Abstract
Mismatch between the external time and the internal circadian time causes loss of circadian organization and is frequently linked to cancer. This chapter describes the role of the molecular circadian clock in the incidence and progression of cancer. The first section will present the strong association between disrupted clock gene expression in either the host or the tumor tissue with cancer progression. Furthermore, it will be evaluated whether timed clock gene expression is a relevant factor for tumor development. Possible processes that are regulated by the circadian clock and may trigger tumor growth during circadian disruption will be summarized in the second section. The last section will highlight the importance of circadian timing for the development of effective cancer therapies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Circadian disruption
- Clock genes
- Tumor growth
- Clock mutants
- Cell cycle
- DNA damage response
- Posttranscriptional modifications
1 Circadian Disruption Is Associated with Cancer
Changes in environmental conditions can disrupt the molecular circadian clock. For example, abrupt shifts in the day/night cycle, as experienced during jetlag or shift work, result in desynchronization within and between circadian clocks in the suprachiasmatic nucleus (SCN) and in peripheral tissues [1]. Furthermore, increasing evidence links dysfunction of the clockwork with tumor progression [2]. Thus, circadian disruption caused by mismatch of the external time with the internal time is believed to be an underlying factor for the risk of cancer. Indeed, in 2007, an agency of the World Health Organization classified shift work with circadian disruption as “probably carcinogenic” to humans [3] based on results from various experimental and epidemiological studies (reviewed by [4]). For example, a higher incidence of endometrial and colorectal cancer was found in nurses exposed to night shift work compared to their colleagues working on day shifts [5]. Another study indicated an increased risk of people working under night shift conditions to develop non-Hodgkin lymphoma [6].
Studies in humans were supported by experiments in rodents, e.g., chronic jetlag condition promotes the incidence of lung cancer in rats following injection of tumor cells [7] and enhances the progression of Glasgow osteosarcoma in mice [8]. These mice show circadian disruption on multiple levels, such as disturbed rhythms of clock gene expression in the SCN, body temperature and hormone levels.
Whether circadian disruption is directly linked to cancer occurrence and enhances tumor progression was further investigated by genetic approaches. Results obtained from studies on mice with genetic defects in clock genes, which lead to circadian dysfunction, should therefore match the results obtained from studies on shift workers. Indeed, numerous studies discovered a close association between single nucleotide polymorphisms (SNPs), deletion, deregulation, or epigenetic silencing of circadian genes in humans or targeted gene ablation in animal models; and increased cancer risk (recently reviewed in [9]).
1.1 Disturbance of Clock Genes in the Host Is Associated with Cancer
1.1.1 Clock Gene Polymorphism in Humans
Recent studies report significant associations between polymorphisms in clock genes and cancer risk, in particular in PER1, PER2, PER3, CRY1, CRY2, TIM, BMAL1 (or ARNTL), CLOCK, NPAS2, REV-ERBα (or NR1D1), DBP, DEC1, DEC2, and CK1ε [10–12]. For instance, a polymorphism in the PER3 gene has been associated with prostate and breast cancer risk [13, 14]. However, other studies did not find such an association [10, 15, 16]. The different outcomes of these studies did not verify a clear association between PER3 and cancer risk; thus, the data have recently been reanalyzed and support an association of PER3 variants with the incidence of breast cancer, but not with glioma, prostate and colorectal cancer [17].
Breast cancer risk has additionally been associated with variants in either BMAL1, CRY1, and NPAS2 [15, 16] or BMAL1, CLOCK, CK1ε, and NPAS2 [10]. Variants of NPAS2 have also been associated with prostate cancer risk [14]. Moreover, prostate cancer risk and lymphomagenesis were linked to variants in CRY2 [18].
Taken together, genetic associations between clock gene variants and cancer risk indicate that variation in clock functions can act as a risk factor for cancer. This is consistent with the possibility that disturbed clock function may enhance the incidence to develop cancer.
1.1.2 Clock Gene Mutations in Mice
Apart from spontaneous genetic variation in human clock genes, cancer cell lines were established and various circadian mutants have been identified and designed in rodents. These mutants show circadian phenotypes of different magnitude, from arrhythmicity to minor changes in their behavior. Here we summarize the implication of mutations in specific clock genes in the development and progression of cancer.
Per1 and Per2 double-knockout (KO) mice lack a functional circadian clock, whereas Per2 mutants (Per2 m/m) have a functional but disturbed circadian clock. However, abnormal cell growth was reported in both of these mouse lines and is further reflected in increased radiation-induced tumor occurrence and progression [2, 19]. Accordingly, Per1 or Per2 overexpression results in slower tumor growth [20, 21]. Thus, Per genes have been characterized as tumor suppressor genes.
Similar to Per1/Per2 double-KO mice, animals lacking both Cry1 and Cry2 have a disrupted circadian clock and show increased tumor development following γ-irradiation [2]. However, a different study by Gauger and Sancar reported that animals with targeted disruption of both Cry genes did not differ from their wild-type (WT) littermates regarding the frequency of tumor development [22].
