Abstract
The growth of population, increasing urbanization and rising standards of human have contributed to increase in both quantity and variety of solid wastes generated by agricultural, domestic and industrial activities. Industrial wastes contributed more than 85 % of solid waste generation globally. Metals are the major component of almost all the industrial activities but their mining, extraction, purification and various manufacturing processes generate mining and metallurgical wastes having enormous environmental and health impacts. This chapter aims to describe the metals in solid wastes from mining and metallurgical industries and their toxicological impacts on plant community. Industrial wastes are composed of a wide range of essential macro- and micronutrients such as Na, K, Ca, Mg, Mn, Fe, Cu, Zn, Ni, Co, and Mo which are required by plants for their growth and development. But the concentrations of micronutrients in plants when they exceed certain thresholds may interfere with plant metabolic activities leading to the reduction in their productivity. Similarly, non-essential metals and metalloids such as Cd, Pb, As, Al, Bi, Cr, Hg, Ti and Si at elevated concentrations in plants cause phytotoxic effects and lead to food chain contamination. These wastes are generated in huge quantities and discarded without any proper pretreatment; therefore, chances of contamination of environmental components are obvious. This chapter also suggests the possible and better management opportunities including site restoration by rehabilitation and phytoremediation of metal-contaminated sites using native and medicinal plant species to reduce food chain contamination and an ultimate risk to human health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
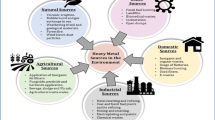
Xenobiotics in residues from agricultural, domestic and industrial sectors are significant environmental pollutants and are the major concerns for safeguarding the human and ecological health of various ecosystems. Common examples of organic xenobiotics are soap, detergents, disinfectants, herbicides, insecticides, vinegar, spices, fats, oils, etc., whereas inorganic xenobiotics include inorganic fertilizers, acidic and basic compounds and metals (Tyus 2012). Metals such as zinc, copper, iron, magnesium, nickel, manganese, molybdenum, etc. are essential for living organism but when present in higher concentration than usual, cause toxic effects (Hodson 2012). Toxic metals include cadmium, lead, chromium and mercury, which are foreign to biological systems are referred as xenobiotic metals (Solenkova et al. 2014). Metals are of significant importance because of their widespread application in manufacturing and infrastructure developments going on throughout the world leading to increased waste generation, and, hence, the metallic wastes from mining and metallurgical industries may pose significant threats to plant species and ecosystems.
Rapid industrialization and urbanization have resulted in an enormous increase in solid wastes due to a variety of activities. Out of ≈ 12 billion tonnes of solid wastes generated during 2002, 11 billion tonnes were contributed by industrial wastes (Yoshizawa et al. 2004). As per the statistics on waste generation in India given by Pappu et al. (2007), the highest proportion of annual solid waste was contributed by the agricultural sector (147.5 MT) followed by thermal power plants (in terms of coal combustion residues) (112 MT), mining and metallurgical industries (99 MT) and municipal solid wastes (48 MT) (Fig. 10.1). Mining and metallurgical industries are of considerable importance in providing great diversity of minerals for industrial and household activities, thus contributing the major proportion of the world’s economy.
Solid waste generation in India from mining and metallurgical industries (Modified from Pappu et al. 2007)
10.2 Mining Industries
Mining is a process where extraction of materials from the ground takes place in order to recover metalliferous (bauxite, lead, zinc, copper, etc.) and non-metalliferous (sand, stone, kaolinite, phosphate, limestone, rock salt, slate and sulphur) ores and fuel (coal and oil). Mining process can be categorized into surface, underground and in situ mining.
10.2.1 Surface Mining
Mining of mineral deposits from either at or close to the earth’s surface involves removing surface vegetation, topsoil and layers of bedrock in order to reach buried mineral deposits.
10.2.2 Underground Mining
Underground mining consists of digging tunnels or shafts into the earth to reach buried ore deposits. Ores for processing and waste rock for disposal are brought to the surface through the tunnels and shafts.
10.2.3 In Situ Mining
In situ mining is a method of extracting minerals from an orebody that is left in place rather than being broken up and removed. The process involves a series of wells that are drilled into the orebody, and solvents are injected through certain wells and withdrawn through others. In situ mining is an advanced technique, providing an alternative with less environmental impact than conventional surface and underground mining.
During the process of mining, large quantities of solid wastes are generated and are categorized as rock wastes, overburden, sludge, tailings and spoils (Fig. 10.2). Mining industries that contribute a major proportion of the gross domestic product (GDP) of the world are bauxite, coal, copper, diamond, gold, iron ore, natural gas, nickel, oil shale, opal, petroleum, rare earth elements, silver, uranium, zinc and lead.
10.3 Metallurgical Industries
A metallurgical industry involves mechanical, physical and chemical methods of producing a pure form of metals or alloys from ores. There are mainly three types of metallurgical operations, namely, pyrometallurgical process, where smelting, refining and roasting of extracted ores are performed; hydrometallurgical operation, where production of phosphoric acid by phosphate digestion takes place and electrometallurgical process which is an electrolytic process of metal refining. Waste materials generated from metallurgical industries are slags, tailings, red mud, sludge and filter residues (Fig. 10.3).
This chapter particularly aims to describe the metals and metalloids in solid wastes from mining and metallurgical industries and their toxic impacts on plant community structure. Information on better management options including phytoremediation, reclaimation of polluted sites and potential reuse of these wastes are also discussed.
10.4 Characterization and Estimation of Metal Contents in Solid Wastes Generated from Mining and Metallurgical Industries
The wastes are characterized, in terms of chemical and mineralogical compositions. Chemical composition is determined by digesting the material in appropriate acids and analysing by atomic absorption spectroscopy (AAS) and inductively coupled plasma and mass spectroscopy (ICP-MS). Other methods of analysis include potentiometric titration, conductometric titration and colorimetric methods using a spectrophotometer (Willard et al. 1988). Mineralogical compositions of the wastes can be determined by X-ray diffraction (XRD), scanning electron microscopy (SEM), microprobe, image analyser (IA), proton-induced X-ray analyser (PIXE), energy-dispersive X-ray analyser (EDX), secondary ion mass spectrometer (SIMS), laser ionization mass spectrometer (LIMS), infrared analysis (IRA) and cathode luminescence (Rao 2011).
Waste materials generated from mining and metallurgical processings (slags, tailings, overburden, rocks, filter residues, sludge and red mud) are heterogeneous geologic materials, which have been deposited in surrounding areas without any proper pretreatments. Physico-chemical properties of these wastes depend upon the mineralogy, geochemistry, particle size of mine materials, moisture content, type of processes used in extraction, purification and refining of materials (Hassinger 1997). Generated wastes are composed of a wide range of particle size fractions varying from coarse mine wastes to slimes (Ritcey 1989). These wastes generally have extreme pH values (acidic to alkaline), reduced concentrations of essential plant nutrients (nitrogen (N), phosphorous (P), potassium (K) and other micronutrients), low organic matter, extremely low microbial activities, high cation exchange capacity, poor water holding capacity, high bulk density and elevated levels of heavy metals (Conesa and Faz 2011).
Metals are an important component in industrial wastes that are dispersed in soil, surface and groundwater leading to environmental risks to adjoining areas (Santos-Jallath et al. 2012; Wójcik et al. 2014). Based on previous studies, concentrations of selected metals such as sodium (Na), potassium (K), calcium (Ca), manganese (Mn), magnesium (Mg), iron (Fe), cobalt (Co), cadmium (Cd), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), lead (Pb), chromium (Cr), mercury (Hg), aluminium (Al), molybdenum (Mo), bismuth (Bi), titanium (Ti) and silica (Si) in mining (metalliferous, non-metalliferous and fuel) and metallurgical wastes are presented in Table 10.1, which shows a wide range of their concentrations in discharged wastes resulting in contamination of not only soil at dumping site but also affecting nearby areas. Essential macro- and micronutrients such as Na, K, Ca, Mg, Fe, Zn, Mn, Co, Cu, Ni and Mo are important for plant growth and development, but their concentrations in either agricultural soil or plants beyond certain limit may cause toxic effects on physico-chemical and biological properties of soil and plant’s performance growing on heavily contaminated soil.
10.5 Toxic Impacts of Metals on Crop and Medicinal Plants
Contamination of agricultural lands with heavy metals in the vicinity of industries has become a major environmental concern. Such toxic elements are considered as soil pollutants due to their acute and (or) chronic toxicity to plants. Metal pollution becomes a persistent problem, as, once released into the environment where organisms may get affected because metals are not destroyed, but they only transform from one oxidation state or organic complex to another or are gradually move into different components of the biosphere (Marques et al. 2009). Surface runoff and leaching from waste dumps pollute the groundwater (Baba and Tayfur 2011), while the dust spread by wind settles on agricultural crops from where they enter the food chain when consumed (Salomons 1995). Like other living organisms, plants are often sensitive both to deficiency and excess availability of some essential micronutrients. Higher concentrations of essential micronutrients are strongly toxic to the metabolic activities of plants (Fig. 10.4). Several researches have been conducted to assess the toxic effects of elevated metal concentrations on plants (Reeves and Baker 2000). Here, the potential implications of some metals on plants are discussed in detail:
10.5.1 Sodium
Sodium is an essential nutrient for the growth of plants, and it plays an important role in maintaining the turgor pressure inside plant cells (Jennings 1976), but its excessive concentrations produce toxic effects on older leaves, cause premature leaf senescence and reduce total photosynthetic leaf area (Munns 2002). Na salts are the major cause of salinity in soil that is a major challenge in many agricultural regions in the world (Pitman and Läuchli 2002). In heavily polluted sites, Na salts precipitate on leaves as water evaporates which in turn may result its higher concentration in plants (Bailey et al. 1999). Phytotoxic symptoms due to higher Na accumulation in plants are leaf burn, scorch and dead tissues which first appear on the outer edges of leaves and then move progressively inward between the veins towards the leaf centre with increase in severity (Table 10.2).
High Na concentration may alter normal growth and physiology of plants. Significant reductions in shoot length, leaf area and dry weight of brinjal (Solanum melongena) were recorded beyond 10 mmol L−1 NaCl concentration, whereas maximum yield reduction (88.0 %) was observed above 150 mmol L−1 NaCl concentration (Chartzoulakis and Loupassaki 1997). Similarly, leaf area development of cotton (Gossypium hirsutum) and bean (Phaseolus vulgaris) was strongly inhibited under Na stress, and such reduction in leaf area altered the photosynthesis and growth of these plants (Brugnoli and Lauteri 1991). Plant height and leaf elongation in tomato (Lycopersicon esculentum) also showed reductions with increase in NaCl in the nutrient solution (Montesano and Van Iersel 2007). In Capsicum annuum, plant height and total leaf area were reduced by 49.0 and 82.0 %, respectively, under NaCl concentrations above 50 mmol L−1 (Chartzoulakis and Klapaki 2000). High levels of NaCl in soil are reported to decrease the number of flowers and stem quality of Gerbera jamesonii (De Kreij and Van Os 1989) and Rosa hybrida (De Kreij and Van Den Berg 1990). Lee and Van Iersel (2008) found that Chrysanthemum sp. receiving 9 gL−1 NaCl showed reduction in shoot dry weight (76.0 %), stomatal conductance (90.0 %) and chlorophyll content (from 42.3 to 29.2 SPAD units), and a 4-day delayed flowering was also observed compared to the control plants. Montesano and Van Iersel (2007) also found significant reductions in leaf photosynthesis and chlorophyll content at NaCl concentrations above 4.1 gL−1. Increased Na+ uptake also interferes with uptake of K+ in plants, thus causing K+ deficiency (Montesano and Van Iersel 2007).
10.5.2 Potassium
Potassium is also an important macronutrient required for plant physiological and metabolic processes. The most important role of K in plants is to activate several enzymes participating in plant metabolism (Evans and Sorger 1966). Most plants require K in the range of 50–100 mM for their normal functioning (Epstein 1980). Due to either K deficiency or excessive uptake, plants show necrosis, chlorosis and leaf curling as visible symptoms (Table 10.2).
Critical concentrations of K for L. esculentum, Helianthus annuus and Zea mays have been found to be 1.0, 2.2 and 1.3 ppm, respectively, which means that K helps to increase their biomass that may reach up to 90 %, but above this concentration, no further increase in biomass takes place (Besford and Maw 1975; Spear et al. 1978; Tyner and Webb 1946). Excessive K uptake may reduce plant’s ability to uptake P from soils as observed by Karlen et al. (1987) in the case of maize/corn (Z. mays). Rodrigues et al. (2016) showed that accumulation of K in Jatropha curcas shoot showed a strong correlation with increase in stomatal conductance and transpiration that ultimately cause reductions in water use efficiency and Na+ content under high relative humidity (80.0 %). The study also showed that the supply of K+ in growing medium strongly restricted Na+ uptake and transport to the shoot because of a strong competitive interaction between K+ and Na+ ions in the growth medium.
10.5.3 Calcium
Calcium is an essential macronutrient which plays a significant role in preserving the structural and functional integrity of cell membranes, stabilizes cell wall structures, regulates ion transport as well as controls cell wall enzymatic activities (Marschner 1995). But excessive Ca uptake by plants may produce phytotoxic effects such as necrosis, chlorosis, leaf curling and puckering (Table 10.2). Studies indicate that higher Ca accumulation in tomato leads to the development of yellowish flecks or gold spots around the calyx and on fruit due to formation of calcium oxalate crystals (De Kreij et al. 1992). Through a hydroponic experiment, Nichols and Beardsell (1981) also showed that increase in levels of Ca induced necrotic spots on leaves and caused reduction in the growth of Grevillea sp.
Addition of CaSO4 (gypsum) to the soil plays a significant role in reducing heavy metal toxicity (Illera et al. 2004), but more than 25.0 % gypsum may cause significant reduction in yield of crop plants due to imbalanced K/Ca and Mg/Ca ratios (Van Alphen and de los Ríos Romero F 1971). Hernando et al. (1965) also reported that higher Ca accumulation caused poor growth of corn at 80.0 % soil moisture in the field, whereas wheat (Triticum aestivum) growth is reduced when gypsum content in soil was 25.0 %. Bureau and Roederer (1960) also suggested that crop cultivation in the soil of Tunisia with 30.0 % gypsum content may cause toxic effects on plant growth and development. Smith and Robertson (1962) observed that wheat grown in soil with higher gypsum input showed wilting during spring time due to reduced uptake of soil moisture by plants. Explants of Chrysanthemum morifolium, a medicinal plant treated with different levels of Ca that showed variable callogenesis and callus growth, were negatively affected by high levels of Ca due to inhibition of enzyme activities, magnesium uptake and protein synthesis (Borgatto et al. 2002).
