Abstract
Alyssum bertolonii is one of the ten European species of Alyssum that hyperaccumulate nickel, and it was the first plant species reported to do so. The species has been suggested to be a useful indicator plant in prospecting for nickel, and the historically well-known connection between lithology and A. bertolonii was already recorded in 1583 by Cesalpino. In the last 20 years, this species has been the subject of intensive physiological, genetic, and botanical researches aimed at exploring the basis of its metal accumulation and tolerance and its life history and the genetic consequences of its restricted distribution being confined to heavy-metal-rich serpentine (ultramafic) substrates.
In the perspective of the present book, A. bertolonii could represent an excellent model and a particularly valuable resource for investigating the biogeochemical interactions in serpentine soils from the genetic/physiological level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
14.1 The Serpentine Factor as a Tool for Studying Biogeochemical Interactions
Serpentine rocks (or ophiolites) derive their name from the olive greenish-gray color, striped in different shades, that looks like the skin of a snake (serpens in latin, oϕiç – ophis in Greek). They originate from metamorphic alterations of peridotites with water and may form near the Earth’s surface or in the upper part of the Earth’s mantle during subduction events. In a wider concept, the same term is extended to all substrates which are derived from the weathering of ultramafic (igneous or metamorphic) rocks that contain at least 70% hydrous magnesium – iron phyllosilicates such as antigorite and chrysotile, minerals with the general formula (Mg, FeII)3Si2O5(OH)4 (Brooks 1987; Kruckeberg 2002). Serpentine outcrops are spread worldwide within 22 of the 35 floristic regions (as defined by Takhtajan 1986), from sea level up to 2,000–3,000 m, ranging from 0 to 70 latitude degrees but cover no more than 1% of total Earth’s surface (Fig. 14.1).
Worldwide distribution of ultramafic outcrops. 1, Western North America (from Alaska to northern California); 2, eastern North America (from Labrador and Newfoundland to South Carolina); 3, Caribbean (Cuba and Puerto Rico); 4, Guyana; 5, Andes; 6, Brazil; 7, Ghana; 8, southern Africa (from Zaire to South Africa); 9, northern Europe (Scotland and Fennoscandia); 10, western Europe and northern Morocco; 11, eastern Europe, Anatolia, and Cyprus; 13, Oman and south-western Asia; 14. central Asia; 15, north-western India and Bangladesh; 16, Ceylon; 17, Japan; 18, Indopacific Islands (from Malay archipelago to Solomon Islands); 19, New Caledonia; 20, south-western Australia; 21, eastern Australia; 22, New Zealand. Black dots indicate localization of the presence of ultramafic outcrop within wider areas defined by gray color
Serpentine soils have such extreme chemical and physical properties to render them potentially toxic and unsuitable for most plant species (Brooks 1987; Brady et al. 2005; Chiarucci et al. 1998) and for many microorganisms (Mengoni et al. 2010) (Table 14.1).
As a general rule, in comparison with other rock types, ultramafites are strongly enriched in elements such as iron, nickel, cobalt, and chromium, whereas they present much lower abundance of plant nutrients, such as calcium, nitrogen, phosphorus, and potassium. The relatively high concentrations of nickel and cobalt in serpentines largely depends on the fact that the ionic radii of their divalent states are very close to that of Mg2+ so that ionic substitution readily takes place into magnesium-rich minerals, which are dominant in ophiolitic rocks. Chromium is enriched because Cr3+ readily substitutes in Fe3+ minerals (Brooks 1987). In any event, the cation concentration of these soils is known to vary markedly as they are derived from world-spread rocks that occur under a wide range of climates (Kruckeberg 2002).
Nickel is often believed to play a major role in determining the flora and vegetation in many serpentine areas (Brooks 1987; Vergnano Gambi 1992; Robinson et al. 1997). Nickel has a relatively high availability in the range of pH values of serpentine soils and values of bioavailable nickel in serpentine soils are often significantly higher than the toxicity threshold (as defined for common plants; see Kabata-Pendias and Pendias 1991). However, not all serpentine soils are ever nickel toxic as shown by early experiments (Slingsby and Brown 1977), suggesting that serpentine adaptation is not always linked to the presence of heavy metals such as Ni. The discovery of a large number of taxa that accumulate Ni in their tissues (Brooks 1987; Bani et al. 2007) may be a further evidence of the high selective pressure that this element exerts on serpentine plants. However, clear evidence for nickel toxicity in any serpentine is sadly lacking in the literature. Although relatively high concentrations of Co are available in plants in ultramafic soils, its accumulation in plant tissues is rare (Robinson et al. 1997). On the other hand, Cr has very low exchangeable concentrations in the soil and few species are known that truly hyperaccumulate this element (Brooks 1987; Robinson et al. 1997; Chiarucci 2003; Zhang et al. 2007). Another possible selective factor is the high concentration of Mg and/or the deficiency of Ca, i.e., the unfavorable ratio of Mg to Ca in serpentine soils. Strong effects of the Mg/Ca quotient (Brooks 1987; Proctor and Woodell 1975; Kruckeberg 2002; Roberts and Proctor 1992) and the toxic influence of Mg (Proctor 1971; Brooks and Yang 1984; Bani et al. 2007) have been found in several studies, and the addition of Ca to serpentine soils may reverse the unfavorable conditions of these soils, at least to some extent (Proctor 1971; Brooks 1987; Brady et al. 2005). Moreover, nonserpentine soils with high Mg concentrations share several floristic elements with ultramafic environments (Mota et al. 2008), suggesting that serpentine adaptation may often be explained as a mere tolerance to the “magnesium factor.”
Another problem can be represented by the low nutrient content of ophiolites (Brooks 1987; Proctor and Nagy 1992). Fertilization with P, K, or N enhanced cover and productivity and resulted in a change in the floristic composition of serpentine plant communities (Huenneke et al. 1990; Proctor and Nagy 1992; Chiarucci et al. 1999; Chiarucci and Maccherini 2007; Bani et al. 2007).
