Abstract
Growing industrialisation, urbanisation and technological advancements have been endlessly increasing the environmentally contaminating heavy metals load. Arsenic contamination as an environmental pollutant has transcended as a major global concern to address. Arsenic contamination of air, soil, water, sediment and crops due to the various anthropogenic (agricultural) and geogenic (geochemical) sources is a major global threat, including India, owing to its hazardous and toxic nature. Primarily, the three and five valency arsenic cause severe human health concerns at an elevated concentration (>0.05 mg/l) affecting millions of people worldwide year-after-year. Generally non-biodegradable, arsenic can be transformed into less toxic forms by adopting chemical, biological and/or composite techniques involving oxidation-reduction, methylation, complexation, precipitation, immobilisation through sorption, etc. Microbial and phyto-remediation of arsenic through adsorption, absorption, extracellular entrapment, precipitation and oxidation-reduction reactions are gaining global attention due to their greater advantages. While phytoremediation includes phytoextraction and phytovolatilisation, microbial biomass remediates through active/passive/combined arsenic binding. The chapter embodies the underlying arsenic toxicity and bioremediation mechanisms for a cleaner and healthier environment. It details the chemical and biological remediation of arsenic highlighting the advantages of biological approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
The delicate environmental balance has been upset due to excess load of numerous inorganics including heavy metals generated through mining, rapid industrialisation, and urbanisation. Amongst the heavy metals, environmental pollution caused due to arsenic contamination is considered as vital for its severe toxicity and potential associated health risks. Arsenic could generate multiple adverse health effects because of its many (inorganic and organic) chemical forms, the most common inorganic trivalent arsenic forms being arsenic trioxide, sodium arsenite, and arsenic trichloride (Adeniji 2004). The acute symptoms of arsenic poisoning, as a consequence of consuming it above the maximum tolerable limit of 0.05 mg/l, are skin discoloration, skin thickening and ultimately skin cancer (Khan et al. 2000; Dey et al. 2017; Banerjee et al. 2011). Arsenic is a metalloid (considered a heavy metal) under group ‘V’ element of the periodic table (Satyapal et al. 2016). It is found in four different oxidation states, i.e., +5, +3, 0, and − 3 in nature. Of these, the pentavalent (AsV; Arsenate) and trivalent (AsIII; Arsenite) exist mostly in inorganic forms (Satyapal et al. 2018). Although both pentavalent and trivalent forms are poisonous but AsIII due to its high mobility is 1000-fold more toxic than its counterpart (Satyapal et al. 2018; Dey et al. 2016). Organic arsenicals derived from pesticides, herbicides and preservatives are also encountered in the environment (Satyapal et al. 2018).
Heavy metals like lead, arsenic, nickel, mercury, and cadmium are not at all beneficial to plants, affecting the plant wellbeing negatively through reduced photosynthesis, nutrient uptake and certain enzymatic malfunctioning (Lim et al. 2014). Low concentration of heavy metals leads to cytotoxicity and higher concentration to cancer (Tak et al. 2013). It occurs due to contamination in food chain at a point and bioaccumulation inside the living organisms (Tak et al. 2013). The cellular damage happens due to the reactive oxygen species ROS, mainly oxygen radicals, damaging the DNA (Chibuike and Obiora 2014). The sources of arsenic contamination, both natural and anthropogenic, have resulted in wide arsenic contamination of the soil, water, air and crops (Satyapal et al. 2016). In certain geographical regions, the animals and humans are constantly exposed to high arsenic concentrations through contaminated drinking water and food crops (Satyapal et al. 2018; Dey et al. 2016). As per World Health Organisation (WHO), the maximum permissible limit for arsenic contaminant in drinking water is 0.01 mg/l (WHO 2011; Aksornchu et al. 2008).
Arsenic’s notoriety is most predominant in groundwater systems as toxic metalloid. The greatest mass poisoning in human history was reportedly due to the drinking of arsenic contaminated groundwater in millions of people (Dey et al. 2017). Various anthropogenic activities have further accelerated arsenic contamination, especially in the South East Asia region. It was estimated that more than six million people in West Bengal, India (Dey et al. 2016; Anyanwu and Ugwu 2010) and 46 million people in Bangladesh are at a risk of arsenic poisoning due to drinking water (Dey et al. 2016; Bachate and L Cavalca 2009). Arsenic contamination in groundwater is at an alarming situation in Indian states of West Bengal, Chhattisgarh, Bihar, Telangana and Uttar Pradesh.
Removing the contaminating heavy metals through (micro)biological means is widely accepted as the most efficient, cost effective, and eco- and health-friendly (Ekperusi and Aigbodion 2015; Ayangbenro and Babalola 2017). The capacity of microbes to remove heavy metals and metalloids is dependent on the suitability of the abiotic factors, such as, temperature, pH, physical and chemical properties of soil, and moisture (Verma and Jaiswal 2016). The chapter deliberates on the chemical and biological remediation of arsenic from contaminated soils, and the phytological/microbial mechanisms involved in heavy metals decontamination.
8.2 Sources of Arsenic Contamination
Arsenic in the environment (air, soil, water, and sediment) originates from both natural (geogenic) and anthropogenic sources (Satyapal et al. 2018; Fig. 8.1). The key origin points of arsenic flow from the natural geogenic sources include volcanic eruptions, weathering, fossil fuels, minerals, parent/sedimentary rock bearing arsenic (Mohapatra et al. 2017a). Various anthropogenic activities like agriculture, mining, smelting, refining, electroplating, coal combustion, painting and chemical manufacturing have added to the arsenic release to the environment (Mohapatra et al. 2017a; Dey et al. 2016; Akhtar et al. 2013). Manufacturing of agricultural chemicals (e.g. pesticides, herbicides, fertilisers, wood preservatives, etc.), dying materials and medical products, are other major sources of arsenic contamination (Mohapatra et al. 2017a; Vishnoi and Singh 2014).