10.5.4 Magnesium
Magnesium is another nutrient essential for plant growth and development. It is a component of chlorophyll and also plays an important role in plant respiration and energy metabolism, but becomes toxic when available in excess. In serpentine soil (soil which is derived from ultramafic rock, have high pH and are rich in Cr, Co, Ni, Fe and Mg, but deficient in macronutrients such as N, P, K and Ca), Mg phytotoxicity is the most common cause of “serpentine syndrome”, resulting in reduction of plant’s growth and development due to high Mg/Ca ratio (Brooks 1987). Certain visible toxic symptoms caused by Mg phytotoxicity are presented in Table 10.2. Oat (Avena sativa) plant in serpentine soil is more susceptible to Mg toxicity, which is caused by lowering of Ca uptake in plants due to their antagonistic behaviour.
Even though it is an essential component of chlorophyll, elevated levels of Mg may impair photosynthesis by inhibiting K+ transport from cytosol to stroma and possibly interfere with Mg homeostasis within the chloroplast (Shaul 2002). Wu et al. (1991) showed that although Mg is very important for tea plants, but its higher concentration may adversely affect the growth and development of plants by altering their metabolic processes. Tea (UPASI-9) plant receiving above 5000 ppm of Mg supplement in soil showed coppery colour development on the leaf surface, and plant death occurred under long-term exposure (Venkatesan and Jayaganesh 2010). Increased concentration of Mg2+ in cytosol blocked K+ channel across the membrane of chloroplasts, thereby inhibiting H+ ion removal from chloroplasts stroma, resulting in its acidification which cause oxidative damage to plant cells (Wu et al. 1991). Wilkinson and Ohki (1988) reported that accumulation of Mg in plants reduced total chlorophyll and carotenoids contents by altering pigment synthetic pathway. Amino acid contents in plant showed a significant decline under its elevated doses above 1000 ppm due to hindered amino acid transport pathway (Ma et al. 2005).
10.5.5 Manganese
Manganese is an essential micronutrient for plant’s growth and development but can be detrimental if available in excessive amounts in soil. There is no regulatory limit for Mn in agricultural soil, whereas for crop and medicinal plants, permissible limits are 500 ppm (FAO/WHO 2001) and 200 ppm (WHO 1998), respectively. Clark (1982) reported that excessive Mn in growth medium may interfere with the absorption, translocation and utilization of other minerals (Ca, Mg, Fe and P) by a plant which may lead to Mn toxicity (Table 10.2). Common phytotoxic symptoms are chlorosis (marginal and interveinal), necrotic brown spots as observed on leaves of Brassica sp., Lactuca sativa, H. vulgäre, G. hirsutum and Tagetes erecta (Bachman and Miller 1995; Albano et al. 1996; Führs et al. 2008). Kang and Fox (1980) reported loss of apical dominance and enhanced formation of auxiliary shoots (witches’ broom) in Vigna unguiculata as symptoms of Mn toxicity.
Maksimović et al. (2012) reported that Mn at 100 μM concentration caused significant reduction in root and shoot biomass of cucumber (Cucumis sativus) as compared to 0.5 μM dose of Mn in the growth medium. Similarly, marked reductions in the dry weight of plant and leaf area were observed in Oryza sativa, Lolium perenne and Populus sp. at 583, 150 and 1000 μM of Mn concentrations, respectively (Lidon and Teixeira 2000; Lei et al. 2007; Mora et al. 2009). Excess Mn in plant tissues may produce errors during the mitochondrial replication by inducing mitochondrial mutations and inhibiting total protein synthesis (Foy et al. 1978). In cotton, Mn toxicity has been associated with increase in the activities of indoleacetic acid oxidase, peroxidase and polyphenol oxidase and reduced catalase, ascorbic acid oxidase and glutathione oxidase activities with lower ATP content and respiration rate (Morgan et al. 1976). Furthermore, in rice (O. sativa) seedlings under Mn stress, superoxide radical was increased preferentially in roots, while H2O2 content was found to be increased in shoots.
10.5.6 Iron
Iron is an essential nutrient for all plants with significant biological role in chlorophyll biosynthesis and photosynthesis; also it is the most limiting nutrient for plant growth primarily due to low solubility of oxidized ferric form in aerobic environment (Guerinot and Yi 1994). No standard maximum allowable limit for Fe in soil has been recommended because of its abundance in mineral soil; however, the expression of Fe toxicity symptoms in leaf tissues may occur under flooded conditions due to reduction of the Fe3+ to Fe2+ (Becker and Asch 2005). Fe is an integral component of many enzymes and proteins including heme and iron sulphur proteins (Marschner 1995). For crop and medicinal plants, permissible limits of Fe are 425.5 ppm (FAO/WHO 2001) and 20 ppm (WHO 1998), respectively. Iron phytotoxicity occurs only when it reaches beyond a threshold level which is characterized by preliminary symptoms such as necrosis, chlorotic stippling, cupping of leaves, bronze speckle, stunted growth, weak stem and delayed flowering (Table 10.2).
Iron content in medicinal plants consumed in UAE ranged between 26.96 and 1046.25 mg kg−1 (Abou-Arab and Abou Donia 2000). High iron levels often cause Mn deficiency in plants because of their competitive behaviour. In wheat, root- and shoot dry weights were found to decrease at 100 ppm Fe concentration in soil (Fageria and Rabelo 1987). Inhibitory effects of elevated Fe concentration on root elongation and photosynthetic pigments in Sinapis alba were reported (Fargašová 2001). Kampfenkel et al. (1995) observed brown spots on the leaf surface of Nicotiana plumbaginifolia and 40.0 % reduction in photosynthetic rate due to foliar accumulation of Fe. Iron toxicity in soybean (Glycine max) caused reduction in photosynthesis rate and yield due to increase in oxidative stress and ascorbate peroxidase activity (Sinha et al. 1997). Excess Fe leads to free radical production which alters the cellular structure irreversibly and damages membranes, DNA and protein structures (de Dorlodot et al. 2005).
10.5.7 Cobalt
Cobalt is a transition element, essential for several enzymes and coenzymes participating in plant metabolism. The maximum allowable range for Co in agricultural soil is 20–50 ppm (Kabata-Pendias and Sadurski 2004), whereas, for crop plants, permissible limit is 50 ppm (FAO/WHO 2001). For medicinal plants, no permissible limit has been specified. Cobalt affects the growth and metabolism of plants by different degrees depending upon its concentration and form in the soil. Toxic effects of Co on morphology include leaf fall, inhibition of greening, discoloured veins, premature leaf closure and reduced shoot weight. The supranormal doses of Co in plants have relatively high toxic effects which are mostly reflected in growth inhibition of plants accompanied by chlorosis of young leaves and other disorders (Table 10.2).
Phytotoxicity study of Co on crop plants such as barley (Hordeum vulgare), oilseed rape (B. napus) and tomato has shown reductions in shoot growth and biomass (Li et al. 2009). The higher foliar concentration of Co leads to lowering of essential mineral nutrients and photosynthesis rate and disturbance in the structural integrity of chloroplasts. High Co concentration (500 ppm) was found to reduce germination percentage and seedling growth of T. aestivum with 97.0 and 83.0 % reductions in root and shoot length, respectively (Gang et al. 2013). Chatterjee and Chatterjee (2000) reported that excess Co in cauliflower (Brassica oleracea) restricted the foliar uptake of Fe, P, S, Mn, Zn and Cu, altered the biosynthesis of chlorophyll and protein and reduced the catalase activity. Water potential and transpiration rate in cauliflower were increased significantly, while diffusive resistance and relative water content increased upon exposure to excess Co (Chatterjee and Chatterjee 2000). Palit et al. (1994) observed that Co affects photosystem (PS-II) by inhibiting either the reaction centre or components of PS-II. Moreover, in C4 and CAM plants, Co hindered the fixation of CO2 by inhibiting the activities of photosynthetic enzymes. Cobalt acts as a preprophase poison and thus retards the process of karyokinesis and cytokinesis, and higher concentrations of Co may hamper RNA synthesis and decrease DNA and RNA contents probably by modifying the activities of endo- and exonucleases (Palit et al. 1994).
10.5.8 Nickel
Nickel is an essential micronutrient required for plant normal functioning, but due to industrial activities, extent of soil contamination with Ni is so high that in some areas it is causing serious damages to agricultural crops (Frank et al. 1982). For agricultural soil, maximum allowable range of Ni is 20–60 ppm (Kabata-Pendias and Sadurski 2004), whereas for crop and medicinal plants, permissible limits are 67 ppm (FAO/WHO 2001) and 1.5 ppm (WHO 2005), respectively. Necrosis, chlorosis, inhibition of seed germination, reduced root and shoot growth, poorly developed branches, deformed plant parts and abnormal flowering are the common symptoms when foliar Ni concentration exceeds its phytotoxic threshold level (Table 10.2).
Ahmad et al. (2011) reported significant reductions in plant biomass, achene yield and foliar concentrations of essential nutrients (Mn, Zn, Cu and Fe) in sunflower (H. annuus), under high Ni concentration. Excessive Ni accumulation in crop and medicinal plants are reported to inhibit photosynthesis and transpiration rates (Sheoran et al. 1990). Progressive impairment of photosynthetic machinery coupled with oxidative damages in Amaranthus paniculatus was observed with increasing Ni treatment in the solution (Pietrini et al. 2015). Nickel concentration ranging from 0.01 to 10 ppm dry weight is considered essential for plant metabolism, regulation of lipid content and as an important constituent of enzymes such as urease, hydrogenase, superoxide dismutase (SOD) and glyoxalases (Küpper and Kroneck 2007). Gajewska et al. (2006) found significant reductions in wheat growth and proline accumulation along with significant decline in SOD and CAT activities at 200 μM Ni concentration. Molas (1998) reported significant decreases in number and size of chloroplasts, grana, thylakoids and plasto globuli in leaves of B. oleracea grown in soil containing NiSO4.7H2O (10–20 g m−3).
10.5.9 Copper
It is an essential trace element for all lower as well as higher plants with several roles in metabolic processes (Narula et al. 2005), but its increased concentration may produce toxic effects on plants (Mittler et al. 2004). The maximum allowable range of Cu for agricultural soil is 60–150 ppm (Kabata‐Pendias and Sadurski 2004), whereas permissible limits for crop and medicinal plants are 73.30 ppm (FAO/WHO 2001) and 10 ppm (WHO 2005), respectively. Chlorosis, necrosis, purple coloration of midrib and reduction in plant growth are common symptoms observed due to Cu phytotoxicity (Table 10.2).
Excessive accumulation of Cu in roots caused root system damage (Atanassova and Zapryanova 2009), photosynthetic inhibition and plasma membrane permeability damage (Narula et al. 2005). Khatun et al. (2008) reported reductions in plant growth parameters, biomass and pigment contents in Withania somnifera above 10 μM of CuSO4.5H20 solution, whereas significant decreases in root and shoot biomass were observed in Z. mays treated with 10 μM of Cu. In Solanum nigrum, relative fresh weight, number of leaves, root and shoot lengths were reduced with increase in CuSO4 level from 50 to 200 μM (Al-Khateeb and Al-Qwasemeh 2014). In barley leaves, Cu inhibited pigment synthesis and retarded chlorophyll integration into photosystems (Caspi et al. 1999). The reduction in pigment contents was attributed to the interaction of Cu to –SH group of enzymes during chlorophyll biosynthesis (Nyitrai et al. 2003). High level of Cu interferes with protein formation, photosynthetic processes and enzyme activities and alters plasma membrane permeability (Al-Khateeb and Al-Qwasemeh 2014). Ouzounidou et al. (1997) reported that Cu affects the ultrastructure of meristematic cells, altering the ribosomal RNA precursor biosynthesis and thus reducing the wheat growth.
10.5.10 Zinc
It is an important constituent of metalloenzyme and acts as a cofactor for several enzymes including anhydrases, dehydrogenases, oxidases and peroxidases (Hewitt 1983). It also plays an important role in regulating the nitrogen metabolism, cell multiplication, auxin synthesis and photosynthesis (Doncheva et al. 2001). The maximum allowable range of Zn for agricultural soil is 100–300 ppm (Kabata-Pendias and Sadurski 2004), whereas for crop and medicinal plants, permissible limits are 99.40 ppm (FAO/WHO 2001) and 50 ppm (WHO 1998), respectively. General symptoms of Zn phytotoxicity when its concentration exceeds the threshold level are chlorosis, necrosis, wilting, purplish colour patches, stunting of shoot, curling and rolling of young leaves and death of leaf tips (Table 10.2).
Zn toxicity is reported to cause nutrient (Fe, Mn and Cu) deficiencies in shoot due to hindered transference of these nutrients from root to shoot of geranium (Lee et al. 1996). Zinc accumulates to a greater extent in roots than in shoot and hence interferes with root growth and elongation and thereby limits plant’s uptake of water and nutrients (Disante et al. 2010). White et al. (1979) reported a 50.0 % reduction in soybean biomass at 450 ppm Zn concentration, whereas 620–860 ppm of Zn caused ≈ 20 % reduction in foliar biomass of trifoliate leaves. Disintegration of cell organelles, disruption of membranes, condensation of chromatin material and increase in number of nucleoli were major events observed in pigeon pea (Cajanus cajan) during Zn toxicity (Sresty and Rao 1999).
10.5.11 Titanium
Although titanium is present in the soil in relatively higher concentrations, majority of Ti is poorly available for plants, due to the insoluble nature of the form of minerals (TiO2 or FeTiO3) in water (Dumon and Ernst 1988). Lower concentration of Ti might participate in plant metabolism as a redox catalyst and has a significant biological role in plant functioning, but at higher concentrations, deleterious effects on plant performances such as reduction in growth, yield and nutrient uptake of Zn, Mg and Fe, alteration in normal physiological functioning and chromosomal aberration with observed phytotoxic symptoms are reported (Geilmann 1920) (Table 10.2).