Bmal1 gene ablation in mice abolishes circadian rhythmicity [23]. A computational model predicted that perturbation of BMAL1-mediated transcription can generate circadian phenotypes similar to those observed in metastatic cell lines [24]. Indeed, Bmal1 KO mice show increased radiation-induced tumor development similar to Per or Cry double-KO mice [2]. Accordingly, knockdown of Bmal1 promoted tumor growth in mice [25], whereas its overexpression inhibited colorectal cancer cell proliferation [26].
Comparable to Bmal1 KO mice, mice with a Clock gene mutation (Clock Δ19) were unable to maintain circadian rhythms in constant darkness [27]. However, these mice only show a moderate cancer-related phenotype [28]. In particular, Clock Δ19 mice do only exhibit increased tumor formation when exposed to long-term γ-irradiation.
Abolished circadian rhythmicity is not necessarily needed to promote cancer. Indeed, deregulation of a single clock gene may be sufficient to change the overall level of other clock genes and account for the cancer-related phenotype. Mice lacking the clock kinases CK1δ, which have an abnormal period, were reported to have an increased incidence to develop mammary carcinogenesis and a shorter life span [29]. Also, as mentioned above, Per2 mutant mice exhibit a cancer phenotype comparable to arrhythmic Per1/Per2 double-KO mice [19].
Taken together, it is necessary to address the role of each clock component within the molecular clockwork at the systems level, especially in relation to an increased risk to develop cancer. In this regard, the expression levels of clock genes have been correlated with the magnitude and prognosis of cancer. In the next section, results are summarized showing a correlation between the gene expression level for specific clock genes and the incidence of cancer.
1.2 Clock Gene Expression Within Tumor Cells Correlates with Cancer Progression
1.2.1 Tumor-Intrinsic Clock Gene Expression Is Associated with Cancer
In humans or WT animals, multiple recurring changes in the light-dark cycle disrupt the molecular clock in the SCN and in peripheral tissues [1]. Aside of circadian disorganization, animals undergoing repeated jetlag exhibit increased tumor frequency and faster tumor growth [7, 8]. Interestingly, circadian disruption has been reported, not only in the host’s tissues, but also in various tumor cell lines and tumor tissues. However, the direction and magnitude of the changes in clock gene expression can be in opposite directions for different cancer types. For example, CRY1 levels are decreased in pancreatic cancer [30], but increased in ovarian cancer [31]. Furthermore, changes in the same clock gene can have opposite effects on tumor growth and prognosis, e.g., PER2 suppression in human pancreatic cancer cells results in reduced proliferation [32], whereas the overall survival in patients with low PER2 levels in pancreatic tumors was found to be reduced [30]. In conclusion, the relationship between clock gene expression and cancer incidence seems to be gene and cancer type specific and does not always match between cancer cell lines and their related tumor tissues. Table 23.1 lists the studies in humans and rodents reporting significant correlations of clock gene expression with the incidence of cancer and overall prognosis.
1.2.2 Circadian Rhythm Disruption in the Tumor Is Associated with Cancer
Various associations of clock gene expression with cancer have been reported (see Table 23.1). However, only an analysis of the time-dependent – circadian – effect of clock genes can allow addressing the role of the circadian rhythms in tumor development and progression. In this regard, disturbed circadian rhythms of clock genes have been documented in various cancer cells and tumor tissue (Table 23.2). Interestingly, circadian rhythmicity of Per1, Per2, Rev-Erbα, and Dbp was significantly reduced in colon tumor tissue, but not in healthy colon tissue surrounding the tumor [33], while the rhythmicity of Bmal1 was abolished on both sides, indicating that tumor-intrinsic circadian rhythms may play a more pronounced role in cancer progression and development than rhythms in the host. Indeed, timed manipulation of circadian rhythms in the tumor was shown to accelerate tumor growth and strongly influence the magnitude of symptoms and prognosis. For example, lack of Per1 or Per2 increases tumor growth only at times when their intrinsic expression levels were high [34]. Thus, tumor-intrinsic circadian rhythms may represent a new target for cancer-related therapies. Indeed, since various tumor tissues and human cancer cell lines harbor a dysfunctional circadian clock, the strategy to improve circadian rhythms in those cells becomes obvious.
1.2.3 Restoring Circadian Rhythm in the Tumor Inhibits Tumor Growth
Only a limited number of studies have addressed whether tumor-intrinsic clock manipulations may become important for cancer prevention or therapy. For example, the inhibitory effect of the drug seliciclib on tumor growth was enhanced when administration was done at times of the day when it stimulates a high amplitude of clock gene expression in the tumor [35]. These results match observations by Li et al. indicating reduced tumor growth when circadian rhythms were restored by time-restricted food access [36]. Both studies are limited by the possible side effects of the drug and the feeding schedule on other peripheral circadian clocks, such as the liver. Whether restoration of circadian oscillations specifically in the tumor is sufficient to inhibit tumor growth needs to be validated in future studies.
Taken together, changes in overall clock gene expression as well as disruption of their rhythmic expression have been documented in the host and in cancer cells. However, a correlation between clock gene alterations, circadian disruption, and their role in delaying cancer development is an indication but not evidence for a causal relationship in either one or both directions. Thus, two distinct hypotheses can be made. A circadian clock gene may be influencing cancer incidence or tumor development (1) due to their gene-specific activities on target genes involved in cancer-related pathways or (2) through their involvement in circadian clock functions regulating cancer-related pathways. In line with the first hypothesis, clock gene alterations or mutations do not necessarily lead to disruption of the circadian system, either centrally or peripherally. Nevertheless, changes in clock gene expression have been correlated to the incidence to develop cancer. In contrast, the best indication for the second hypothesis is that WT mice with an environmentally disrupted circadian system show enhanced tumor progression. Moreover, deregulated clock genes are frequent in human cancer cells and tumor tissue.
