectively, and t is the duration of the arsenic exposure (Zeng et al. 2009). The specific growth rate inhibition (I) was calculated from the μ for the sample and μ 0 for the control with the equation: I% = (μ 0 − μ) / μ 0 × 100.
Inorganic As treatment of M. aeruginosa cultures
The inoculum of cells from the stock cultures was designed to give an initial cell density of 105 cells mL−1. Each culture, just after inoculation with M. aeruginosa, was treated with arsenate or arsenite at six concentrations (0, 5, 10, 20, 50, and 100 μM) for a 15-day uptake period to examine As bioaccumulation, biotransformation, and release by living cells. Cultures without As were used as controls. Periodically, each flask was sampled eight times by removing 5-mL aliquots of culture solution beginning 24 h after inoculation and then every 2 days thereafter. Additionally, separate flasks with all treatments in BG11, but no algae, were also monitored to detect the stability of AsIII and AsV at the onset and end of the experiments.
Each sample was analyzed for optical density, total As, and As speciation in the media. Chlorophyll a quantification was performed every 2 days in M. aeruginosa starting 8 days after the beginning of the experiment in order to attain sufficient cell density. For total As determination, chlorophyll a and speciation analysis of media samples, alga were sampled and centrifuged to create an algal pellet. Then, the algal pellet was used for analysis of chlorophyll a, while the supernatant (media) was used for analysis of total As and As speciation. For total As and As speciation analyses in the media, the supernatant was filtered through 0.45-μm cellulose acetate syringe filters. Samples for total As measurements were acidified to a final concentration of 1 % acid with guaranteed reagent 10 M nitric acid for storage. These samples were then analyzed for total As concentration using inductively coupled plasma mass spectrometry (ICP-MS, 7500a Agilent). Supernatant that was analyzed for As speciation was stored at −20 °C for no more than 10 days. Arsenic species were analyzed using anion exchange chromatography coupled to an inductively coupled plasma mass spectrometer as a detector. At the end of the experiment, two algal suspensions of 25 mL were centrifuged into a pellet. Both algal pellets were washed twice with sterile Milli-Q water (18.2 mΩ cm−2; Millipore) and then immersed in an ice-cold phosphate buffer (1 mM K2HPO4, 5 mM MES, and 0.5 mM Ca (NO3)2) for 15 min to completely remove apoplastic As. Eventually, one was dried in an oven at 60 °C and the other was freeze-dried at a vacuum freeze dryer (Labconco, USA). Next, both of them were digested separately. Then, the oven-dried alga samples were used to analyze total As and the freeze-dried alga for As speciation (Zhang et al. 2009). To get better insights into the cellular concentrations of As speciation and possible biotransformation in alga, the cell concentrations of different As species (AsIII, AsV, MMA, and DMA) were measured in the dissolved and intracellular fractions following a 15-day exposure.
Chlorophyll a measurements
Chlorophyll a was extracted from the algal pellet using 5 mL of cold, neat ethanol overnight at 4 °C (Knudson et al. 1977). The extract was filtered using cellulose filters (0.45 μm) and then measured with a Thermo ultraviolet visible light spectrophotometer for light absorbance at 649 and 665 nm for chlorophyll a, using the previously empirical correction equation: chlorophyll a (μg L−1) = 13.7A 665 nm − 5.76A 649 nm (Knudson et al. 1977).
Total As determination
For the estimation of the intracellular total As, the samples of alga were prepared and analyzed according to the method of Yin et al. (2011a). About 0.02 g oven-dried alga samples were digested with 5 mL concentrated HNO3 (65 %, guaranteed reagent, Fairfield, OH, USA) overnight and heated in a microwave-accelerated reaction system (CEM Microwave Technology Ltd., Matthews, NC, USA). The details were similar to the report by Xue et al. (2012).
The total As in the media and alga were measured by ICP-MS operating in the helium gas mode to remove possible interference of 40Ar35Cl on m/z 75. The masses monitored were 75As with 115In and 103Rh as internal standards for signal stability.