Burke et al. (2015) reported no negative effects of TiO2 at low concentrations, but had strong negative effects on plant growth such as reduced root growth and elongation under elevated Ti concentration in crop and medicinal plants (Boonyanitipong et al. 2011). Phytotoxic effects on oat biomass at the concentration of 18 ppm Ti in nutrient solution were observed by Kužel et al. (2003). Yield in terms of grain weight in barley was reduced under foliar spray of 18 ppm Ti solution (Tlustoš et al 2005). The length of the petiole of strawberries was reduced, and hardness of fruits was increased under increasing applications of Ti (50, 100 or 150 mg kg−1) (Choi et al. 2015). Feizi et al (2012) found reductions in plant growth and leaf carbon content in soybean plants under TiO2 treatments. Also, the hydraulic conductivity of cell wall and diameter of root cell wall pores were reduced from 6.6 to 3.0 nm. Ghosh et al. (2010) found negative effects of Ti on plant growth, cell elongation and transpiration. Pakrashi et al. (2014) showed that TiO2 nanoparticle is capable of inducing genotoxicity in plants even at a low concentration (12.5 μg mL−1) due to internalization of particles, resulting in oxidative stress due to ROS generation which can damage cell structures and DNA. A dose-dependent decrease in the mitotic index (69 to 21) and increase in chromosomal aberrations, DNA damage and ROS generation were observed in onion (Allium cepa) root tips treated with Ti nanoparticles at four different concentrations (12.5, 25, 50, 100 μg mL−1) (Pakrashi et al. 2014).
10.5.12 Lead
Lead is one of the most abundant toxic elements in soil with the half-life of 740–5900 years (Iimura et al. 1977). Its allowable range in agricultural soil is 20–300 ppm (Kabata‐Pendias and Sadurski 2004), whereas permissible limits for crop and medicinal plants are 0.3 ppm (FAO/WHO 2001) and 10 ppm (WHO 1998), respectively. General symptoms due to Pb toxicity in plants are chlorosis, necrosis, inhibition of root growth, blackening of root system and underdeveloped shoot (Table 10.2).
Increase in treatments of Pb from 0 to 3 mM in aqueous solution is known to reduced seed germination and biomass in T. aestivum, with 50.0 % reduction in their values at 3 mM of Pb (Lamhamdi et al. 2011). Inhibition of seedling growth under high Pb levels was reported in rice (Mishra and Choudhuri 1999), maize (Małkowski et al. 2002) and medicinal plants (Street et al. 2007). In Sinapis arvensis, Pb at 1500 μM reduced seed germination and plant biomass by 10.23 and 23.0 %, respectively, whereas Pb treatments beyond 400 μM showed more than 50.0 % reduction in biomass as well as water content of B. juncea (Zaier et al. 2010). Lead accumulation beyond its permissible level caused inhibition of leaf expansion, root and stem elongation in A. cepa (Gruenhage and Jager 1985) and H. vulgare (Juwarkar and Sinde 1986). Low amount of Pb (0.005 ppm) has also been reported to cause significant reduction in the growth of lettuce (L. sativa) and carrot (Daucus carota) roots, primarily due to Pb-induced simulation of indol-3 acetic acid (IAA) oxidation (Barker 1972). Toxicity due to Pb alters photosynthetic and enzymatic activities, water balance and mineral uptake by the plant, which affects their normal physiological functioning (Sharma and Dubey 2005). A high Pb level in soil induced abnormal plant morphology such as irregular radial thickening in pea (Pisum sativum) roots, cell walls of the endodermis and lignification of the cortical parenchyma (Paivoke 1983). High Pb concentrations inhibited the activities of enzymes at cellular level by reacting with their sulfhydryl groups and induced oxidative stress by increasing the production of ROS in plants (Reddy et al. 2005).
10.5.13 Cadmium
Ranked seventh amongst top toxins affecting environment and living beings, Cd is of major environmental concern to agriculture system in the vicinity of industries because of its longer residence period (>1000 years) in soil (Nazar et al. 2012). Although Cd is a non-essential metal for medicinal and crop plants, it is an extremely significant pollutant due to its high toxicity and large solubility in water, resulting in an easy uptake by plants when grown in soil either supplemented or contaminated with Cd. For uncontaminated or agricultural soil, allowable range of Cd is 1–5 ppm (Kabata-Pendias and Sadurski 2004), whereas for crop and medicinal plants, permissible limits are 0.2 ppm (FAO/WHO 2001) and 0.3 ppm (WHO 2005), respectively. Cadmium, beyond its phytotoxic thresholds for crop and medicinal plants cause chlorosis, growth inhibition, leaf rolls and stunting, browning of root tips, biomass reduction and finally death which are the main and easily visible symptoms of Cd toxicity when grown in soil containing high levels of Cd (Table 10.2).
Accumulation of Cd in edible plants may cause several physiological, biochemical and structural changes (Feng et al. 2010). In Rhazya stricta, a traditional medicine used in treatment of diabetes mellitus, skin infections and stomach disorders, total concentration of Cd was found 9.63 ppm which caused chlorosis and growth reduction of the plant (Nawab et al. 2015b). In C. sativus, Cd at 5 M concentration or higher induced Fe(II) deficiency by inhibiting root Fe(III) reductase, which affects photosynthesis (Alcantara et al. 1994). Cd caused alteration in uptake and transport of essential nutrients by plants either by affecting the availability of minerals from the soil or by reducing the soil microbial population (Moreno et al. 1999). Cd toxicity can affect the plasma membrane permeability thereby altering water balance and stomatal opening in mung bean (Hossain et al. 2010). Fodor et al. (1995) observed reduced ATPase activity in roots of wheat and sunflower and altered membrane functionality inducing lipid peroxidation at higher Cd levels. Cd accumulation at 200 μM reduced nitrogen fixation and primary ammonia assimilation in nodules of soybean plants (Chikile et al. 2013), while 100 mM Cd uptake by mustard and soybean plants inhibited nitrate reductase activity (Balestrasse et al. 2003).
10.5.14 Bismuth
Bismuth exists in number of oxidation states but its trivalent forms (bismuth, bismuthinite and bismite) are most stable, abundant and rarely occur alone (Das et al. 2006). Very little is known about the phytotoxic effects of Bi on crop and medicinal plants due to less information on biocoordination chemistry of Bi(III) with proteins, enzymes and cell membranes. Galindo et al. (1999) observed an inhibition of radical growth due to heterocyclic Bi compounds with necrotic effects on foliage of lettuce and cucumber. Similar effects were observed on Sorghum bicolor by Rimando et al. (1998). Seed germination was inhibited by 50 % in lettuce at 16.1, 34.0 and 49.7 \( \mu M \) of different Bi compounds, whereas it was 4.6, 7.5 and 12.4 \( \mu M \) in Trifolium pratense. Cespedes et al. (2003) reported an inhibition of hypocotyl growth of L. sativa and T. pratense beyond 15 \( \mu M \) of Bi treatment. Nagata (2015) showed higher germination rate, total dry weight and root length of Arabidopsis thaliana under low concentration of Bi; however, these parameters reduced significantly above 1.0 \( \mu M \) Bi concentrations.
10.5.15 Arsenic
Arsenic is the most toxic metalloid widely distributed in environment as As(III) and As(V) which are ubiquitous and toxic to many life forms (Tripathi et al. 2007). The range of maximum allowable concentration of As for agricultural soil is 15–20 ppm (Kabata‐Pendias and Sadurski 2004), whereas for crop and medicinal plants, permissible limits are 1.0 ppm (WHO 1992) and 5.0 ppm (WHO 1998), respectively. Growth reduction, interveinal necrosis and chlorosis have been reported as easily visible symptoms due to As phytotoxicity (Table 10.2).
Plants exposed to As undergo severe stress such as growth inhibition, improper physiological functioning and finally leading to death (Stoeva et al. 2005). It is an analogue of phosphate (P) and transported across the plasmalemma through phosphate transport systems (Smith et al. 2010). Liu et al. (2005) reported a significant decline in seed germination, biomass production and grain yield with an increase in As concentrations (0–16 mgL−1) in growing medium for six varieties of T. aestivum. The straight head disease is a physiological disorder of O. sativa due to As toxicity characterized by sterile florets/spikelets leading to reduced grain yield (Smith et al. 2010). Mazumdar et al. (2015) showed significant reductions in shoot length (34.6 %), shoot biomass (27.0 %) and essential oil yield (0.08 %) in Ocimum basilicum under As stress. Arsenic accumulation in plants caused chloroplast membrane damage, reduced stomatal conductance and interfered with water and essential nutrient uptake by plant leading to improper functioning of photosynthetic process (Stoeva and Bineva 2003) and altered plant metabolic activities (Mokgalaka-Matlala et al. 2008). Elevated levels of As in plants thus cause considerable cellular damage through lipid peroxidation and protein and deoxyribonucleic acid damage (Pitzschke et al. 2006). In thylakoids, As may create a condition where energy level exceeds the amounts that can be dissipated by metabolic pathways of chloroplasts; as a result the electron transport system in the thylakoid membranes is impeded, and toxic symptoms develop (Stoeva et al. 2003).
10.5.16 Aluminium
Aluminium is a metalloid contributing about 7.0 % of the earth’s crust and exists in non-reactive state and produces no toxic effects on plants. However, in acidic environment, Al turns to soluble forms and readily uptake by plants thus producing phytotoxic effects with certain visible symptoms such as chlorosis, yellowing, curling or rolling of young leaves and stunted growth (Table 10.2). Aluminium toxicity has been considered as a main limiting factor in crop production due to inhibition of root growth and metabolic alteration in plant cells (Inostroza-Blancheteau et al. 2012). Significant reductions in fresh weights of cotyledons, hypocotyl and radicles were observed in J. curcas at 2 and 3 mM of Al treatments (Ou-yang et al. 2014). It alters morphology of root cells, resulting in thick, stunted, brittle and poorly developed root system thereby affecting nutrient and water uptake (Matsumoto 2000). Net photosynthetic rate, transpiration rate and stomatal conductance reduced significantly under Al stress which could be attributed to significant reductions in length, width and area of stomata in leaves of Scutellaria baicalensis (YaMin et al. 2011). Greatest cell damage and ROS generation were found in the distal transition zone in roots of Z. mays and S. bicolor (Sivaguru et al. 2013). Based on previous report, cell wall is considered a major site of Al accumulation with 85–90 % of total Al accumulation roots of H. vulgare (Zhu et al. 2013). Higher hemicellulose content was found in wheat on exposure to 10 μM Al for 6 h (Tabuchi and Matsumoto 2001). Aluminium ions possess higher affinity (560-folds) for phosphatidylcholine by replacing Ca2+resulting in inhibition of H+-ATPase activity, alteration of membrane fluidity and phospholipid packing (Ahn et al. 2001). Binding of Al to nuclear materials results in their condensation and inhibition of cell division, nuclear aberration and micronuclear and binuclear cells in H. vulgare (Zhang 1995).
10.5.17 Molybdenum
Molybdenum is a transition element, which exists in several oxidation states ranging from zero to VI. Mo(VI) form is most commonly found in agricultural soils and is essential for growth of plants (Bergeaux 1976). The maximum allowable range of Mo for agricultural soil is 4–10 ppm (Kabata-Pendias and Sadurski 2004), whereas no permissible limits are specified for crop and medicinal plants. Importance of Mo in growth and development of higher plants was first shown by Arnon and Stout (1939). Though required only in small amounts, it has a large role within the plant system. Molybdenum itself is not biologically active but is rather predominantly found to be an integral part of an organic pterin complex called the molybdenum cofactor (MoCo) (Mendel and Hansch 2002). Brenchley (1948) reported that heavier dressing of molybdate in soil suppressed the growth of plants with an appearance of golden colour toxic symptoms due to molybdenum poisoning. Mo toxicity leads to marginal leaf scorch, abscission, yellowing and browning of leaves and depressed tillering (Table 10.2).
For barley and oats, toxic effects of Mo were observed when it reached 135 and 200 ppm, respectively (Davis et al. 1978; Hunter and Vergnano 1953). Significant reductions in maize seedling growth (Kovács et al. 2015) and grain yield of wheat and barley (Gupta 1971) were observed in soil containing excessive levels of Mo. Foliar application of 40 g Mo ha−1 at 25 days after plant emergence resulted in higher reduction in acetylene and nitrate reductase activities in bean (Vieira et al. 1998).
10.5.18 Chromium
This is one of the most common contaminant in soil, water and sediments mainly due to industrial activities. Amongst its different valance states, Cr (III) and Cr (VI) are most stable and common in terrestrial environment (Kimbrough et al. 1999). In agricultural soil, 20–500 ppm is a maximum allowable range of Cr (Kabata-Pendias and Sadurski 2004), whereas permissible limits set by WHO are 2.3 and 1.5 ppm for crop (FAO/WHO 2001) and medicinal plants (WHO 1998), respectively. It is a non-essential element which produces toxic effects on plant’s growth and development as its concentrations reaches beyond the phytotoxic threshold. Phytotoxic effects of Cr are characterized by reduced plant growth and chlorosis in young leaves followed by wilting.
Seed germination is a first physiological process affected by Cr (Peralta et al. 2001). Reductions of 51.1 and 57 % in seed germination of Hibiscus esculentus (Amin et al. 2013) and sugarcane (Saccharum officinarum) bud germination (Jain et al. 2000) were observed under 100 and 80 ppm Cr treatments, respectively. Similarly, seed germination and total plant biomass in different cultivars of T. aestivum showed significant reductions with increase in concentrations of Cr from 0 to 125 ppm (Datta et al. 2011). Reduced seed germination under Cr stress could be ascribed to inhibition of amylases and enhancement in protease activities (Zeid 2001). Elevated Cr accumulation by Vetiveria zizanoides caused reductions in root length, biomass and essential oil yield (Prasad et al. 2014). Chromium stress affects photosynthesis in terms of CO2 fixation, electron transport, photophosphorylation and enzyme activities (Clijsters and Van Assche 1985). Higher Cr exposure to plants may disrupt the defence mechanism by inactivating enzymatic and non-enzymatic antioxidants (Gwóźdź et al. 1997). Chromium stress alters normal plant metabolism by altering pigment’s production such as chlorophyll and anthocyanin (Boonyapookana et al. 2002), elevating production of glutathione and ascorbic acid (Shanker et al. 2005) and by altering metabolic pool to channelize the production of new metabolites, which either exhibit resistance or tolerance to Cr stress (Schmfger 2001).