2 Possible Mechanisms Linking the Circadian Clock with Cancer
Cancer development may be pronounced when tumor suppressor functions of either the circadian clock or specific clock genes are lacking. Transcriptome analysis has revealed that many important genes involved in cancer-related pathways, such as the cell cycle, cell proliferation, apoptosis, and DNA damage, are targets of the circadian clock [37]. This section will describe the current knowledge regarding the involvement of the circadian clock in processes which are dysregulated during tumorigenesis. Another possible mechanism involves the circadian control of immune responses, in particular NK cell cytotoxicity. The reader is referred to Chap. 22 for more details.
2.1 The Circadian Clock Controls the Cell Cycle
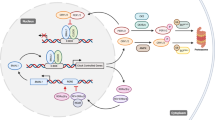
The cell cycle is the process by which a cell prepares for and accomplishes its division into two daughter cells. This occurs through different phases: G1, where the cell prepares for DNA replication; S, where DNA replication occurs; G2, where the cell prepares for mitosis; and M (mitosis), where the cell divides to give rise to the two daughter cells. The cell cycle involves a network of cyclin-dependent kinases (CDKs) forming complexes with cyclins, which regulate each phase of the cell cycle by controlling major checkpoints. This gene network is coupled to the circadian clock through circadian variations in the levels of major players regulating cell cycle checkpoints, such as WEE1, c-MYC, p21, and cyclin E [19, 38] (see Fig. 23.1).
The circadian clock controls the cell cycle on multiple levels. The circadian clock (a) interacts with all cell cycle phases by controlling major cell cycle factors, such as WEE1, MYC, p21, cyclin A, and cyclin D (b). The cell cycle network consists of cyclin-dependent kinases (CDKs) forming complexes with cyclins and regulating each phase of the cell cycle by controlling major checkpoints: G1 and G1/S phase transition are regulated by cyclin D/CDK4–6 and cyclin E/CDK2, respectively; entry into S phase and thereby DNA replication as well as S-G2 transition are controlled by cyclin A/CDK1; cyclin B/CDK1 finally elicits G2/M phase transition [21, 38, 39]
The important checkpoint kinase Wee1 shows rhythmic expression, established through gene activation by CLOCK/BMAL1 and repression by PERs/CRYs [21, 38]. WEE1 inhibits the entry into the M phase by suppression of CDK1 and CDK2 activity [38, 39]. Consequently, reduced synthesis of WEE1 favors the entry into mitosis and may even shorten its duration. Dysregulated circadian expression of WEE1 in turn could induce disruption of the cell cycle, which may result in uncontrolled fast proliferation. Indeed, low and arrhythmic levels of Wee1 have been reported in Clock mutant mice [40] and after Bmal1 deletion in cancer cells [41]. In contrast, loss of Cry genes increased Wee1 expression, which inhibited the G2/M transition and may account for slower liver regeneration [38].
Another important cell cycle regulator, the oncogene c-Myc, is rhythmically regulated via promoter elements for CLOCK/BMAL1 [19]. In contrast to Wee1, c-Myc expression is repressed by CLOCK/BMAL1 and REV-ERBα but elevated by PER1 [21]. Cell proliferation is regulated by c-MYC via activation of cyclin E/CDK2 and cyclin D/CDK4–6 in parallel, to inhibition of cell cycle inhibitors p21 and p27 [39]. Importantly, c-Myc expression was found to be highly elevated in many human tumors [42] and mouse mutants [19]. For example, enhanced expression of c-Myc correlates with increased γ-irradiation-induced cell proliferation and tumor development in Per2 mutant mice, which exhibit suppressed Bmal1 expression. Accordingly, overexpression of BMAL1 can suppress elevated c-MYC levels and restore its rhythmic activity in ovarian cancer cells [43]. c-MYC is required for G0/G1 transition and elicits S-phase entry, and thus overexpression in cancer cells may be a crucial link for enhanced tumor development [42].
Circadian rhythmicity of the CDK inhibitor p21 is achieved via inhibition by REV-ERB factors and activation by ROR factors [44]. Additionally, p21 harbors CLOCK-binding elements [45], and Per1 overexpression was reported to repress p21 [21]. P21 inactivates various cyclin/CDK complexes, which induce cell cycle phase entries, such as S-phase transition by cyclin E/CDK2. Other than Wee1, p21 is upregulated and arrhythmic in Clock mutant and Bmal1 KO mice [40, 44]. Consequently, decreased proliferation rate was observed in Bmall KO hepatocytes, which can be rescued by p21 knockdown. In contrast and despite enhanced p21 levels, increased cell proliferation was found in the epidermis of Bmal1 KO mice [46], and enhanced tumor development after tissue-specific ablation of Bmal1 has been documented [25]. An explanation might be that the regulatory function of p21 in clock gene KO mice may be masked by other cell cycle regulators also regulated by CLOCK/BMAL1, such as c-MYC or WEE1. This is seen, for example, in pleural mesothelioma cells, where P21 and WEE1 and CYCLIN E levels were altered upon knockdown of BMAL1 [41].
