Abstract
Rhodiola is one of the important plants studied for its medicinal properties in ancient time. Some of the well-known and mostly evaluated species of genus Rhodiola are Rhodiola rosea, Rhodiola imbricata, Rhodiola heterodonta, Rhodiola quadrifida, etc. These species are known to possess potent biological/pharmacological activities such as antioxidant, leprosy, anti-inflammatory, antistress, etc. These plants grow at a height of around 4000–5000 m above the sea level with a low temperature of around −10 °C, thus surviving in very harsh conditions. Their survival in such harsh conditions is due to their adaptation in that environment as well as the kind of compounds these plants produce in their biological mechanism. The present chapter deals with the phytochemical composition, bioactivity, and in vitro analysis of some important Rhodiola species.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Plants, on the planet earth, are one of the most important natural resources available for the human life. They provide oxygen, food, essential pigments, and ornamental and medicinal components that provide support to humans for sustenance of life and combat with the harmful and life-threatening conditions on the planet. There are more than 300–315 species of medicinal plants which have been identified by the various botanists of the world (Sundriyal et al. 2004). The main and foremost use of the plant by humans is in the form of medicines. Since ancient times it has been recognized that there are many plant species which have huge medicinal value and have healing potential against various fatal diseases. Plants like Azadirachta indica (Neem), Ocimum tenuiflorum (Tulsi), Mentha arvensis (Menthol), Psidium guajava (guava), Aleo vera, Rheum sp., Hypericum sp., Rhodiola sp., etc. were not only used by the ancient people for treatment of diseases like stomachache, headache, paralysis, fever, etc., but in the present day also, they are being used in their raw as well as in the mixture form with other compounds for various human-related abnormalities like high free radical production in the body, inflammation, tumor, etc. (Sikkink 2009) The knowledge to use these plant species is available to us through various Vedas in which they are properly classified and designated for their potential use as medicine. Besides these well-known species of plants, there are many other species which are known for their high medicinal values and are maintained by various local traditional medicinal systems like Ayurveda, Siddha medicine, Unani, and Ancient Iranian medicinal system. All over the world, there are various practitioners who practice the medicinal plants for the treatment. In some African and Asian countries, around 80% of the their population relies on these traditional medicinal systems. Amchi system (sowa-rigpa) is also one of the well-developed medicinal system of India. In Amchi system of medicine, they have provided a very deep knowledge of medicinal uses of various plants which are found in the high mountain ranges of Ladakh region of Jammu and Kashmir. There are more than 200 species of plant species like Rhodiola, Rheum, Podophyllum, Artemisia, Centurea, etc. from these regions which have extensive application for the treatment against diseases like malaria, cancer, etc. and also show antimicrobial-, anticancer-, anti-inflammatory-, antihypotensive-, and anticholinergic-like activities. In various traditional formulations, these plant species were dried and mixed with the butter and applied as the ointment to relieve pain and swelling (Halldorsson and Grasnytjar 1783) and also being used as a supplement for long journey (Alm 2004). These plants contain a plethora of various classes of bioactive compounds like flavonoids, glycosides, phenylpropanoid, etc., which have a very high value toward the human health (Chaurasia et al. 2007). Rhodiola is one of the perennial herbs which belongs to the Campanulaceae family and also resembles to the sebum which is known as stonecrops. This genus is made up of about 93 species. It has a few distinguishing characters which includes the series of stamen and has a stout rhizome from which the plant arises. This plant makes a whorl and contain red or yellow color top which includes seeds of plants. Rhodiola has adapted to harsh and almost unforgiving climate. These Rhodiola species were reported to find in the northern hemisphere in countries like China, Mongolia, Korea, Sakhalin, the Kuriles, Japan, Sweden, Norway, Finland, India, and Pakistan. In China, it is distributed in the northwest and the southwest region, and it is locally known as Hong Jing Tian (Bassa et al. 2016). In India, it is distributed in Jammu and Kashmir region, Himachal Pradesh and Arunachal Pradesh, and almost in complete Himalayan belt. Tibet is one of the places which are rich in its production (Kumar et al. 2010a). Rhodiola species are also reported to find in Russia, the United States, and Canada (Lei et al. 2003).
In the Ladakh region of Jammu and Kashmir, Rhodiola imbricata and Rhodiola heterodonta have a diverse distribution. Out of the five different valleys of Ladakh (Suru, Zanskar, Nubra, Indus, and Changthang), these plants are available in Changthang, Nubra, Zanskar, and Indus valley. The major population of the plant found in the passes such as Khardungla pass (between Indus and Nubra valley), Changla pass (between Nubra and Changthang), Pensi-la pass (between Zanskar and Suru valley), etc. are present in between these valleys; they join these valleys to one another (Chaurasia et al. 2007). Few of the commonly known species of this family are Rhodiola rosea (known as roseroot), Rhodiola imbricata (recently known as a sanjivani), Rhodiola heterodonta, Rhodiola quadrifida, etc. All these species were reported to have very high medicinal values like antioxidant, anti-inflammatory, leprosy, antistress, etc. The kind of environment in which these plants grow is very harsh with a low temperature of around −10 °C and at a height of around 4000–5000 m above the sea level. In such hard survival conditions, these plants survive as well as propagate and have successfully adapted to that environment. The key component for their survival in those conditions is the adaptation of these plants and also the kind of compounds these plants produce in their biological mechanism which are not only helpful to the plants but for human also. Historically, these plants have been used in various traditional medicine systems of India, China, Europe, etc. In the modern world, these plants of Campanulaceae genus are extensively used in various formulations and basically include the roots of these plants which contain a very high content of secondary metabolites. The key secondary metabolites which have been reported and extensively studied in these plants are rosin, rosavin, salidroside, etc. The present chapter discusses about the distribution of the plants in various countries, its bioactive compounds, and their bioactivity (Chaurasia et al. 2007).
Distribution of Rhodiola Species
Rhodiola rosea
Rhodiola rosea is an inhabitant of subarctic area of the northern hemisphere. It is mainly available in high altitudes over rocks and on Arctic sea cliffs in Europe, Asia, and North America, including Britain, further south in mountains, and China (Zhang et al. 2016). Mountain Altai and south region of foothill Altai, mainly in Ust-Kansky, Ust-Koksinsky, and Charishki regions, the availability of commercial roots and rhizome is in great abundance (Saratikov and Krasnov 2004).
Rhodiola imbricata
Rhodiola imbricata, found in Sinai Himalayas, Nepal, Qinghai, Xizang, and India, is also found in the hilly region of western Himalaya (Kanupriya et al. 2005). The major distribution of Rhodiola imbricata is in three different valleys (Zanskar (N33.95 and E76.46), Indus (N34.29 and E77.86), and Changthang (N 34.26 and E 78.14)) of Ladakh region of Trans-Himalayas (Chaurasia et al. 2007).
Rhodiola heterodonta
Rhodiola heterodonta species are endemic to the mountain range of Central Asia and mainly distributed in East Europe and Asia (Grace et al. 2009).
Rhodiola crenulata
The plant Rhodiola crenulata is native to the Qinghai-Tibet Plateau and the only original plant, according to the “Pharmacopoeia of China” (Chinese Pharmacopoeia Commission 2010). This plant has shown a distribution in Hengduan Mountains Region of China, Tibet, and Yunnan (Lei et al. 2003).
Rhodiola kirilowii
The plant is mainly prevalent in Gansu, Hebei, Qinghai, Shaanxi, Shanxi, Sichuan, Xinjiang, Xizang, and Yunnan (Kazakhstan, Myanmar). In Central Asia it is found in Narynskiy Range, Terskey Alatau, Alayskiy Range, northern China, and Tibet (Maximowicz 2007).
Rhodiola bupleuroides
The plant R. bupleuroides is aboriginal to western Tibet Autonomous Region, locally known as “Sheng-Chang Hong Jing Tian” or “Bu-Dan Hong Jing Tian, northwest of Yunnan, and Sichuan (Li et al. 2007). It is also found in Pakistan, Kumaon, Nepal, Sikkim, Bhutan, Myanmar, and SW China, at altitudes of 2750–3700 m (Hooker and Thomson 1998).
Rhodiola dumulosa
Rhodiola dumulosa is a perennial plant species that are found in various regions of China that includes Northern, Northwestern, and Central China. It is distributed as fragmented populations across Northern, Northwestern, and Central China (Hou and Lou 2011).
Rhodiola algida
Rhodiola algida is mainly distributed in the Qinghai Plateau in China (Qi et al. 2015). It is also present in large amount in Tibet. Rhodiola algida helps to improve oral mucositis which were induced in breast cancer patients (Loo et al. 2010).
Rhodiola sachalinensis
Rhodiola sachalinensis is a herbaceous plant (perennial) of the Crassulaceae family, predominantly found in the polar region of Arctic and Alpine (Seo et al. 2001) and high rocky mountain areas of East Asian countries (Ohwi 1984).