8.3 Arsenic Toxicity
Presence of Arsenic in the environment beyond the permissible limit of 0.01 mg/l can generate multiple acute and chronic health disorders (Dey et al. 2016). AsIII compound such as arsenic trioxide, sodium arsenite and arsenic trichloride could cause neurotoxicity of both the peripheral and the central nervous system (Adeniji 2004). On the other hand, AsV inorganic forms such as arsenic pentoxide, arsenic acid and Arsenates could also affect the enzyme activity of human metabolism (Klaassen and Watkins III 2003). Trivalent or pentavalent organoarsenic, specially the methylated, forms cause biomethylation and are potential health hazards in humans, animals and other higher living organisms as they enter the food chain (Mateos et al. 2006; Adeniji 2004). Excess exposure to arsenic even at a low concentration could create acute health issues including skin itching, skin discoloration, hyperkeratosis, skin thickening leading to skin cancer, fever, anorexia, melanosis, weight loss, appetite loss, gastrointestinal disorders (e.g. nausea, anorexia, stomach irritation, abdominal pain, enlarged liver and spleen), anaemia, weakness, lethargy, granulocyopenia cardiac arrhythmia, and cardiovascular failure (Mohapatra et al. 2017a; Taran et al. 2013; Cavalca et al. 2013; Dey et al. 2016). Long-term arsenic exposure could cause chronic respiratory disorders, lungs irritation, immune-suppression, arsenicosis, sensory loss, changes in skin epithelium, ultimately leading to cancer due to DNA damage (Mohapatra et al. 2017a; Adeniji 2004). High arsenic intake could cause infertility, fatal health issues, miscarriages in women, type-II diabetes, brain damage, cardiovascular problems including hypertension, coronary artery diseases, peripheral vascular disease and atherosclerosis (Mohapatra et al. 2017a). AsIII could also cease protein functions by binding to the sulfhydryl groups of cysteine residues (Cavalca et al. 2013).
8.4 Soil Reclamation Strategies
The various remediation measure for heavy metal contamination are functional now days (Fig. 8.2). The measures for removal of arsenic from the contaminated environment should follow several minimal technical standards that consists effectiveness, no adverse impact on the environment, and no health hazard for the neighbouring organism or the system. Presently, three main reclamation practices are functional namely, physical, chemical, and biological/bioremediation (Lim et al. 2014; Duarte et al. 2009). There are varieties of conventional methods have been used for removal of arsenic from the contaminated aqueous system such as membrane filtration, precipitation, reverse osmosis, coagulation, oxidation-reduction, adsorption etc. (Dey et al. 2017; Dey et al. 2016; Bahar et al. 2012). In addition to these physicochemical remediation processes, microbial bioremediation is also proven as a potential eco-friendly approach for clean-up of the arsenic contaminated area because of its low operation cost, less energy requirements, non and high removal efficiency (Voica et al. 2016; Dey et al. 2017; Das and Dash 2014).
Complex make of the microbial cell and their metabolic specialities make them potential agents to address arsenic toxicity by transforming it to a less-toxic form, and/or remove them during the cellular metabolic process, biosorption and accumulation phenomena (Mohapatra et al. 2017a; Dey et al. 2016). Table 8.1 describes the advantages and disadvantages of some of the reported physicochemical and biological arsenic remediation approaches.
8.4.1 Arsenic Removal from Contaminated Waters
Conventional and advanced successful treatment approaches to remove arsenic from groundwater under both laboratory and field conditions have been reported, which include: (i) coagulation/flocculation, (ii) adsorption, (iii) ion-exchange and (iv) membrane processes (Mondal et al. 2013). A potentially scalable bioremediation technology involving green synthesis of nano-adsorbents using bacteria, yeasts, fungi and plant extracts is also reported (Mondal et al. 2006; Bahar et al. 2012).
8.4.2 Arsenic Removal from Contaminated Soils
There are many arsenic removal approaches that could be divided primarily into three categories, physical, chemical, and biological (Lim et al. 2014).
8.4.3 Physical Approach
One of the popular approaches is, mixing both the uncontaminated and contaminated soils together till the arsenic concentration reaches an acceptable level (Lim et al. 2014; Mahimairaja et al. 2005). Soil washing is a physicochemical approach whereby the soil contaminated with arsenic is washed in presence of chemicals such as sulphuric/nitric/phosphoric acids, and/or hydrogen bromide (Lim et al. 2014).
8.4.4 Chemical Approach
The chemical approach employed for the purpose as extractant is costly and often is restricted to soil washing at smaller-scale operations (Mahimairaja et al. 2005). Cement could also immobilise soluble Arsenites and has been successfully used to stabilise arsenic-rich sludge (Sullivan et al. 2010). Furthermore, additives, such as, surfactants, cosolvents, etc. could also enhance the soil flushing efficiencies using aqueous solutions. Surfactant alone was about 80–85% efficient in laboratory conditions, while more complex processes such as polymer injection enhanced the efficiency (Atteia et al. 2013). Available chemical remediation approaches involve methods such as adsorption by using specific media, immobilisation, modified coagulation along with filtration, precipitations, immobilisations and complexation (Duarte et al. 2009; Mahimairaja et al. 2005). Coagulation along with filtration for arsenic removal is quite economical but often displayed lower (<90%) efficiencies (Lim et al. 2014).
8.4.5 Biological Approach
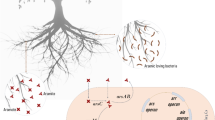
Biological measures are broadly distinguished as phytoremediation and microbially-mediated remediation. Plants and microbes, especially the ones thriving in arsenic-rich environment, have evolved themselves to sustain and metabolise arsenic and their metalloids. Some bacteria convert the inorganic and organic arsenic to trimethyl-arsine (less toxic gaseous arsenic), particularly under anaerobic conditions. This could be accomplished through various ways including biomethylation, biotransformation and biooxidation (Srivastava et al. 2011; Liu et al. 2009; Casarett et al. 2008). With several limitations of non-biological approaches, biological approach is gaining popularity, particularly due to its cost effectiveness. Biological remediation approach is primarily divided into two subcategories, intrinsic and engineered. Intrinsic bioremediation is mainly meant to address low-level contamination by specialised natural/wild microbes, whereas engineered bioremediation is useful in addressing critically contaminated soils by engineered microbes (Lim et al. 2014) (Fig. 8.3).