Adding to this picture, other cell cycle genes were identified as targets of the circadian clock, such as cyclin D1 and A [19, 47, 48].
Taken together, the clock-controlled cell cycle proteins have different sometimes even antagonistic effects on cell cycle progression. This may explain why different clock gene mutants exhibit different cell cycle- and cancer-related phenotypes. Nevertheless, this complex interaction between the circadian clock and cell cycle events probably underlies at least in part the key role for circadian rhythm alterations in carcinogenesis.
2.2 DNA Damage Response
Errors during repair of damaged DNA can cause mutations [49]. Genomic instability or accumulation of mutations within the genome is a hallmark of cancer. When damaged DNA is detected by sensor kinases, such as ATM or ATR, cells activate the cell cycle checkpoint kinases CHK1 and CHK2, which stabilize the oncogene p53. P53 in turn activates a series of genes that restrict cell cycle progression and stimulates DNA repair or, in the case of irreparable damage, triggers apoptosis [50]. Important p53 targets are Mdm2, Gadd45α, and the CDK inhibitor p21, a major cell cycle player which mediates G1 arrest and is controlled by the circadian clock (see above). Additionally, p53 and its targets Mdm2 and Gadd45α are regulated by the circadian clock [19]. MDM2 activates cell cycle arrest, but it also feeds back on p53 and inhibits its functions [37, 38, 51]. GADD45 inhibits the G2/M transition and triggers apoptosis [37].
Daily oscillation of DNA excision repair was documented in mouse skin [52]. At the clock gene level, BMAL1 was found to suppress p53 functions in human fibroblast cells and thus to induce the release from cell cycle arrest [51]. Accordingly, overexpression of BMAL1 inhibited DNA damage sensitivity [26], whereas tissue-specific ablation of Bmal1 increased the risk of genomic instability and cell cycle arrest in the epidermis [46].
In Per2 mutant mice, p53 induction and Gadd45α and Mdm2 rhythmicity were deregulated [19]. Accordingly, daytime-dependent DNA damage-induced apoptosis was perturbed in thymocytes of Per2 mutant mice [53]. Recent studies demonstrated that PER proteins modulate apoptosis and cell cycle arrest by controlling ATM and CHK2 [21, 54]. In this regard, overexpression of PER1 triggered DNA damage-induced apoptosis, whereas inhibition of PER1 blocked apoptosis in human cancer cells [21]. In contrast, CLOCK knockdown increased γ-irradiation-induced cell cycle arrest and apoptosis in human glioma cells [49].
Interestingly, p53, Gadd45α, and Mdm2 are additionally controlled by c-Myc, which is in turn regulated by BMAL1 [37]. Thus, clock-controlled c-Myc expression may be another important factor for tumorigenesis by integrating the cell cycle and DNA damage response with the circadian clock. Indeed, jetlag in mice was sufficient to uncouple p53 and c-MYC signaling in the thymus and induce tumor development [2].
Taken together, the circadian clock has been implicated in both DNA damage-induced apoptosis and DNA repair. Consequently, in cases of circadian deregulation, the occurrence of DNA mutations may increase. Moreover, mutated cells may bypass their apoptosis, which would result in accumulation of mutated cells and thereby inducing cancer. Consequently, a reasonable explanation for how clock gene manipulation leads to enhanced cancer incidence or growth rate could be their interaction with transcriptional regulators controlling cell proliferation, DNA repair, and apoptosis.
3 Does the Tumor Disrupt Circadian Rhythms?
The interconnection between the circadian clock and the cell cycle does not allow conclusions about the cause of cell cycle deregulation and circadian perturbation in cases of cancer. An intriguing hypothesis is that disturbance of one of the cycles during cancerogenesis, either the circadian clock or the cell cycle, can in turn disrupt the function of the other one. In this section, we address the possible mechanisms for the disruption of clock function in tumors.
3.1 Cancer-Related Genes
Interestingly, recent evidence supports the idea that cancer-related signals may interfere with the circadian clock machinery. The clock-controlled major cell cycle regulator MYC can induce circadian malfunction by indirectly repressing Bmal1 through upregulation of REV-ERBα and PER2 [55]. Importantly, previous studies mentioned an upregulation of Myc in human tumor tissues [42] and circadian mouse mutants [19]. Consequently, MYC is an even more important candidate for cancerogenesis by integrating disturbed circadian rhythms and cell cycle dysfunctions and thus is considered as an important target for the development of cancer therapies.
Perturbation of another oncogene, RAS, has been reported to cause circadian clock disruption. RAS transformation induced major phase shifts of Bmal1 promoter-driven luminescence in human keratinocytes, mouse fibroblasts, and human colorectal cancer cells [24]. Moreover, decreased PER2 levels and upregulated CRY1 expression were observed after RAS transformation, supporting the possibility that the activity of RAS might modulate the circadian disruption in cancer cells by influencing CLOCK/BMAL1.