Rhodiola quadrifida
Rhodiola quadrifida is a grassy plant occurring predominantly in some highland regions of the East Siberia, former USSR (Altai-Sayan), mountainous regions of China (Sichuan), and Mongolia (Hentii, Hangai, Hovsgol, Hovd, and Mongol Altai) (Wiedenfeld et al. 2007).
Rhodiola alsi
The data related to the distribution of this species is not extensively available, but it is only reported to be found in Qinghai-Tibet Plateau of China (Ma et al. 2008).
Chemoprofiling of Rhodiola Species
From age-old times, Asia and Europe have been utilizing Rhodiola species as medicinal resource which is endemic to the northern hemisphere’s subarctic areas. Their usages include valuable functions as adaptogen, anti-inflammatory, and antidepressant drugs. In the process to establish the therapeutic/pharmacological uses of these plants in modern medicine, the effects of Rhodiola sp. have been extensively studied. Out of all the species, Rhodiola rosea has been shown to possess greater amount of activities like angiomodulatory, antioxidant, antimicrobial, adaptogenic, antistress, immunomodulatory, and antitumoral effects. From a chemotaxonomical’s view, eight compounds which include the phenylpropanoids rosarin, rosavin, and rosin, the phenylethanoids salidroside and tyrosol, the flavonol rhodionin, as well as catechin and gallic acid have been proposed as reference markers (Recio et al. 2016). These are monoterpene alcohols and their glycosides (cyanogenic and aryl glycosides), phenylethanoids and their glycosides, proanthocyanidins, flavonoids and gallic acid derivatives, flavonolignans. In chemical nature of the adaptogens, they are typically tetracyclic triterpenoids/steroids complex or phenolics. Salidroside (p-hydroxyphenethyl-β-D-glucoside), which is a major compound in Rhodiola, seems to be accountable for many observed with Rhodiola extract’s effects (Table 1).
Figure 1 represents the structure of salidroside, rosavin, and rhodionin compounds (Panossian et al. 2010).
Structure of salidroside, rosavin, and rhodionin reported by Panossian et al. (2010)
Rhodiola rosea
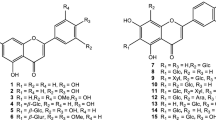
The phenolic compounds included in this species are based on phenylpropanoids and phenylethane derivatives class, such as rosavin, tyrosol, salidroside (rhodioloside), syringin, and triandrin. Few of the reported lignans are eleutherosid E and schisandrin B. Figure 2 represents the structure of important compounds from Rhodiola rosea (tyrosol, syringin, rosiridine, ginsinoside, sitoindoside) (Panossian et al. 2010).
Structure of some important phenylpropanoids and phenylethane derivatives reported by Panossian et al. (2010)
Rhodiola imbricata
Rhodiola imbricata contains large amount of bioactive compounds. The Chemoprofile of Rhodiola imbricata’s root revealed the presence of 63 phyto-chemotypes (Fig. 3), among them, 1-pentacosanol; hexadecanoic acid; 2-hydroxy-1-(hydroxymethyl)ethyl ester; stigmast-5-en-3-ol, (3β,24S); 1-tetracosanol; 1-hentetracontanol; 9,12,15-octadecatrienoic acid, 2,3-dihydroxypropyl ester,(Z,Z,Z); thujone; 9,12-octadecadienoic acid(Z,Z)-,; 17-pentatriacontene; 13-tetradecen-1-ol acetate; bis(2-ethylhexyl) phthalate; 7,8-dimethylbenzocyclooctene; ethyl linoleate; 3-methoxy-5-methylphenol; camphor; 1,3-dimethoxybenzene; methyl tri-butyl ammonium chloride; 1,3-benzenediol, 5-methyl; 1-heptacosane; benzenemethanol, 3-hydroxy, 5-methoxy; cholest-4-ene-3,6-dione; dodecanoic acid, 3-hydroxy; octadecane, 1-chloro; ethanone, 1-(4-hydroxyphenyl); a-tocopherol; d-tocopherol; campesterol; 1-dotriacontane; heptadecane, 9 hexyl;1-hentriacontane; 1-tericosanol; 13-docosen-1-ol; 1,30-triacontanediol; Stigmast-4-en-3-one; Stigmast-3,5-dien-7-one; stigmastanol; 1-tetratetracontane; 1-pentatriacontane; bacteriochlorophyll-c-stearyl; ascaridole; a-D- glucopyranoside, O-b-D- glucopyranosyl-(1.fwdarw.3)-b-D-fructofuranosyl; benzene sulfonic acid, 4-amino-3-nitro; Cis-9- eicosen-1-ol;(4-carboxymethoxy) benzoyl, methanol; oleic acid; hexadecanoic acid, bis(2-ethylhexyl) ester; hexadecanoic acid, methyl ester; bacteriochlorophyll-c-stearyl; eucalyptol; 1-(2,6-dihydroxy-4-methoxyphenyl) ethanone; linalyl isovalerate; 1-chloro-2,4-dimethoxybenzene; borneol; 4-chloro benzenethiol; phenol,3,5-dimethoxy acetate; 2,4-bis(1,1dimethylethyl) phenol; b-fenchyl alcohol; 5-pentadecyl −1,3-benzenediol; A-D-glucopyranoside,O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl; 1-pentatricontene; 3,7,11-trimethyl- 1-dodecanol; 1-dodecane; 3-methoxy-5-methyl phenol; and di-butyl phthalate were found to be present (Tayade et al. 2013). Figure 4, 3,5-dihydroxybenzyl alcohol; 3-methoxy-5-hydroxybenzyl alcohol; orcinol; O-methylorcinol; p-hydroxybenzaldehyde; p-hydroxyacetophenone, p-hydroxybenzyl alcohol; 4-methoxyphenethyl alcohol, 3-hydroxy-5-methylphenyl-β-D-glucopyranoside, methoxyphenyl-β-D-glucopyranoside,2-hydroxymethyl-6-methoxy- β-D-glucopyranoside, phenyl- β-D-glucopyranoside,3,5-dimethoxyphenyl- β-D-glucopyranoside, trimethoxyphenyl- β-D-glucopyranoside,3-hydroxy-2-(3-methyl-2-buten-1-yl)-benzoic acid, 2-(hydroxymethyl(−6-methoxy-3-acetylphenyl- β-D-glucopyranoside,2-(hydroxymethyl)-6-methoxyphenyl β-D-glucopyranoside,2-hydroxy-4-methylphenyl- β-D-glucopyranoside (Choudhary et al. 2015).
63 Phyto-chemotypes reported by Tayade et al. (2013)
Phytocomponents reported by Choudhary et al. (2015)
Rhodiola heterodonta
Rhodiola heterodonta contains a wide range of secondary metabolites. Figure 5 represents the structure of tyrosol, viridoside, salidroside, and tyrosol methyl ester. Heterodontoside, mongrhoside, and Rhodiocynanoside A were found in the ethanol extract (Grace et al. 2009). In case of the proanthocyanidins fraction, the class of compounds are epigallocatechin – epigallocatechin-3-O- gallate, epigallocatechin gallate, and 3-O- galloyl-epigallocatechin-epigallocatechin-3-O gallate. Figure 6 shows the chemical compounds reported by Yousef et al. (−)-EGCG-4β-benzylthioether and (−)- Epigallocatechin-3-O-gallate (Yousef et al. 2006).
Phytocomponents reported by Grace et al. (2009)
Phytocomponents reported by Yousef et al. (2006)
Rhodiola crenulata
This species of Rhodiola also contain many medicinally important phytochemicals. A total of around 48 chemical compounds were found which includes 12 flavonoids and their glycosides, 5 flavanols and gallic acid derivatives, 26 alcohols and their glycosides, and 4 organic acids and 1 cyanogenic glycoside (Han et al. 2016). Figure 7 salidroside; tyrosol; p-hydroxyphenacyl-b-D-glucopyranoside; picein; icariside D2; rutin; lotaustralin; rhodiocyanoside A; daucosterol; crenulatin; rhodionin; b-sitosterol; gallic acid; creosides I,II, III, IV, V (Grech-Baran et al. 2015). Figure 8 kenposide A; rhodioloside E; isopentyl-3-O-β-glycopyranoside; rhodiooctanoside; coniferoside; dihydroconiferin; Icariside D2; 4-hydroxybenzyl- β-D-glycopyranoside; triandrin; vimalin; caffeic acid; pollenitin; rhodiosin; kaempferol; clemastanin A (Nakamura et al. 2008). The other various phenolic compounds identified from R. crenulata are (Fig. 9) 5,7,3′,5′-tetrahydroxydihydroflavone; luteolin; kaempferol-7-O-α-L-rhamnoside; ternatumoside II; crenuloside; (+)-isolarisiresinol; (+)-dihydrodehydrodiconiferyl alcohol; methyl gallate; (7β,7′β”,8α,8′α′)-3′-methoxy-9-oxo-7,9′,7,9”-diepoxylignan-3,4,4”-triol; (7R,8R)-3-methoxy-8′-carboxy-7′-en-3′,7-epoxy-8,4′-oxyneolignan-4,9-diol; (7R,8R)-3-methoxy-8′-carboxy-7′-en-3′,8-epoxy-7,4′-oxyneolignan-4,9-diol; 2-(4-hydroxyphenyl) ethyl 3,4,5-trihydroxybenzoate; herbacetin-7-methyl ether; and rhodiolate (Zhou et al. 2015). Some different phytochemicals isolated from R. crenulata includes 4′-hydroxyacetophenone; salidroside; p-tyrosol epicatechin-(4β,8)-epicatechin gallate (B2–3′-O-gallate) (Fig. 10) (Chu et al. 2014). (1R)-1-O-(β-d-glucopyranosyl)-phenylethylene glycol; (3R,5R,8R)-3-O-[α-l-arabinopyranosyl (1 → 6)-β-d-glucopyranosyl]-5-hydroxymegastigma-6,7-dien-9-one (Fig. 11) (Ma et al. 2008). n-octanol; 3-methyl-2-buten-1-ol ; 2-methyl-3-buten-2-ol; citronellol; myteolp ; Geraniol; and linalool (Fig. 12) (Lei et al. 2003).