8.4.5.1 Phytoremediation
Phytoremediation is an efficient way to bioremediate contaminated soils and water bodies (Mishra et al. 2000). Several hyperaccumulating plant varieties (1 kg biomass accumulating up to one-gram arsenic) are reported. The cheapest technology for heavy metal removal, this approach is time saving and also decreases the volume of the contaminated biomass (Chattopadhyay et al. 2017). Phosphorus helped in mobilising and enhancing the uptake capacity of arsenic in sunflower which could sustain 250 mg of arsenic/kg plant biomass in soil, whereas Chinese brake (Pteris vittata L.) could tolerate up to 22,600 mg of arsenic/kg plant biomass on a dry weight basis (Jang et al. 2016). The nonprotein thiols, phytochelatins, phytochelatins and glutathione produced by plants as a defense mechanism help in decontaminating arsenic-rich soil (Dixit et al. 2016). The mechanisms involved arsenic decontamination involve phytoextraction, rhizofiltration and phytovolatilization (Fig. 8.4). Microbial associations with plants often play an important role as the facilitator in arsenic cycling.
The internal resistant mechanisms to reduce metal toxicity in plants include sequestration of metals and phytochelatins. Phytochelatins are cysteine rich peptides formed by glutathione at high arsenic concentration (Mesa et al. 2017). Further, plants are divided into the following groups on the basis of their metal removal efficiencies.
8.4.5.1.1 Excluders
These group of plants restrict the uptake and translocation of arsenic on the terminal parts by tolerating the existing high concentration of arsenic through intracellular chelators. The excess arsenic segregated and is stored in the non-sensitive plant parts, a phenomenon known as compartmentalisation (Sun et al. 2009).
8.4.5.1.2 Accumulators
Accumulator plant performs remediation via the uptake and translocation arsenic into the terminal parts without any discernible plant symptoms. These can uptake upto 1000 mg As/kg dry shoot.
8.4.5.2 Microbially-Mediated Remediation
Microbes (bacteria, fungi and algae) are very effective and efficient bioremediating agents. Their small life span and adaptative abilities help thrive well even in harsh environments. Below is an account of the usefulness of each group as bioremediation agents.
8.4.5.2.1 Bacterial Remediation
Bacteria possess multiple bioremediation potentials and hence certainly are beneficial agents, from both environmental and economic point of view, for toxic pollutants cleanup. Such bioremediation (of toxic pollutants including metals/metalloids) is achieved by using native bacteria isolated from the contaminated sites and stimulating their detoxification ability by process and product engineering (Das and Dash 2014). The use of suitable non-native and/or genetically engineered microbes suited for arsenic bioremediation has been successfully demonstrated at least at research-scale if not at field-scale (Das and Dash 2014).
8.4.5.2.1.1 Mechanism of Arsenic Bioremediation
The mechanisms in arsenic bioremediation are majorly biotransformation and biosorption.
8.4.5.2.1.1.1 Biotransformation Mechanism
In this, the microbes could decrease the toxicity of the contaminants by using them as energy sources while transforming them through the energy-yielding oxidation-reduction reactions utilising oxygen, carbon dioxide, nitrates, sulphate acetate, lactate and glucose as electron acceptors/donors during metabolism (Dey et al. 2017; Akhtar et al. 2013). Dey et al. (2017) reported that the bacteria having the capacity to resist toxic metals can chemically transform heavy metals/metalloids through their common cellular metabolism through oxidation, reduction, methylation, demethylation, precipitation etc. Bacteria could exploit arsenic in their metabolic process either as an electron acceptor as in case of anaerobic respiration or as an electron donor as in case of chemoautotrophic fixation of CO2 into cell carbon (Akhtar et al. 2013). Dissimilatory arsenate-reducing bacteria use arsenate as an electron acceptor and reduce it to arsenite. Chemoautotrophic arsenite oxidising bacteria use CO2 as the carbon source and arsenite as an electron acceptor, oxidising it to arsenate for energy, whereas heterotrophic arsenite oxidisers use oxygen as an electron acceptor to oxidise arsenite to arsenate (Fig. 8.3; Akhtar et al. 2013).
8.4.5.2.1.1.2 Biosorption of Arsenic
Microbial arsenic biosorption involves the sorption of arsenate ions (sorbate) present in aqueous form on to the surface of a solid microbial biomass (biosorbent). Due to their higher affinity towards charged ions which further dependent on the chemical constituents of the cell wall, biosorbents facilitate the binding of the contaminant ions. Further, the degree of biosorption differs according to the distribution of arsenic ions between the solid biosorbent and the liquid (aqueous) phase. This process continues till establishment of equilibrium between the amount of contaminant-bound biosorbent and the free ions in the solution. The extracellular polymeric substances (EPSs) such as the peptidoglycan, phospholipids, lipopolysaccharides, proteins, teichoic and teichuronic acids of the bacterial cell, primarily having a role in quorum sensing, play a key role in binding and adsorption of the toxic arsenic ions. Several (carboxylic, amino, thiol, hydroxyl and hydrocarboxylic) functional groups present in the biomass also actively participate in the binding process (Mohapatra et al. 2017a, b).