The relative available concentrations of all the above-mentioned elements in water matrices are mutually influenced, as a consequence of their direct chemical interactions and their indirect contribution to the soil organic matter content and pH values. As a consequence, the effects of fertilization with a certain nutrient elements may lead to the misinterpretation of its actual role in producing the “serpentine factor” (Brooks 1987).
The physical conditions of serpentine are also hostile to many plants. Serpentine outcrops are often steep and relatively rocky, making them particularly vulnerable to erosion, which results in shallow freely draining soils. In addition, they generally have negligible contents of silt and clay. Combined, these factors yield an environment with little moisture and depressed nutrient levels (Kruckeberg 2002; Proctor and Woodell 1975; Walker 1954). Furthermore, the scarce plant cover also promotes erosion and elevated soil thermal excursions (Kruckeberg 2002). Each of these factors poses an additional stress to plant life. As a collective result, three traits can be identified as strictly characteristic of serpentine environments: poor plant productivity, high rates of endemism, and vegetation types distinct from those of neighboring areas (Whittaker 1954).
Jointly in the still elusive “serpentine factor,” the chemical, physical, and biotic components of such soils produce what Jenny (1980) defined as the “serpentine syndrome,” i.e., the cumulative effect of these components on plant form, development, and distribution. Such a “syndrome” is the key for the evolution of endemic taxa (Pichi Sermolli 1948; Kruckeberg 1954; Kruckeberg and Kruckeberg 1990), and the reason is that serpentine outcrops have to be considered as “ecological islands” (Lefèbvre and Vernet 1990), taking also into account that they are ubiquitous but patchily distributed. Because of all the above-mentioned reasons, the linkage between the ophiolites and their flora generates an extremely valuable and irreplaceable tool for studying bio–geo interactions.
14.2 Plants that “Like” Metals
The notion that species are indicators of particular environments is a time-honored one in plant science (Kruckeberg 2002). This concept was widely exploited even in mineral exploration, so that the first practical geobotanists can really be identified in the mediaeval miners and metallurgists. In fact, the biological method for prospecting (geobotany), depending only on visual observation of vegetation cover, has a very long history dating back at least to Roman times, whereas biogeochemical procedures, depending on advances in analytical chemistry, date back only to the last century (Brooks 1998). As a consequence, the connection of a specific flora to a specific environment seemed to be so strong to have allowed a whole profit-based discipline, such as the mineral exploration itself, to develop; this depends on the amazing ability of some plants to evolve tolerance to unfavorable substrates like the metal-enriched ones. Metal-adapted genotypes are the result of the Darwinian natural selection of metal-tolerant individuals selected from surrounding nonmetallicolous populations (Antonovics et al. 1971; Baker 1987; Ernst 2006). Once tolerance evolved, a tight link between plants and the metal-rich environment is established, depending on the fact that these plant populations are competitive only in such environments, where the fitness takes advantage from the acquired tolerance mechanisms. Such selection can lead ultimately to speciation and the evolution of endemic taxa.
These unique plants with an ability to tolerate metal toxicities and survive and reproduce on metalliferous soils are called metallophytes. The majority of them are able to tolerate specific metals in the substrate by physiologically restricting the entry of metals into the root and/or their transport to the shoot (termed “excluders” by Baker 1981). A few species, however, have extremely specialized biological mechanisms in that they are able to accumulate, or even “hyperaccumulate,” metals in their shoots at concentrations that can exceed 2% of their dry weight (DW) (Baker et al. 2000). These latter plants are the so-called metal “hyperaccumulators,” a term first coined by Brooks et al. (1977) to define particular plants living on nickel-rich serpentine substrates with nickel concentrations >1,000 μg g−1 DW in their above-ground parts. Hyperaccumulators may be at a disadvantage when resources are abundant, but thrive in disturbed habitats because, for example, the high concentration of metals in their organs deters some animals from grazing upon them (Pollard and Baker 1997; Boyd 2004; Jiang et al. 2005). Among the metallophytes, metal hyperaccumulators can represent the most indicative case of the linkage between a certain plant and a certain soil, considering also that for some hyperaccumulators the presence of the metal at high concentration in the soil is essential for a normal growth (Küpper et al. 2001). Hyperaccumulators have therefore been studied as peculiar interesting examples of evolution and adaptation, and as useful indicators in prospecting for metals (Brooks 1983). Recently, the development of new plant-based technologies for the remediation of polluted sites (Vassilev et al. 2004) has stimulated new research interest on metallophytes (Whiting et al. 2004), and on the underlying physiological mechanisms that enable some of these plants to take up such extraordinary amounts of metals.
14.3 Evolution of Serpentine Plants
Serpentine habitats are geologic islands in a “sea” of other soil types. When these rocks were exposed, new species spread on to them from the surrounding substrates. In due course, those that could colonize and survive on serpentines evolved on a different route from their nonserpentine relatives. In several cases, the new species survived on a patch of serpentine because they were poor competitors on other substrates. As a result of this “island” effect, serpentine soils show a large number of species that are found only on such substrates and have highly restricted geographical ranges.
The subject of adaptation of plant species to the total environment of serpentine soils has occupied scientist for many years. Plant biologists have studied in depth the ecology, physiology, phylogeny, and taxonomy of plants occurring on serpentine soils, the so-called serpentinophytes (for a review see Brady et al. 2005). The ecological island model has boosted much research on evolution and adaptation and provoked discussion on the microevolutionary dynamics of metal tolerance in plants, from the population to the single-gene level (for examples see Nyberg Berglund et al. 2004; Kazakou et al. 2010; Quintela-Sabarís et al. 2010; Mengoni et al. 2000, 2001, 2003a, b, c; Rajakaruna et al. 2003; Vekemans and Lefèbvre 1997).
The tight bond between these plants and their environment has been proved from a long time ago by Kruckeberg (1950, 1954), through experiments showing that serpentine-tolerant species and races are limited to serpentine soils because of their inability to compete in nonserpentine environments. This suggests that along the evolutionary trajectory toward serpentine tolerance, genetic trade-offs occur, rendering the serpentine-adapted plant species or ecotypes unable to re-colonize their parental habitat. Moreover, the self-fertility of metal-tolerant populations has proved to be usually much greater than that of nontolerant taxa, presumably as a strategy to reduce reduction of tolerance by flow of nontolerant genes from the surrounding populations (Brady et al. 2005; Brooks 1987) or could be a side effect of previous history of higher self-pollination rates because of the expected low number of first colonizers. Thus, serpentine-tolerant taxa are often endemic to serpentine regions (Brady et al. 2005) and, indeed, the occurrence of plant species restricted to serpentine substrates was documented as long ago as the sixteenth century (Vergnano Gambi 1992).