3.2 Epigenetic and Posttranscriptional Modifications
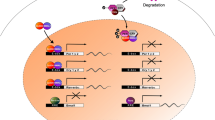
DNA methylation plays an important role in modifying gene expression posttranscriptionally, and promoter hypermethylation is a hallmark of cancer. Most core clock genes are predominantly downregulated in various cancer cell lines and tumor tissues (see Table 23.1). Thus, improper DNA methylation may suppress clock gene expression, contributing to the development and progression of cancer. Interestingly, methylated DNA immunoprecipitation microarray identified BMAL1 among the genes that are differentially methylated in ovarian cancer cells [43]. Cancer cell growth could be restored by rescuing BMAL1 expression, indicating that DNA methylation may be an important mechanism to suppress the circadian clock in cancer cells and induce cancer proliferation. Indeed, hypermethylation has been found on the promoters of core clock genes, such as PER1, PER2, CRY1, and BMAL1 in breast cancer tissue [56].
Another tumor-intrinsic mechanism which may disrupt circadian rhythms is ubiquitination. For example, transfection with the oncogenes E6/E7 in mouse fibroblasts led to BMAL1 ubiquitination and degradation after the action of the UBE3A ubiquitin ligase on this clock protein and suppression of circadian rhythms in these cells [57].
Histone modifications such as acetylation/deacetylation can also control gene expression and in turn underlie circadian clock control [58]. Interestingly, the histone deacetylase sirtuin 1 (SIRT1) was identified as a circadian clock component, as it deacetylates BMAL1 and PER2 [59]. Low levels of SIRT1 were documented in various colorectal cancer cell lines and tumor tissues [60], and a correlation was found between expression levels of SIRT1, altered clock gene expression and the outcome of pancreatic adenocarcinoma in patients [61]. Collectively, these data indicate that tumor components may direct epigenetic modifications, leading to disruption of circadian rhythms.
4 Conclusions and Perspectives
In conclusion, circadian disruption within tumor tissues and in the host enhances cancer progression, and a poorer prognosis was documented in cancer patients with altered circadian rhythms. Thus, improving circadian rhythms in the host and in the tumor may be an important strategy to address cancer therapy.
Also important is cancer chronotherapy – the timed administration of anticancer drugs. The circadian regulation of physiological processes, such as metabolism or detoxification, has severe consequences on the outcome of anticancer therapies [62]. For example, the treatment efficacy and patient survival were improved by rhythmic delivery of the therapeutic into colorectal cancer [63]. Studying the timing of anticancer therapies will allow maximal therapeutic effect with minimal cytotoxic side effects, which may dramatically enhance the life quality of cancer patients.
However, the molecular mechanism linking circadian disruption and cancer should be examined based on specific cancer subtypes. The alteration of clock gene expression differs between cancer subtypes and thus does not allow generalizations about the function of circadian clock genes or the overall circadian system on the development of cancer. Precise characterization of specific cancer subtypes could be used to develop therapeutic approaches involving circadian control, tailored for each cancer type.
References
Kiessling S, Eichele G, Oster H (2010) Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest 120(7):2600–2609
Lee S, Donehower LA, Herron AJ, Moore DD, Fu L (2010) Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One 5(6):e10995
Straif K et al (2007) Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8(12):1065–1066
Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J (2013) Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 138(1):291–301
Lie JA et al (2011) Night work and breast cancer risk among Norwegian nurses: assessment by different exposure metrics. Am J Epidemiol 173(11):1272–1279
Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E (2008) Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer J Int Cancer 123(9):2148–2151
Logan RW et al (2012) Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol 188(6):2583–2591
Filipski E et al (2004) Effects of chronic jet lag on tumor progression in mice. Cancer Res 64(21):7879–7885
Kettner NM, Katchy CA, Fu L (2014) Circadian gene variants in cancer. Ann Med 46(4):208–220
Zienolddiny S et al (2013) Analysis of polymorphisms in the circadian-related genes and breast cancer risk in Norwegian nurses working night shifts. Breast Cancer Res: BCR 15(4):R53
Truong T et al (2014) Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr Relat Cancer 21(4):629–638
Zhu Y et al (2009) Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res 69(24):9315–9322
Zhu Y, Brown HN, Zhang Y, Stevens RG, Zheng T (2005) Period3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol Biomarkers Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 14(1):268–270
Chu LW et al (2008) Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis 11(4):342–348
Grundy A et al (2013) Shift work, circadian gene variants and risk of breast cancer. Cancer Epidemiol 37(5):606–612
Zhu Y et al (2008) Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat 107(3):421–425
Geng P et al (2015) Genetic association between PER3 genetic polymorphisms and cancer susceptibility: a meta-analysis. Medicine 94(13):e568