8.4.5.2.1.1.3 Biochemistry of Bacterial Arsenic Removal
Studies have demonstrated that plant could sustain in high arsenic contaminated soils when the phosphorus concentration was also high (Rosen et al. 2011). The arsenic in soil and water occurring naturally generally enters the plant via phosphate transporters and facilitate bacterial survival under arsenic stress condition. A common bacterial defence mechanism is the three detoxifying operons, ArsR, ArsC, and ArsB (Musingarimi et al. 2010; Yang et al. 2012a, b). The transportation of arsenate to cell and its reduction to arsenite is accomplished by ArsC gene and the outward transportation of arsenite from cell by ArsB gene (Musingarimi et al. 2010).
Arsenic-tolerant bacteria Acinetobacter from the rhizospheric soil of Pteris vittata, a fern, oxidises AsIII, whereas a few others (Flavobacterium, Pseudomonas, and Staphylococcus) could oxidise as well as reduce arsenic (Wang et al. 2012). Agrobacterium radiobacter in the roots of Populus deltoids makes the plant tolerant to 300 mg/kg Arsenic in soil, with a 54% removal efficiency (Wang et al. 2011). Reports suggest volatilisation (Sphingomonas desiccabilis and Cyanobacteria), adsorption (Ralstonia eutropha) and oxidation (Rhodococcus equi, Thiomonas arsenivorans and Ensifer adhaerens) of arsenic from the contaminated soil (Table 8.2; Liu et al. 2011; Mondal et al. 2008; Yin et al. 2011; Bag et al. 2010; Dastidar and Wang 2012; Ito et al. 2012). A few of the siderophore-producing arsenic-tolerant bacteria are Pseudomonas fluorescens, Micrococcus luteus and Bacillus licheniformis. They are also active in solubilising phosphorus and fixing nitrogen (Ivan et al. 2017). A genetically modified Rhizobium leguminosarum incorporated with AsIII S-adenosylmethionine methyltrasnferase gene (CrarsM) from Chlamydomonas reinhardtii was useful in arsenic detoxification through the methylation of AsIII (Zhang et al. 2017). Some microbial mechanisms enhance the plant growth by producing indole-3-acetic and other organic acids. These metabolites metabolise the heavy metal through the bacterial 1-ammino-cyclopropane-1-carboxylic acid deaminase (Ma et al. 2011).
8.4.5.2.2 Algal Remediation
Algae from the groups Cyanophyta and Chlorophyta help in absorption and accumulation of arsenic from contaminated water (Mitra et al. 2017). The prokaryotic (such as cyanobacteria) and eukaryotic (such as Chlorella) algae usually bioremediate arsenic (arsenate and arsenite) via the phosphate transportation and plasma membrane-based hexose permeases and aqua-glyceroporins pathways (Zhang et al. 2014). Arsenate is transported by competitive inhibition of phosphate due to their chemical similarity (between AsO43− and PO43−). The different functional groups present on the cell wall of algae help in adsorbing the metal. Wang et al. (2013) and Zhang et al. (2013) reported more than 60% of arsenic removal by algae from contaminated water through adsorption. With regard the biochemistry of arsenic biotransformation to reduce its toxicity, the two biochemical conversion pathways occurring inside the algal cells, viz. oxidation and methylation, are discussed below.
8.4.5.2.2.1 Oxidation of Arsenic
Few algae such as Synechocystis and Cynidiales could oxidise AsIII to AsIV inside the cells. Zhang et al. (2011) reported a process of detoxification through the uptake, accumulation and transformation of arsenic in Synechocystis sp. inside the cell. A few other reports confirm that the oxidation of AsIII happens outside with the help of extracellular phosphatases (Mitra et al. 2017). The role of the enzyme involved in the process particularly in the oxidation process is hitherto obscure (Mitra et al. 2017; Zhang et al. 2014).
8.4.5.2.2.2 Methylation of Arsenic
This mechanism involves the conversion of toxic AsIII arsenic to a less toxic monomethyl and dimethyl arsenates with the help of arsenite methyltransferases (Ye et al. 2012). Qin et al. (2009) confirmed that Cyanidioschyzon sp. (an extremophilic alga) could alone oxidise AsIII to AsV, reduce AsV to AsIII and methylate AsV to monomethyl arsenate and dimethyl arsenate.
8.4.5.2.3 Fungal Remediation
In terms of bioactive compound production, fungi are the most prominent and potent biomass in soil. The fungal cell wall is made up of polysaccharide molecules and proteins with hydroxyl, phosphate, sulphate and amino functional groups that could bind to the metal ions and metalloids relatively easily (Maheswari and Murugesan 2011). Most fungi, viz., Trichoderma, Candida, Aspergillus, Fusarium and Penicillium, help in methylating inorganic arsenic to its organic counterpart (Upadhyay et al. 2018). The advantages of fungi over bacteria as bioremediation agents are their longer life-span, higher biomass content and a complex hyphal network (Singh et al. 2016). Additionally, metal savouring fungi can compete with native bacteria in relatively inhospitable conditions (Sun et al. 2012).
Trichoderma is another filamentous Ascomycete fungus of great significance in plant growth promotion (Waghunde et al. 2016). It improves soil fertility and has the ability to induce stress-tolerance, a peculiar characteristic unlike the competing neighbouring rhizospheric microbes. It could promote hormone production, nutrient release from the soil, and rhizosphere development (de Souza et al. 2017). It contains a variety of functional groups on the outer layer of cell wall that could bind to metal ions and metalloids (Tripathi et al. 2017). Westerdykella aurantiaca, a soil fungus, bears arsenic methyl-transferase (WaarsM) gene which could be expressed in Saccharomyces cerevisiae (Verma and Jaiswal 2016). Such bioengineered yeasts capable of expressing the WaarsM gene demonstrated a higher arsenic methylation property. Laboratory studies confirmed an enhanced arsenic tolerance in paddy when such yeast cells were cocultured/inoculated in paddy (Verma and Jaiswal 2016).