10.5.19 Mercury
Mercury is a rare element, ubiquitously distributed in the environment in trace amounts in two ionic forms Hg2+ and Hg+ amongst which Hg2+ is highly water-soluble and strongly phytotoxic (Goldwater 1971). In agricultural soil, maximum allowable range of Hg is 0.5–5 ppm (Kabata-Pendias and Sadurski 2004), whereas for crop and medicinal plants, its permissible limits are 0.3 (FAO/WHO 2001) and 0.2 ppm (WHO 1998), respectively. Above permissible limit, it produces toxic effects on human health through crop and medicinal plants leading to contamination of food chain and causing disease such as “Minamata”. Concentrations of Hg beyond its phytotoxic levels can induce visible injuries such as chlorosis and reduction in growth and yield (Table 10.2).
Seed germination of bean showed a significant reduction when exposed to 2-methoxy ethyl mercuric chloride and mercurous chloride (Semu et al 1985). Use of mercury-based pesticide treatments in agricultural fields caused damaging effects on wheat crops characterized by hypertrophy of roots and coleoptile of seedlings, inhibition of cell division in the apical meristem of plumule and extreme cell enlargement of existing cells (Purdy 1956). These adverse effects of Hg on seed germination and growth could be attributed to Hg interference with –SH system in living cells (Sass 1937). Exposure of green gram (Vigna radiata) to Hg caused reductions in its biomass by 95.0 % with significant inhibition of α- amylase activity (Varshney 1990). Godbold (1991) reported significant reductions in K, Mg and Mn contents in root due to Hg induced Fe and Ca accumulation. Substitution of Hg in central atom of chlorophyll is a damaging mechanism thus affecting light and dark photosynthesis (Krupa and Baszymski 1995). An elevated level of Hg uptake by plants can bind to water channel proteins, thus inducing leaf stomatal closure and physical obstruction in water flow. Also, anatomical distortion in root and stem structures was observed at 2.0 mM HgCl2 treatment to T. aestivum (Zhang and Tyerman 1999). Setia et al. (1994) reported reductions in cell sizes, cell wall thickness and number of vascular bundles in plants. Vijay et al. (1988) showed marked inhibitions in amino-transferase and β-amylase activities in H. vulgare at 50 ppm Hg treatment. Similarly, Mahajan and Dua (1993) showed a significant inhibition in endo- β-1-3-glucanase activity in Brassica campestris due to Hg toxicity. Somatic mutation, inhibition in spindle formation during cell division and chromosomal aberration were observed in monocot as well as dicot plants under Hg stress (De Flora et al. 1994). A high level of Hg interferes with mitochondrial activity and induced oxidative stress by triggering ROS generation which led to disruption of biomembrane lipids and cellular metabolism in cucumber seedlings (Cargnelutti et al. 2006).
10.5.20 Silicon
Silicon is the second most abundant element after oxygen both on the surface of the earth’s crust and in soils in the form of silicic acid at concentrations normally ranging from 0.1 to 0.6 mM (Epstein 1999). Although, Si is not recognized as an essential element for plant but its beneficial effects on the growth, development, yield and disease resistance in many crop plants such as maize, rice and some cyperaceous plants have been observed within certain limits (Liang et al. 2005; Ma and Yamaji 2006).
Like all the metals we discussed above, silica compounds when present in excess start interfering with the plant’s normal development and defence mechanisms. Côté-Beaulieu et al. (2009) observed yellow streaks on foliage and brittle leaves of wheat treated with monomethyl and dimethyl silicic acid followed by a stunted growth after 10 days of treatments (Table 10.2). Dimethyl silicic acid at 0.1 mM concentration was found sufficient to reduce growth and development of wheat plant (Côté-Beaulieu et al. 2009). Reduction in length of third and second leaves of rice and wheat plants, respectively, was observed at 20 mM of silicic acid which could be due to the formation of polymer and then changes to gel at higher concentration of Si resulting in later period of Si deficiency (Hossain et al. 2002). It has been reported that Si promotes cell wall extensibility in the growing zone and decreased the cell wall extensibility in the basal zone of isolated stellar tissues in the roots of S. bicolor, implying that Si plays a significant role in enhancing root elongation and protecting the stele by hardening the cell wall of the stele and endodermal tissues (Hattori et al. 2003). Similar observation was made in roots of rice plant by Hossain et al. (2002).
10.6 Alteration in Plant Community Structure in Response to Metals from Mining and Metallurgical Industries
During mining and metallurgical activities, significant land areas are degraded, and existing habitats are replaced by solid wastes such as tailings, slags, red mud and sludge that contain several metals. Soil is the main terrestrial sink for such toxic and persistent industrial pollutants, and it cause alteration in the vegetation structure and physico-chemical properties of the soil (Adriano 2001). These activities are directly responsible for destruction of vegetation cover and deterioration of soil quality (Conesa et al. 2011).
Major visible symptoms of environmental stress due to industrial activities are changes in vegetation structure which also variably or invariably alters animal communities and threatens the natural biodiversity in the area. Hence, research on changes in vegetation pattern, structure of plant communities and their dynamics are useful in assessing the degree of environmental contamination and degradation. Dutta and Agrawal (2003) observed the plant growth performance, biomass accumulation and net primary productivity (NPP) of some exotic species on wasteland of coal mining area and observed significant biochemical responses in Eucalyptus hybrid and Acacia auriculiformis, whereas toxic components of mine spoils absorbed through the roots of Cassia siamea resulted in its reduced above-ground biomass. Impact of mining on plant communities in the district of Villa de la Paz was studied by Espinosa-Reyes et al. (2014) where they observed that the plants in the proximity of 0.3 km from mining industry were characterized by lower diversity with species richness of 13 compared to the reference site (10 km from mining industry). The most polluted sites were dominated by plant species such as Parthenium incanum, Larrea tridentata, Zaluzania triloba, Jatropha dioica, Dyssodia acerosa, Zinnia acerosa and Bahia absinthifolia (Espinosa-Reyes et al. 2014). Pandey et al. (2014) showed the effects of coal mining activities on plant community structure where minimum numbers of herbaceous species (19) were found in both Raniganj and Jharia coalfields compared to the reference site (Central Institute of Mining and Fuel Research (CIMFR)). Both the coalfields were dominated by Alternanthera paronychioides and Cynodon dactylon, whereas Achyranthes aspera, Convolvulus sp., Dichanthium sp., Eclipta alba and Solanum sp. were the most sensitive species present only at reference site and completely vanished from coal mining areas. Eragrostis cynosuroides and Setaria glauca were identified as polluphilic species only found at coal fields. Pandey et al. (2014) also observed a significant reduction in numbers of woody species in coal fields, while Butea monosperma, Ficus benghalensis, Ficus religiosa and Psidium guajava were dominant species. Moreover, canonical correspondence analysis of the study revealed that main drivers of herbaceous community structure in mining affected areas were soil total organic carbon and nitrogen, whereas woody layer community was influenced mainly by soil sulphate and phosphorus contents. The changes in species diversity indicated an increase in proportion of resistant herbs and grasses in response to altered soil characteristics due to mining activities (Pandey et al. 2014). Morrey et al. (1988) performed an analysis of vegetation composition in relation to physico-chemical variation in soil near metalliferous mining industry and found that soil pH was a main driving component in determining the species distribution affecting 51.0 % of floristic variation, whereas 43.8, 19.7 and 44.6 % of floristic variations were influenced by soil concentrations of phytoavailable Zn, Ca and Pb, respectively.
Koptsik et al. (2003) reported reductions in number, height, diameter at breast height and crown density of living trees, whereas number of standing dead increased with decrease in distance from Zn-Cu smelter in the Kola Peninsula, Russia. Moreover, reductions in the number of plant species from 13 to 5 per 100 m2, plant cover from 100 to 20 % and total plant biomass from 1.0 to 0.15 kg m−2 were found at highly contaminated sites near to Zn-Cu smelter compared to reference site. Narayan et al. (1994) assessed the vegetation characteristics at different distances from HINDALCO Industries Ltd., Renukoot, an important aluminium smelter in India. It was found that important value index (IVI) of sensitive species such as Achyranthes aspera, Cassia tora and Eclipta alba decreased and those of tolerant species such as Alternanthera sp., Cynodon sp., Cyprus sp. and Sida sp. increased with decrease in distance from the industry. Species richness and Shannon-Wiener index though increased, while concentration of dominance reduced on moving from 1 to 11 km from the industrial premise. A quadrat study of vegetation cover was carried out by Remon et al. (2005) at solid waste dumps from the iron and steel industry at Firminy, Loire, France. It was found that 30 plant species belonging to 11 families were present, and most of these species were perennial forbs and grasses from family Asteraceae and Poaceae. Despite of the taxonomic diversity at that site, the vegetation cover was not uniform, and, inside each quadrant, the covered ground surface, the number of taxons and the type of dominant species were highly variable (Remon et al. 2005).
10.7 Risk of Food Chain Contamination
Soil contamination by anthropogenic activities results in multiparametric consequences on the quality of living beings. Disposal of residues from these industries has resulted in contamination of surrounding areas thus converting them into land not suitable for agricultural practice. The productivity of crop and medicinal plants growing in contaminated agricultural soil or cultivation of these plants in soil amended with industrial wastes can be reduced due to elevated metal uptake (Alloway et al. 1990; Pruvot et al. 2006). Solid waste dumps which are naturally invaded by endemic species pose a potential threat of transfer of toxic metals into food chain through the accumulation of metals in above-ground plant parts. Amongst plants with special reference to high added values are medicinal plants which are commonly consumed in countries like Greece and some Mediterranean regions which are collected from contaminated sites (Pullaiah 2006). This raises a question of how safe it is for consumption of crop and medicinal plants collected from such contaminated areas (Fig. 10.5). Cultivation of edible plants mainly crop and vegetables for human or livestock consumption on contaminated sites can potentially lead to uptake, accumulation and biomagnification of toxic metals such as Cd, Pb, Hg, As, Cr, etc. with a resultant risk to human and animal health leading to serious systemic health issues (Gautam et al. 2016; Sharma and Agrawal 2005).
Zhuang et al. (2009) reported that in Asia’s staple crop “rice”, cultivated in the vicinity of Dabaoshan Mine, it contained many folds higher Pb (8-folds) and Cd (6.5-folds) concentrations in comparison to their maximum permissible limit (MPL) as per national safety standard for milled rice (NPSF 2002). Similarly, in corn grains grown in the vicinity of Pb-Zn mine, Liaoning, concentrations of Cd and Pb were found 1.5 and 2 times higher than their MPL, respectively (Gu et al. 2005). Concentration of Cd was assessed in leafy vegetables grown in a village near Dabaoshan Mine where it was found 2.5 times higher than its MPL in spinach and 3.8 times of MPL in carrot (Zhuang et al. 2009; Hernandez et al. 2003). Ok et al. (2011) reported 0.90 ppm of Cd accumulation in rice grain, grown in metal-contaminated paddy field in the vicinity of an abandoned metal mine in South Korea. Bose and Bhattacharyya (2012) found significantly higher concentration of Zn, an essential micronutrient in wheat grain grown in JNU and Chattarpur soil amended with industrial waste. In Bulgaria, large areas of agricultural land in the vicinity of Zn-Cu smelter (0.8–3 km) are contaminated with heavy metals resulting in contamination of medicinal plants with several times higher concentrations of Cd, Pb and Zn than their allowable limits. Also, essential oil yields from sage, basil, dill, cham, coriander, lemon balm and hyssop plants were increased with increase in distance from the source (Zheljazkov et al. 2008). Similarly, Angelova et al. (2006) showed that concentrations of Pb, Cu, Zn and Cd in root, stem and leaves were manyfolds higher in Mentha piperita, Salvia officinalis and Salvia sclera grown at 0.1 km from non-ferrous metal industry near Plovdiv, Bulgaria, compared to reference site (15 km from the industrial premise). Linalool content in volatile oil of sweet basil was found to reduce under concentrations of Cr (10 and 20 ppm), Cd (25 and 50 ppm), Pb (25 and 50 ppm) and Ni (25 and 50 ppm) compared to uncontaminated soil (Prasad et al. 2011). Affholder et al. (2013) also assessed the effects of heavy metals on Rosmarinus officinalis growing in the vicinity of the former metallurgical industry (1851–1952) compared to the reference site (a suburban area) and found that of the total volatile compounds, more than 50.0 % of compounds in essential oil were reduced significantly in plants grown at contaminated site. On contrary to this, 15 compounds including alpha pinene, camphene, myrcene, limonene, etc. were increased in plants at contaminated soil.
It is clear from the studies that metal accumulation not only cause quantitative and qualitative changes in yield of economically important plants but also affect their nutritional qualities. Edible parts from crop plants grown in the vicinity of industry often contain toxic levels of potentially toxic metals, whereas in case of medicinal plants, essential oil yield and composition were found less or unaffected by metal contaminants (Zheljazkov et al. 2008).
10.8 Technological Innovations in Management of Mining and Metallurgical Solid Wastes
In recent years, almost every country is facing the challenge of managing the huge quantity of wastes generated from mining and metallurgical industries because of their accumulation and suitable storage space constraints. Therefore, the wastes are usually dumped on land, either in wet or dry forms without proper pretreatments which occupy larger land areas leading to various environmental problems within and surrounding areas. Disposal of such a huge quantity of waste poses a big challenge because of lack of their cost-effective management practices. Several technological innovations on the applications of mining and metallurgical wastes have been suggested such as manufacture of ceramics, building materials, pigments, paints, adsorbents and catalysts (Pappu et al. 2007; Wang et al. 2008).
Mining wastes such as Fe, Cu, Zn and Al tailings, coal washeries and overburden wastes are used as raw materials in the recovery of expensive minerals and manufacture of construction materials for embankments of roadways, railways, rivers, dams, bricks, tiles, lightweight aggregates and fuel (Skarżyńska1995). The Pb-Zn tailings from upper Mississippi Valley mining district were used to prepare foamed building blocks, concrete beams and tiles, dense silicate bricks and aerated concrete (Hansen et al. 1968). Dean et al. (1986) also reported the utilization of Cu mill tailings for making building bricks. In the USA, Canada and Britain, mine wastes are used in manufacturing glasses and ceramics (Jacobi 1975). In India also, mine wastes are utilized in manufacture of glasses (IBM 2002; Kumar 2000). Metallurgical wastes such as slags, red mud and galvanizing residues are used in making cement, bricks, tiles, ceramics, blocks, paints and boards (Skarżyńska 1995). Slags from non-ferrous metal industries are used in improving the strength, morphology and abrasion resistance of cement, whereas ferroalloy industrial wastes are used in making high-strength and lightweight concrete (Bhattacharya et al. 2004). Gorai and Jana (2003) reported the use of Cu slags in preparing tiles, mine backfill and granular materials. Similarly, red mud from aluminium industries has been utilized as a substitute for ordinary clay for producing bricks, polymer, composites, wood substitute products, ceramic glazes and in metal recovery (Sglavo et al. 2000; Saxena and Mishra 2004).