Hoffman AE et al (2009) Clock-cancer connection in non-Hodgkin’s lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res 69(8):3605–3613
Fu L, Pelicano H, Liu J, Huang P, Lee C (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111(1):41
Chen-Goodspeed M, Lee CC (2007) Tumor suppression and circadian function. J Biol Rhythms 22(4):291–298
Gery S et al (2006) The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22(3):375–382
Gauger MA, Sancar A (2005) Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res 65(15):6828–6834
Bunger MK et al (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103(7):1009–1017
Relogio A et al (2014) Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet 10(5):e1004338
Zeng ZL et al (2010) Effects of the biological clock gene Bmal1 on tumour growth and anti-cancer drug activity. J Biochem 148(3):319–326
Zeng ZL et al (2014) Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 20(4):1042–1052
Vitaterna MH et al (1994) Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 264(5159):719–725
Antoch MP et al (2008) Disruption of the circadian clock due to the clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle 7(9):1197–1204
Hirner H et al (2012) Impaired CK1 delta activity attenuates SV40-induced cellular transformation in vitro and mouse mammary carcinogenesis in vivo. PLoS One 7(1):e29709
Relles D et al (2013) Circadian gene expression and clinicopathologic correlates in pancreatic cancer. J Gastrointest Surg: Off J Soc Surg Aliment Tract 17(3):443–450
Tokunaga H et al (2008) Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand 87(10):1060–1070
Suzuki T et al (2008) Period is involved in the proliferation of human pancreatic MIA-PaCa2 cancer cells by TNF-alpha. Biomed Res 29(2):99–103
Sotak M, Polidarova L, Ergang P, Sumova A, Pacha J (2013) An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Int J Cancer J Int Cancer 132(5):1032–1041
Yang X, Wood PA, Ansell C, Hrushesky WJ (2009) Circadian time-dependent tumor suppressor function of period genes. Integr Cancer Ther 8(4):309–316
Iurisci I et al (2006) Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer Res 66(22):10720–10728
Li XM et al (2010) Cancer inhibition through circadian reprogramming of tumor transcriptome with meal timing. Cancer Res 70(8):3351–3360
Sotak M, Sumova A, Pacha J (2014) Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med 46(4):221–232
Matsuo T et al (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302(5643):255–259
Perez-Roger I, Solomon DL, Sewing A, Land H (1997) Myc activation of cyclin E/Cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27(Kip1) binding to newly formed complexes. Oncogene 14(20):2373–2381
Miller BH et al (2007) Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A 104(9):3342–3347
Elshazley M et al (2012) The circadian clock gene BMAL1 is a novel therapeutic target for malignant pleural mesothelioma. Int J Cancer J Int Cancer 131(12):2820–2831
Jamerson MH, Johnson MD, Dickson RB (2004) Of mice and Myc: c-Myc and mammary tumorigenesis. J Mammary Gland Biol Neoplasia 9(1):27–37
Yeh CM et al (2014) Epigenetic silencing of ARNTL, a circadian gene and potential tumor suppressor in ovarian cancer. Int J Oncol 45(5):2101–2107
Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F (2008) The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem 283(8):4535–4542
Alhopuro P et al (2010) Mutations in the circadian gene CLOCK in colorectal cancer. Mol Cancer Res: MCR 8(7):952–960
Geyfman M et al (2012) Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A 109(29):11758–11763
Liu Y et al (2013) The transcription factor DEC1 (BHLHE40/STRA13/SHARP-2) is negatively associated with TNM stage in non-small-cell lung cancer and inhibits the proliferation through cyclin D1 in A549 and BE1 cells. Tumour Biol: J Int Soc Oncodev Biol Med 34(3):1641–1650
Wang Y, Kojetin D, Burris TP (2015) Anti-proliferative actions of a synthetic REV-ERBalpha/beta agonist in breast cancer cells. Biochem Pharmacol 96(4):315–322
Wang F, Li C, Yongluo, Chen L (2015) The circadian gene clock plays an important role in cell apoptosis and the dna damage response in vitro. Technol Cancer Res Treat 15(3):480–486
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S (2004) Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73:39–85
Mullenders J, Fabius AW, Madiredjo M, Bernards R, Beijersbergen RL (2009) A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One 4(3):e4798
Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A (2011) Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A 108(46):18790–18795
Lee CC (2006) Tumor suppression by the mammalian period genes. Cancer Causes Control 17(4):525–530
Yang X, He X, Yang Z, Jabbari E (2012) Mammalian PER2 regulates AKT activation and DNA damage response. Biochem Cell Biol = Biochim Biol Cell 90(6):675–682
Altman BJ et al (2015) MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab 22(6):1009–1019
Kuo SJ et al (2009) Disturbance of circadian gene expression in breast cancer. Virchows Archiv: Int J Pathol 454(4):467–474
Gossan NC et al (2014) The E3 ubiquitin ligase UBE3A is an integral component of the molecular circadian clock through regulating the BMAL1 transcription factor. Nucleic Acids Res 42(9):5765–5775
Etchegaray JP, Lee C, Wade PA, Reppert SM (2003) Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421(6919):177–182