HPLC–ICP-MS for As speciation analysis
Approximately 0.02-g freeze-dried alga samples were placed in 15-mL centrifuge tubes and treated in 5 mL of 1 % HNO3 overnight. Then, they were digested in a microwave-accelerated reaction system (the working program was similar to the measurement of intracellular As). The digests were filtered through 0.45-μm cellulose acetate syringe filters. The alga extracts as well as the solutions were analyzed by HPLC–ICP-MS (Agilent LC1100 series and Agilent ICP-MS 7500a; Agilent Technologies) for As speciation with anion exchange columns as detailed by Zhu and Zhang et al. ( Zhu et al. 2008; Zhang et al. 2012). Chromatographic columns were obtained from Hamilton and consisted of a precolumn (11.2 mm, 12–20 μm) and a PRP-X100 10-μm anion exchange column (250 × 4.1 mm). As species (AsIII, AsV, DMA, and MMA) were separated with a mobile phase of 10 mM NH4H2PO4 and 10 mM NH4NO3, adjusted to pH 6.2 with nitric acid or ammonia, and run isocratically at 1.0 mL min−1. Germanium (Ge) as an internal standard was mixed continuously with the postcolumn solution through a peristaltic pump. Signals at m/z 75 (As) and 72 (Ge) were collected with a dwell time of 300 and 100 ms, respectively.
Quality control and data analysis
For quality control, two standard solutions of 5 and 50 μg L−1 As were analyzed every 10 samples, and results were accepted only if recovery was in an acceptable range (e.g., 90–110 %). The deviation of the instrument was below 1.3 % during the analysis. Total As was certified by the Laver, a standard reference materials (GBW08521 from the National Research Center for Standard Materials in China), which was used to represent phytoplankton tissues. The measured recovery (n = 3) was 105 % with the concentration of 43 ± 1 mg kg−1, which was similar to the certified concentration (41 ± 3 mg kg−1).
The software OriginPro 8.5.0 SR1 (OriginLab Corporation, 1991–2010) was used to measure the concentration of each compound identified. The limits of detection of the instrument were, respectively, 0.6, 0.5, 0.5, and 0.6 μg kg−1 for AsIII, DMA, MMA, and AsV with anion exchange column. All data were subjected to analysis of variance (ANOVA) using windows-based SPSS 15.0 and GraphPad Prism 6.0.
Results
Algal growth and inorganic As toxicity
The toxic effect of inorganic As on the algal cells (M. aeruginosa) was determined. Algal-specific growth inhibition rate increased with the increasing concentration of As. This algae showed great tolerance to AsV with 72-h IC50 value of 3,582 μM and to AsIII with 72-h IC50 of 133 μM, indicating that AsIII was more toxic than AsV to M. aeruginosa. 72-h IC50 was many orders of magnitude greater than the expected environmental As concentrations.
Variation of alga cell density and chlorophyll a concentrations of M. aeruginosa exposed to different concentrations of inorganic As were shown in Fig. 1. There was an insignificant difference of alga cell density and chlorophyll a between the control and different AsV concentration treatments during 15 days of culture (Fig. 1). Both cell density and chlorophyll a increased gradually with the increase of incubation time in the log phase of growth, indicating that unnoticeable growth-stimulating or toxic effects of AsV on this alga at the tested concentrations occurred. No significant difference of alga cell density and chlorophyll a was detected between the control and 5–20 μM AsIII treatments (Fig. 1). Nevertheless, 50 μM AsIII treatment inhibited the alga growth at the preliminary stage. It regenerated slightly on day 7 and was multiplied on subsequent days, although both cell count and chlorophyll a content decreased obviously compared to the control or other treatments. Approximately complete death of alga at 100 μM AsIII after 7 days demonstrated its sensitivity to AsIII stress.
Accumulation of As by M. aeruginosa
The intracellular As concentrations of the alga after 15-days AsIII exposures were significantly higher than that after AsV exposures (Fig. 2). In the control, As concentration in the alga was only 4 μg g−1 DW, indicating the original algae were not contaminated by As. During 15 days of incubation period, total As concentrations accumulated by M. aeruginosa were proportional to the increasing concentrations of As added to the growth media (P < 0.05). Compared to the maximum As accumulation (96.6 μg g−1 DW) in the AsV treatments, the intracellular As amount was higher in AsIII exposures with the maximum value of 260 μg g−1 DW. The percentage of the intracellular As contents to the total As supplemented in the media was up to 12 % for 100-μM AsV exposures and 21 % for 50-μM AsIII exposures after 15 days. No data about the intracellular concentration was shown in 100-μM AsIII exposures due to the death of algal cells after 5 days (Fig. 2).