8.5 Approach Involving Plant-Microbe Associations
Phytoremediation is a selective way used by plants to clean heavy metals from the environment through modified rhizospheric PGPR and PGPM. Several studies have been performed to select hyper-accumulating plants to assess the consequence of metal stress on the useful rhizospheric microbes (PGPMs) that can further facilitate the development of a more promising bioremediation strategy (Tak et al. 2013). The efficacy of phytoremediation is limited by the major factors, such as, tolerance level for the contaminant by the plant, selection of the plant variety to be employed for bioremediation, and its capacity to uptake and translocate the heavy metals (Jutsz and Gnida 2015). Phytoremediation, as indicated earlier, is an economically feasible bioremediation strategy as it produces the utilisable biomass while removing the toxic metals (Angelova et al. 2016).
Most plant species harbour vesicular-arbuscular mycorrhizae (VAM) that primarily help in phosphate solubilisation and uptake thereby enhancing their stress tolerance ability (Sharma et al. 2017). Upadhyay et al. (2018) reported that VAM supplementation helped overcome arsenic-induced phosphate deficiency in wheat. VAM also helps in maintaining a good ratio of arsenic and phosphate by translocating arsenic to inside the plant cells, particularly in soils with low arsenic contamination. In a similar study, Li et al. (2016) observed a decrease in the inorganic and organic ratio of arsenic in seeds when rice was inoculated with Rhizophagus irregularis.
It is important to note that arsenic volatilisation and methylation depends on the structure, organic content, the degree of the contamination and the chemical status of the soil (Mestrot et al. 2011). Upadhyay et al. (2018) recorded an annual 0.002–0.13% of net arsenic biovolatilisation in rice fields, with an about 4 μg/kg/year rate of volatilisation.
8.6 Challenges in Field-Scale Replication of the Strategy
The challenges met particularly by the translational (lab-to-land) researchers are manifold. These challenges include ecological, environmental, biotic and abiotic. For instance, in situ bioremediation could be a huge challenge when the arsenic concentration and the soil characteristics are adversely positioned. As every technology has an associated risk so is the bioremeation. For example, useful more efficient genetically modified microbes and plants could be employed to remediate arsenic contamination but its on-field application remains a topic of concern with biosafety consequences. The pollens of the genetically engineered plants and the plasmid of the genetically modified microbes could be major challenges to address the biosafety concern.
8.7 Future Research Directions
The role of genetically modified microbes in expediting the removal and remediation of contaminating arsenic and their survival when transferred for in situ bioremediation need to be addressed. Factors like temperature, lesser available nutrients and other related factors that are not easy to restore, may impact bioremediation potential negatively (Freitas et al. 2013). Furthermore, access to the genetically engineered plants and microbes to evaluate their role in heavy metals decontamination needs to be more focussed. Hyperaccumulative plants producing high biomass must be identified and could be further improved genetically to enhance their remediation efficiency. Similarly, the bioremediation ability of the these microbes to compete with the indigenous microbiota for efficient bioremediation by demonstrating an upper hand in the competitive-exclusion ecological principle calls for technological insights.
8.8 Conclusion
Several mechanisms and biochemical interactions, and their role in bioremediation have been detailed, with an attempt to expose the practicality of chemical- and bio-remediation strategies and their effect on the scaled-up remediation processes. As it is time consuming, generates harmful byproducts, and a costly proposition, chemical approach has slowly taken a backseat in the recent technological advancements. In that place, biological strategies for arsenic removal are slowly gaining popularity as they are eco-friendly. Plants and microbes have their own adaptive mechanisms to survive and sustain in contaminated soils and waters. Microbes bioremediate by oxidation, reduction, biosorption, and degradation of metals with the help of extracellular transformation and phytoremediation is based on phytoextraction and phytovolatilisation which have been very useful. Nevertheless, a bioremediation to be better accomplished would require friendly environmental conditions. Phytoremediation depends on the concentration of the contaminant, and the physical and chemical properties of soil.
References
Adeniji A (2004) Bioremediation of arsenic, chromium, lead, and mercury. National network of environmental management studies fellow for US Environmental Protection Agency Office of Solid Waste and Emergency Response Technology Innovation Office, Washington, DC, pp 14–19
Ahmed I, Hayat S, Pichtel J (2005) Heavy metal contamination of soil: problems and remedies. Science Publishers Inc, Enfield
Akhtar MS, Chali B, Azam T (2013) Bioremediation of arsenic and lead by plants and microbes from contaminated soil. Res Plant Sci 1(3):68–73
Aksornchu P, Prasertsan P, Sobhon V (2008) Isolation of arsenic-tolerant bacteria from arsenic-contaminated soil. Sonklanakarin J Sci Technol 30(1):95–102