Serpentinophytes comprise facultative taxa, plants that will grow quite well on serpentine soils without having a specific requirement for any of the edaphic or physical properties of the substrate and obligate taxa that are presumed to grow on serpentine because of a specific nutritional or other requirement which only such soils can provide, mainly the protection from biotic factors present in nonserpentine substrates (Brooks 1987; Boyd 2004). Widespread serpentine endemics can act as flag species, because they are loyal to the substrate; they are thus good indicator plants for serpentine. Furthermore, these species often display unusual and characteristic features in their habitus. In fact, studies of serpentine floras have noted the so-called serpentinomorphoses (Novák 1928), morphological differences between populations or taxa growing on serpentine and nonserpentine soils (Kruckeberg 2002) that concur to plant adaptation to the serpentine factor. The most frequent serpentinomorphoses are xeromorphic foliage, including reduced leaf size and sclerophylls, development of a large root system, dwarfism, plagiotropism, glabrescence or pubescence, glaucescence, and erythrism (Vergnano Gambi 1993; Brady et al. 2005).
Serpentine plants also show a wide range of physiological strategies to adapt to the particular substrate they colonize. Indeed, the most intriguing ones are those related to overcoming the often high heavy metal concentrations present. In relation to such strategies, the two main categories, excluders and accumulators sensu Baker (1981), can be found. Tolerant plants are often excluders, limiting the entry and root-to-shoot translocation of trace metals. Differential uptake and transport between root and shoot in excluders leads to more-or-less constant low shoot levels over a wide range of external concentrations. On the other hand, accumulators concentrate metals in plant parts from low or high background levels. Among the latter, a class of rare plants shows extreme behavior in metal uptake and translocation to the shoots are the so-called hyperaccumulators (Brooks et al. 1977) as mentioned above. Inevitably, metal hyperaccumulation is associated with a strongly enhanced ability to detoxify the metal accumulated in above-ground tissues, and thus with metal hypertolerance (Krämer 2010; Rascio and Navari-Izzo 2011). Metal hyperaccumulation requires complex alterations in the plant metal homeostasis network. Briefly, the main processes supposed to be involved are increased root metal uptake rates, enhanced rates of metal loading from the root symplasm into the apoplastic xylem, highly effective metal detoxification, and sequestration in the leaves (Krämer 2010).
Hyperaccumulators occur in over 54 different families of angiosperms, and very few species among conifers and pteridophytes (see Krämer 2010 for a comprehensive list). Because Ni hyperaccumulation occurs in a broad range of unrelated families, it is certainly of polyphyletic origin (Macnair 2003). The Brassicaceae family is relatively rich in Ni hyperaccumulators, in particular the genera Alyssum and Noccaea (Thlaspi s.l.). In Sect. 14.5 a phylogenetic discussion about the evolution of hyperaccumulation in tribe Alysseae is presented.
The selective factors causing the evolution of hyperaccumulation are unknown and difficult to identify retrospectively. Increased metal tolerance, protection against herbivores or pathogens, inadvertent uptake, drought tolerance, and allelopathy are the different nonmutually exclusive hypotheses formulated so far (Boyd and Martens 1992). Anyway, the supposition of defense against natural enemies is certainly the most accepted one (Boyd 2004, 2007). Whatever the reason of the evolution of this particular phenomenon was, metal hyperaccumulators can surely be the most representative emblem of the link between geology and plant life, thus representing a valuable model system for studying biogeochemical interactions.
14.4 Hyperaccumulation as a Variable Trait
Whatever the physiological strategies for nickel hyperaccumulation are, it is of fundamental importance to investigate bio–geo interactions, i.e., if possible variations in the soil, in terms of chemical characteristics of the substrates and spatial distribution, can affect plant variability both in terms of phenotype and of selective pressure on target genes for tolerance and hyperaccumulation. In the field, individual plants of a metal-hyperaccumulating species exhibit wide phenotypic variation, even within a single population (Boyd et al. 1999; Macnair 2002). Obviously, the two most important determinants are bioavailable soil metal concentration and individual genotype. Plant metal concentrations may be expected to be related to soil metal levels. However, it is also possible that they could be relatively insensitive to those of the soil, especially when the curve relating plant metal uptake to the soil concentrations suggests saturation at quite a low external metal concentration (Baker 1981).
Molecular variability in genetic and biochemical pathways (Krämer 2010; Verbruggen et al. 2009) involved in metal accumulation and metal tolerance can also lead to variation in plant metal concentrations. In Noccaea (Thlaspi) caerulescens and Cardaminopsis (Arabidopsis) halleri, the variability of Zn and Cd accumulation has been widely investigated (Assunçao et al. 2003, 2008; Bert et al. 2002; Macnair, 2002; Taylor and Macnair 2006), showing that there is heritable variation in degree of metal accumulation between local populations and that microevolutionary adaptation plays important role on the onset of the enhanced tolerance in metallicolous populations. In particular, it has been reported that metallicolous populations are of polyphyletic origin (Verbruggen et al. 2009). In other hyperaccumulators such as Sedum alfredii (Crassulaceae), both Zn and Cd hyperaccumulations are not constitutive at the species level but confined to metallicolous populations (Deng et al. 2007; Yang et al. 2006). The distinct intraspecific variations in S. alfredii provide very useful potential material for genetic and physiological dissection of the hyperaccumulation trait in a species not belonging to the Brassicaceae family. In N. caerulescens the variation in Cd accumulation among populations is correlated with the variation in Zn accumulation suggesting the hypothesis of common determinants for Cd and Zn hyperaccumulation (Verbruggen et al. 2009). However, N. caerulescens populations from southern France do not show such a correlation (Escarré et al. 2000), indicating that molecular mechanisms correlating Zn and Cd accumulation are variable among populations and no simple conclusions can be drawn about the hyperaccumulating phenotype even in a constitutive hyperaccumulator.