Arsenic accumulation and influx into M. aeruginosa following 15 days of exposures to AsV (open circles) or AsIII (open squares) (mean values ± SD, n = 3). Concentrations are shown following the subtraction of the background As concentration. Linear regression (solid line) was used to determine the uptake rate constants ((μg g−1 day−1), and nonlinear regression (Michaelis–Menten) fits were used to derive metal binding characteristics
Kinetic constants for As uptake were calculated by fitting hyperbolic models to the data of As accumulation using the nonlinear regression module (Kahle and Zauke 2002; Clason et al. 2003). Arsenic influx can be well described by the Michaelis–Menten equation with R 2 values of 0.940 for AsV and 0.973 for AsIII (Fig. 2, dotted line). The Michaelis–Menten calculations suggested that the maximum uptake rate V max (micrograms per gram per day ± SE) for AsIII (27.53 ± 3.185) was twofold greater than the V max for AsV (10.66 ± 0.997). The half saturation concentrations (K m (micromolars) ± SE) were 27.66 ± 6.484 for AsIII and 25.15 ± 6.241 for AsV (not significantly different with P = 0.26, two-tailed t test).
Arsenic speciation in M. aeruginosa
Four As species including AsV, AsIII, MMA, and DMA were detected in algal cells in different AsIII treatments (Fig. 3). However, no MMA was observed in AsV treatments, while the range of 0.10 to 0.15 μg g−1 for MMA was observed in cells in AsIII treatments. Analysis of As speciation showed that AsV was the predominant species in AsIII or AsV exposures, accounting for 78–93 % of total As, while AsIII, the other inorganic form, was the minor species that accounted for 6–21 % of the total As. Additionally, small amounts of organoarsenicals accounted for <1 % of total As in different treatments. This alga accumulated relatively more AsV from AsIII solutions than that from AsV solutions. Arsenate accumulation in cells increased linearly with increasing concentrations of AsIII and AsV added in the test media. The cellular concentrations of DMA were similar in both AsIII and AsV exposures, and reached a plateau at 10 μM concentrations (about 0.75 μg g−1). In contrast to AsV accumulation, AsIII accumulation in AsV treatments ranged largely from 8.9 to 20.3 μg g−1 with a significant difference from the control. However, in AsIII treatments, the tendency of AsIII accumulation was similar to AsV concentrations in cells, which remarkably increased with increasing addition of AsIII.
Arsenic speciation in M. aeruginosa after 15 days of exposures to arsenate (a) or arsenite (b) (mean values (μg g−1 (dw)) ± SD, n = 3); MMA momomethylarsonous acid, DMA dimethylarsinous acid. Regression(s) have been fit to reflect “One site-specific binding with Hill slope” using GraphPad Prism. a The coefficients of regression (R 2) for AsV, AsIII, and DMA are 0.9770, 0.4620, and 0.5889, respectively. b The coefficients of regression (R 2) for AsV, AsIII, MMA, and DMA are 0.9695, 0.9758, −0.3265, and 0.9403, respectively
Arsenic speciation changes in the growth media
Although small amounts of AsV were found in the growth media containing AsIII (about 15 %), neither of the tested As species showed any transformation in the algae-free controls after 15-day incubation (data not shown). This confirmed their relative stability in the growth media throughout the test period. Only trace amounts of AsIII were present in test media containing M. aeruginosa and decreased with increasing time during the first 5 days in AsV exposures (Fig. 4). AsIII reduced from the initial AsV and increased with increasing initial AsV concentrations. However, no AsIII was detected in the controls (no algae), indicating that the presence of AsIII in the growth media containing M. aeruginosa was due to biological reduction. Reduction to AsIII in the media also occurred insignificantly on other days (Fig. 4). As for AsV treatments, AsV concentrations in the media decreased with increasing time. Although the lowest values were observed on the 13th day, AsV remained relatively stable and predominated in the media over the 15-day period. Methylated As species including DMA and MMA were also detected at lower concentrations in these media. Additionally, both of them increased with time and had insignificant differences among different concentration treatments.