Angelova VR, Perifanova-Nemska M, Uzunova G, Ivanov K, Lee H (2016) Potential of sunflower (Helianthus annuus L.) for phytoremediation of soils contaminated with heavy metals. World. J Sci Eng Technol 10:1–11. https://doi.org/10.5281/zenodo.1126371
Anyanwu CU, Ugwu CE (2010) Incidence of arsenic resistant bacteria isolated from a sewage treatment plant. Int J Basic Appl Sci 10:64–78
Atteia O, Estrada EDC, Bertin H (2013) Soil flushing: a review of the origin of efficiency variability. Rev Environ Sci Biotechnol 12:379–389. https://doi.org/10.1007/s11157-013-9316-0
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94. https://doi.org/10.3390/ijerph14010094
Bachate SP, L Cavalca V (2009) Andreoni, arsenic-resistant bacteria isolated from agricultural soils of Bangladesh and characterization of arsenate-reducing strains. J Appl Microbiol 107:145–156. https://doi.org/10.1111/j.1365-2672.2009.04188
Bag P, Bhattacharya P, Chowdhury R (2010) Bio-detoxification of arsenic laden ground water through a packed bed column of a continuous flow reactor using immobilized cells. Soil Sediment Contam 19:455–466. https://doi.org/10.1080/15320383.2010.486050
Bahar MM, Megharaj M, Naidu R (2012) Arsenic bioremediation potential of a new arsenite-oxidizing bacterium Stenotrophomonas sp. MM-7 isolated from soil. Biodegradation 23:803–812. https://doi.org/10.1007/s10532-012-9567-4
Banerjee S, Datta S, Chattyopadhyay D, Sarkar P (2011) Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. J Environ Sci Health Part A 4:1736–1747. https://doi.org/10.1080/10934529.2011.623995
Casarett LJ, Doull J, Klaassen CD (2008) Casarett and Doull’s toxicology: the basic science of poisons, 7th edn. McGraw-Hill, New York, p 1331
Cavalca L, Corsini A, Zaccheo P, Andreoni V, Muyzer G (2013) Microbial transformations of arsenic: perspectives for biological removal of arsenic from water. Future Microbiol 8(6):753–768. https://doi.org/10.2217/fmb.13.38
Chakraborti D, Basu GK, Biswas BK, Chowdhury UK, Rahman MM, Paul K, Roy Chowdhury T, Chanda CR, Lodh L, Ray SL (2001) Characterization of arsenic bearing sediments in Gangetic Delta of West Bengal-India. In: Chappell WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects 4. Elsevier Science, New York, pp 27–52
Chattopadhyay A, Singh AP, Rakshit A (2017) Bioreclamation of arsenic in the soil-plant system: a review. Sci Int 5(1):30–41. https://doi.org/10.3923/sciintl.2017.30.41
Chibuike G, Obiora S (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:752708–752712. https://doi.org/10.1155/2014/752708
Chipirom K, Tanasupawat S, Akaracharanya A, Leepepatpiboon N, Prange A, Kim KW, Lee KC, Lee JS (2012) Comamonas terrae sp. nov., an arsenite-oxidizing bacterium isolated from agricultural soil in Thailand. J Gen Appl Microbiol 58(3):245–251
Das S, Dash HR (2014) Microbial bioremediation: a potential tool for restoration of contaminated areas. In: Microbial biodegradation and bioremediation. Elsevier, pp 1–21
Dastidar A, Wang YT (2012) Modelling arsenite oxidation by chemoautotrophic Thiomonas arsenivorans strain b6 in a packed-bed bioreactor. Sci Total Environ 432:113–121. https://doi.org/10.1016/j.scitotenv.2012.05.051
de Souza Vandenberghe LP, Garcia LM, Rodrigues C, Camara MC, de Melo Pereira GV, de Oliveira J, Soccol CR (2017) Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol 3(3):629
Dey U, Chatterjee S, Mondal NK (2016) Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol Rep 10:1–7. https://doi.org/10.1016/j.btre.2016.02.002
Dey U, Chatterjee S, Mondal NK (2017) Investigation of bioremediation of arsenic by Bacteria isolated from an arsenic contaminated area. Environ Process 4(1):183–199
Dixit G, Singh AP, Kumar A, Mishra S, Dwivedi S, Kumar S, Trivedi PK, Pandey V, Tripathi RD (2016) Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol Biochem 99:86–96. https://doi.org/10.1016/j.plaphy.2015.11.005
Duarte AALS, Cardoso SJA, Alcada AJ (2009) Emerging and innovative techniques for arsenic removal applied to a small water supply system. Sustainability 1:1288–1304. https://doi.org/10.3390/su1041288
Ekperusi O, Aigbodion F (2015) Bioremediation of petroleum hydrocarbons from crude oil-contaminated soil with the earthworm: Hyperiodrilus africanus. 3 Biotech 5:957–965. https://doi.org/10.1007/s13205-015-0298-1
Fazi S, Amalfitano S, Casentini B, Davolos D, Pietrangeli B, Crognale S, Lotti F, Rossetti S (2016) Arsenic removal from naturally contaminated waters: a review of methods combining chemical and biological treatments. Rendiconti Lincei 27(1):51–58
Freitas EV, Nascimento CW, Souza A, Silva FB (2013) Citric acid-assisted phytoextraction of lead: a field experiment. Chemosphere 92:213–217. https://doi.org/10.1016/j.chemosphere.2013.01.103
Ito A, Miura JI, Ishikawa N, Umita T (2012) Biological oxidation of arsenite in synthetic groundwater using immobilised bacteria. Water Res 46:4825–4831. https://doi.org/10.1016/j.watres.2012.06.013
Ivan FP, Salomon MV, Berli F, Bottini R, Piccoli P (2017) Characterization of the As(III) tolerance conferred by plant growth promoting rhizobacteria to in vitrogrown grapevine. Appl Soil Ecol 109:60–68. https://doi.org/10.1016/j.apsoil.2016.10.003
Jang YC, Somanna Y, Kim H (2016) Source, distribution, toxicity and remediation of arsenic in the environment–a review. Int J Appl Environ Sci 11(2):559–581
Jutsz AM, Gnida A (2015) Mechanisms of stress avoidance and tolerance by plants used in phytoremediation of heavy metals. Arch Environ Prot 2015(41):104–114. https://doi.org/10.1515/aep-2015-0045