Phytocomponents reported by Grech-Baran et al. (2015)
Phytocomponents reported by Nakamura et al. (2008)
Phytocomponents reported by Zhou et al. (2015)
Phytocomponents reported by Chu et al. (2014)
Phytocomponents reported by Ma et al. (2008)
Phytocomponents reported by Lei et al. (2003)
Rhodiola kirilowii
The compounds isolated from R. kirilowii were arbutin, epigallocatechin gallate, rhodiocyanoside A, fructopyran(1–4)-glycopyranose, and lotaustralin (Fig. 13) (Wiedenfeld et al. 2007). 3,3′-Digalloylprocyanidin B2; 3,3′-Digalloylproprodelphinidin B2 (Rhodisin); epicatechin-3-O-gallate (Fig. 14) (Wojcik et al. 2009). Beta-sitosterol; trans-hydroxycinnamic acid; geranyl beta-glucopyranoside; neryl beta-glucopyranoside; sacranoside B; hexyl beta-glucopyranoside; tyrosol; gallic acid; rhodiolgin; isolariciresinol-9-O-beta-glucopyranoside; rhodiooctanoside (Fig. 15) (Wong et al. 2008).
Phytocomponents reported by Wiedenfeld et al. (2007)
Phytocomponents reported by Wojcik et al. (2009)
Phytocomponents reported by Wong et al. (2008)
Rhodiola bupleuroides
The various compounds isolated from R. bupleuroides were gallic acid; kaempferol-7-O-α-L-rhamnopyranoside; rhodiosin; quercetin; syringic acid; and β-sitosterol (Fig. 16) (Li et al. 2007). Rhobupcyanoside B (Fig. 17) (Wang et al. 2016).
Phytocomponents reported by Li et al. (2007)
Phytocomponents reported by Wang et al. (2016)
Rhodiola dumulosa
The various bioactive compounds isolated from Rhodiola dumulosa were β-sitosterol; sexangularetin; kaempferol-7-O-α-L-rhamnoside; herbacetin-7-α-L-rhamnoside; kaempferol ; and β-sitosterol glucoside (Fig. 18). The compounds which were obtained from this plant for the first time are (Dingqiang et al. 2005) quercetin; gallic acid; (±) -Isolariciresinol-3-alpha-O-beta-D-glucopyranoside; rutin; kaempferol-3-O-beta-D-glucopyranoside-7-alpha-O-L-rhamnoside (Fig. 19) (Liu et al. 2008).
Phytocomponents reported by Dingqiang et al. (2005)
Phytocomponents reported by Liu et al. (2008)
Rhodiola algida
The marker compounds found in Rhodiola algida were salidroside and tyrosol (Fig. 20) (Lu et al. 2011). The other bioactive compounds reported in Rhodiola algida were rhodalgin, acetylrhodalgin, diacetylrhodalgin, and triacetylrhodalgin (Fig. 21) (Pangarova and Zapesochnaya 1975).
Phytocomponents reported by Lu et al. (2011)
Phytocomponents reported by Pangarova and Zapesochnaya (1975)
Rhodiola sachalinensis
The major active constituent of Rhodiola sachalinensis is salidroside (Li and Chen 2001). Several other bioactive compounds are glycosides such as rhodiocyanosides (Yoshikawa et al. 1995), sacranosides (Yoshikawa et al. 1997), and phenolic components (Fig. 22) (Lee et al. 2000). Kaempferol, cinnamyl alcohol, and daucosterol (Song et al. 2003).
Rhodiola qundrifida
Rhodiacyanosides A and B; octyl α-L-arabinopyranosyl(1-6)-β-D-glucopyranoside; tricetin and gossypetin 7-O-β-D glucopyranosyl(1-3)-α-L-rhamnopyranoside (Fig. 23) (Yoshikawa et al. 1995), two flavonols (quercetin and kaempferol); p-tyrosol and rhodioloside (Fig. 24) (Troshchenko and Kutikova 1967) were major compounds of this species of Rhodiola.
Phytocomponents reported by Yoshikawa et al. (1995)
Phytocomponents reported by Troshchenko and Kutikova (1967)
Bioactivity of Rhodiola Species
Rhodiola rosea
Antioxidant, adaptogenic, antistress, antimicrobial, immunomodulatory, angiomodulatory, and antitumor effects were the activities reported for Rhodiola rosea. p-Hydroxyphenethyl-β-D-glucoside is one of the major compounds found in Rhodiola that is responsible for many of the effects observed with Rhodiola extracts (Recio et al. 2016). Salidroside can be used as an effective agent against diabetes due to repression of adipogenesis and inflammation in eWAT and stimulation in hypothalamus of leptin signal transduction (Wang et al. 2016). The compounds from Rhodiola rosea showed RRL-induced protective effect on pulmonary fibrosis (PF) in rats. The treated rats had less lung fibrosis and inflammation than those in BLM-treated rats. Significant reduction of MMP-9 and α-SMA expression in the (bleomycin) BLM-induced PF rat mode was found after RRL treatment. Consistently, the expression of matrix TGF-β1 was inhibited significantly, while metalloproteinase-9 increased in the lungs of rats. These results strongly suggest that RRL attenuated BLM-induced fibrotic lung injury in rats (Zhang et al. 2016). The compound salidroside found to have protective effects toward the pulmonary arterial hypertension (PAH) induced chronic hypoxia. It has the potential to inhibit chronic hypoxia-induced pulmonary arterial smooth muscle cells (PASMCs) proliferation and reverse apoptosis resistance via AMPKα1-P53-P27/P21 pathway and via adenosine monophosphate-activated protein kinase (AMPK) α1-P53-Bax/Bcl-2-caspase 9-caspase 3 pathway (Chen et al. 2016). The extracts of R. rosea promote the host’s immune response showing antitumoral properties, weak and medium-strength mutagens, and protecting tissues against free radicals. Even the Rhodiola extracts have the ability to inhibit angiogenesis. Extracts and salidroside stimulated specific and nonspecific immunity in in vivo as well as in vitro. It seems that they ameliorate immunity by enhancing Th1 cytokines without affecting the Th2 profile (Recio et al. 2016). The studies on R. rosea indicate that R. rosea extract was also characterized by unique pharmacological properties and stimulate positive effect on ATP synthesis in mitochondria of skeletal muscles in rat and stimulated reparative energy processes after intense exercise. R. rosea was found to be most effective for stimulating and increasing physical endurance. Treatment with R. rosea found to decrease the ammonia concentration in mouse muscles, thus reducing acidosis (Abidov et al. 2003). Based on numerous studies conducted over recent 35 years, R. rosea is recommended as the means of improving strength and endurance and replenishing the energy resources of the body (Seifulla 1999). R. rosea acts as an adaptogens by improving the physical endurance of male athletes, reducing blood lactate level, and accelerating recovery after exhausting exercise (Abidov et al. 2003; Azizov and Seifulla 1998; Maimeskulova et al. 1997). Administration of Rhodiola rosea (SHR-5) prior to acute stress produce favorable results and helps to prevent stress-induced disruptions in performance (Panossian et al. 2010). Rhodiola rosea extract found to have an anti-inflammatory effect and protected muscle tissue during exercise (Abidov et al. 2004). Various preclinical studies revealed the adaptogenic effect of Rhodiola root water-alcoholic extract (Abidov et al. 2003; Saratikov 1976; Saratikov et al. 1968; Aksenova et al. 1968; Panossian and Wagner 2005; Jafari et al. 2007; Perfumi and Mattioli 2007; Mattioli et al. 2008; Diermen et al. 2009; Qin et al. 2008; Siwicki et al. 2007; Wang et al. 2009; Pooja et al. 2009; Bany et al. 2009).