The engineered techniques in management of industrial wastes are although advanced and highly efficient but are still at their initial stages of development. Particularly in developing countries like India, these technologies do not offer a cost-effective option at the moment. Conventional mechanical or physico-chemical treatments such as excavation, soil washing, solidification/stabilization, electrokinetic remediation and soil incineration also suffer from limitations like cost ineffective, require intensive labour, cause irreversible soil disturbances, etc. Therefore, rehabilitation of industrial waste dumps by revegetation is an environmentally benign process to safeguard the environment.
The approach of ecological restoration is the most accepted and cost-effective way to restore the ecological integrity of disturbed land due to mining and metallurgical activities. The goal of restoration is usually to develop a long-term sustainable management of residual dumps in industrial areas. It includes the management of all types of physical, chemical and biological disturbances in soils such as soil pH, fertility, microbial community and various soil nutrient cycles. Revegetation and reclamation of waste dumps are extremely difficult, due to physical or chemical limitations to plant growth and presence of potentially toxic concentrations of heavy metals in the spoil (Conesa et al. 2011). Such constraints can be resolved by adding suitable soil amenders such as sawdust, wood residues, sewage sludge and animal manures, as these amendments stimulate the microbial activities and add up nutrients (N, P, K) and organic carbon to the soil thereby reducing the phytotoxic effects of metals (Juwarkar and Jambhulkar 2008). Suitable soil amenders or microbial assisted revegetation minimizes the damages and helps in recovering the waste dumps by stabilization through development of extensive root systems. Once vegetation gets established on waste dumps, it improves soil organic matter and nutrient status, lower soil bulk density, moderate soil pH and enhances nutrient bioavailability in soil (Conesa et al. 2007; Mendez and Maier 2008). For revegetation, it is necessary to choose drought-resistant, metal-tolerant, fast-growing plants with dense canopies and root systems (Mendez and Maier 2008). Dutta and Agrawal (2002) suggested that indigenous plants should be preferred over exotic species for reclamation of the coal mine spoil dumps because indigenous plants easily fit into a fully functional ecosystem and adopt climatically also. Annual grasses are considered as a nurse crop for an early vegetation purpose offering superior tolerance to drought, low soil nutrients and other climatic stresses. Roots of grasses are fibrous that can slow erosion, and their soil-forming tendencies eventually produce a layer of organic soil, stabilize soil, conserve soil moisture and enable them to compete with weedy species. The initial vegetation cover must be allowed for the development of diverse self-sustaining plant communities (Singh et al. 2002; Xiuzhen et al. 2004).
Revegetation is a widely used technique for stabilization of dumps (Singh 1996) and maintaining ecological equilibrium of mining and metallurgical areas (Jørgensen 1994). Restoration or reclamation of industrial dumps coupled with phytoremediation triggers the stabilization of dumps with further reduced risk of environmental contamination (Salt et al. 1998). Juwarkar and Jambhulkar (2008) attempted a phytoremediation of coal mine spoil dumps by using effluent treated plant sludge, organic amendments and biofertilizer (Rhizobium sp., Azotobacter sp. and VAM spores) inoculation along with suitable plant species, to improve the physico-chemical properties of coal mine spoil and to reduce the metal toxicity in spoil. Microbial inoculation with organic amendments helped in reducing the concentrations of heavy metals such as chromium, zinc, copper, iron, manganese, lead, nickel and cadmium by 41.0, 43.0, 37.0, 37.0, 34.0, 39.0, 37.0 and 40 %, respectively. For the process of phytoremediation, it is preferable to use the plants which are metallophytes, pseudometallophytes and hyperaccumulators (Meagher 2000) as these plants have evolved biological mechanism to resist, tolerate and thrive in metalliferous soils. They are an optimal choice for restoration of mining and metallurgical closure for rehabilitation of metal-contaminated sites and underpinning for the development of environmental technologies such as phytoremediation of metals and making the substrate favourable for the flourishment of sensitive plant species (Adams and Lamoureux 2005).
Despite of the fact of human health risk, several researchers recommended many edible crops for phytoremediation purposes (Gupta et al. 2013). But utilization of crop plants for phytoremediation does not seem to be an intelligent option because heavy metals may enter into food chain through consumption by humans and animals (Vamerali et al. 2010). Contrary to this, many aromatic plants are not being consumed directly by humans and animals and hence can be grown for the production of essential oil, in case oils can strictly qualify the safety limits for toxic contaminants as have been shown in some studies (Lal et al. 2013; Zheljazkov et al. 2008). Gupta et al. (2013) also suggested the use of aromatic plants rather than non-aromatic edible crops for cultivation in metal-polluted land as a sustainable and environmental-friendly technique.
10.9 Conclusion
Mining and metallurgical industries are crucial for development of any country; however, wastes generated from these industries pose a threat to human and biological welfare. It is evident from several reports that extreme physico-chemical and biological properties and excessive amounts of metals affect adversely to native as well as exotic plant species including crop and medicinal plants. Both plant essential and non-essential metals when exceeding their phytotoxic threshold interfere with several metabolic processes, causing toxicity to the plants as exhibited by reduced growth, chlorosis, impaired photosynthesis and finally plant death. As industrial development continues, sustainable and environmental-friendly ways to manage these wastes remain a big challenge, but restoration of such polluted sites by rehabilitation and phytoremediation using native and medicinal plants could be a sustainable and environmental viable option for better management of these wastes. Such studies, however, need to be designed cautiously with utmost care to prevent any further contamination of food chain or environment.
References
Abou-Arab AAK, Abou Donia MA (2000) Heavy metals in Egyptian spices and medicinal plants and the effect of processing on their levels. J Agric Food Chem 48(6):2300–2304
Adamo P, Dudka S, Wilson MJ, McHardy WJ (2002) Distribution of trace elements in soils from the Sudbury smelting area (Ontario, Canada). Water Air Soil Pollut 137(1–4):95–116
Adams PW, Lamoureux S (2005) A literature review of the use of native northern plants for the re-vegetation of arctic mine tailings and mine waste. Environ Nat Resour, Northwest Territories, pp 21–22. http://www.enr.gov.nt.ca/sites/default/files/wkssnorthern plants_re-vegetation-2005.pdf. Accessed on 12 Mar 2016
Adriano DC (ed) (2001) Trace elements in the terrestrial environments: biogeochemistry, bioavailability and risks of heavy metals, 2nd edn. Springer, New York
Affholder MC, Prudent P, Masotti V, Coulomb B, Rabier J, Nguyen-The B, Laffont-Schwob I (2013) Transfer of metals and metalloids from soil to shoots in wild rosemary (Rosmarinus officinalis L.) growing on a former lead smelter site: human exposure risk. Sci Total Environ 454:219–229
Ahmad MSA, Ashraf M, Hussain M (2011) Phytotoxic effects of nickel on yield and concentration of macro-and micro-nutrients in sunflower (Helianthus annuus L.) achenes. J Hazard Mater 185(2):1295–1303
Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H (2001) Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. J Plant Physiol 126(4):1381–1390
Albano JP, Miller WB, Halbrooks MC (1996) Iron toxicity stress causes bronze speckle, a specific physiological disorder of marigold (Tagetes erecta L.). J Am Soc Hortic Sci 121:430–437
Alcántara E, Romera FJ, Cañete M, Manuel D (1994) Effects of heavy metals on both induction and function of root Fe (lll) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. J Exp Bot 45(12):1893–1898
Al-Khateeb W, Al-Qwasemeh H (2014) Cadmium, copper and zinc toxicity effects on growth, proline content and genetic stability of Solanum nigrum L., a crop wild relative for tomato; comparative study. J Physiol Mol Biol Plants 20(1):31–39
Alloway B, Ayres DC (eds) (1997) Chemical principles of environmental pollution, 2nd edn. CRC Press/University of London, London
Alloway BJ, Jackson AP, Morgan H (1990) The accumulation of cadmium by vegetables grown on soils contaminated from a variety of sources. Sci Total Environ 91:223–236
Amin H, Arain BA, Amin F, Surhio MA (2013) Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am J Plant Sci 4(12):2431–2439
Angelova V, Ivanov K, Ivanova R (2006) Heavy metal content in plants from family Lamiaceae cultivated in an industrially polluted region. J Herbs Spices Med Plants 11(4):37–46
Arizona Cooperative Extension (ACE) (1998) Diagnosing plant damage: key to symptoms of chemical disorders on individual plants. College of Agriculture, University of Arizona https://ag.arizona.edu/pubs/garden/mg/damage/key.html. Accessed on 12 Mar 2016
Arnon DI, Stout PR (1939) Molybdenum as an essential element for higher plants. J Plant Physiol 14(3):599
Atanassova B, Zapryanova N (2009) Influence of heavy metal stress on growth and flowering of Salvia Splendens Ker.-Gawl. Biotechnol Biotechnol Equip 23(1):173–176
Baba A, Tayfur G (2011) Groundwater contamination and its effect on health in Turkey. Environ Monit Assess 183:77–94
Bacchetta G, Cappai G, Carucci A, Tamburini E (2015) Use of native plants for the remediation of abandoned mine sites in Mediterranean semiarid environments. B Environ Con Tox 94(3):326–333
Bachman GR, Miller WB (1995) Iron chelate inducible iron/manganese toxicity in zonal geranium. J Plant Nutr 18(9):1917–1929
Bailey D, Bilderback T, Bir D (1999) Water considerations for container production of plants. In: Horticulture information leaflet. Raleigh: North Carolina State University, NC Cooperative Extension Service. http://faculty.caes.uga.edu/pthomas/hort4050.web/Hort4050web/HIL557.pdf.Accessed 12 Mar 2016
Balestrasse KB, Benavides MP, Gallego SM, Tomaro ML (2003) Effect of cadmium stress on nitrogen metabolism in nodules and roots of soybean plants. Funct Plant Biol 30(1):57–64
Barker WG (1972) Toxicity levels of mercury, lead, copper, and zinc in tissue culture systems of cauliflower, lettuce, potato, and carrot. Can J Bot 50(5):973–976
Becker M, Asch F (2005) Iron toxicity in rice – conditions and management concepts. J Plant Nutr Soil Sci 168(4):558–573
Bergeaux PJ (1976) The need for micronutrients in Georgia agriculture. Bull No 784, pp 1–18
Besford RT, Maw GA (1975) Effect of potassium nutrition on tomato plant growth and fruit development. Plant Soil 42(2):395–412
Bhattacharyya JK, Shekdar AV, Gaikwad SA (2004) Recyclability of some major industrial solid wastes. J Ind Assoc Environ Mange 31:71–75
Boonyanitipong PKB, Kumar P, Baruah S, Dutta J (2011) Toxicity of ZnO and TiO2nanoparticles on germinating rice seed O ryza sativa L. Int J Biosci Biochem Bioinform 1:282–285
Boonyapookana B, Upatham ES, Kruatrachue M, Pokethitiyook P, Singhakaew S (2002) Phytoaccumulation and phytotoxicity of cadmium and chromium in duckweed Wolffia globosa. Int J Phytorem 4(2):87–100
Borgatto F, Dias CTDS, Amaral AFC, Melo M (2002) Calcium, potassium and magnesium treatment of Chrysanthemum morifolium cv. “Bi Time” and callogenesis in vitro. Sci Agricola 59(4):689–693
Bose S, Bhattacharyya AK (2012) Effect of industrial sludge application on soil nitrogen and wheat plant response. Open J Soil Sci 2(02):133
Boulet MP, Larocque AC (1998) A comparative mineralogical and geochemical study of sulfide mine tailings at two sites in New Mexico, USA. Environ Geol 33(2–3):130–142
Brenchley WE (1948) The toxic action of molybdenum in relation to soils and crops. Ann App Biol 35(2):139–160
Brooks RR (1987) Serpentine and its vegetation: a multidisciplinary approach. Dioscorides Press. http://agris.fao.org/agris-search/search.do?recordID=US8918938. Accessed on 12 Mar 2016
Broschat TK, Moore KK (2004) Phytotoxicity of several iron fertilizers and their effects on Fe, Mn, Zn, Cu, and P content of African marigolds and zonal geraniums. Hortic Sci 39(3):595–598
Brugnoli E, Lauteri M (1991) Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. J Plant Physiol 95:628–635
Bureau P, Roederer O (1960) Contribution a Fetude des sols gypseux du Sud Tunisien, Croutes et encroutements gypseux de la partie Sud de Golfe de Gabes. Bull Assoc Franc Etude Sol 8:145–149
Burke DJ, Pietrasiak N, Situ SF, Abenojar EC, Porche M, Kraj P, …, Samia ACS (2015) Iron oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int J Mol Sci 16(10):23630–23650
Cabala J, Teper L (2007) Metalliferous constituents of rhizosphere soils contaminated by Zn–Pb mining in southern Poland. Water Air Soil Pollut 178(1–4):351–362
Cargnelutti D, Tabaldi LA, Spanevello RM, de Oliveira Jucoski G, Battisti V, Redin M, …, Morsch VM (2006) Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere 65(6):999–1006
Caspi V, Droppa M, Horváth G, Malkin S, Marder JB, Raskin VI (1999) The effect of copper on chlorophyll organization during greening of barley leaves. Photosynth Res 62(2):165–174
Cele EN, Maboeta M (2016) A greenhouse trial to investigate the ameliorative properties of biosolids and plants on physicochemical conditions of iron ore tailings: implications for an iron ore mine site remediation. J Environ Manag 165:167–174