Significant changes of As speciation occurred in the media containing AsIII (Fig. 5). With the onset of exponential algal growth, AsIII concentration decreased rapidly with time and almost completely disappeared on the seventh day due to the biologically and chemically mediated oxidation of AsIII to AsV. However, AsV immediately appeared in the initial stage in the media and then became dominant on the seventh day. At the end of the experiment, 99 % of total As remaining in the solution was presented as AsV. Two other As species, MMA and DMA, which were measured at the same time on the fifth day, increased similarly with increasing time during 15-day incubation in the media containing different concentrations of AsIII. Additionally, DMA showed higher concentrations in the media than MMA. In our experiments, there were significant differences between the sum of two reduced transformation products and total As concentrations remaining in the media at the end of the exposure period (two-way ANOVA, P < 0.01), indicating that there were other major unidentified As species released into the solution. Supportively, there was a little loss of volatile As speciation, which was verified by ancient Gutzeit method in our experiment.
As can be seen from Fig. 6a, the release rate of AsIII, averaging from 4.7 × 10−3 to 4.3 × 10−1 μM g−1 day−1, dramatically increased with increasing AsV concentration. This suggested that a significant fraction of AsIII was produced and released by M. aeruginosa after AsV exposure. The average release rate of MMA reached peak value with 2.6 × 10−2 μM g−1 day−1 at 10 μM AsV concentration, but DMA was at 20 μM AsV concentration with 7.7 × 10−2 μM g−1 day−1. Notably, only AsIII was found in solution on the first 5 days during AsV exposure, of which average release rate was higher than that of organoarsenicals, especially on the second day. As can be seen from Fig. 6b, all the average release rates of AsV, DMA, and MMA increased with increasing AsIII concentration during 15-day incubations, particularly at 50 μM AsIII concentration. This suggested the coexistence of bio-oxidation and methylation of As metabolism.
Discussion
Accumulation of As by M. aeruginosa
In this study, the accumulation of As by M. aeruginosa typically increased with increasing AsV and AsIII concentrations in the test media. The active uptake was considered to play major roles in As accumulation in alga (Maeda et al. 1985). Surely, similar studies have already documented the findings in Synechococcus leopoliensis and Chlorella vulgaris (Budd and Craig 1981; Maeda et al. 1992a, b).
The V max for AsIII was twice more than that of AsV, suggesting that as an analog of phosphate structure, AsV uptake in M. aeruginosa cells is achieved via fewer available sites (i.e., lower binding site density) in such excess phosphate regimes (Guo et al. 2011). AsIII can be easily transported via aquaporins in some plants because it exists predominantly as an undissociated neutral molecule at pH 6.0 (Zhao et al. 2009). Therefore, the influx rate of AsIII was greater than that of AsV in solutions with higher phosphate concentrations in this study (Fig. 2) (Huang et al. 2011). Subsequently, AsIII exposures significantly elevated intracellular As concentrations compare to AsV exposures.
Additionally, M. aeruginosa exhibited a stronger capacity of As bioaccumulation compared to other freshwater algae (Levy et al. 2005; Yin et al. 2011b). It was supported by the results that intracellular As in M. aeruginosa was more than 7 % of total As supplemented in the media on the 15th day. Surely, previous reports have stated that much greater accumulation can occur in C. vulgaris and Synechocystis from an As-polluted environment (Maeda et al. 1992a, b; Yin et al. 2011a). Consequently, As bioaccumulation in alga bloom should be given more attention due to a potential hazard to animals and humans.