Many studies demonstrated that the regulation of key mediator like molecular chaperones (e.g., Hsp70) (Lishmanov et al. 1996; Prodius et al. 1997; Panossian et al. 2007, 2008, 2009; Wiegant et al. 2008; Olsson et al. 2009), cortisol (Olsson et al. 2009), nitric oxide (Panossian et al. 2007), Forkhead box O (FOXO) transcription factor DAF-16 (Wiegant et al. 2009), stress-activated c-Jun N-terminal protein kinase 1 (JNK1) (Panossian et al. 2007), and beta-endorphin by Rhodiola rosea is associated with the stress response (Lishmanov et al. 1987; Maslov et al. 1997; Arora et al. 2005). The studies reveal that the administration of Rhodiola rosea promotes a moderate increase in serum immune reactive beta-endorphin in rats under basal conditions which is equivalent to rats adapted to exercise. When Rhodiola rosea-treated rats were subjected to a 4 h period of nonspecific stress, the expected elevation in beta-endorphin was either not observed or substantially decreased. Consequently resulting in the characteristic perturbations of the hypothalamic-pituitary-adrenal axis was decreased or totally prevented (Lishmanov et al. 1987). Rhodiola rosea, with its potential to act as an anticancer agent, might be useful in conjunction with some pharmaceutical antitumor agents, and even supplementation of Rhodiola rosea extract inhibits the growth of both tumor types, extended survival times in rats with transplanted solid Ehrlich’s adenocarcinoma and metastasizing rat Pliss lymphosarcoma and decreased metastasis to the liver. The studies reveal that the extract also directly suppressed the lung carcinomas (Udintsev and Shakhov 1991). R. rosea’s protective effect against the antioxidant stress is not totally because of its antioxidant or prooxidant effects (Wiegant et al. 2008; Schriner et al. 2009) because it does not elevate the major antioxidant defenses but due to activation of the antioxidant response element or degrading H2O2 (Schriner et al. 2009).
Rhodiola imbricata
Rhodiola imbricata is known to have many biological effects. There was a significant decrease in the cytotoxicity in comparison to control created by the tert-BHT (250 μM) by using the aqueous and alcoholic extract. Extracts have also reduced the ROS production which was developed by tert-BHT in the mitochondria which is comparable to the vitamin C. Addition of aqueous and alcoholic extract has no effect in GSH level in the tert-BHT-exposed macrophages. Treatment with the extract has six times increased the early apoptotic cells and three times increased the late apoptotic cells which were significantly low when treated with tert-BHT (500 μM). Comet assay revealed that 500 μM tert-BHT has applicably increased the single-strand break which has been reduced by the use of alcoholic and aqueous extract (Kanupriya et al. 2005). The DPPH assay study has reported to show significant inhibition of DPPH activity at 4.391 μg/ml in comparison to quercetin (3.824 μg/ml) and BHT (4.743 μg/ml) for 50% inhibition. The lipid peroxidation activity shows that Rhodiola aqueous extract has maximum scavenging activity at 500 μg/ml and minimum at 0.5 μg/ml where α-Tocopherol was used as a standard. The IC50 of extract was 5.12 and of standard was 4.89. For the superoxide ion radical, the IC50 of the extract, ascorbic acid, α-Tocopherol, and quercetin was 4.78, 3.36, 4.53, and 4.33 μg/ml. The aqueous extract has also reported to show ferric ion chelating activity; IC50 of the extract, α-Tocopherol, and quercetin was 5.33, 6.13, and 3.123 μg/ml. The hydrogen peroxide inhibition study revealed that the extract has a very high inhibition activity for hydrogen peroxide which is comparable to α-Tocopherol. The total flavonoid content in extract was reported to be 66.7 μg quercetin equivalent/mg, and total phenolic content was 240 ± 10 mg of gallic acid equivalent (Gupta et al. 2009). The acetone extract of R. imbricata was found nontoxic up to 2000 mg/ml and had shown no mortality in mice. This extract is reported to increase hematological count like RBC count, hemoglobin, hematocrit, MCV, MCH, MCHC, RDW, leukocytes, and platelets at conc. of 400 mg/ml. It also has shown a comparable recovery to standard silymarin for paracetamol-induced hepatic damage; it has decreased the SGPT (88.43 ± 0.3 U/l), ALP(193.53 ± 0.3 U/l), and SGOT(79.56 ± 0.3 U/l) liver marker and had increased the concentration of total protein and enzymatic antioxidants. It has also prevented the oxidation of the liver cells after the administration of paracetamol (Senthilkumar et al. 2014). It is reported that Rhodiola extract stimulates interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) increase in human PBMCs and RAW 264.7 cell line. Reports also show an increase in the production of nitric oxide simultaneously which also activates the nuclear translocation of NF-κB in human PBMCs, which is comparable to the LPS which is a positive stimulant (Mishra et al. 2006). MTT assay of U87 cell line showed an increased survival of cells at doses between 25 and 125 μg/ml in case of drug +radiation group. In vivo evaluation of revealed that intraperitoneal administration of hydro-ethanol extract rendered 83.3% survival (maximally effective dose, 400 mg/kg b.w.) 30 min prior to lethal (10 Gy) total-body γ-irradiation. The ability of hydro-ethanol extract to reduce the effect of lipid peroxidation induced by iron/ascorbate, radiation (250 Gy), and their combination was also analyzed, and it was found that it decreases in a dose-dependent manner. In a study aqueous extract of Rhodiola showed antiproliferative against K-562 cell line in 72 h incubation at a dose of 100 and 200 mg/mlin comparison to normal human peripheral blood lymphocytes or mouse macrophage cell line RAW-264.7 where there is no suppression. Aqueous extract was also found to induce intracellular reactive oxygen species which leads to apoptosis and arrested the cell progression at G2/M phase in K-562 cells. It also shows the anticancer activity which leads to an elevated NK cell cytotoxicity (Mishra et al. 2008). In vitro antioxidant activity was investigated by DPPH radical R. imbricata hydroalcoholic root extract which shows a greater correlation of inhibiting the free radicals with the increase in conc. of extract (Kumar et al. 2010b).
Rhodiola heterodonta
Study of the 80% EtOH extract of R. heterodonta (40 mg/kg) increased the survival rate of the treated mice under hypoxic conditions by 1.9 times compared with the untreated control have been reported. The survival time of mice under hypoxia increased by 192%, by injecting 80% ethanol extract of R. heterodonta which acts as an indication of adaptogenic activity (Grace et al. 2009). The research revealed that Rhodiola heterodonta extract has moderate adaptogenic effect and may be used as adaptogen. Also, low toxicity of Rhodiola heterodonta preparations has been demonstrated by histomorphological analysis of internal organs (Yunuskhodjaev et al. 2014). The total phenol and flavonoids of Rhodiola heterodonta root extract was found to be 79.21 ± 0.26 mg GAE/g and 269.3 ± 0.82 mg Qc/g, respectively (Kumar et al. 2010b).