Céspedes CL, Lemus A, Salazar JR, Cabrera A, Sharma P (2003) Herbicidal, plant growth inhibitory, and cytotoxic activities of bismuthines containing aromatic heterocycles. J Agric Food Chem 51(10):2923–2929
Chang YC, Grace-Martin K, Miller WB (2004) Efficacy of exogenous calcium applications for reducing upper leaf necrosis in Lilium Star Gazer. Hortic Sci 39(2):272–275
Chartzoulakis K, Klapaki G (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Hortic 86:247–260
Chartzoulakis KS, Loupassaki MH (1997) Effects of NaCl salinity on germination, growth, gas exchange and yield of greenhouse eggplant. Agric Water Manag 32(3):215–225
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109(1):69–74
Chikile SJ, Sharma R, Mogallapu NR (2013) Cadmium toxicity on Soybean (Glycine max L.). Ind J App Pure Biol 28(2):257–264
Choi HG, Moon BY, Bekhzod K, Park KS, Kwon JK, Lee JH, …, Kang NJ (2015) Effects of foliar fertilization containing titanium dioxide on growth, yield and quality of strawberries during cultivation. Hortic Environ Biotechnol 56(5):575–581
Clark RB (1982) Plant response to mineral element toxicity and deficiency [Potentially arable soils in the world]. In: Breeding plants for less favorable environments. International Council on Lethal Yellowing, USA. http://agris.fao.org/agris-search/search.do?recordID=US19830863455. Accessed on 12 Mar 2016
Clijsters H, Van Assche F (1985) Inhibition of photosynthesis by heavy metals. Photosynth Res 7(1):31–40
Conesa HM, Faz Á (2011) Metal uptake by spontaneous vegetation in acidic mine tailings from a semiarid area in south Spain: implications for revegetation and land management. Water Air Soil Pollut 215(1-4):221–227
Conesa HM, Schulin R, Nowack B (2007) A laboratory study on revegetation and metal uptake in native plant species from neutral mine tailings. Water Air Soil Pollut 183(1-4):201–212
Conesa HM, María-Cervantes A, Álvarez-Rogel J, González-Alcaraz MN (2011) Influence of soil properties on trace element availability and plant accumulation in a Mediterranean salt marsh polluted by mining wastes: implications for phytomanagement. Sci Total Environ 409(20):4470–4479
Costa G, Polettini A, Pomi R, Stramazzo A (2016) Leaching modelling of slurry-phase carbonated steel slag. J Hazard Mater 302:415–425
Côté-Beaulieu C, Chain F, Menzies JG, Kinrade SD, Bélanger RR (2009) Absorption of aqueous inorganic and organic silicon compounds by wheat and their effect on growth and powdery mildew control. Environ Exp Bot 65(2):155–161
Das AK, Chakraborty R, Cervera ML, de la Guardia M (2006) Analytical techniques for the determination of bismuth in solid environmental samples. Trends Anal Chem 25:599–608
Datta JK, Bandhyopadhyay A, Banerjee A, Mondal NK (2011) Phytotoxic effect of chromium on the germination, seedling growth of some wheat (Triticum aestivum L.) cultivars under laboratory condition. J Agric Technol 7(2):395–402
Davis RD, Beckett PHT, Wollan E (1978) Critical levels of twenty potentially toxic elements in young spring barley. J Plant Soil 49(2):395–408
Dayan FE, Hernandez A, Allen SN, Moraes R, Vroman JA, Avery MA, Duke SO (1999) Comparative phytotoxicity of artemisinin and several sesquiterpene analogues. Phytochemistry 50:607–614
De Dorlodot S, Lutts S, Bertin P (2005) Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J Plant Nutr 28(1):1–20
De Flora S, Bennicelli C, Bagnasco M (1994) Genotoxicity of mercury compounds: a review. Mutat Res/Rev Gen Tox 317(1):57–79
De Kreij C, Van Den Berg THJM (1990) Nutrient uptake, production and quality of Rosa hybrida in rockwool as affected by Electrical Conductivity of the Nutrient Solution. In: Van Beusichem ML (ed) Plant nutrition: physiology and applications. Springer, Dordrecht, pp 519–523
De Kreij C, Van Os PC (1989) Production and quality of Gerbera in rockwool as affected by electrical conductivity of the nutrient solution. In: Proceedings of the seventh international congress on soilless culture, Flevohof, Netherlands, 13–21 May 1988
De Kreij C, Janse J, Van Goor BJ, Van Doesburg JDJ (1992) The incidence of calcium oxalate crystals in fruit walls of tomato (Lycopersicon esculentum Mill.) as affected by humidity, phosphate and calcium supply. J Hortic Sci 67(1):45–50
Dean KC, Froisland LJ, Shirts MB (1986) Utilization and stabilization of mineral waste. Bulletin 688. United States: Department of Interior Bureau of Mines, p 46
Disante KB, Fuentes D, Cortina J (2010) Sensitivity to zinc of Mediterranean woody species important for restoration. Sci Total Environ 408(10):2216–2225
Doncheva S, Stoynova Z, Velikova V (2001) Influence of succinate on zinc toxicity of pea plants. J Plant Nutr 24(6):789–804
Douay F, Pruvot C, Waterlot C, Fritsch C, Fourrier H, Loriette A, …, Scheifler R (2009) Contamination of woody habitat soils around a former lead smelter in the North of France. Sci Total Environ 407(21):5564–5577
Dube BK, Sinha P, Gopal R, Chatterjee C (2004) Chromium phytotoxicity alters metabolism in radish. J Veg Crop Prod 10(2):61–71
Dumon JC, Ernst WHO (1988) Titanium in plants. J Plant Physiol 133(2):203–209
Dutta RK, Agrawal M (2002) Effect of tree plantations on the soil characteristics and microbial activity of coal mine spoil land. J Trop Ecol 43(2):315–324
Dutta RK, Agrawal M (2003) Restoration of opencast coal mine spoil by planting exotic tree species: a case study in dry tropical region. Ecol Eng 21(2):143–151
Ene A, Pantelică A (2011) Characterization of metallurgical slags using low-level gamma-ray spectrometry and neutron activation analysis. Rom J Phys 56:7–8
Epstein E (1980) In: Spanswik RM, Lucas WJ, Dainty J (eds) Plant membrane transport: current conceptual Carlen issues. Elsevier/North Holland Biomedical Press, Amsterdam, p 32
Epstein E (1999) Silicon. Annu Rev Plant Biol 50(1):641–664
Espinosa-Reyes G, González-Mille DJ, Ilizaliturri-Hernández CA, Mejía-Saavedra J, Cilia-López VG, Costilla-Salazar R, et al (2014) Effect of mining activities in biotic communities of Villa de la Paz, San Luis Potosi, Mexico BioMed Res Int, pp 1–13
Evans HJ, Sorger GJ (1966) Role of mineral elements with emphasis on the univalent cations. Annu Rev Plant Physiol 17(1):47–76
Fageria NK, Rabelo NA (1987) Tolerance of rice cultivars to iron toxicity. J Plant Nutr 10(6):653–661
FAO/WHO (Codex Alimentarius Commission) (2001) Food additives and contaminants. Joint FAO/WHO Food Standards Program, ALINORM 01/12A:1-289
Fargašová A (2001) Phytotoxic effects of Cd, Zn, Pb, Cu and Fe on Sinapis alba L. seedlings and their accumulation in roots and shoots. Biol Planta 44(3):471–473
Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A (2012) Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol Trace Elem Res 146(1):101–106
Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci Hortic 123(4):521–530
Filippi M, Drahota P, Machovič V, Böhmová V, Mihaljevič M (2015) Arsenic mineralogy and mobility in the arsenic-rich historical mine waste dump. Sci Total Environ 536:713–728
Fodor E, Szabó-Nagy A, Erdei L (1995) The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J Plant Physiol 147(1):87–92
Foy CD, Chaney RLT, White MC (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29(1):511–566
Frank R, Stonefield KI, Suda P, Potter JW (1982) Impact of nickel contamination on the production of vegetables on an organic soil, Ontario, Canada, 1980–1981. Sci Total Environ 26(1):41–65
Führs H, Hartwig M, Buitrago L, Heintz D, Van Dorsselaer A, Braun H, Horst W (2008) Early manganese-toxicity response in Vigna unguiculata L. – a proteomic and transcriptomic study. Proteomics 8:149–159
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Planta 50(4):653–659
Galindo JC, Hernandez A, Dayan FE, Tellez M, Macias FA, Paul RN, Duke SO (1999) Dehrydozaluzanin C, a natural sesquiterpenolide, causes rapid plasma membrane leakage. Phytochemistry 52:805–813
Galindo R, Padilla I, Rodríguez O, Sánchez-Hernández R, López-Andrés S, López-Delgado A (2015) Characterization of solid wastes from aluminum tertiary sector: the current state of Spanish industry. J Miner Mater Charact Eng 3(02):55
Gang A, Vyas A, Vyas H (2013) Toxic effect of heavy metals on germination and seedling growth of wheat. J Environ Res Dev 8(2):206
Gao S, Ou-yang C, Tang L, Zhu JQ, Xu Y, Wang SH, Chen F (2010) Growth and antioxidant responses in Jatropha curcas seedling exposed to mercury toxicity. J Hazard Mater 182(1):591–597
Gautam M, Pandey D, Agrawal M (2016) Bioprocessing of metals from packaging wastes. In: Muthu SS (ed) Environmental footprints of packaging. Springer, Singapore, pp 139–164
Geilmann (1920) The occurrence of titanium in soil and plants. Zeitschrift für Landwirtschaft 68:107–124
Gholizadeh A, Borůvka L, Vašát R, Saberioon M, Klement A, Kratina J, …, Drábek O (2015) Estimation of potentially toxic elements contamination in anthropogenic soils on a brown coal mining dumpsite by reflectance spectroscopy: a case study. PloS one 10(2):117–457
Ghosh M, Bandyopadhyay M, Mukherjee A (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 81:1253–1262
Godbold DL (1991) Mercury-induced root damage in spruce seedlings. Water Air Soil Pollut 56:823–831
Goldwater LJ (1971) Mercury in the environment. Sci Am, United States 224(5)
Gorai B, Jana RK (2003) Characteristics and utilisation of copper slag – a review. Resour Conserv Recycl 39(4):299–313
Gruenhage L, Jager HJ (1985) Effect of heavy metals on growth and heavy metal content of Allium porrum and Pisum sativum. Angew Bot 59:11–28
Gu JG, Lin QQ, Hu R, Zhuge YP, Zhou QX (2005) Translocation behavior of heavy metals in soil–plant system: a case study from Qingchengzi lead–zinc mine in Liaoning Province. J Agro-Environ Sci 24:634–637
Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. J Plant Physiol 104(3):815
Guo X, Wang K, He M, Liu Z, Yang H, Li S (2014) Antimony smelting process generating solid wastes and dust: characterization and leaching behaviors. J Environ Sci 26(7):1549–1556
Gupta UC (1971) Boron and molybdenum nutrition of wheat, barley and oats grown in Prince Edward Island soils. Can J Soil Sci 51(3):415–422
Gupta AK, Verma SK, Khan K, Verma RK (2013) Phytoremediation using aromatic plants: a sustainable approach for remediation of heavy metals polluted sites. Int J Environ Sci Technol 47(18):10115–10116
Gutiérrez-Gutiérrez SC, Coulon F, Jiang Y, Wagland S (2015) Rare earth elements and critical metal content of extracted landfilled material and potential recovery opportunities. Waste Manag 42:128–136
Gwóźdź EA, Przymusiński R, Rucińska R, Deckert J (1997) Plant cell responses to heavy metals: molecular and physiological aspects. Acta Physiol Plant 19(4):459–465
Hamzah Z, Saat A, Bakar ZA, Wood AK (2011) Anthropogenic heavy metals, U-238 and Th-232 profiles in sediments from an abandoned tin mining lake in Malaysia. In: Proceedings of 3rd International Conference on Chemical, Biological and Environmental Engineering, Singapore, 2011
Hansen TC, Richards CW, Mindess S (1968) Production of high pressure steam-cured calcium silicate building materials from mining industry waste products. Part I: Program for Investigation and Report of Field Survey of Siliceous Mine Waste in California, Department of Civil Eng, Stanford University, p 83
Hassinger BW (1997) Erosion. In: Marcus JJ (ed) Mining environmental handbook: effects of mining on the environment and American environmental controls on mining. Imperial College Press, London, pp 136–140
Hattori T, Inanaga S, Tanimoto E, Lux A, Luxová M, Sugimoto Y (2003) Silicon-induced changes in viscoelastic properties of Sorghum root cell walls. Plant Cell Physiol 44(7):743–749
Hernandez L, Probst A, Probst JL, Ulrich E (2003) Heavy metal distribution in some French forest soils: evidence form atmospheric contamination. Sci Total Environ 312:195–219
Hernando V, Sanchez Conde MP, Contreras JG (1965) Study of the mineral nutrition of maize on soils rich in gypsum. Zolfo in Agricoltura, Palermo 1964:398–411
Hewitt EJ (1983). In: Robb DA, Pierpoint WS (eds) Metals and micronutrients: uptake and utilization by plants. Academic, London, pp 277–300
Hodson MJ (2012) Metal toxicity and tolerance in plants. Biochemical Society, Oxford Brooks University, United Kingdom. http://www.hodsons.org/MartinHodson/Biochemist12.pdf. Accessed on 22 May 2016
Horiguchi T (1988) Mechanism of manganese toxicity and tolerance of plants: IV. Effects of silicon on alleviation of manganese toxicity of rice plants. Soil Sci Plant Nutr 34(1):65–73
Hossain MT, Mori R, Soga K, Wakabayashi K, Kamisaka S, Fujii S, …, Hoson T (2002) Growth promotion and an increase in cell wall extensibility by silicon in rice and some other Poaceae seedlings. J Plant Res 115(1): 0023–0027
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plant 16(3):259–272
Huaiwei Z, Xin H (2011) An overview for the utilization of wastes from stainless steel industries. Resour Conserv Recycl 55(8):745–754
Hunter JG, Vergnano O (1953) Trace‐element toxicities in oat plants. Ann App Biol 40(4):761–777
Iimura K, Ito H, Chino M, Morishita T, Hirata H (1977) Behavior of contaminant heavy metals in soil plant system. In: Proceedings of the international seminar on soil environment and fertility management in intensive agriculture, AGRIS, FAO of United Nations
Illera V, Garrido F, Serrano S, García‐González MT (2004) Immobilization of the heavy metals Cd, Cu and Pb in an acid soil amended with gypsum‐and lime‐rich industrial by‐products. Eur J Soil Sci 55(1):135–145
Indian Bureau of Mines. (2002) Indian minerals year book, Ministry of Mines, Government of India, India. http://ibm.nic.in/. Accessed on 12 Mar 2016
Inostroza-Blancheteau C, Rengel Z, Alberdi M, de la Luz MM, Aquea F, Arce-Johnson P, Reyes-Díaz M (2012) Molecular and physiological strategies to increase aluminum resistance in plants. Mol Biol Repor 39(3):2069–2079
Ishtiaq S, Mahmood S (2012) Phytotoxicity of nickel and its accumulation in tissues of three Vigna species at their early growth stages. J App Bot Food Qual 84(2):223