Ni hyperaccumulation in N. caerulescens also shows considerable variation and seems to be confined to serpentine populations. Moreover, Ni tolerance and Ni accumulation are not correlated (Richau and Schat 2009). Concerning the preference for Zn and Ni, the Turkish serpentine endemics Masmenia rosularis, Noccaea violascens, and Thlaspiceras oxyceras (all species formerly included in Thlaspi s.l.), contrarily to N. caerulescens, do not seem to take up Zn over Ni, suggesting that different strategies for Ni hyperaccumulation may have been evolved within the tribe Noccaeeae (Peer et al. 2003). Recently (Kazakou et al. 2010), in an effort to characterize the Ni hyperaccumulation capacity of the serpentine endemic Alyssum lesbiacum over all its distribution area, large inter-population differences were recorded and related to soil Ni availability. Extreme intra-specific variation for Ni has also been found in the South African hyperaccumulator Senecio coronatus (Boyd et al. 2008) for which differences in elemental content of, e.g., Ca, Fe, Mn, and Zn have recorded. Intra-specific variability in metal uptake has also been shown for other metals such as Mn in Gossia bidwillii (Myrtaceae) (Fernando et al. 2007).
14.5 Phylogenetic Pattern of Ni Hyperaccumulation in Alyssum and Its Relatives
Hyperaccumulation of nickel is a rare physiological adaptation shared worldwide by a small number of serpentine endemic or subendemic plants (ca. 360 species), especially at tropical and subtropical latitudes. Despite the fact that a large amount of tropical flora is still waiting to be studied, the relatively few Ni hyperaccumulator species we know, even in the richest and best known serpentine floras of Northern hemisphere, suggest this ability is unlikely shared by more than a small percentage of the metallophytes all over the world.
The Brassicaceae is undoubtedly the widest and most diversified group of Ni hyperaccumulators, with up to 83 species distributed in 8–12 genera (see Checchi et al. 2010 and references therin). The traditional morphological classification of this family, mainly based on homoplasic characters such as the fruit shape or dehiscence, was recently shown as widely artificial by a molecular phylogenetic approach (reviewed in Koch and Al-Shehbaz 2009). Following the deep ongoing rearrangement of intrafamilial taxonomy, inspired by a monophyly criterion, hyperaccumulators of Ni in the Brassicaceae can be now referred to only 5 out of the 35 natural tribes (Koch and Al-Shehbaz 2009; Cecchi et al. 2010): Aethionemaeae (1 species), Alysseae (56), Cardamineae (1), Noccaeeae (24), and Schizopetaleae (1). Within the Alysseae they are circumscribed to the genera Alyssum (50), Bornmuellera (5), and Leptoplax (1), and their main specific diversity occurs in Anatolia and the Balkans, which include some of the largest serpentine outcrops in Europe and one of the richest serpentine floras in the world (Brooks 1987; Stefanović et al. 2003).
As already noted above, the unusual behavior of such plants with respect to the presence of Ni does not bear necessarily to a true “dependence” on that metal or tolerance of it, but rather to a facultative advantage in synecological dynamics. Nevertheless, because Ni hyperaccumulators are almost absent on ultramafics which were involved by glacial phenomena during the Quaternary (Proctor and Nagy 1992), it could be suggested that they need a long time to develop either such physiological adaptations or a preadaptive genetic pattern (from which they can easily differentiate when metalliferous soils outcrop). Indeed, one of the most intriguing topics concerning metallophytes is their evolution from nonmetallophyte ancestors, which is also a good starting point to approach the genetic bases of such specialization. The distribution of hyperaccumulators (not only Ni hyperaccumulators) through the angiosperms is highly uneven, with a few groups covering the main percentage of the total alone, but it is unclear how many times hyperaccumulation of a given metal evolved in a given group, and whether this specialization represents a widespread a homoplasic character or is mainly a synapomorphic trait restricted to given lineages. Today, a very few researches are dedicated to the origin of serpentine and heavy metal adaptation at the superspecific level (Broadley et al. 2001; Jansen et al. 2002, 2004; Patterson and Givnish 2004; Cecchi and Selvi 2009), and the only ones regarding Ni hyperaccumulation just deal with Alyssum species and their relatives (Mengoni et al. 2003a; Cecchi et al. 2010).
In order to assess the actual relationships among Ni-hyperaccumulating Alysseae and the significance of physiological adaptation from an evolutionary point of view, nuclear ribosomal internal transcribed spacer (ITS nDNA) sequences have been recently obtained for comparison from a wide sampling of species and populations, and their phylogenetic pattern was reconstructed at the tribal, generic, and specific levels (Cecchi et al. 2010). This also allows the development of a clearer and more practical taxonomy of European hyperaccumulators in this group, and the identification of suitable model systems consisting of phylogenetically related taxa for comparative studies of the molecular mechanisms of metal hyperaccumulation, and for practical applications.
Both morphological, caryological, and molecular data agree with a double origin of this specialization within the tribe, namely the clades of Bornmuellera/Leptoplax and that of Alyssum. Despite the fact that several metal-tolerant species in the latter are able to grow on serpentine soils, hyperaccumulation is restricted to the monophyletic sect. Odontarrhena, a widely polymorphic group which accounts some 50 species in the Mediterranean and Irano-Turanian regions. It has been suggested that this should deserve the position of an independent genus because of the paraphyletic structure of Alyssum s.l. (Warwick et al. 2008).
In the clade including the Greek endemic Leptoplax emarginata and the very closely related west Mediterranean and Irano-Turanian species in the genus Bornmuellera, Ni hyperaccumulation must be probably considered as one of the traits they inherited from a common ancestor, thus reducing the total number of natural groups where this physiological character has occurred. By contrast, in Odontarrhena it must have evolved multiple times as a consequence of a complex of preadaptive, genetic traits shared by all the taxa of this group.