Rhodiola crenulata
Rhodiola crenulata was reported to show increase in the glycogen synthesis and inhibits the lipogenesis by regulating the genes (glycogen synthase, glycogen synthase kinase 3β, CCAAT/enhancer-binding protein, fatty acid synthase, sterol-regulating element-binding protein 1c) related to the metabolism and process (Lin et al. 2016). It has been reported that the water and ethanol extract of R. crenulata has shown α-amylase inhibitory activity with an IC50 value of 98.1 μg total phenolic/ml and 120.9 μg total phenolic/ml. Besides this it also has reported to show an α-glycosidase inhibitory activity at an IC50 value of 60.3 μg total phenolic/ml and 60.2 μg total phenolic/ml (Kwon et al. 2006). A finding tells that its extract improved the functioning of the brain of rat model of Alzheimer’s disease through protecting neural stem cells by its key component salidroside which scavenged intracellular free radicals (Qu et al. 2012). Rhodiola crenulata was used in the treatment of chronic intermittent hypoxia-decreased cardiac fractional shortening and has shown a significant effective improvement, based on decreases in Fas, activated caspase 8, and FADD, activated caspase 3, compared to the hypoxia group. With treatment of Rhodiola crenulata, the cardiac mitochondrial-based apoptotic pathway in mice with chronic intermittent hypoxia was significantly decreased, which leads to decreases in pro-apoptotic protein levels like t-Bid, Bad, Bax, activated caspase-9, and activated caspase 3 as well as increases in anti-apoptotic protein levels p-Bad, Bcl-xL, and Bcl-2. Another pathway which is cardiac VEGF-related leads to a significant increase in protein p-PI3k, VEGF, and p-AKT level compared to the hypoxia group with treatment of Rhodiola crenulata, which is based on increased in pro-survival (Lai et al. 2015). Rhodiola crenulata extract and its bioactive components have reported to show a significant decrease in hypoxia-mediated endocytosis of the Na and K-ATPase because of the inhibition of the ROS-AMPK-PKC pathway in A549 cell line (Chen et al. 2015). It is reported that the extract also has estrogenic activity (Bassa et al. 2016). There is a reduction in proliferation, which stimulates differentiation and eliminates tumorsphere formation of in vitro glioblastoma multiforme cells with the effect of Rhodiola crenulata. The effects were associated with inhibition of Wnt/β-catenin signaling pathway (Guo et al. 2014). It also has effect on gluconeogenic gene expression by increasing the phosphorylation of AMPK level. It also reduced the plasma glucose level (Lee et al. 2015). Its treatment reduces the level of IFN-γ, high-sensitivity C-reactive protein, and CD8 (+) but increases in expression of CD4(+) CD25(+) FOXP3(+) and CD4(+) CD25(+) CD45(+) FOXP3(+) in the blood (Chen et al. 2015). A study for the survival rate of Drosophila melanogaster against the gut immunity raised by pathogen demonstrate that R. crenulata has increased the survival rates of adult flies and expression of antimicrobial peptide genes after pathogen or toxic compound ingestion. Moreover, it improved intestinal morphology and decreased levels of reactive oxygen species (Zhu et al. 2014). The compounds from Rhodiola crenulata extract were tested for xanthine oxidase (XO) inhibition activity in comparison to a known XO inhibitor allopurinol which has an IC50 value of 12.21 ± 0.27 μM. The compound B2-3′-O-gallate and 4-HAP reported an XO inhibitory effect, the half maximal inhibitory concentration values of compound were 15.62 ± 1.19 and 24.24 ± 1.80 μM, respectively, and there inhibition constants were 8.41 ± 1.03 and 6.16 ± 1.56 μM, respectively. These results suggest that β-2-3′-O-gallate and 4-HAP are potent XO inhibitors (Chu et al. 2014). Its use in Chinese prescription significantly decreases the level of serum glucose, lipid profile, blood urea nitrogen, urine albumin excretion, and urease activity which improves renal function. Chinese prescription could also affect oxidative stress. It could reduce the renal damage induced by hyperglycemia in type 1 diabetic rats. Its effects work by regulating the metabolism of glucose and lipid, the oxidative stress, and the microcirculation disturbance (Fu et al. 2013). R. crenulata phenolic-enriched extract was capable of inhibiting the proliferation, motility, and invasion of human-derived MDA-MB-231 and mouse-derived V14 breast cancer cell lines. The extract also leads to death of the tumor cell lines by inducing autophagic-like vesicles but not the immortal human mammary epithelial cells (Tu et al. 2008). By activation of AMPK signaling, Rhodiola crenulata root extract (RCE) can regulate hepatic gluconeogenesis (Lin et al. 2016). The root extract of R. crenulata was found to improve insulin sensitivity and attenuate abnormal lipid metabolism in a rodent model of diabetes (Wang et al. 2012). Increase in glycogen synthesis and inhibition of lipogenesis, while regulating genes included in glycogen metabolism like glycogen synthase (GS), glycogen synthase kinase 3β (GSK3β), CCAAT/enhancer-binding protein (C/EBP), fatty acid synthase (FAS), and sterol regulatory element-binding protein 1c (SREBP-1c), have been reported by Lin et al. (Lin et al. 2016). The various phenolic compounds were found to be potent as antioxidants and could moderately stimulate IFN-γ expression (Zhou et al. 2015).
Rhodiola kirilowii
Rhodiola kirilowii extract was reported to protect against problems related to the heart and lungs while moving to high altitude, anticoagulative property and decrease the level of blood sugar (Zhang et al. 1989). Rhodiola kirilowii was found to have in vitro inhibitory activity against serine protease (NS3-SP). Serine protease is a potent target of antiviral screening against HCV (Zuo et al. 2007). It also have a great potential as cellular immunity enhancers. The in vitro studies revealed that the extracts stimulate activity of granulocyte and increased lymphocyte response toward mitogens, and in vivo experiment leads to enhance the ability of lymphocytes derived from parental strain mice which were fed with R. kirilowii aqueous and hydroalcoholic extracts, to induce local cutaneous graft-versus-host reaction in F1 hybrids (Wojcik et al. 2009). The in vitro activities against Mycobacterium tuberculosis of its extracts and pure components were evaluated by testing their minimal inhibitory concentration and minimal bactericidal concentration, and the compounds gallic acid and epigallocatechin gallate exhibited an in vitro inhibitory and bactericidal activities against Mycobacterium tuberculosis in different extent (Wong et al. 2008).
Rhodiola bupleuroides
The data available related to the species is very less. Still the report which is available in relation to this species shows that it has compounds which were evaluated for its inhibitory activity against α-glucosidase, and the results show that it has an IC50 of 278.28 ± 0.55 μM in comparison to the positive control (acarbose) at 210.40 ± 0.32 μM (Wang et al. 2016).
Rhodiola algida
Rhodiola algida found to increase immunity which was receiving chemotherapy post-mastectomy and also decreases oral ulcers. Thus Rhodiola algida has the potential to be used concurrently with chemotherapy to alleviate the occurrence of oral ulcers. The optimal concentration of Rhodiola algida favored the proliferation of lymphocytes (Loo et al. 2010). The clinical reports suggest that it regulates IL-2 in Th1 cells and IL-4, IL-6, and IL-10 in Th2 cells which effectively stimulate human peripheral blood lymphocytes, enhancing immune responses and its underlying immunomodulatory effects (Li et al. 2009). Its anticarcinogenic effect on MCF-7 breast cancer cells can lead to downregulation of protein levels of HIF-1α and HIF-2α, which are overexpressed under hypoxic conditions and increasing cell apoptosis. R. algida has a high potential to be antitumor agent (Iaremii and Grigoreva 2002). Beside its antitumor role, R. algida also have immunomodulatory agent (Li et al. 2009).
Rhodiola sachalinensis
The studies reported the hypnotic activity and sedative of salidroside from Rhodiola sachalinensis (Li et al. 2007). Rhodiola sachalinensis has found to have stimulating role for the nervous system, enhancing working efficacy, decreasing depression and microwave radiation, resisting anoxia, eliminating fatigue, improving sleep, preventing high altitude sickness, etc. (Khanum et al. 2005; Ming et al. 1988). Salidroside, which is a phenylpropanoid glycoside, has been reported to have anti-inflammatory activity (Lu et al. 2003). Crude extracts of Rhodiola sachalinensis was found to have high DDPH radical scavenging activity (Zhang et al. 2007). In the study with mice, salidroside showed that it enhances the sleep, by shortening the effect on the sleep latency and also prolonging the effect on the sleeping time in mice treated with hypnotic dosage of pentobarbital sodium (Li et al. 2007). The extract of Rhodiola sachalinensis was found to promote endurance and to increase the body’s resistance against mental and physical stresses (Xu et al. 1998). The aqueous extract found to activate NF-ķB which enhances the induction of iNOS gene in RAW264.7 macrophages (Li et al. 2012). The root has been used to treat cold and flu-like symptoms, but the underlying mechanism is not known (Li et al. 2012). It has been also found to be hepatoprotective against cytotoxicity induced by tacrine in human liver-derived Hep G2 cells (Mishra et al. 2010). Kaempferol is a representative flavonol, and its derivative, kaempferol-6′-O-acetate, was reported to have hepatoprotective effect against cell death induced by TNF-α (Lu et al. 2003).
Rhodiola qundrifida
The plant extract was applied for the treatment of fatigue, blood pressure, dysentery, and genital diseases of women and as a stimulator of the nervous system (Saratikov et al. 1967, 1978; Rohloff 2008; Mora et al. 2015; Yoshikawa et al. 1996). Rhodiola qundrifida reported to be antiallergic; rhodiacyanosides have inhibition effect on the histamine which were released from the rat peritoneal exudate cells which were sensitized with anti-DNP-IgE (Mora et al. 2015). The extract of Rhodiola qundrifida was found to be stimulatory in cell-mediated immunity (Rozewska et al. 2008a). Rhodiola quadrifida hydroalcoholic and aqueous extracts induce an immune modulatory effect on the mouse granulocytes activity which is evaluated by chemiluminescence test (Rozewska et al. 2008b). Its extract response toward the cell-mediated immunity is also evaluated by other tests like respiratory burst activity (RBA) and potential killing activity (PKA) tests (Siwicki et al. 2007; Rozewska et al. 2008a). Rhodiola quadrifida also have inhibitory in the highest (50 μg/ml) dose and granulocytes activity in lower doses (Wojcik et al. 2008).
In Vitro Propagation/Culture
In vitro study was done to develop the plant in lab condition to use for various medicinal purposes besides collecting the raw material from the wild environment and to develop the germplasm. The explant selection is a very crucial factor for the success of morphogenic potential of the isolated cells. There is an extensive use of biotechnological approach to increase the various metabolites of the plant (Grech-Baran et al. 2015). The explants will determine the organogenic as well as the genetic stability of the progeny after cloning. Leaves or leaf disks were the most potent and preferable explants for callus, shoot, and bud formation (Tasheva and Kosturkowa 2010). In a procedure for in vitro propagation of roseroots (Rhodiola rosea), a medicinal plant, was developed by using a RITA bioreactor system which includes liquid medium in combination with a gelled medium. Three clones were established on a basal medium (BM) for germinated seedlings on half strength Murashige and Skoog (MS) salts. Shoots of all three clones rooted in vitro in the growth regulator-free basal medium within 5–6 week of culturing with a frequency of 90–95% in all three clones. Plantlets obtained in vitro were adapted and transferred to soil with a survival rate of 85–90% (Debnath 2009). In a report a callus culture was established to produce the cinnamyl glycosides (Gyorgy et al. 2004). An in vitro micropropagation study was conducted for the plant in 24 modified Murashige and Skoog media (Tasheva and Kosturkowa 2010). A change in the media composition can effect in the metabolite production of the plant. The addition of methyl jamsonite in the callus culture of Rhodiola sachalinensis had increased the salidroside and polysaccharide content (Yu et al. 2011). A change in composition of media is also used for the establishment of the in vitro culture of Rhodiola henryi (Kang et al. 2010). The preservation study of callus has shown that the melatonin has improved the survival of callus Rhodiola crenulata (Zhao et al. 2011).