Arsenic speciation in M. aeruginosa
The distribution of intracellular As speciation (Fig. 3) clearly demonstrated that AsV was the predominant species followed by AsIII, DMA, and MMA after 15 days of algae exposure to AsIII or AsV at concentrations of 5–100 μM. The findings also clearly demonstrated that bio-oxidation of AsIII to AsV was the predominant transformation process in algal cells in freshwater enriched with AsIII and phosphate. Similar findings were also observed in C. vulgaris and Synechocystis (Suhendrayatna et al. 1999; Yin et al. 2011a). Maeda et al. (1992a) similarly found that 95 % of As was accumulated by freshwater algae, C. vulgaris, as inorganic species, and dimethylated As was the major methylated As compound. In our study, significant changes of intracellular As speciation occurred after 15 days of exposure to different concentrations of AsV or AsIII (Fig. 3). With the increase of exposure of As concentrations, intracellular AsV concentrations as the dominant species increased dramatically. However, for methylated As, the contents seemed to be limitation to the alga, just as Maeda inferred in the survey of C. vulgaris (Maeda et al. 1992b). Arsenite concentrations in cells showed a similar tendency after AsIII treatments, but it fluctuated strongly in AsV treatments. Nevertheless, the accumulation of AsIII in cells occupied a higher proportion for AsV treatments compared to AsIII treatments. These results demonstrated that M. aeruginosa have the ability to biotransform AsV into reduced As species as a precursor for methylation (Hellweger and Lall 2004).
Arsenic speciation changes in the growth media
Rapid changes of As speciation that occurred in the growth media containing inorganic As species indicated that M. aeruginosa was able to oxidate, reduce, methylate, and release As to the growth media. Although some of the additional As was incorporated into cells, much of it was apparently metabolized and rapidly released to the surrounding water. The reduction of AsV to AsIII was rapid in the initial stage, which exhibited positive correlations with the exposure concentrations of AsV, just as Sanders and Cullen documented (Sanders and Windom 1980; Cullen et al. 1994). The result of a peak of AsIII in the media occurred in the initial stage and preceded algal bloom as reported by Hellweger (2005). It is generally known that the amount of organo-As species in the media depends on the rates of methylation/demethylation and release processes. The discovery of MMA in the growth media with little or no MMA in cells also supported the methylation of inorganic As as well as a rapid and ready excretion of MMA and DMA in AsV exposures.
AsIII oxidation rates in the media containing AsIII dominated by the alga were significant (Fig. 6), especially at 50 μM AsIII concentration. The average release rate of AsIII increased with increasing AsV concentration, indicating that AsV reduction by algae was enhanced with increasing As concentrations in the media. The observed reduction rates of M. aeruginosa with 2.0 × 10−2 to 4.3 × 10−1 μM g−1 day−1 were similar to net reduction rates previously estimated from green alga Chlorella sp. in culture (Knauer and Hemond 2000).
Generally, bio-oxidation was the first and primary transformation mechanism for AsIII biotransformation, while bioreduction together with subsequent methylation may play an important role in AsV biotransformation. Additionally, the results also showed that the alga was more sensitive to AsIII. Similar results were also often reported (Carbonell et al. 1998; Levy et al. 2005; Duester et al. 2011; Yin et al. 2011a). These similar findings supported that this algal bloom species from As-polluted water could tolerate higher As concentrations in freshwater environments and might become the dominant species in As-contaminated waters. Therefore, As toxicity depends on not only As speciation in the media but also the specific mechanism of bioaccumulation and biotransformation of the algal species of interest.
Conclusion
The aims of this study were to investigate the bioaccumulation and biotransformation of inorganic As in M. aeruginosa as well as the changes of As speciation in the media caused by the dominant species in cyanobacterial bloom. M. aeruginosa came from As-polluted lake has high tolerance to AsV with little influence on its growth. Although different As species transformations occurred during AsV or AsIII exposures, AsV was the prominent species in the media, eventually with unremarkable DMA and MMA. It can be concluded that the transformations of As species in M. aeruginosa were seldom affected by external As concentrations, especially in high concentrations of As pollution.