Such a different frequency of evolution of metal tolerance, depending on the phylogenetic depth, has been observed for obligate serpentinophytism even in tribe Lithospermeae of Boraginaceae (Cecchi and Selvi 2009), and is in line with the results of similar phylogenetic inferences for serpentine adaptation in Calochortus (Patterson and Givnish 2004) or Al accumulation in the Ericales (Jansen et al. 2002, 2004). Obligate serpentine taxa in Lithospermeae and Ni hyperaccumulators in Alysseae also share a similar evolutionary pattern regarding their ancestry among nonserpentine taxa; in both the cases, these sister groups of serpentine endemics are strictly basophilous and xerophytic plants growing on limestone, or even dolomite rocks, with a high magnesium content. Thus, there is evidence that the combined tolerance to an ultrabasic pH value, dry environments, and, especially, high levels of magnesium in the soil may be key factors for the evolution of serpentinophile (then hyperaccumulator) plants.
14.6 Alyssum bertolonii
One of the earliest reports in the scientific literature about the strict connection between plants and geology dates back to over four centuries (Cesalpino 1583), when the Italian botanist Andrea Cesalpino observed the crucifer currently known as A. bertolonii (Fig. 14.2) growing on black stony soils of the Upper Tiber Valley in Tuscany (Vergnano Gambi 1992). Its discovery as a curious and bizarre case of evolution was in the late 1940s, when Minguzzi and Vergnano (1948) discovered its uncommonly high concentration of nickel in its leaves. Since then, some studies have attempted to investigate the physiological mechanisms of its Ni tolerance and hyperaccumulation. For example, Gabbrielli et al. (1991) found that A. bertolonii is characterized by a higher Ni tolerance as compared to other serpentine nonaccumulator species. The hypertolerance strategy confers high costs, but is important for surviving in unfavorable serpentine conditions, thus determining the inseparable connection between hyperaccumulators and serpentine soils. Gabbrielli and Pandolfini (1984) showed, instead, that in A. bertolonii the internal Ca and Mg concentrations possibly counteract Ni toxicity or in any case enhance Ni tolerance, through physiological mechanisms still unknown. However, Marmiroli et al. (2004) investigated the Ni distribution in its tissues and found a specific pattern of nickel distribution, with the highest concentrations present in parenchyma and sclerenchyma cells for the roots, while in the shoots, the highest amounts of nickel were found in the stem epidermis, the leaf epidermal surface, and the leaf trichome base.
In terms of biogeochemical interactions, serpentine soils are well known to markedly differ in their cation concentrations as they are derived from rocks occurring under a wide range of climates, from temperate to tropical regions (Kruckeberg 2002). Galardi et al. (2007a) showed that even at the local scale of the distribution of A. bertolonii, mainly central Italy, there could be statistically significant heterogeneity in the levels of cations of these soils, probably due both to microclimatic factors and to differences in the composition of the original rocks between different outcrops. In that study, for example, Ni concentration spanned a wide range of values, from around 1,000 μg g−1 to more than threefold higher, while an Ni mean value of 2,000 μg g−1 DW was reported by Brooks (1987) for serpentine outcrops. That heterogeneity in soil Ni concentrations was shown to generate substantial differences in mineral element concentration between A. bertolonii populations, as Ni shoot concentrations showed a fivefold range, from 4,000 to 21,000 μg g−1 DW. The scale of concentration variation of the other elements was similar to that of nickel, irrespective of their absolute values. Moreover, in the study of Galardi et al. (2007a) it was also demonstrated that A. bertolonii was not only a well-known faithful indicator of serpentine soils for geobotanical prospecting but also a useful tool for biogeochemistry as, in the case of nickel and cobalt, it is representative of the degree of mineralization of the soil. A previous population genetic study (Mengoni et al. 2003b) showed that A. bertolonii populations are strongly genetically distinct from each other and that a relatively high genetic heterogeneity does exist within the same population (Fig. 14.3). Furthermore, in the same study a clear relationship between geographical isolation and genetic differentiation of populations has been found. Evaluating the relationship between soil and plant metal concentration differences among outcrops and population genetic diversity, at the intra-population level, a hypothetical edaphic effect on the genetic diversity of populations was suggested, i.e., the more variable the soil Ni concentrations were, the more genetically variable were the plant populations. Thus, Ni concentration variability of soil seems to be an important factor shaping A. bertolonii genetic diversity. Considering the geographical distribution of the outcrops (Fig. 14.4), Galardi et al. (2007a) suggested also that the center of diversity, then possibly the center of origin, of A. bertolonii was in the outcrop with the lowest Ni concentration and that from there, plants might have diffused into the other outcrops with higher Ni concentrations.
Pattern of genetic relationships among Tuscan A. bertolonii populations. The neighbor joining method was applied to an average squared distance matrix among populations. Scale bar indicates average squared distance (Microsat 1.5). Original data from Mengoni et al. (2003b)
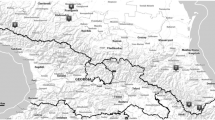
The patchy distribution of serpentine outcrops in Tuscany (Central Italy). Highlighted areas and names in bold indicate localities where A. bertolonii populations were sampled in the study by Mengoni et al. (2003b)
After having demonstrated for the first time that there can be significant variation in Ni tolerance and hyperaccumulation in populations of a species endemic to metalliferous soils, such as A. bertolonii, and that their relationship was positive, Galardi et al. (2007b) compared data obtained in hydroponic tests with data on metal concentration collected in the field, in order to assess the effects of local soil and plant metal concentration on Ni tolerance and accumulation. In the field, a positive correlation has been found between soil Ni concentration and shoot Ni concentration (Galardi et al. 2007a), but neither of these measures seemed to be related to the considerable differences in Ni tolerance and accumulation levels measured under controlled conditions. Hence, in contrast to the general notion that the least tolerant populations are found on the least metalliferous soils, with tolerance being a result of adaptive evolution in response to soil toxicity (Pollard et al. 2002), A. bertolonii populations do not show this particular feature. Variation in Ni tolerance and accumulation also shows no relationship to the variation in genetic diversity that was found by Mengoni et al. (2003b) for A. bertolonii populations. So genotypic differences in Ni tolerance and accumulation do not seem to be the main cause for generating the differences in shoot Ni concentrations shown by A. bertolonii populations in the field (Galardi et al. 2007a), whereas the nickel “serpentine factor” has been demonstrated to play a significant role (Galardi et al. 2007a).