References
Abidov M, Crendal F, Grachev S, Seifulla R, Ziegenfuss T (2003) Effect of extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) roots on ATP content in mitochondria of skeletal muscles. Bull Exp Biol Med 136:585–597
Abidov M, Grachev S, Seifulla RD, Ziegenfuss TN (2004) Extract of Rhodiola rosea radix reduces the level of C-reactive protein and creatinine kinase in the blood. Bull Exp Biol Med 138:63–74
Aksenova RA, Zotova MI, Nekhoda MF, Cherdintsev SG (1968) Comparative characteristics of the stimulating and adaptogenic effects of Rhodiola rosea preparations. In: Saratikov AS (ed) Stimulants of the Central Nervous System, vol 2. Tomsk University Press, Tomsk, pp 3–12
Alm T (2004) Ethnobotany of Rhodiolarosea (Crassulaceae) in Norway. SIDA Contrib Bot 21:321–344
Arora R, Chawla R, Sagar R, Prasad J, Singh S, Kumar R et al (2005) Evaluation of radioprotective activities of Rhodiola imbricata Edgew – a high altitude plant. Mol Cell Biochem 273:209–223
Azizov AP, Seifulla RD (1998) The effect of elton, leveton, fitoton and adapton on the work capacity of experimental animals. Eksp Klin Farmakol 61:61–63
Bany J, Zdanowska D, Skopinskaroewska E, Sommer E, Siwicki AK, Wasiutynski A (2009) The effect of Rhodiola rosea extracts on the bacterial infection in mice. Centr Eur J Immunol 34:35–37
Bassa LM, Jacobs C, Gregory K, Henchey E, Ser-Dolansky J, Schneider SS (2016) Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomed 23:87–94
Chaurasia OP, Ahmed Z, Ballabh B (2007) Ethnobotany and plants of trans-Himalaya. Satish Serial Publishing House. ISBN: 81-89304-33-X
Chen S-P, Liu RH, Tsong-Ming L, Wei JC-C, Tzu-Chin W, Tsai W-Y, Yang C-C (2015) Complementary usage of Rhodiola crenulata (L.) in chronic obstructive pulmonary disease patients: the effects on Cytokines and T cells. Phytother Res 29:518–525
Chen M, Cai H, Yu C, Wu P, Fu Y, Xu X et al (2016) Salidroside exerts protective effects against chronic hypoxia-induced pulmonary arterial hypertension via AMPKα1-dependent pathways. Am J Transl Res 8:12–27
Choudhary A, Kumar R, Srivastava RB, Surapaneni SK, Tikoo K, Singh IP (2015) Isolation and characterization of phenolic compounds from Rhodiola imbricata, a Trans-Himalayan food crop having antioxidant and anticancer potential. J Funct Foods 16:183–193
Chu YH, Chen CJ, Wu SH, Hsieh JF (2014) Inhibition of xanthine oxidase by Rhodiola crenulata extracts and their phytochemicals. J Agric Food Chem 62:3742–3749
Debnath SC (2009) Zeatin and TDZ-induced Shoot proliferation and use of bioreactor in clonal propagation of medicinal herb, Roseroot (Rhodiola rosea L). J Plant Biochem Biotechnol 18:245–248
Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, Hostettmann K (2009) Monoamine oxidase inhibition by Rhodiola rosea L. roots. J Ethnopharmacol 122:397–401
Dingqiang L, Xiangyu Z, Junxian W (2005) Studies on the Chemical Constituents from Rhodiola dumulosa. J Chin Med Mater. 2:98–99
Fu JY, Zhang XL, Tian JY, Huang LW, Zhang PC, Ye F (2013) Investigation of compound, compatibility of Rhodiola crenulata, Cordyceps militaris, and Rhum palmatum, on metabolic syndrome treatment VI-improving hyperglycemia-mediated renal damage. Zhon Zhong Yao ZaZhi 38:3961–3966
Grace MH, Yousef GG, Kurmukov AG, Raskin I, Lila MA (2009) Phytochemical characterization of an adaptogenic preparation from Rhodiola heterodonta. Nat Prod Commun 4:1053–1058
Grech-Baran M, Sykłowska-Baranek K, Pietrosiuk A (2015) Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochem Rev 14:657–674
Guo N, Zhu M, Han X, Sui D, Wang Y, Yang Q (2014) The metabolism of salidroside to Its Aglycone p-Tyrosol in rats following the administration of Salidroside. Plos One 9:e103648
Gupta V, Lahiri SS, Sultana S, Kumar R (2009) Mechanism of action of Rhodiola imbricata Edgew. during exposure to cold, hypoxia and restraint (C-H-R) stress induced hypothermia and post stress recovery in rats. Food Chem Toxicol 47:1239–1245
Gyorgy Z, Tolonen A, Pakonen M, Neubauer P, Hohtola A (2004) Enhancing the production of cinnamyl glycosides in compact callus aggregate cultures of Rhodiola rosea by biotransformation of cinnamyl alcohol. Plant Sci 166:229–236
Halldorsson B, Grasnytjar AF. Stein and Copenhagen, 1783 (reprinted in Akureyri 1983, pp 241–242)
Han F, Li Y, Mao X, Xu R, Yin R (2016) Characterization of chemical constituents in Rhodiola Crenulate by high-performance liquid chromatography coupled with Fourier-transform ion cyclotron resonance mass spectrometer (HPLC-FT-ICR MS). J Mass Spectrom 51:363–368
Hooker F, Thomson T (1998) J Linn Soc Bot, Clarke in Hooker, “Flor Brit India” 2:418
Hou Y, Lou A (2011) Population genetic diversity and structure of a naturally isolated plant species, Rhodiola dumulosa (Crassulaceae). Plos One 6:1–10
Iaremii IN, Grigoreva NF (2002) Hepatoprotective properties of liquid extract of Rhodiola rosea. Eksp Klin Farmakol 65:57–59
Jafari M, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR, Vince-Cruz C, Mueller LD (2007) Rhodiola: a promising anti-aging Chinese herb. Rejuvenat Res 10:587–602
Kang L, Li C, Wang Z (2010) Tissue culture and plant regeneration of Rhodiola henryi. Chin J Chin Mater Med 35:3250–3254
Kanupriya DP, Sai Ram M, Kumar R, Sawhney RC, Sharma SK, IIavazhagan G, Kumar D, Banerjee PK (2005) Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol Cell Biochem 275:1–6
Khanum F, Bawa AS, Singh B (2005) Rhodiola rosea: a versatile adaptogen. Compr Rev Food Sci Food Safety 4:55–62
Kumar R, Tayade A, Chaurasia OP, Hota S, Singh SB (2010a) Evaluation of anti-oxidant activities and total phenol and flavonoid content of the hydro-alcoholic extracts of Rhodiola sp. Pharmaco J 2:431–435
Kumar R, Kumar GP, Chaurasia OP (2010b) In vitro antioxidant activity of methanolic extract of Rhodiola imbricata Edgew. Pharmaco J 2:157–161
Kwon YI, Jang HD, Shetty K (2006) Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac J Clin Nutr 15:425–432
Lai MC, Lin JG, Pai PY, Lai MH, Lin YM, Yeh YL, Cheng SM, Liu YF, Huang CY, Lee SD (2015) Effects of Rhodiola crenulata on mice hearts under severe sleep apnea. BMC Complement Altern Med 15:198
Lee MW, Lee YA, Park HM, Toh SH, Lee EJ, Jang HD, Kim YH (2000) Antioxidant phenolic compounds from the roots of Rhodiola sachalinensis. Arch Pharm Res 23:455–458
Lee SY, Lai FY, Shi LS, Chou YC, Yen IC, Chang TC (2015) Rhodiola crenulata extract suppresses hepatic gluconeogenesis via activation of the AMPK pathway. Phytomed 22:477–486
Lei Y, Nan P, Tsering T, Bai Z, Tian C, Zhong Y (2003) Chemical composition of the essential oils of two Rhodiola species from Tibet. Naturforsch C. 58:161–164
Li HB, Chen F (2001) Preparative isolation and purification of salidroside from the Chinese medicinal plant Rhodiola sachalinensis by high-speed counter-current chromatography. J Chromatogr A 132:91–95
Li T, Xu G, Wu L, Sun C (2007) Pharmacological studies on the sedative and hypnotic effect of salidroside from the Chinese medicinal plant Rhodiola sachalinensis. Phytomed 14:601–604
Li HX, Sze SC, Tong Y, Ng TB (2009) Production of Th1- and Th2-dependent cytokines induced by the Chinese medicine herb, Rhodiola algida, on human peripheral blood monocytes. J Ethnopharmacol 123:257–266
Li X, Sipple J, Pang Q, Du W (2012) Salidroside stimulates DNA repair enzyme Parp-1 activity in mouse HSC maintenance. Blood 119:4162–4173