References
Alava P, Tack F, Du Laing G, Van de Wiele T (2012) HPLC–ICP-MS method development to monitor arsenic speciation changes by human gut microbiota. Biomed Chromatogr 26:524–533
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35:647–654
Budd K, Craig SR (1981) Resistance to arsenate toxicity in the blue-green alga Synechococcus leopoliensis. Can J Bot 59:1518–1521
Carbonell AA, Aarabi MA, DeLaune RD, Gambrell RP, Patrick WH (1998) Arsenic in wetland vegetation: availability, phytotoxicity, uptake and effects on plant growth and nutrition. Sci Total Environ 217:189–199
Caumette G, Koch I, Estrada E, Reimer KJ (2011) Arsenic speciation in plankton organisms from contaminated lakes: transformations at the Base of the Freshwater Food Chain. Environ Sci Technol 45:9917–9923
Clason B, Duquesne S, Liess M, Schulz R, Zauke GP (2003) Bioaccumulation of trace metals in the Antarctic amphipod Paramoera walkeri (Stebbing, 1906): comparison of two-compartment and hyperbolic toxicokinetic models. Aquat Toxicol 65:117–140
Cullen WR, Li H, Pergantis SA, Eigendorf GK, Harrison LG (1994) The methylation of arsenate by a marine alga Polyphysa peniculus in the presence of L-methionine-methyl-d3. Chemosphere 28:1009–1019
Dopp E, von Recklinghausen U, Diaz-Bone RA, Hirner AV, Rettenmeier AW (2010) Cellular uptake, subcellular distribution and toxicity of arsenic compounds in methylating and non-methylating cells. Environ Res 110:435–442
Duester L, van der Geest HG, Moelleken S, Hirner AV, Kueppers K (2011) Comparative phytotoxicity of methylated and inorganic arsenic- and antimony species to Lemna minor, Wolffia arrhiza and Selenastrum capricornutum. Microchem J 97:30–37
Duncan E, Foster S, Maher W (2010) Uptake and metabolism of arsenate, methylarsonate and arsenobetaine by axenic cultures of the phytoplankton Dunaliella tertiolecta. Bot Mar 53:377–386
Francesconi KA, Edmonds JS, Stick RV (1992) Arsenic compounds from the kidney of the giant clam Tridacna maxima—isolation and Identification of an arsenic-containing nucleoside. J Chem Soc Perkin Trans 1:1349–1357
Guo PR, Gong Y, Wang C, Liu X, Liu JT (2011) Arsenic speciation and effect of arsenate inhibition in a Microcystis aeruginosa culture medium under different phosphate regimes. Environ Toxicol Chem 30:1754–1759
Hellweger FL (2005) Dynamics of arsenic speciation in surface waters: as(III) production by algae. Appl Organomet Chem 19:727–735
Hellweger FL, Lall U (2004) Modeling the effect of algal dynamics on arsenic speciation in lake Biwa. Environ Sci Technol 38:6716–6723
Hellweger FL, Farley KJ, Lall U, Di Toro DM (2003) Greedy algae reduce arsenate. Limnol Oceanogr 48:2275–2288
Huang Y, Hatayama M, Inoue C (2011) Characterization of As efflux from the roots of As hyperaccumulator Pteris Vittata L. Planta 234:1275–1284
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332
Kahle J, Zauke GP (2002) Bioaccumulation of trace metals in the copepod Calanoides acutus from the Weddell Sea (Antarctica): comparison of two-compartment and hyperbolic toxicokinetic models. Aquat Toxicol 59:115–135
Karadjova IB, Slaveykova VI, Tsalev DL (2008) The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat Toxicol 87:264–271
Knauer K, Hemond H (2000) Accumulation and reduction of arsenate by the freshwater green alga Chlorella sp. (Chlorophyta). J Phycol 36:506–509
Knudson LL, Tibbitts TW, Edwards GE (1977) Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol 60:606–608
Levy JL, Stauber JL, Adams MS, Maher WA, Kirby JK, Jolley DF (2005) Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ Toxicol Chem 24:2630–2639
Maeda S, Nakashima S, Takeshita T, Higashi S (1985) Bioaccumulation of arsenic by freshwater algae and the application to the removal of inorganic arsenic from an aqueous phase. Part II. By Chlorella vulgaris isolated from arsenic-polluted environment. Sep Sci Technol 20:153–161
Maeda S, Kusadome K, Arima H, Ohki A, Naka K (1992a) Biomethylation of arsenic and its excretion by the alga Chlorella vulgaris. Appl Organomet Chem 6:407–413