14.7 Conclusions
Recent studies on the special features of interactions played between serpentine hyperaccumulators and soil substrate are highlighting more and more the role of genetic background, physiological constraints, and facilitated variation in the evolution of metal hyperaccumulation from nonaccumulating relatives. Very recently, evidence has shown that Cd tolerance and accumulation are not independent in Cardaminopsis halleri (Willems et al. 2010), as well as the important role played by both genes (sets of genes) and environmental interactions in the evolution of Zn tolerance and hyperaccumulation (Frérot et al. 2010). Similar evidence has also been suggested by field and population studies on A. bertolonii which have shown a high degree of heterogeneity of population metal concentrations, Ni tolerance, and hyperaccumulation capacities as well as a strong positive linear relationship between Ni tolerance and hyperaccumulation and Ni in plants and soils. These features render A. bertolonii an attractive model for studying evolution, both physiological and molecular, of the most striking feature produced by the interactions of biological systems with the geological substrate–metal hyperaccumulation.
References
Antonovics J, Bradshaw AD, Turner RG (1971) Heavy metal tolerance in plants. Adv Ecol Res 7:1–85
Assunçao AGL, ten Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO (2003) Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol 159:411–419
Assunçao AGL, Bleeker P, Ten Bookum WM, Vooijs R, Schat H (2008) Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary metal exposures. Plant Soil 303:289–299
Baker AJM (1981) Accumulators and excluders – strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, FL, pp 85–107
Bani A, Echevarria G, Sulçe S, Morel JL, Mullai A (2007) In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania). Plant Soil 293:79–89
Bert V, Bonnin I, Saumitou-Laprade P, De Laguérie P, Petit D (2002) Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol 155:47–57
Boyd RS (2004) Ecology of metal hyperaccumulation. New Phytol 162:563–567
Boyd RS (2007) The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant Soil 293:153–176
Boyd RS, Davis MA, Balkwill K (2008) Elemental patterns in Ni hyperaccumulating and non-hyperaccumulating ultramafic soil populations of Senecio coronatus. S Afr J Bot 74:158–162
Boyd RS, Jaffré T, Odom JW (1999) Variation in nickel content in the nickel-hyperaccumulating shrub Psychotria douarrei (Rubiaceae) from New Caledonia. Biotropica 31:403–410
Boyd RS, Martens SN (1992) The raison d'être for metal hyperaccumulation by plants. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept, Andover, pp 279–289
Brady KU, Kruckeberg AR, Bradshaw HD (2005) Evolutionary ecology of plant adaptation to serpentine soils. Ann Rev Ecol Evol Syst 36:243–266
Broadley MR, Willey NJ, Wilkins JC, Baker AJM, Mead A, White PJ (2001) Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytol 152:9–27
Brooks RR (1983) Biological methods of prospecting for minerals. Wiley, New York
Brooks RR (1998) Plants that hyperaccumulate heavy metals. CAB International, Wallingford
Brooks RR (1987) Serpentine and its vegetation. A multidisciplinary approach. Dioscorides, Portland
Brooks RR, Lee J, Reeves RD, Jaffré T (1977) Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J Geochem Explor 7:49–57
Brooks RR, Yang XH (1984) Elemental levels and relationships in the endemic serpentine flora of the Great Dyke, Zimbabwe and their significance as controlling factors for this flora. Taxon 33:392–399
Cecchi L, Gabbrielli R, Arnetoli M, Gonnelli C, Hasko A, Selvi F (2010) Evolutionary lineages of nickel hyperaccumulation and systematics in European Alysseae (Brassicaceae): evidence from nrDNA sequence data. Ann Bot 106(5):751–767
Cecchi L, Selvi F (2009) Phylogenetic relationships of the monotypic genera Halacsya and Paramoltkia and the origins of serpentine adaptation in circummediterranean Lithospermeae (Boraginaceae): insights from ITS and matK DNA sequences. Taxon 58:700–714
Cesalpino A (1583) De plantis libri XVI, Florentiae
Chiarucci A (2003) Vegetation ecology and conservation on Tuscan ultramafic soils. Bot Rev 69(3):252–268
Chiarucci A, Maccherini S (2007) Long-term effects of climate and phosphorus fertilisation on serpentine vegetation. Plant Soil 293:133–144
Chiarucci A, Maccherini S, Bonini I, De Dominicis V (1999) Effects of nutrient addition on community productivity and structure of serpentine vegetation. Plant Biol 1:121–126
Chiarucci A, Robinson BH, Bonini I, Petit D, Brooks RR, De Dominicis V (1998) Vegetation of tuscan ultramafic soils in relation to edaphic and physical factors. Folia Geobot 33:113–131
Deng DM, Shu WS, Zhang J, Zou HL, Lin Z, Ye ZH, Wong MH (2007) Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environ Pollut 147:381–386
Ernst WHO (2006) Evolution of metal tolerance in higher plants. For Snow Landsc Res 80:251–274
Escarré J, Lefèbvre C, Gruber W, Leblanc M, Lepart J, Rivière Y, Delay B (2000) Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implications for phytoremediation. New Phytol 145:429–437
Fernando DR, Woodrow IE, Bakkaus EJ, Collins RN, Baker AJM, Batianoff GN (2007) Variability of Mn hyperaccumulation in the Australian rainforest tree Gossia bidwillii (Myrtaceae). Plant Soil 293:145–152
Frérot H, Faucon M-P, Willems G, Godé C, Courseaux A, Darracq A, Verbruggen N, Saumitou-Laprade P (2010) Genetic architecture of zinc hyperaccumulation in Arabidopsis halleri: the essential role of QTL x environment interactions. New Phytol 187:355–367