Lin KT, Hsu SW, Lai FY, Chang TC, Shi LS, Lee SY (2016) Rhodiola crenulata extract regulates hepatic glycogen and lipid metabolism via activation of the AMPK pathway. BMC Complement Altern Med 16:127
Lishmanov IB, Trifonova ZV, Tsibin AN, Maslova LV, Dementeva LA (1987) Plasma beta-endorphin and stress hormones in stress and adaptation. Biull Eksp Biol Med 103:422–424
Lishmanov YB, Krylatov AV, Maslov LN, Nariznayaand NV, Zamotrinskii AV (1996) Effect of Rhodiola rosea on the level of inducible Hsp-70 in miocard in stress. Bull Exp Biol Med 121:235–237
Liu Q, Liu ZL, Tian X (2008) Phenolic components from Rhodiola dumulosa. Zhongguo Zhong Yao ZaZhi 33:411–413
Loo WT, Jin LJ, Chow LW, Cheung MN, Wang M (2010) Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expert Opin Investig Drugs Suppl 19:91–100
Lu C, Chen Y, Jian L (2003) Role of mucilage cells and glycoprotein at mesophyll cell surface in the freeze tolerance of a alpine plant, Rhodiola algida via. Tangutica. Chin J Appl Environ Biol 9:16–20
Lu DX, Zhang SN, Wang WP, Zhen J (2011) The study of cytostatic effect on MCF-7 cells of the alcohol extract of Rhodiola Algida Var. Tangutica. Proc Environ Sci 8:615–619
Ma CY, Tang J, Wang HX, Gu XH, Tao GJ (2008) Simultaneous determination of six active compounds in Rhodiola L. by RP-LC. Chromatographia 67:383–388
Maimeskulova LA, Maslov LN, Lishmanov IB, Krasnov EA (1997) The participation of the mu-, delta- and kappa-opioid receptors in the realization of the anti-arrhythmia effect of Rhodiola rosea. Eksp Klin Farmakol 60:38–39
Maslov LN, Lishmanov IB, Naumova AV, Lasukova TV (1997) Do endogenous ligands of peripheral mu- and delta-opiate receptors mediate anti-arrhythmic and cardioprotective effects of Rhodiola rosea extract? Biull Eksp Biol Med 124:151–153
Mattioli L, Funariand C, Perfumi M (2008) Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J Psycopharmacol 23:130–142
Maximowicz M (2007) Eleutherococcus Maximowicz, Mém Acad Imp Sci St.-Pétersbourg Divers Savans 9 [Prim. Fl. Amur.]: 132. 1859, Flora of China 13:466–472
Ming HQ, Zia GC, Jheng RZ (1988) Advanced research on Rhodiola. Chin Tradit Herb Drugs 19:229–234
Mishra KP, Chauhan UK, Naik S (2006) Effect of lead exposure on serum immunoglobulins and reactive nitrogen and oxygen intermediate. Hum Exp Toxicol 25:661–665
Mishra KP, Padwad YS, Dutta A, Ganju L, Sairam M, Banerjee PK, Sahwney RC (2008) Aqueous extract of Rhodiola imbricata rhizome inhibits proliferation of an erythroleukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Immunobiol 213:125–131
Mishra KP, Chanda S, Shukla K, Ganju L (2010) Adjuvant effect of aqueous extract of Rhodiola imbricata rhizome on the immune responses to tetanus toxoid and ovalbumin in rats. Immunopharmacol Immunotoxicol 32:141–146
Mora MC, Bassa LM, Wong KE, Tirabassi MV, Arenas RB, Schneider SS (2015) Rhodiola crenulata inhibits Wnt/β-catenin signaling in glioblastoma. J Surg Res 197:247–255
Nakamura S, Li X, Matsuda H, Yoshikawa M (2008) Bioactive constituents from Chinese natural medicines. XXVIII. Chemical structures of acyclic alcohol glycosides from the roots of Rhodiola crenulata. Chem Pharm Bull 56:536–540
Ohwi J (1984) Flora of Japan. Smithsonian Institution, Washington, DC, p 495
Olsson EMG, von Scheele B, Panossian AG (2009) A randomized double-blind placebo controlled parallell group study of SHR-5 extract of Rhodiola rosea roots as treatment for patients with stress related fatigue. Planta Med 75:105–112
Pangarova TT, Zapesochnaya GG (1975) The structure of the flavonoids from Rhodiola algida. II. Chem Nat Compd 11:744–750
Panossian A, Wagner H (2005) Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res 19:819–838
Panossian A, Hambartsumyan M, Hovanissian A, Wikman G (2007) The adaptogens rhodiola and schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress-activated protein kinase, nitric oxide and cortisol. Drug Targets Insights 2:39–54
Panossian A, Nikoyan N, Chanyan N, Hovhannisyan A, Abrahamyan H, Gabnelyan E, Wikman G (2008) Comparative study of Rhodiola preparations on behavioral despair of rats. Phytomed 15:84–91
Panossian A, Wikman G, Kaur P, Asea A (2009) Adaptogens exert a stress protective effect by modulation of expression of molecular chaperons. Phytomed 16:617–622
Panossian A, Wikman G, Sarris J (2010) Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomed 17:481–493
Perfumi M, Mattioli L (2007) Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res 21:37–43
Pooja, Bawa AS, Khanum F (2009) Anti-inflammatory activity of Rhodiola rosea-“a second-generation adaptogen”. Phytother Res 23:1099–1102
Prodius PA, Manukhina EB, Bulanov AE, Wikman G, Malyshev II (1997) Adaptogen ADAPT modulates synthesis of inducible stress protein HSP 70 and increases organism resistance to heat shock. Biull Eksp Biol Med 123:629–631
Qi YJ, Cui S, Lu DX, Yang YZ, Luo Y, Ma L, Ma Y, Wuren T, Chang R, Qi L, Ben BJ, Han J, Ge RL (2015) Effects of the aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica on proliferation and HIF-1α, HIF-2α expression in MCF-7 cells under hypoxic condition in vitro. Cancer Cell Int 15:81. https://doi.org/10.1186/s12935-015-0225-x
Qin YJ, Zeng YS, Zhou CC, Li Y, Zhong ZQ (2008) Effects of Rhodiola rosea on level of 5-hydroxytryptamine, cell proliferation and differentiation, and number of neuron in cerebral hippocampus of rats with depression induced by chronic mild stress. ZhongguoZhong Yao ZaZhi 33:2842–2846
Qu ZQ, Zhou Y, Zeng YS, Lin YK, Li Y, Zhong ZQ, Chan WY (2012) Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. Plos One 7:e29641
Recio MC, Giner RM, Manez S (2016) Immunmodulatory and antiproliferative properties of Rhodiola species. Planta Med 82:952–960
Rohloff J (2008) Volatiles from rhizomes of Rhodiola rosea L. Phytochem 59:655–661
Rozewska ES, Wojcik R, Siwicki AK, Somer E, Wasiutynski A, Furmanowa M, Malinowski M, Mazurkiewcz M (2008a) The effect of Rhodiola quadrifida extracts on cellular immunity in mice and rats. Pol J Vet Sci 11:105–111
Rozewska ES, Wasiutynski A, Sommer E, Mielcarek S, Scisz AM, Patan AK, Mazurkiewicz M, Pastewka K (2008b) The influence of Rhodiola rosea, Rhodiola kirilowii, and Rhodiola quadrifida extracts on cutaneous angiogenesis induced in mice after grafting of human kidney cancer tissue. Centr Eur J Immunol 33:185–189
Saratikov AS (1976) Adaptogenic action of Eleutherococcus and golden root preparations. In: Brekhman II (ed) Adaptation processes and biologically active compounds, pp 54–62
Saratikov AS, Krasnov EA (2004) Rhodiolarosea (Golden root): a valuable medicinal plant. Tomsk University Press, Tomsk, pp 1–205
Saratikov AS, Krasnov EA, Khnikina LA, Duvidson LM (1967) Isolation and chemical analysis of individual biologically active constituents of Rhodiola rosea. Proc Siberian Acad Sci Biol 1:54–60
Saratikov AS, Krasnov EA, Chnikina LA, Duvidson LM, Sotova MI, Marina TF, Nechoda MF, Axenova RA, Tscherdinzeff SG (1968) Rhodiolosid, a new glycoside from Rhodiola rosea and its pharmacological properties. Pharmazie 23:392–395
Saratikov A, Marina TF, Fisanova LL (1978) Effect of golden root extract on processes of serotonin synthesis in CNS. J Biol Sci 6:142
Schriner SE, Avanesian A, Liu Y, Luesch H, Jafari M (2009) Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defenses. Free Radic Biol Med 47:577–584