Jacobi JS (1975) Recovery and reuse of metals. In: Proceedings of Minerals and the environment. The institute of mining and metallurgy proceedings of international symposium, pp 291–301
Jacobs JA, Testa SM (2005) Overview of chromium (VI) in the environment: background and history. In: Guertin J, Jacobs JA, Avakian CP (eds) Chromium (VI) handbook. Independent environmental technical group, pp 1–21
Jain R, Srivastava S, Madan VK (2000) Influence of chromium on growth and cell division of sugarcane. Indian J Plant Physiol 5(3):228–231
Jellali S, Wahab MA, Anane M, Riahi K, Bousselmi L (2010) Phosphate mine wastes reuse for phosphorus removal from aqueous solutions under dynamic conditions. J Hazard Mater 184(1):226–233
Jennings BD (1976) The effects of sodium chloride on higher plants. Biol Rev 51(4):453–486
Jørgensen SE (1994) Models as instruments for combination of ecological theory and environmental practice. Ecol Model 75:5–20
Juwarkar AA, Jambhulkar HP (2008) Phytoremediation of coal mine spoil dump through integrated biotechnological approach. Biores Technol 99(11):4732–4741
Juwarkar AS, Sinde GB (1986) Interaction of Cd, Pb: effect on growth, yield and content of Cd, Pb in barley, Hordeum vulgare. Ind J Environ Health 28:218–234
Kabata‐Pendias A, Sadurski W (2004) Trace elements and compounds in soil. In: Elements and their compounds in the environment: occurrence, analysis and biological relevance, 2nd edn, pp 79–99
Kampfenkel K, Van Montagu M, Inzé D (1995) Effects of iron excess on Nicotiana plumbaginifolia plants (implications to oxidative stress). J Plant Physiol 107(3):725–735
Kang BT, Fox RL (1980) A methodology for evaluating the manganese tolerance of cowpea (Vigna unguiculata) and some preliminary results of field trials. Field Crops Res 3:199–210
Karlen DL, Sadler EJ, Camp CR (1987) Dry matter, nitrogen, phosphorus, and potassium accumulation rates by corn on Norfolk loamy sand. J Agro 79(4):649–656
Khatun S, Ali MB, Hahn EJ, Paek KY (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64(3):279–285
Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Int J Environ Sci Technol 29(1):1–46
Kitano T, Sasaki K, Amako K, Takahashi M (1997) PCR-assisted differential display of the expression of genes that are induced by manganese-deficiency in tomato roots. In: Plant nutrition for sustainable food production and environment. Springer, Dordrecht, pp 225–226
Koptsik S, Koptsik G, Livantsova S, Eruslankina L, Zhmelkova T, Vologdina Z (2003) Heavy metals in soils near the nickel smelter: chemistry, spatial variation, and impacts on plant diversity. J Environ Monitor 5(3):441–450
Kovács B, Puskás-Preszner A, Huzsvai L, Lévai L, Bódi É (2015) Effect of molybdenum treatment on molybdenum concentration and nitrate reduction in maize seedlings. J Plant Physiol Biochem 96:38–44
Kříbek B, Majer V, Veselovský F, Nyambe I (2010) Discrimination of lithogenic and anthropogenic sources of metals and sulphur in soils of the central-northern part of the Zambian Copperbelt Mining District: a topsoil vs. subsurface soil concept. J Geochem Explor 104(3):69–86
Krupa Z, Baszynski T (1995) Some aspects of heavy metals toxicity towards photosynthetic apparatus: direct and indirect effects on light and dark reactions. Acta Physiol 17:177–190
Kumar A (2000) Conveners report. In: The souvenir of mines environment and mineral conservation week (Goa and parts of Karnataka), p 8
Küpper H, Kroneck PM (2007) Nickel in the environment and its role in the metabolism of plants and cyanobacteria. Metal Ions Life Sci 2:31–62
Kužel S, Hruby M, Cígler P, Tlustoš P, Van Nguyen P (2003) Mechanism of physiological effects of titanium leaf sprays on plants grown on soil. Biol Trace Elem Res 91(2):179–189
Lal K, Yadav RK, Kaur R, Bundela DS, Khan MI, Chaudhary M, …, Singh G (2013) Productivity, essential oil yield, and heavy metal accumulation in lemon grass (Cymbopogon flexuosus) under varied wastewater–groundwater irrigation regimes. Indus Crops Prod 45:270–278
Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F (2011) Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. Comptes Rendus Biol 334(2):118–126
Lee MK, Van Iersel MW (2008) Sodium chloride effects on growth, morphology, and physiology of Chrysanthemum (Chrysanthemum × morifolium). Hortic Sci 43(6):1888–1891
Lee CW, Choi JM, Pak CH (1996) Micronutrient toxicity in seed geranium (Pelargonium × hortorum Bailey). J Am Soc Hortic Sci 121(1):77–82
Lei Y, Korpelainen H, Li C (2007) Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 68:686–694
Leonard RP (1978) Hazardous solid waste from metallurgical industries. Environ Health Persp 27:251
Li HF, Gray C, Mico C, Zhao FJ, McGrath SP (2009) Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 75(7):979–986
Li Z, Feng X, Bi X, Li G, Lin Y, Sun G (2014) Probing the distribution and contamination levels of 10 trace metal/metalloids in soils near a Pb/Zn smelter in middle China. Environ Sci Pollut Res 21(6):4149–4162
Liang Y, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58(4):475–483
Lidon FC, Teixeira MG (2000) Rice tolerance to excess Mn: implications in the chloroplast lamellae and synthesis of a novel Mn protein. Plant Physiol Biochem 38:969–978
Liu X, Zhang S, Shan X, Zhu YG (2005) Toxicity of arsenate and arsenite on germination seedling growth and amylolytic activity of wheat. Chemosphere 61:293–301
Liu Y, Lin C, Wu Y (2007) Characterization of red mud derived from a combined Bayer Process and bauxite calcination method. J Hazard Mater 146(1):255–261
Liu W, Yang J, Xiao B (2009) Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J Hazard Mater 161(1):474–478
López I, Balocchi O, Lailhacar P (1997) Caracterización de sitios de crecimiento de seis especies pratenses nativas y naturalizadas del dominio húmedo de Chile. Agro Sur 25(1):62–80
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11(8):392–397
Ma L, Ruan JY, Yang Y, Han W, Shi Y (2005) Effect of magnesium nutrition of the formation and transport of free amino acids in tea plants. In: Proceedings of international symposium on innovation in tea science and sustainable development in tea industry, pp 10–15
Mahajan A, Dua S (1993) Some characteristics of Indian rapeseed acid phosphatase. Physiol Biochem 20:86–89
Mahmood T, Islam KR (2006) Response of rice seedlings to copper toxicity and acidity. J Plant Nutrition 29(5):943–957
Maksimović JD, Mojović M, Maksimović V, Römheld V, Nikolic M (2012) Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J Exp Bot 63(7):2411–2420
Małkowski E, Kita A, Galas W, Karcz W, Kuperberg JM (2002) Lead distribution in corn seedlings (Zea mays L.) and its effect on growth and the concentrations of potassium and calcium. Plant Growth Regul 37(1):69–76
Marques AP, Rangel AO, Castro PM (2009) Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit Rev Int J Environ Sci Technol 39(8):622–654
Marschner H (1995) Functions of mineral nutrients: macronutrients. In: Mineral nutrition of higher plants, 2nd edn. Academic, New York, pp 299–312
Mathiyazhagan N, Natarajan D, Suresh K (2015) Impact of waste dumps of mines on plants in genomic & bio molecules level. Mesop Environ J 2(1):33–45
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46
Mazumdar M, Christiani DC, Biswas SK, Ibne-Hasan OS, Kapur K, Hug C (2015) Elevated sweat chloride levels due to arsenic toxicity. New Engl J Med 372(6):582–584
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3(2):153–162
Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43(4):519–524
Mendel RR, Hänsch R (2002) Molybdoenzymes and molybdenum cofactor in plants. J Exp Bot 53(375):1689–1698
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ Health Persp 116(3):278
Mishra A, Choudhuri MA (1999) Monitoring of phytotoxicity of lead and mercury from germination and early seedling growth indices in two rice cultivars. Water Air Soil Pollut 114(3-4):339–346
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Mohanpuria P, Rana NK, Yadav SK (2007) Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environ Tox 22(4):368–374
Mohanty M, Dhal NK, Patra P, Das B, Reddy PSR (2010) Phytoremediation: a novel approach for utilization of iron-ore wastes. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol 206. Springer, New York, pp 29–47
Mokgalaka-Matlala NS, Flores-Tavizón E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL (2008) Toxicity of arsenic (III) and (V) on plant growth, element uptake, and total amylolytic activity of mesquite (Prosopis juliflora x P. velutina). Int J Phytorem 10(1):47–60
Molas J (1998) Changes in morphological and anatomical structure of cabbage (Brassica oleracea L.) outer leaves and in ultrastructure of their chloroplasts caused by an in vitro excess of nickel. Photosynth 34(4):513–522
Montesano F, Van Iersel MW (2007) Calcium can prevent toxic effects of Na+ on tomato leaf photosynthesis but does not restore growth. J Am Soc Hortic Sci 132:310–318
Mora M, Rosas A, Ribera A, Rengel R (2009) Differential tolerance to Mn toxicity in perennial ryegrass genotypes: involvement of antioxidative enzymes and root exudation of carboxylates. J Plant Soil 320:79–89
Moreno JL, Hernández T, Garcia C (1999) Effects of cadmium-contaminated sewage sludge compost on dynamics of organic matter and microbial activity in an arid soil. Biol Fert Soils 28(3):230–237
Morgan PW, Taylor DM, Joham HE (1976) Manipulation of IAA‐oxidase activity and auxin‐deficiency symptoms in intact cotton plants with manganese nutrition. Physiol Planta 37(2):149–156
Morrey DR, Baker AJM, Cooke JA (1988) Floristic variation in plant communities on metalliferous mining residues in the northern and southern Pennines, England. Environ Geochem Health 10(1):11–20
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250
Nagata T (2015) Growth inhibition and IRT1 induction of Arabidopsis thaliana in response to bismuth. J Plant Biol 58(5):311–317
Narayan D, Agrawal M, Pandey J, Singh J (1994) Changes in vegetation characteristics downwind of an aluminium factory in India. Ann Bot 73(5):557–565
Narendrula R, Nkongolo KK, Beckett P (2012) Comparative soil metal analyses in sudbury (Ontario, Canada) and lubumbashi (Katanga, DR-Congo). Bull Environ Con Tox 88(2):187–192
Narula A, Kumar S, Srivastava PS (2005) Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep 24(4):250–254
National Programme on Safety Food (NPSF) (2002) Safety milled rice criteria of PR China (NY5115-2002). China Ministry of Agriculture, Beijing [In Chinese]
Nawab J, Khan S, Shah MT, Khan K, Huang Q, Ali R (2015a) Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species. Int J Phytorem 17(9):801–813
Nawab J, Khan S, Shah MT, Qamar Z, Din I, Mahmood Q, …, Huang Q (2015b) Contamination of soil, medicinal, and fodder plants with lead and cadmium present in mine-affected areas, Northern Pakistan. Environ Monit Asses 187(9):1–14
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3(10):1476–1489
Neelima P, Reddy KJ (2002) Interaction of copper and cadmium with seedling growth and biochemical responses in Solanum melongena. Nat Environ Pollut Technol 1(3):285–290
Nichols DG, Beardsell DV (1981) Interactions of calcium, nitrogen and potassium with phosphorus on the symptoms of toxicity in Grevillea cv. ‘poorinda firebird’. J Plant Soil 61(3):437–445
Niemeyer JC, Moreira-Santos M, Nogueira MA, Carvalho GM, Ribeiro R, Da Silva EM, Sousa JP (2010) Environmental risk assessment of a metal-contaminated area in the Tropics. Tier I: screening phase. J Soils Sediment 10(8):1557–1571
Nyitrai P, Bóka K, Gáspár L, Sárvári É, Lenti K, Keresztes Á (2003) Characterization of the stimulating effect of low-dose stressors in maize and bean seedlings. J Plant Physiol 160(10):1175–1183
Ok YS, Kim SC, Kim DK, Skousen JG, Lee JS, Cheong YW, …, Yang JE (2011) Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environ Geochem Health 33(1):23–30
Osman KT (ed) (2012) Soils: principles, properties and management. Springer, Chittagong
Ou-yang C, Gao S, Mei LJ, Chung TW, Tang L, Wang SH, Chen F (2014) Effects of aluminum toxicity on the growth and antioxidant status in Jatropha curcas seedlings. J Med Plants Res 8(3):178–185
Ouzounidou G, Moustakas M, Eleftheriou EP (1997) Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Arch Environ Con Tox 32(2):154–160
Päivöke A (1983) The long-term effects of lead and arsenate on the growth and development, chlorophyll content and nitrogen fixation of the garden pea. Ann Bot Fenn 20(3):297–306
Pakrashi S, Jain N, Dalai S, Jayakumar J, Chandrasekaran PT, Raichur AM, …, Mukherjee A (2014) In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PloS one 9(2):87789
Palit S, Sharma A, Talukder G (1994) Effects of cobalt on plants. The Bot Rev 60(2):149–181
Palumbo-Roe B, Klinck B, Cave M (2007) Arsenic speciation and mobility in mine wastes from a copper–arsenic mine in Devon, UK: a SEM, XAS, sequential chemical extraction study. Trace Metals Other Con Environ 9:441–471
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17(1):95–102
Pandey B, Agrawal M, Singh S (2014) Assessment of air pollution around coal mining area: emphasizing on spatial distributions, seasonal variations and heavy metals, using cluster and principal component analysis. Atmos Pollut Res 5(1):79–86
Pandey B, Agrawal M, Singh S (2016) Ecological risk assessment of soil contamination by trace elements around coal mining area. J Soils Sediment 16(1):159–168
Pappu A, Saxena M, Asolekar SR (2007) Solid wastes generation in India and their recycling potential in building materials. Build Environ 42(6):2311–2320
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422
Peralta JR, Gardea-Torresdey JL, Tiemann KJ, Gomez E, Arteaga S, Rascon E, Parsons JG (2001) Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bul Environ Con Tox 66(6):727–734
Pietrini F, Iori V, Cheremisina A, Shevyakova NI, Radyukina N, Kuznetsov VV, Zacchini M (2015) Evaluation of nickel tolerance in Amaranthus paniculatus L. plants by measuring photosynthesis, oxidative status, antioxidative response and metal-binding molecule content. Environ Sci Pollut Res 22(1):482–494