Gabbrielli R, Mattioni C, Vergnano O (1991) Accumulation mechanisms and heavy metal tolerance of a nickel hyperaccumulator. J Plant Nutr 14:1067–1080
Gabbrielli R, Pandolfini T (1984) Effect of Mg2 and Ca2 on the response to nickel toxicity in a serpentine and nickel accumulating species. Physiol Plant 62:540–544
Galardi F, Corrales I, Mengoni A, Pucci S, Barletti L, Arnetoli M, Gabbrielli R, Gonnelli C (2007a) Intra-specific differences in nickel tolerance and accumulation in the Ni-hyperaccumulator Alyssum bertolonii. Environ Exp Bot 60:377–384
Galardi F, Mengoni A, Pucci S, Barletti L, Massi L, Barzanti R, Gabbrielli R, Gonnelli C (2007b) Intra-specific differences in mineral element composition in the Ni-hyperaccumulator Alyssum bertolonii: a survey of populations in nature. Environ Exp Bot 60:50–56
Huenneke LF, Hamburg SP, Koide R, Mooney HA, Vitousek PM (1990) Effects of soil resources on plant invasion and community structure in Californian serpentine grassland. Ecology 71:478–491
Jansen S, Broadley M, Robbrecht E, Smets E (2002) Aluminium hyperaccumulation in angiosperms: a review of its phylogenetic significance. Bot Rev 68:235–269
Jansen S, Watanabe T, Caris P, Geuten K, Lens F, Pyck N, Smets E (2004) The distribution and phylogeny of aluminium accumulating plants in the Ericales. Plant Biol 6:498–505
Jenny H (1980) The soil resource: origin and behavior. Ecol Stud 37:256–259
Jiang RF, Ma DY, Zhao FJ, McGrath SP (2005) Cadmium hyperaccumulation protects Thlaspi caerulescens from leaf feeding damage by thrips (Frankliniella occidentalis). New Phytol 167:805–814
Kabata-Pendias A, Pendias H (1991) Trace elements in soils and plants, 2nd edn. CRC, Boca Raton, FL
Kazakou E, Adamidis GC, Baker AJM, Reeves RD, Godino M, Dimitrakopoulos PG (2010) Species adaptation in serpentine soils in Lesbos Island (Greece): metal hyperaccumulation and tolerance. Plant Soil 332:369–385
Koch M, Al-Shehbaz IA (2009) Phylogeny of Brassica and wild relatives. In: Gupta SK (ed) Biology and breeding of crucifers. Taylor & Francis, Boca Raton, FL, pp 1–19
Krämer U (2010) Metal hyperaccumulation in plants. Ann Rev Plant Biol 61:517–534
Kruckeberg AR (1950) An experimental inquiry into the nature of endemism on serpentine soils. Ph.D. thesis. University of California, Berkeley, p 154
Kruckeberg AR (1954) The ecology of serpentine soils: a symposium III. Plant species in relation to serpentine soils. Ecology 35:267–274
Kruckeberg AR (2002) Geology and plant life. University Press, Washington
Kruckeberg AR, Kruckeberg AL (1990) Endemic metallophytes: their taxonomic, genetic and evolutionary attributes. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC, Boca Raton, FL, pp 301–312
Küpper H, Lombi E, Zhao F-J, Wieshammer G, McGrath SP (2001) Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J Exp Bot 52:2291–2300
Lefèbvre C, Vernet P (1990) Microevolutionary processes on contaminated deposits. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC, Boca Raton, FL, pp 286–297
Macnair MR (2002) Within and between population genetic variation for zinc accumulation in Arabidopsis halleri. New Phytol 155:59–66
Macnair MR (2003) The hyperaccumulation of metals by plants. Adv Bot Res 40:63–105
Marmiroli M, Gonnelli C, Maestri E, Gabbrielli R, Marmiroli N (2004) Localisation of nickel and mineral nutrients Ca, K, Fe, Mg with scanning electron microscopy microanalysis in tissues of the nickel-hyperaccumulator Alyssum bertolonii Desv. and the non-accumulator Alyssum montanum L. Plant Biosyst 138:231–243
Mengoni A, Baker AJM, Bazzicalupo M, Reeves RD, Adigüzel N, Chianni E, Galardi F, Gabbrielli R, Gonnelli C (2003a) Evolutionary dynamics of nickel hyperaccumulation in Alyssum revealed by ITS nrDNA analysis. New Phytol 159:691–699
Mengoni A, Barabesi C, Gonnelli C, Galardi F, Gabbrielli R, Bazzicalupo M (2001) Genetic diversity of heavy metal tolerant populations of Silene paradoxa L.: a chloroplast microsatellite analysis. Mol Ecol 10:1909–1916
Mengoni A, Gonnelli C, Brocchini E, Galardi F, Pucci S, Gabbrielli R, Bazzicalupo M (2003b) Chloroplast genetic diversity and biogeography in the serpentine endemic Ni-hyperaccumulator Alyssum bertolonii. New Phytol 157:349–356
Mengoni A, Gonnelli C, Galardi F, Gabbrielli R, Bazzicalupo M (2000) Genetic diversity and heavy metal tolerance in populations of Silene paradoxa L. (Caryophyllaceae): a RAPD analysis. Mol Ecol 9:1319–1324
Mengoni A, Gonnelli C, Hakvoort HW, Galardi F, Bazzicalupo M, Gabbrielli R, Schat H (2003c) Evolution of copper-tolerance and increased expression of a 2b-type metallothionein gene in Silene paradoxa L. populations. Plant Soil 257:451–457
Mengoni A, Grassi E, Barzanti R, Biondi EG, Gonnelli C, Kim CK, Bazzicalupo M (2004) Genetic diversity of bacterial communities of serpentine soil and of rhizosphere of the nickel-hyperaccumulator plant Alyssum bertolonii. Microb Ecol 48:209–217
Mengoni A, Schat H, Vangronsveld J (2010) Plants as extreme environments? Ni-resistant bacteria and Ni-hyperaccumulators of serpentine flora. Plant Soil 331:5–16
Minguzzi C, Vergnano O (1948) Il contenuto di nichel nelle ceneri di Alyssum bertolonii. Atti Soc Tosc Sci Nat 55:49–74
Mota JF, Medina-Cazorla JM, Navarro FB, Pérez-García FJ, Pérez-Latorre A, Sánchez-Gómez P, Torres JA, Benavente A, Blanca G, Gil C, Lorite J, Merlo ME (2008) Dolomite flora of the Baetic Ranges glades (South Spain). Flora 203:359–375