Seifulla SD (1999) Sport pharmacology. Sport-Farma Press, Moscow, p 120
Senthilkumar R, Chandranand R, Parimelazhagan T (2014) Hepatoprotective effect of Rhodiola imbricata rhizome against paracetamol-induced liver toxicity in rats. Saudi J Biol Sci 21:409–416
Seo WG, Pae HO, Oh GS, Kim NY, Kwon TO, Shin MK et al (2001) The aqueous extract of Rhodiola sachalinensis root enhances the expression of inducible nitric oxide synthase gene in RAW264.7 macrophages. J Ethnopharmacol 76:119–123
Sikkink L (2009) Med Anthropol Appl Perspect. ISBN-13: 978-0-495-10017-1. ISBN-10: 0-495-10017-X
Siwicki AK, Skopinska-Różewska E, Hartwich M (2007) The influence of Rhodiola rosea extracts on non-specific and specific cellular immunity in pigs, rats and mice. Centr Eur J Immunol 32:84–91
Song EK, Kim JH, Kim JS, JI-Xing Nan HC, Sohn DH, Ko G, Oh H, Kim YC (2003) Hepatoprotective phenolic constituents of Rhodiola sachalinensis on tacrine-induced cytotoxicity in Hep G2 cells. Phytother Res 17:563–565
Sundriyal M, Sundriyal RC, Sharma E (2004) Dietary use of wild plant resources in the Sikkim Himalaya, India. Econ Bot 58:626–638
Tasheva K, Kosturkowa G (2010) Rhodiola rosea L. in vitro cultures peculiarities scientific. Proceedings of the 3rd International Symposium. New Researches in Biotechnology, Bucharest, Romania, 2010
Tayade AB, Dhar P, Kumar J, Sharma M, Chauhan RS, Chaurasia OP, Srivastava RB (2013) Chemometric profile of root extracts of Rhodiola imbricata Edgew with hyphenated gas chromatography mass spectrometric technique. Plos One 13:1–15
Troshchenko AT, Kutikova GA (1967) Rhodioloside from Rhodiola rosea and Rh. quadrifida. I. Chem Nat Compd 3:204–207
Tu Y, Roberts L, Shetty K, Schneider SS (2008) Rhodiola crenulata induces death and inhibits growth of breast cancer cell lines. J Med Food 11:413–423
Udintsev SN, Shakhov VP (1991) The role of humoral factors of regenerating liver in the development of experimental tumors and the effect of Rhodiola rosea extract on this process. Neoplasma 38:323–331
Wang H, Ding Y, Zhou J, Sun X, Wang S (2009) The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomed 16:146–155
Wang J, Rong X, Li W, Yang Y, Yamahara J, Li Y (2012) Rhodiola crenulata root ameliorates derangements of glucose and lipid metabolism in a rat model of the metabolic syndrome and type 2 diabetes. J Ethnopharmacol 142:782–788
Wang H, Dong L, Ge JQ, Deng LN, Lan XZ, Liao ZH, Chen M (2016) A new cyanoside from Rhodiola bupleuroides. J Asian Nat Prod Res 1:1–7
Wiedenfeld H, Zych M, Buchwald H, Furmanowa M (2007) New compounds from Rhodiola kirilowii Scientia. Pharm Sci Pharm 75:29–34
Wiegant FAC, Limandjaja G, de Poot SAH, Bayda LA, Vorontsova ON, Zenina TA et al (2008) Plant adaptogens activate cellular adaptive mechanisms by causing mild damage. In: Lukyanova L, Takeda N, Singal PK (eds) Adaptation biology and medicine: health potentials, vol 5. Narosa Publishers, New Delhi, pp 319–332
Wiegant FA, Surinova S, Ytsma E, Makkinje M, Wikman G, Post JA (2009) Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 10:27–42
Wojcik R, Siwicki AK, Roewska ES, Mrozikiewicz PM (2008) Experimental immunology: The in vitro influence of Rhodiola quadrifida extracts on non-specific cellular immunity in pigs. Centr Eur J Immunol 33:193–196
Wojcik R, Siwicki AK, Skopińska-Różewska E, Wasiutyński A, Sommer E, Furmanowa M (2009) The effect of Chinese medicinal herb Rhodiola kirilowii extracts on cellular immunity in mice and rats. Pol J Vet Sci 12:399–405
Wong YC, Zhao M, Zong YY, Chan CY, Che CT (2008) Chemical constituents and anti-tuberculosis activity of root of Rhodiola kirilowii. Zhongguo Zhong Yao ZaZhi 33:1561–1565
Xu JF, Su ZG, Feng PS (1998) Activity of tyrosol glucosyltransferase and improved salidroside production through biotransformation of tyrosol in Rhodiola sachalinensis cell cultures. J Biotechnol 61:69–73
Yoshikawa M, Shimada H, Shimoda H, Matsuda H, Yamahar J, Murakami N (1995) Rhodiocyanoside-A and Rhodiocyanoside-B, new antiallergic cyanoglycosides from Chinese natural medicine Si-LiHong-Jing-Tian, the underground part of Rhodiola quadrifida (Pall). Fisch Et Mey. Chem Pharm Bull 43:1245–1247
Yoshikawa M, Shimada H, Shimoda H, Murakami N, Yamahara J, Matsuda H (1996) Bioactive constituents of Chinese natural medicines. II. Rhodiolae radix. (1). Chemical structures and Antiallergic activity of Rhodiocyanosides A and B from the underground Part of Rhodiola guadrifida (PALL.) FISCH. et MEY. (Crassulaceae). Chem Pharm Bull 44:2086–2091
Yoshikawa M, Shimada H, Horikawa S, Murakami T, Shimoda H, Yamahara J et al (1997) Bioactive constituents of Chinese natural medicines. 4. Rhodiolae radix. 2. On the histamine release inhibitors from the underground part of Rhodiola sacra (Prain ex Hamet) S.H. Fu (Crassulaceae): chemical structures of rhodiocyanoside D and sacranosides A and B. Chem Pharm Bull 45:1498–1503
Yousef GG, Grace MH, Cheng DM, Belolipov IV, Raskin I, Lila MA (2006) Comparative phytochemical characterization of three Rhodiola species. Phytochem 67:2380–2391
Yu HS, Mab LQ, Zhang JX, Shib GL, Hua YH, Wang YN (2011) Characterization of glycosyltransferases responsible for salidroside biosynthesis in Rhodiola sachalinensis. Phytochem 72:862–870
Yunuskhodjaev AN, Iskandarova SF, Kurmukov A, Saidov SA (2014) Study of adaptogenic properties and chronic toxicity of extract of Rhodiola heterodonta. Eur J Nat History 2:35–38
Zhang ZH, Feng SH, Hu GD, Cao ZK, Wang LY (1989) Effect of Rhodiola kirilowii (Regel.) Maxim on preventing high altitude reactions. A comparison of cardiopulmonary function in villagers at various altitudes. Zhon Zhong Yao ZaZhi 14:687–690
Zhang SQ, Bi HM, Liu CJ (2007) Extraction of bio-active components from Rhodiola sachalinensis under ultrahigh hydrostatic pressure. Sep Purif Technol 57:277–282
Zhang K, Si SP, Huang J, Han J, Liang X, Xu XB, Wang YT, Li GY, Wang HY, Wang JH (2016) Preventive effects of Rhodiola rosea L. on Bleomycin-induced pulmonary fibrosis in rats. Int J Mol Sci 17:1–20
Zhao Y, Qi LW, Wang WM, Saxena PK, Liu CJ (2011) Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J Pineal Res 50:83–88
Zhou JT, Li CY, Wang CH, Wang YF, Wang XD, Wang HT, Jiang MM, Gao XM (2015) Phenolic compounds from the roots of Rhodiola crenulata and their antioxidant and inducing IFN-γ production activities. Molecules 20:13725–13739
Zhu C, Guan F, Wang C, Jin LH (2014) The protective effects of Rhodiola crenulata extracts on Drosophila melanogaster gut immunity induced by bacteria and SDS toxicity. Phytother Res 28:1861–1866
Zuo G, Li Z, Chen L, Xu X (2007) Activity of compounds from Chinese herbal medicine RodiolaKirilowii (Regal) Maxim against HCV NS3 serine protease. Anim Res 76:86–92
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhardwaj, P., Bhardwaj, G., Raghuvanshi, R., Thakur, M.S., Kumar, R., Chaurasia, O.P. (2018). Rhodiola: An Overview of Phytochemistry and Pharmacological Applications. In: Singh, B., Peter, K. (eds) New Age Herbals. Springer, Singapore. https://doi.org/10.1007/978-981-10-8291-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-8291-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8290-0
Online ISBN: 978-981-10-8291-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)