Pitman MG, Läuchli A (2002) Global impact of salinity and agricultural ecosystems. In: Salinity: environment-plants-molecules. Springer, Dordrecht, pp 3–20
Pitzschke A, Forzani C, Hirt H (2006) Reactive oxygen species signaling in plants. Antioxid Redox Signal 6:1757–1764
Prasad KVSK, Saradhi PP, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot 42(1):1–10
Prasad A, Kumar S, Khaliq A, Pandey A (2011) Heavy metals and arbuscular mycorrhizal (AM) fungi can alter the yield and chemical composition of volatile oil of sweet basil (Ocimum basilicum L.). Biol Fert Soils 47(8):853–861
Prasad MSR, Babu BR, Ramesh KV, Trinath K (2014) Structural and magnetic studies on chromium substituted Ni-Zn nano ferrite synthesized by citrate Gel auto combustion method. J Supercond Nov Magn 27(12):2735–2745
Pruvot C, Douay F, Herve F, Waterlot C (2006) Heavy metals in soil, crops and grass as a source of human exposure in the former mining areas. J Soils Sediments 6:215–220
Pullaiah T (2006) Encyclopaedia of world medicinal plants. In: Daya books, VoI-IIl, Department of Botany, Shri Krishnadevaraya University, Anantpur, A.P. 515003. Regency Publication, New Delhi
Purdy LH (1956) The nature and extent of mercury injury and associated anatomical responses in wheat seedlings. Rev App Mycol 36:391
Qureshi A, Maurice C, Öhlander B (2016) Potential of coal mine waste rock for generating acid mine drainage. J Geochem Explor 160:44–54
Rao SR (2011) Resource recovery and recycling from metallurgical wastes. In: Waste manag series, vol 7. Department of mining, metals and materials engineering, McGill University, Montreal, Quebec, Canada. Elsevier, pp 13–34
Reddy AM, Kumar SG, Jyothsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60(1):97–104
Reeves RD, Baker AJ (2000) Metal-accumulating plants. Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193–229
Remon E, Bouchardon JL, Cornier B, Guy B, Leclerc JC, Faure O (2005) Soil characteristics, heavy metal availability and vegetation recovery at a former metallurgical landfill: implications in risk assessment and site restoration. Environ Pollut 137(2):316–323
Rimando AM, Dayan F, Czarnota MA, Weston LA, Duke SO (1998) A new photosystem II electron-transfer inhibitor from Sorghum bicolor. J Nat Prod 61:927–930
Ritcey GM (1989) Tailings management: problems and solutions in the mining industry. Elsevier, Amsterdam/New York. ISBN 0-444-87374-0
Rodrigues CR, Silveira JA, Viégas RA, Moura RM, Aragão RM, Silva EN (2016) Combined effects of high relative humidity and K+ supply mitigates damage caused by salt stress on growth, photosynthesis and ion homeostasis in J. curcas plants. Agric Water Manag 163:255–262
Rola K, Osyczka P, Nobis M, Drozd P (2015) How do soil factors determine vegetation structure and species richness in post-smelting dumps? Ecol Eng 75:332–342
Romero‐Puertas MC, Rodríguez‐Serrano M, Corpas FJ, Gomez MD, Del Rio LA, Sandalio LM (2004) Cadmium‐induced subcellular accumulation of O2·− and H2O2 in pea leaves. Plant Cell Environ 27(9):1122–1134
Salomons W (1995) Environmental impact of metals derived from mining activities: processes, predictions, prevention. J Geochem Explor 52(1):5–23
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Biol 49(1):643–668
Samal S, Ray AK, Bandopadhyay A (2015) Characterization and microstructure observation of sintered red mud–fly ash mixtures at various elevated temperature. J Clean Prod 101:368–376
Santos-Jallath J, Castro-Rodríguez A, Huezo-Casillas J, Torres-Bustillos L (2012) Arsenic and heavy metals in native plants at tailings impoundments in Queretaro, Mexico. Phys Chem Earth Parts A/B/C 37:10–17
Sarkar S, Mazumder D (2015) Solid waste management in steel industry-challenges and opportunities, world academy of science, engineering and technology. Int J Soc Behav Edu Econ Busi Indus Eng 9(3):984–987
Sass JE (1937) Histological and cytological studies of ethyl mercury phosphate poisoning in corn seedlings. J Phytopat 27:95–99
Saxena M, Mishra CR (2004) Processing of red mud for development of wood substitutes. In: Recycling, waste treatment and clean technology, vol 1. TMS Min Metals Mater, Spain, pp 371–380
Schmfger MEV (2001) Phytochelatins: complexation of metals and metalloids, studies on the phytochelatin synthase. Doctoral dissertation, PhD thesis, Munich University of Technology, Munich, Germany
Semu E, Singh BR, Selmer Olsen AR, Steenberg K (1985) Uptake of mercury from mercury-203-labeled mercury compounds by wheat and beans grown on an oxisol. Plant Soil 87:347–355
Setia RC, Rajna B, Bala R (1994) Anatomical changes in root and stem of wheat (Triticum aestivum L.) in response to different heavy metals. J Phytomor 44:95–104
Sglavo VM, Campostrini R, Maurina S, Carturan G, Monagheddu M, Budroni G, Cocco G (2000) Bauxite ‘red mud’in the ceramic industry. Part 1: thermal behaviour. J Eur Ceram Soc 20(3):235–244
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31(5):739–753
Sharma RK, Agrawal M (2005) Biological effects of heavy metals: an overview. J Environ Biol 26(2):301–313
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17(1):35–52
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15(3):307–321
Shen H, Forssberg E (2003) An overview of recovery of metals from slags. Waste Manag 23(10):933–949
Sheoran IS, Singal HR, Singh R (1990) Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.). Photosynth Res 23(3):345–351
Singh JS (1996). In: An integrated ecological study on revegetation of mine spoil, Banaras Hindu University, Department of Botany
Singh VP (2005) Trace metals in plants. In: Metal toxicity and tolerance in plants and animals, Edition 1. Swaroop and Sons, New Delhi, p 5
Singh SP, Hendry MJ (2013) Solid-phase distribution and leaching behaviour of nickel and uranium in a uranium waste-rock piles. Water Air Soil Pollut 224(1):1–11
Singh AN, Raghubansh AS, Singh JS (2002) Plantations as a tool for mine spoil restoration. Curr Sci 82(12):1436–1441
Sinha S, Gupta M, Chandra P (1997) Oxidative stress induced by iron in Hydrilla verticillata (lf) royle: response of antioxidants. Ecotoxicol Environ Saf 38(3):286–291
Sivaguru M, Liu J, Kochian LV (2013) Targeted expression of Sb Mate in the root distal transition zone is responsible for Sorghum aluminum resistance. J Plant 76(2):297–307
Skarżyńska KM (1995) Reuse of coal mining wastes in civil engineering – Part 2: Utilization of minestone. Waste Manag 15(2):83–126
Smith R, Robertson VS (1962) Soil irrigation classification of shallow soils overlying gypsum beds, Northern Iraq. J Soil Sci 13:106–115
Smith SE, Christophersen HM, Pope S, Smith FA (2010) Arsenic uptake and toxicity in plants: integrating mycorrhizal influences. J Plant Soil 327:1–21
Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA (2014) Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J 168:812–822
Spear SN, Asher CJ, Edwards DG (1978) Response of cassava, sunflower, and maize to potassium concentration in solution I. Growth and plant potassium concentration. Field Crops Res 1:347–361
Sresty TVS, Rao KM (1999) Ultrastructural alterations in response to zinc and nickel stress in the root cells of pigeonpea. Environ Exp Bot 41(1):3–13
Stoeva N, Bineva T (2003) Oxidative changes and photosynthesis in Oat plants grown in As-contaminated soil. Bulg J Plant Physiol 29(1–2):87–95
Stoeva N, Berova M, Zlatev Z (2003) Physiological response of maize to arsenic contamination. Biol Planta 47(3):449–452
Stoeva N, Berova M, Vassilev A, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Planta 49(2):293–296
Street RA, Kulkarni MG, Stirk WA, Southway C, Van Staden J (2007) Toxicity of metal elements on germination and seedling growth of widely used medicinal plants belonging to Hyacinthaceae. Bull Environ Con Tox 79(4):371–376
Tabuchi A, Matsumoto H (2001) Changes in cell‐wall properties of wheat (Triticum aestivum) roots during aluminum‐induced growth inhibition. Physiol Planta 112(3):353–358
Tlustoš P, Cígler P, Hrubý M, Kužel S, Száková J, Pavlíková D, Balík J (2005) The role of titanium in biomass production and its influence on essential elements contents in field growing crops. Plant Soil Environ 51:19–25
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ (2007) Arsenic hazards: strategies for tolerance and remediation by plants. J Trends Biotechnol 25(4):158–165
Tyner EH, Webb JR (1946) Relation of corn yields to nutrient balance as revealed by leaf analysis. J Am Soc Agron, pp 173–185
Tyus HM (2012) Ecology and conservation of fishes. CRC Press/Taylor and Francis Group, Boca Raton/London/New York, pp 439. https://books.google.co.in/books?isbn=143989759X. Accessed on 10 Mar 2016
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land: a review. Environ Chem Lett 8(1):1–17
Van Alphen JG, de los Ríos Romero F (1971) Gypsiferous soils: notes on their characteristics and management ILRI (Bulletin/International Institute for Land Reclamation and Improvement 12), p 44. http://library.wur.nl/WebQuery/wurpubs/personal/453924. Accessed on 12 Mar 2016
Varshney AK (1990) Differential response of Phaseolus aureus cultivars to mercury pretreatment on mobilization of N and protease activity during germination and seedling growth. Geobios 17:82–87
Venkatesan S, Jayaganesh S (2010) Characterisation of magnesium toxicity, its influence on amino acid synthesis pathway and biochemical parameters of Tea. Res J Phytochem 4:67–77
Vieira RF, Vieira C, Cardoso EJBN, Mosquim PR (1998) Foliar application of molybdenum in common bean. II Nitrogenase and nitrate reductase activities in a soil of low fertility. J Plant Nutr 21(10):2141–2151
Vijay S, Kant S, Bohra SP (1988) Ecophysiological studies on Indian desert plants, II: Physiological response to heavy metals in Sorghum vulgare. Pers Trans Indian Soc Desert Technol 59–67
Wang S, Ang HM, Tade MO (2008) Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 72(11):1621–1635
White MC, Chaney RL, Decker AM (1979) Differential cultivar tolerance in soybean to phytotoxic levels of soil Zn. II. Range of Zn additions and the uptake and translocation of Zn, Mn, Fe, and P. J Agro 71(1):126–131
Wilkinson RE, Ohki K (1988) Influence of manganese deficiency and toxicity on isoprenoid syntheses. J Plant Physiol 87(4):841–846
Willard HH, Merritt LL Jr, Dean JA, Settle FA Jr (1988) Instrumental methods of analysis, 7th edition. Wadsworth Publishing Company, United States. http://www.osti.gov/scitech/biblio/5209599. Accessed in 12 Mar 2016
Wissemeier AH (1993) Marginal bract necrosis in poinsettia cultivars and the relationship to bract calcium nutrition. Gartenbauwissenschaft 58:158–163
Wójcik M, Sugier P, Siebielec G (2014) Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci Total Environ 487:313–322
World Health Organization (WHO) (1992) Guideline for drinking water quality, recommendation, vol 1, 2nd edn. World Health Organization, Geneva, p 41
World Health Organization (WHO) (1998) Quality control methods for medicinal plant materials. World Health Organization, Geneva
World Health Organization (WHO) (2005) Quality control methods for medicinal plant materials. World Health Organization, Geneva
Wu SH (1994) Effect of manganese excess on the soybean plant cultivated under various growth conditions. J Plant Nutr 17(6):991–1003
Wu W, Peters J, Berkowitz GA (1991) Surface charge-mediated effects of Mg2+ on K+ flux across the chloroplast envelope are associated with regulation of stromal pH and photosynthesis. J Plant Physiol 97(2):580–587
Xiuzhen H, Dongmei ZHOU, Yujun WANG (2004) Study of ryegrass growth in copper mine tailing treated with peat and chemical fertilizer. Acta Pedol Sinica 41(4):645–648
YaMin L, JianZhong L, YongBo S, Feng Z (2011) Effects of aluminum stress on the stomata characteristics and photosynthesis of Scutellaria baicalensis Georgi seedlings. Med Plant 2(1):5–8
Yoshizawa S, Tanaka M, Shekdar AV (2004) Global trends in waste generation. Recyc Waste Treat Clean Technol. TMS Mineral, Metals and Materials Publishers, Spain, pp 1541–1552
Zaier H, Mudarra A, Kutscher D, de la Campa MF, Abdelly C, Sanz-Medel A (2010) Induced lead binding phytochelatins in Brassica juncea and Sesuvium portulacastrum investigated by orthogonal chromatography inductively coupled plasma-mass spectrometry and matrix assisted laser desorption ionization-time of flight-mass spectrometry. Analy Chimica Acta 671(1):48–54
Zeid IM (2001) Responses of Phaseolus vulgaris chromium and cobalt treatments. Biol Planta 44(1):111–115
Zhang Y (1995) Effects of aluminum chloride on the nucleus and nucleolus in root tip cells of Hordeum vulgare. Mut Res/Environ Mutag Rel Sub 335(2):137–142
Zhang WH, Tyerman SD (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. J Plant Physiol 120(3):849–858
Zheljazkov VD, Jeliazkova EA, Kovacheva N, Dzhurmanski A (2008) Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ Exp Bot 64(3):207–216
Zhu XF, Lei GJ, Wang ZW, Shi YZ, Braam J, Li GX, Zheng SJ (2013) Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. J Plant Physiol 162(4):1947–1955
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, south China. Sci Total Environ 407(5):1551–1561
Acknowledgements
Divya Pandey and Meenu Gautam are thankful to the AXA Junior Research (Post-doctoral) Fellowship, UK, and Council of Scientific and Industrial Research (CSIR), India, respectively, in the form of Research Associateship and Senior Research Fellowship, respectively.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gautam, M., Pandey, D., Agrawal, S.B., Agrawal, M. (2016). Metals from Mining and Metallurgical Industries and Their Toxicological Impacts on Plants. In: Singh, A., Prasad, S., Singh, R. (eds) Plant Responses to Xenobiotics. Springer, Singapore. https://doi.org/10.1007/978-981-10-2860-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-2860-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2859-5
Online ISBN: 978-981-10-2860-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)