Chang HC, Guarente L (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153(7):1448–1460
Pazienza V et al (2012) SIRT1 and the clock gene machinery in colorectal cancer. Cancer Invest 30(2):98–105
Tavano F et al (2015) SIRT1 and circadian gene expression in pancreatic ductal adenocarcinoma: effect of starvation. Chronobiol Int 32(4):497–512
Levi F, Schibler U (2007) Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47:593–628
Innominato PF, Levi FA, Bjarnason GA (2010) Chronotherapy and the molecular clock: clinical implications in oncology. Adv Drug Deliv Rev 62(9–10):979–1001
Pogue-Geile KL, Lyons-Weiler J, Whitcomb DC (2006) Molecular overlap of fly circadian rhythms and human pancreatic cancer. Cancer Lett 243(1):55–57
Sato F et al (2009) PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem 146(6):833–838
Karantanos T et al (2013) Expression of clock genes in patients with colorectal cancer. Int J Biol Markers 28(3):280
Mostafaie N et al (2009) Correlated downregulation of estrogen receptor beta and the circadian clock gene Per1 in human colorectal cancer. Mol Carcinog 48(7):642–647
Oshima T et al (2011) Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep 25(5):1439–1446
Krugluger W et al (2007) Regulation of genes of the circadian clock in human colon cancer: reduced period-1 and dihydropyrimidine dehydrogenase transcription correlates in high-grade tumors. Cancer Res 67(16):7917–7922
Mazzoccoli G et al (2011) Clock gene expression levels and relationship with clinical and pathological features in colorectal cancer patients. Chronobiol Int 28(10):841–851
Zhao H et al (2014) Prognostic relevance of Period1 (Per1) and Period2 (Per2) expression in human gastric cancer. Int J Clin Exp Pathol 7(2):619–630
Cadenas C et al (2014) Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 13(20):3282–3291
Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL (2007) Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia 9(10):797–800
Kolomeichuk SN, Gurov EV, Piskunova TS, Tyndyk ML, Anisimov VN (2011) Expression of circadian Per1 and Per2 genes in the liver and breast tumor tissues of HER2/neu transgenic mice of different age. Bull Exp Biol Med 151(2):227–229
Lin YM et al (2008) Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog 47(12):925–933
Geusz ME, Blakely KT, Hiler DJ, Jamasbi RJ (2010) Elevated mPer1 gene expression in tumor stroma imaged through bioluminescence. Int J Cancer J Int Cancer 126(3):620–630
Gery S et al (2007) Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 13(5):1399–1404
Xia HC et al (2010) Deregulated expression of the Per1 and Per2 in human gliomas. Can J Neurol Sci J Can Sci Neurol 37(3):365–370
Kovacheva VP et al (2009) Raising gestational choline intake alters gene expression in DMBA-evoked mammary tumors and prolongs survival. FASEB J: Off Publ Fed Am Soc Exp Biol 23(4):1054
Cao Q et al (2009) A role for the clock gene per1 in prostate cancer. Cancer Res 69(19):7619–7625
Yeh KT et al (2005) Abnormal expression of period 1 (PER1) in endometrial carcinoma. J Pathol 206(1):111–120
Shih HC et al (2005) Disturbance of circadian gene expression in endometrial cancer: detection by real-time quantitative RT-PCR. Oncol Rep 14(6):1533–1538
Lengyel Z et al (2013) Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol: J Int Soc Oncodev Biol Med 34(2):811–819
Hsu CM, Lin SF, Lu CT, Lin PM, Yang MY (2012) Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol: J Int Soc Oncodev Biol Med 33(1):149–155
Roe OD et al (2009) Genome-wide profile of pleural mesothelioma versus parietal and visceral pleura: the emerging gene portrait of the mesothelioma phenotype. PLoS One 4(8):e6554
Oda A et al (2009) Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res 29(4):1201–1209
Wood PA et al (2008) Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol Cancer Res: MCR 6(11):1786–1793
Yang X et al (2009) Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res Treat 117(2):423–431
Hua H et al (2007) Inhibition of tumorigenesis by intratumoral delivery of the circadian gene mPer2 in C57BL/6 mice. Cancer Gene Ther 14(9):815–818
Mazzoccoli G et al (2012) Altered expression of the clock gene machinery in kidney cancer patients. Biomed Pharmacother = Biomed Pharmacother 66(3):175–179
Wang F, Luo Y, Li C, Chen L (2014) Correlation between deregulated expression of PER2 gene and degree of glioma malignancy. Tumori 100(6):e266–e272
Miyazaki K, Wakabayashi M, Hara Y, Ishida N (2010) Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells: Devoted Mol Cell Mech 15(4):351–358
Cheng AY et al (2015) Construction of a plasmid for overexpression of human circadian gene period2 and its biological activity in osteosarcoma cells. Tumour Biol: J Int Soc Oncodev Biol Med 36(5):3735
Yu H et al (2013) Cryptochrome 1 overexpression correlates with tumor progression and poor prognosis in patients with colorectal cancer. PLoS One 8(4):e61679
Jung CH et al (2013) Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol Rep 29(6):2109
Xue X et al (2014) Silencing NPAS2 promotes cell growth and invasion in DLD-1 cells and correlated with poor prognosis of colorectal cancer. Biochem Biophys Res Commun 450(2):1058–1062
Yi C et al (2010) The circadian gene NPAS2 is a novel prognostic biomarker for breast cancer. Breast Cancer Res Treat 120(3):663–669
Madden MH et al (2014) Circadian pathway genes in relation to glioma risk and outcome. Cancer Causes Control 25(1):25–32