Khan AH, Rasul SB, Munir A, Habibuddowla M, Alauddin M, Newaz SS, Hussan A (2000) Appraisal of a simple arsenic removal method for groundwater of Bangladesh. J Environ Sci Health A 35:1021–1041. https://doi.org/10.1080/10934520009377018
Klaassen CD, Watkins JB III (2003) Absorption, distribution, and excretion of toxicants. Karl K Rozman Essen Toxicol
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides–a review. Environ Pollut 172:9–22. https://doi.org/10.1016/j.envpol.2012.07.045
Leiva ED, dP Rámila C, Vargas IT, Escauriaza CR, Bonilla CA, Pizarro GE, Regan JM, Pasten PA (2014) Natural attenuation process via microbial oxidation of arsenic in a high Andean watershed. Sci Total Environ 466:490–502. https://doi.org/10.1016/j.scitotenv.2013.07.009
Li H, Chen XW, Wong MH (2016) Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chemosphere 145:224–230
Lim KT, Shukor MY, Wasoh H (2014) Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed Res Int 2014. https://doi.org/10.1155/2014/503784
Liu Y, Zheng BH, Fu Q, Meng W, Wang YY (2009) Risk assessment and management of arsenic in source water in China. J Hazard Mater 170:729–734. https://doi.org/10.1016/j.jhazmat.2009.05.006
Liu S, Zhang F, Chen J, Sun G (2011) Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J Environ Sci 23:1544–1550. https://doi.org/10.1016/S1001-0742(10)60570-0
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258. https://doi.org/10.1016/j.biotechadv.2010.12.001
Maheswari S, Murugesan AG (2011) Removal of arsenic(III) ions from aqueous solution using Aspergillus flavus isolated from arsenic contaminated site. Ind J Chem Technol 18:45–52
Mahimairaja S, Bolan NS, Adriano DC, Robinson B (2005) Arsenic contamination and its risk management in complex environmental settings. Adv Agron 86:1–82
Mateos LM, Ordóñez E, Letek M, Gil JA (2006) Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int Microbiol 9:207–215
Mesa V, Navazas A, González-Gil R, González A, Weyens N, Lauga B, Peláez AI (2017) Use of endophytic and rhizosphere bacteria to improve phytoremediation of arsenic-contaminated industrial soils by autochthonous Betula celtiberica. Appl Environ Microbiol 83(8):03411–03416. https://doi.org/10.1128/AEM.03411-16
Mestrot A, Feldmann J, Krupp EM, Hossain MS, Roman-Ross G, Meharg AA (2011) Field fluxes and speciation of arsines emanating from soils. Environ Sci Technol 45:1798–1804. https://doi.org/10.1021/es103463d
Mishra S, Barik SK, Ayyappan S, Mohapatra BC (2000) Fish bioassays for evaluation of raw and bioremediated dairy effluent. Bioresour Technol 72(3):213–218
Mitra A, Chatterjee S, Gupta DK (2017) Uptake, transport, and remediation of arsenic by algae and higher plants. In: Arsenic contamination in the environment. Springer, Cham, pp 145–169
Mohapatra RK, Parhi PK, Patra JK, Panda CR, Thatoi HN (2017a) Biodetoxification of toxic heavy metals by marine metal resistant Bacteria-a novel approach for bioremediation of the polluted saline environment. Microb Biotechnol 1:343–376
Mohapatra RK, Parhi PK, Thatoi H, Panda CR (2017b) Bioreduction of hexavalent chromium by Exiguobacterium indicum strain MW1 isolated from marine water of Paradip port, Odisha, India. Chem Ecol 33(2):114–130
Mondal P, Majumder CB, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137:464–479. https://doi.org/10.1016/j.jhazmat.2006.02.023
Mondal P, Majumder CB, Mohanty B (2008) Treatment of arsenic contaminated water in a batch reactor by using Ralstonia eutropha MTCC2487 and granular activated carbon. J Hazard Mater 153:588–599. https://doi.org/10.1016/j.jhazmat.2007.09.028
Mondal P, Bhowmic S, Chatterjee D, Figoli A, Bruggen B (2013) Remediation of inorganic arsenic in groundwater for safe water supply: a critical assessment of technological solutions. Chemosphere 92:157–170. https://doi.org/10.1016/j.chemosphere.2013.01.097
Musingarimi W, Tuffin M, Cowan D (2010) Characterisation of the arsenic resistance genes in Bacillus sp. UWC isolated from maturing fly ash acid mine drainage neutralised solids. S Afr J Sci 106(1–2):59–63. https://doi.org/10.4102/sajs.v106i1/2.17
Porter EK, Peterson PJ (1975) Arsenic accumulation by plants on mine waste (United Kingdom). Sci Total Environ 4(4):365–371. https://doi.org/10.1016/0048-9697(75)90028-5
Qin J, Lehr CR, Yuan CG, Le XC, Mc Dermott TR, Rosen BP (2009) Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc Natl Acad Sci U S A 106:5213–5217. https://doi.org/10.1073/pnas.0900238106
Rosen BP, Ajees AA, McDermott TR (2011) Life and death with arsenic: arsenic life: an analysis of the recent report “A bacterium that can grow by using arsenic instead of phosphorus”. BioEssays 33(5):350–357. https://doi.org/10.1002/bies.201100012
Satyapal GK, Rani S, Kumar M, Kumar N (2016) Potential role of arsenic resistant bacteria in bioremediation: current status and future prospects. J Microb Biochem Technol 8(3):256–258. https://doi.org/10.4172/1948-5948.1000294
Satyapal GK, Mishra SK, Srivastava A, Ranjan RK, Prakash K, Haque R, Kumar N (2018) Possible bioremediation of arsenic toxicity by isolating indigenous bacteria from the middle Gangetic plain of Bihar, India. Biotechnol Rep 17:117–125. https://doi.org/10.1016/j.btre.2018.02.002
Sharma S, Anand G, Singh N, Kapoor R (2017) Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front Plant Sci 8:906
Shrivastava A, Ghosh D, Dash A, Bose S (2015) Arsenic contamination in soil and sediment in India: sources, effects, and remediation. Current Pollution Reports 1(1):35–46
Silva GMI, Gonzaga SJA, Qiying ML (2006) Arsenic phytoextraction and hyperaccumulation by fern species. Sci Agric. https://doi.org/10.1590/S0103-90162006000100015