Novák FA (1928) Quelques remarques relative au problème de la végétation sur les terrains serpentiniques. Preslia 6:42–71
Nyberg Berglund AB, Dahlgren S, Westerbergh A (2004) Evidence for parallel evolution and site-specific selection of serpentine tolerance in Cerastium alpinum during the colonization of Scandinavia. New Phytol 161:199–209
Patterson TB, Givnish TJ (2004) Geographic cohesion, chromosomal evolution, parallel adaptive radiations, and consequent floral adaptations in Calochortus (Calochortaceae): evidence from a cpDNA phylogeny. New Phytol 161:253–264
Peer WA, Mamoudian M, Lahner B, Reeves RD, Murphy AS, Salt DE (2003) Identifying model metal hyperaccumulating plants: germplasm analysis of 20 Brassicaceae accessions from a wide geographical area. New Phytol 159:421–430
Pichi Sermolli R (1948) Flora e vegetazione delle serpentine e delle altre ofioliti dell’alta valle del Tevere (Toscana). Webbia 17:1–380
Pollard AJ, Baker AJM (1997) Deterrence of herbivory by zinc hyperaccumulation in Thlaspi caerulescens (Brassicaceae). New Phytol 135:655–658
Pollard AJ, Dandridge Powell K, Harper FA, Smith JAC (2002) The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci 21:539–566
Proctor J (1971) The plant ecology of serpentine II. Plant responses to serpentine soils. J Ecol 59:397–410
Proctor J, Nagy L (1992) Ultramafic rocks and their vegetation: an overview. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept, Andover, pp 469–494
Proctor J, Woodell SRJ (1975) The ecology of serpentine soils. Adv Ecol Res 9:255–365
Quintela-Sabarís C, Vendramin G, Castro-Fernández D, Fraga M (2010) Chloroplast microsatellites reveal that metallicolous populations of the Mediterranean shrub Cistus ladanifer L. have multiple origins. Plant Soil 334:161–174
Rajakaruna N, Baldwin BG, Chan R, Desrochers AM, Bohm BA, Whitton J (2003) Edaphic races and phylogenetic taxa in the Lasthenia californica complex (Asteraceae: eliantheae): an hypothesis of parallel evolution. Mol Ecol 12:1675–1679
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Richau KH, Schat H (2009) Intraspecific variation of nickel and zinc accumulation and tolerance in the hyperaccumulator Thlaspi caerulescens. Plant Soil 314:253–262
Roberts BA, Proctor J (1992) The ecology of areas with serpentinized rocks: a world view. Kluwer, Dordrecht
Robinson BH, Brooks RR, Kirkman JH, Gregg PEH, Alvarez HV (1997) Edaphic influences on a New Zealand ultramafic (“serpentine”) flora: a statistical approach. Plant Soil 188:11–20
Slingsby DR, Brown BH (1977) Nickel in British serpentine soils. J Ecol 65:597–618
Stefanović V, Tan K, Iatrou G (2003) Distribution of the endemic Balkan flora on serpentine I. – obligate serpentine endemics. Plant Syst Evol 242:149–170
Takhtajan AL (1986) The floristic regions of the World. University of California Press, Berkeley
Taylor SI, Macnair MR (2006) Within and between population variation for zinc and nickel accumulation in two species of Thlaspi (Brassicaceae). New Phytol 169:505–514
Vassilev A, Schwitzguébel JP, Thewys T, Van der Lelie D, Vangronsveld J (2004) The use of plants for remediation of metal-contaminated soils. Sci World J 4:9–34
Vekemans X, Lefèbvre C (1997) On the evolution of heavy metal tolerant populations in Armeria maritima: evidence from allozyme variation and reproductive barriers. J Evol Biol 10:175–191
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776
Vergnano Gambi O (1992) The distribution and ecology of the vegetation of ultramafic soils in Italy. In: Roberts BA, Proctor J (eds) The ecology of areas with serpentinized rocks – a world view. Kluwer, Dordrecht, The Netherlands, pp 217–247
Vergnano Gambi O (1993) Gli adattamenti delle piante. In: Le ofioliti dell’Appennino Emiliano (ed) Regione Emilia-Romagna, pp 103–128
Walker RB (1954) The ecology of serpentine soils: a symposium II. Factors affecting plant growth on serpentine soils. Ecology 35:259–266
Warwick SI, Sauder CA, Al-Shehbaz IA (2008) Phylogenetic relationships in the tribe Alysseae (Brassicaceae) based on nuclear ribosomal ITS DNA sequences. Botany 86:315–336
Whiting SN, Reeves RD, Richards D, Johnson MS, Cooke JA, Malaisse F, Paton A, Smith JAC, Angle JS, Chaney RL, Ginocchio R, Jaffré T, Johns R, McIntyre T, Purvis OW, Salt DE, Schat H, Baker AJM (2004) Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Rest Ecol 12:106–116
Whittaker RH (1954) The ecology of serpentine soils: a symposium. I. Introduction. Ecology 35:258–259
Willems G, Frérot H, Gennen J, Salis P, Saumitou-Laprade P, Verbruggen N (2010) Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri x Arabidopsis lyrata petraea F2 progeny grown on cadmium-contaminated soil. New Phytol 187:368–379
Yang X, Li T, Yang J, He Z, Lu L, Meng F (2006) Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 224:185–195
Zhang X-H, Liu J, Huang H-T, Chen J, Zhu Y-N, Wang D-Q (2007) Chromium accumulation by the hyperaccumulator plant Leersia hexandra Swartz. Chemosphere 67:1138–1143
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Mengoni, A., Cecchi, L., Gonnelli, C. (2012). Nickel Hyperaccumulating Plants and Alyssum bertolonii: Model Systems for Studying Biogeochemical Interactions in Serpentine Soils. In: Kothe, E., Varma, A. (eds) Bio-Geo Interactions in Metal-Contaminated Soils. Soil Biology, vol 31. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-23327-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-23327-2_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23326-5
Online ISBN: 978-3-642-23327-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)