Wu Y et al (2012) The BHLH transcription factor DEC1 plays an important role in the epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol 41(4):1337–1346
Liu Y et al (2013) DEC1 is positively associated with the malignant phenotype of invasive breast cancers and negatively correlated with the expression of claudin-1. Int J Mol Med 31(4):855–860
Shi XH et al (2011) DEC1 nuclear expression: a marker of differentiation grade in hepatocellular carcinoma. World J Gastroenterol: WJG 17(15):2037–2043
Wei H et al (2014) MicroRNA target site polymorphisms in the VHL-HIF1alpha pathway predict renal cell carcinoma risk. Mol Carcinog 53(1):1–7
Nishiwaki T, Daigo Y, Kawasoe T, Nakamura Y (2000) Isolation and mutational analysis of a novel human cDNA, DEC1 (deleted in esophageal cancer 1), derived from the tumor suppressor locus in 9q32. Genes Chromosomes Cancer 27(2):169–176
Wong VC et al (2011) Abrogated expression of DEC1 during oesophageal squamous cell carcinoma progression is age- and family history-related and significantly associated with lymph node metastasis. Br J Cancer 104(5):841–849
Jia YF et al (2013) Differentiated embryonic chondrocyte-expressed gene 1 is associated with hypoxia-inducible factor 1alpha and Ki67 in human gastric cancer. Diagn Pathol 8:37
Yunokawa M et al (2007) Differential regulation of DEC2 among hypoxia-inducible genes in endometrial carcinomas. Oncol Rep 17(4):871–878
Muscat GE et al (2013) Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol 27(2):350–365
Davis LM et al (2007) Amplification patterns of three genomic regions predict distant recurrence in breast carcinoma. J Mol Diagn: JMD 9(3):327
Chin K et al (2006) Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10(6):529–541
Kourtidis A et al (2010) An RNA interference screen identifies metabolic regulators NR1D1 and PBP as novel survival factors for breast cancer cells with the ERBB2 signature. Cancer Res 70(5):1783–1792
Mond M et al (2014) Nuclear receptor expression in human differentiated thyroid tumors. Thyroid: Off J Am Thyroid Assoc 24(6):1000–1011
Kottorou AE et al (2012) Altered expression of NFY-C and RORA in colorectal adenocarcinomas. Acta Histochem 114(6):553–561
Knower KC et al (2013) Distinct nuclear receptor expression in stroma adjacent to breast tumors. Breast Cancer Res Treat 142(1):211–223
Zhang S et al (2012) ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One 7(3):e31127
Moretti RM, Montagnani Marelli M, Sala A, Motta M, Limonta P (2004) Activation of the orphan nuclear receptor RORalpha counteracts the proliferative effect of fatty acids on prostate cancer cells: crucial role of 5-lipoxygenase. Int J Cancer J Int Cancer 112(1):87–93
Xiong G, Wang C, Evers BM, Zhou BP, Xu R (2012) RORalpha suppresses breast tumor invasion by inducing SEMA3F expression. Cancer Res 72(7):1728–1739
Karasek M, Gruszka A, Lawnicka H, Kunert-Radek J, Pawlikowski M (2003) Melatonin inhibits growth of diethylstilbestrol-induced prolactin-secreting pituitary tumor in vitro: possible involvement of nuclear RZR/ROR receptors. J Pineal Res 34(4):294–296
Chen ST et al (2005) Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis 26(7):1241–1246
Maronde E, Motzkus D (2003) Oscillation of human period 1 (hPER1) reporter gene activity in human neuroblastoma cells in vivo. Chronobiol Int 20(4):671
Yang MY et al (2011) Altered expression of circadian clock genes in human chronic myeloid leukemia. J Biol Rhythms 26(2):136–148
Ye H, Yang K, Tan XM, Fu XJ, Li HX (2015) Daily rhythm variations of the clock gene PER1 and cancer-related genes during various stages of carcinogenesis in a golden hamster model of buccal mucosa carcinoma. Onco Targets Ther 8:1419–1426
Xiang S et al (2012) Oscillation of clock and clock controlled genes induced by serum shock in human breast epithelial and breast cancer cells: regulation by melatonin. Breast Cancer: Basic Clin Res 6:137–150
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Key Questions of Interest and Suggested Readings
Key Questions of Interest and Suggested Readings
-
Is circadian clock disruption the cause of cancerogenesis or does cancer induce circadian clock disruption? Hints exist to support both hypotheses, but further studies are required to address this key question.
-
How could circadian clock disruption enhance tumor growth? Dysregulation of the cell cycle by altered expression of cell cycle regulators such as WEE1 or c-MYC in circadian clock mutant mice affects the speed of the cell cycle and thus may regulate cancer progression [19, 38].
-
What tumor-intrinsic mechanism could downregulate clock genes? Possible factors are DNA methylation [43], ubiquitination [57], or histone modifications [59].
-
How can we take advantage of the link between the circadian clock and cancer? Improving circadian rhythms in the host and tumor tissue may reduce cancer progression [35, 36]. Cancer chronotherapy [63] uses the circadian time to treat cancer most effectively.
Rights and permissions
Copyright information
© 2017 Springer (India) Pvt. Ltd.
About this chapter
Cite this chapter
Kiessling, S., Cermakian, N. (2017). Clock Genes and Cancer. In: Kumar, V. (eds) Biological Timekeeping: Clocks, Rhythms and Behaviour. Springer, New Delhi. https://doi.org/10.1007/978-81-322-3688-7_23
Download citation
DOI: https://doi.org/10.1007/978-81-322-3688-7_23
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-3686-3
Online ISBN: 978-81-322-3688-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)