Maeda S, Kusadome K, Arima H, Ohki A, Naka K (1992b) Uptake and excretion of total inorganic arsenic by the freshwater alga Chlorella vulgaris. Appl Organomet Chem 6:399–405
Markley CT, Herbert BE (2010) Modeling phosphate influence on arsenate reduction kinetics by a freshwater cyanobacterium. Environ Model Assess 15:361–368
Naranmandura H, Suzuki KT (2008) Formation of dimethylthioarsenicals in red blood cells. Toxicol Appl Pharmacol 227:390–399
Nordstrom DK (2002) Public health—worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Oberholster PJ, Botha AM, Grobbelaar JU (2004) Microcystis aeruginosa: source of toxic microcystins in drinking water. Afr J Biotechnol 3:159–168
Pawlik-Skowronska B, Pirszel J, Kalinowska R, Skowronski T (2004) Arsenic availability, toxicity and direct role of GSH and phytochelatins in As detoxification in the green alga Stichococcus bacillaris. Aquat Toxicol 70:201–212
Qin J, Lehr CR, Yuan CG, Le XC, McDermott TR, Rosen BP (2009) Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc Natl Acad Sci USA 106:5213–5217
Rahman M (2002) Arsenic and contamination of drinking-water in Bangladesh: a public-health perspective. J Health Popul Nutr 20:193–197
Rahman MA, Hasegawa H (2012) Arsenic in freshwater systems: influence of eutrophication on occurrence, distribution, speciation, and bioaccumulation. Appl Geochem 27:304–314
Sanders JG, Windom HL (1980) The uptake and reduction of arsenic species by marine algae. Estuar Coast Mar Sci 10:555–567
Sharma VK, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Int 35:743–759
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Suhendrayatna OA, Kuroiwa T, Maeda S (1999) Arsenic compounds in the freshwater green microalga Chlorella vulgaris after exposure to arsenite. Appl Organomet Chem 13:127–133
Sun GX, Van de Wiele T, Alava P, Tack F, Du Laing G (2012) Arsenic in cooked rice: effect of chemical, enzymatic and microbial processes on bioaccessibility and speciation in the human gastrointestinal tract. Environ Pollut 162:241–246
Wu ZX, Gan NQ, Song LR (2007) Genetic diversity: geographical distribution and toxin profiles of Microcystis strains (Cyanobacteria) in China. J Integr Plant Biol 49:262–269
Xue PY, Yan CZ, Sun GX, Luo ZX (2012) Arsenic accumulation and speciation in the submerged macrophyte Ceratophyllum demersum L. Environ Sci Pollut Res 19:3969–3976
Yin XX, Wang LH, Bai R, Huang H, Sun GX (2011a) Accumulation and transformation of arsenic in the blue-green alga Synechocystis sp. PCC6803. Water Air Soil Pollut 223:1183–1190
Yin XX, Wang LH, Duan GL, Sun GX (2011b) Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii. J Environ Sci 23:1186–1193
Zeng J, Yang L, Wang WX (2009) Cadmium and zinc uptake and toxicity in two strains of Microcystis aeruginosa predicted by metal free ion activity and intracellular concentration. Aquat Toxicol 91:212–220
Zhang X, Zhao FJ, Huang Q, Williams PN, Sun GX, Zhu YG (2009) Arsenic uptake and speciation in the rootless duckweed Wolffia globosa. New Phytol 182:421–428
Zhang X, Uroic MK, Xie WY, Zhu YG, Chen BD, McGrath SP, Feldmann J, Zhao FJ (2012) Phytochelatins play a key role in arsenic accumulation and tolerance in the aquatic macrophyte Wolffia globosa. Environ Pollut 165:18–24
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, Meharg AA, Williams PN (2008) High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol 42:5008–5013
Acknowledgments
We thank the anonymous reviewers for their helpful comments. This work was supported by International Science & Technology Cooperation Program of China (2011DFB91710), the National Nature Science Foundation of China (Project Nos. 21277136 and 41271484), and the Fujian Provincial Education Department Foundation (JA12214).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
About this article
Cite this article
Wang, Z., Luo, Z. & Yan, C. Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa . Environ Sci Pollut Res 20, 7286–7295 (2013). https://doi.org/10.1007/s11356-013-1741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1741-7