Singh N, Marwa N, Mishra SK, Mishra J, Verma PC, Rathaur S, Singh N (2016) Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol Environ Saf 125:25–34. https://doi.org/10.1016/j.ecoenv.2015.11.020
Srivastava PK, Vaish A, Dwivedi S, Chakrabarty D, Singh N, Tripathi RD (2011) Biological removal of arsenic pollution by soil fungi. Sci Total Environ 409:2430–2442. https://doi.org/10.1016/j.scitotenv.2011.03.002
Sullivan C, Tyrer M, Cheeseman CR, Graham NJD (2010) Disposal of water treatment wastes containing arsenic—a review. Sci Total Environ 408(8):1770–1778. https://doi.org/10.1016/j.scitotenv.2010.01.010
Sun Y, Zhou QX, Liu WT, Wang L (2009) Joint effects of arsenic, cadmium on plant growth and metal bioaccumulation: a potential Cd hyperaccumulator and As-excluder Bidens pilosa. L. J Hazard Mater 161(2–3):808–814. https://doi.org/10.1016/j.jhazmat.2008.10.097
Sun J, Zou X, Ning Z, Sun M, Peng J, Xiao T (2012) Culturable microbial groups and thallium-tolerant fungi in soils with high thallium contamination. Sci Total Environ 441:258–264. https://doi.org/10.1016/j.scitotenv.2012.09.053
Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol 223. Springer, New York, pp 33–52
Taran M, Safari M, Monaza A, Reza JZ, Bakhtiyari S (2013) Optimal conditions for the biological removal of arsenic by a novel halophilic archaea in different conditions and its process optimization. Pol J Chem Technol 15(2):7–9
Tripathi P, Singh PC, Mishra A, Srivastava S, Chauhan R, Awasthi S, Mishra S, Dwivedi S, Tripathi P, Kalra A, Tripathi RD (2017) Arsenic tolerant Trichoderma sp. reduces arsenic induced stress in chickpea (Cicer arietinum). Environ Pol 223:137–145
Upadhyay MK, Yadav P, Shukla A, Srivastava S (2018) Utilizing the potential of microorganisms for managing arsenic contamination: a feasible and sustainable approach. Front Environ Sci 6:24. https://doi.org/10.3389/fenvs.2018.00024
Verma JP, Jaiswal DK (2016) Book review: advances in biodegradation and bioremediation of industrial waste. Front Microbiol 6:1555. https://doi.org/10.3389/fmicb.2015.01555
Vishnoi N, Singh DP (2014) Biotransformation of arsenic by bacterial strains mediated by oxido-reductase enzyme system. Cell Mol Biol (Noisy-le-Grand, France) 60(5):7–14
Voica DM, Bartha L, Banciu HL, Oren A (2016) Heavy metal resistance in halophilic bacteria and archaea. FEMS Microbiol Lett 363(14):146. https://doi.org/10.1093/femsle/fnw146
Waghunde RR, Shelake RM, Sabalpara AN (2016) Trichoderma: A significant fungus for agriculture and environment. African J Agric Res 11(22):1952–1965
Wang Q, Xiong D, Zhao P, Yu X, Tu B, Wang G (2011) Effect of applying an arsenic-resistant and plant growth-promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoides LH05-17. J Appl Microbiol 111:1065–1074. https://doi.org/10.1111/j.1365-2672.2011.05142.x
Wang X, Rathinasabapathi B, Oliveira LMD, Guilherme LR, Ma LQ (2012) Bacteria-mediated arsenic oxidation and reduction in the growth media of arsenic hyperaccumulator Pteris vittata. Environ Sci Technol 46:11259–11266. https://doi.org/10.1021/es300454b
Wang ZH, Luo ZX, Yan CZ (2013) Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ Sci Pollut Res 20:7286–7295. https://doi.org/10.1007/s11356-013-1741-7
WHO (2011) Guidelines for drinking-water quality. WHO Chron 38:104–108
Xiong J, Wang W, Fan H, Cai L, Wang G (2006) Arsenic resistant bacteria in mining wastes from Shangrao coal mine of China. Environ Sci Technol 1:535–540
Yang HC, Fu HL, Lin YF, Rosen BP (2012a) Pathways of arsenic uptake and efflux. In: Current topics in membranes, vol 69. Academic Press, pp 325–358
Yang Q, Tu S, Wang G, Liao X, Yan X (2012b) Effectiveness of applying arsenate reducing bacteria to enhance arsenic removal from polluted soils by Pteris vittata L. Int J Phytoremediation 14(1):89–99
Ye J, Rensing C, Rosen BP, Zhu YG (2012) Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci 17:155–162. https://doi.org/10.1016/j.tplants.2011.12.003
Yin XX, Chen J, Qin J, Sun GX, Rosen BP, Zhu YG (2011) Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol 156:1631–1638. https://doi.org/10.1104/pp.111.178947
Zhang B, Wang LH, Xu YX (2011) Study on absorption and transformation of arsenic in blue alga (Synechocystis sp. PCC6803). Asian J Ecotoxicol 6:629–633
Zhang JY, Ding TD, Zhang CL (2013) Biosorption and toxicity responses to arsenite (As(III)) in Scenedesmus quadricauda. Chemosphere 92:1077–1084. https://doi.org/10.1016/j.chemosphere.2013.01.002
Zhang SY, Rensing C, Zhu YG (2014) Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments. Environ Sci Technol 489:994–1000. https://doi.org/10.1021/es403836g
Zhang J, Xu Y, Cao T, Chen J, Rosen BP, Zhao FJ (2017) Arsenic methylation by a genetically engineered Rhizobium-legume symbiont. Plant Soil 416(1):259–269
Acknowledgements
The technical, logistics and administrative support received by the authors from their respective affiliated organisations are duly acknowledged.
Declaration
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Parhi, P.K. et al. (2021). Arsenic Contamination: Sources, Chemistry and Remediation Strategies. In: Rajendran, S., Naushad, M., Cornejo Ponce, L., Lichtfouse, E. (eds) Metal, Metal-Oxides and Metal-Organic Frameworks for Environmental Remediation. Environmental Chemistry for a Sustainable World, vol 64. Springer, Cham. https://doi.org/10.1007/978-3-030-68976-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-68976-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68975-9
Online ISBN: 978-3-030-68976-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)