Abstract
Accumulations of potentially toxic metals were investigated in soils and five North Caucasian Alyssum species from metalliferous areas and non-metalliferous areas in Karachay-Cherkessia, Kabardino-Balkaria, Dagestan and the Krasnodar region. Analyses of field samples showed that chemical features of the soils significantly affected the concentrations of Ni, Co, Zn, but had less effect on Cu and Pb concentrations in the shoots of Alyssum. Variations in the degree of accumulating ability were found in the studied species, including hyperaccumulation of Ni in Alyssum murale (up to 12,100 mg kg−1), and significant accumulation of Zn in A. gehamense (up to 1700 mg kg−1). A comparative molecular genetic analysis of two A. murale populations, both Ni-hyperaccumulating population from Karachay-Cherkessia and non-hyperaccumulating population from Dagestan, indicated considerable genetic difference between them. This result supports the hypothesis that the selection of metal hyperaccumulator species with enhanced phytoremediation efficiency should be considered at the population level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metalliferous soils, owing to their extreme chemical characteristics, are hostile habitats and often unfavourable to normal plant growth. However, plants growing on naturally metal-enriched soils are adapted to cope with bioavailable levels of potentially toxic metals. Many of these plants have metal content higher than for plants on non-metalliferous soils. A small number of these metallophytes are hyperaccumulators. Hyperaccumulators are unusual plants that accumulate particular metals or metalloids in their living tissues to levels that may be hundreds or thousands of times greater than is normal for most plants (van der Ent et al. 2013). The criterion for hyperaccumulation varies for different metals and represents a leaf concentration > 100 mg kg−1 for Cd, > 1000 mg kg−1 for Ni, Co, Cu, Pb, > 3000 mg kg−1 for Zn, and > 10,000 mg kg−1 for Mn (Reeves et al. 2018).
The phenomenon of metal hyperaccumulation by plants aroused much interest because its biochemical and physiological uniqueness may be exploitable economically in the clean-up of metal-contaminated soils. Soil decontamination by hyperaccumulator cropping might represent a low-cost, environmentally friendly technique of detoxifying soils contaminated with metals (Baker et al. 1994). Hyperaccumulator plants often appear to be restricted in their distribution to metalliferous soils, from which they always exhibit hyperaccumulation of some element; these are described as ‘obligate’ hyperaccumulators (Pollard et al. 2002). However, some are more widespread, with populations that hyperaccumulate from metalliferous soils, and other populations, from non-metalliferous soils, that do not show unusual accumulation; these are ‘facultative’ hyperaccumulators (Pollard et al. 2014).
Among hyperaccumulators, the largest number of species possessing the ability to hyperaccumulate Ni. Species of Euphorbiaceae and Brassicaceae families include about 25% of the known Ni hyperaccumulators (Krämer 2010). Most of these species are almost completely restricted to soils enriched with Ni, Cr and sometimes Co derived from ultramafic (serpentine) rocks. The genus Alyssum (Brassicaceae) has attracted the interest of botanists because of the association of many of its species with substrata with potentially toxic metals (Baker and Brooks 1989). Species of this genus found on soils derived from ultramafic rocks in southern Europe can accumulate concentration of Ni in excess of 2% of dry weight (Bani et al. 2010). Among them, Alyssum murale is a well-known Ni hyperaccumulator that has been developed as a commercial crop for phytoremediation/phytomining Ni from metal-enriched soils (Tappero et al. 2007; Bani et al. 2015; Osmani and Bani 2018; Saad et al. 2018).
In fact, hyperaccumulators can be exploited not only as natural remediation species but also as sources of genes for improvement of other remediation species (Galardi et al. 2007a). Many studies are devoted to assessing the genetic diversity of populations of plant species native to areas where soils are naturally enriched with chemical elements (Deng et al. 2007; Galardi et al. 2007a). The estimation of genetic variation between plants in their ability to accumulate metals is of both practical and theoretical importance (Pollard et al. 2002). Genetic study of A. bertolonii populations showed that they are strongly genetically distinct from each other and that a relatively high genetic heterogeneity does exist within the same population (Galardi et al. 2007a). Moreover, in the same study a clear relationship between geographical isolation and genetic differentiation of populations has been found. A field study at the population level can join the theoretical research on the estimation of differences between plants in levels of metal concentration to the practical advantage of being a possible first step towards the selection of populations with the highest extraction ability (Pollard et al. 2002). Recently, Bani et al. (2010) demonstrated that different populations of A. murale living in their natural habitats, present differences in Ni hyperaccumulation related to soil Ni concentration. Intra-specific variation in Ni hyperaccumulation have presented also for other Ni-hyperaccumulating species, e.g. A. lesbiacum (Adamidis et al. 2014). In this context, the most effective populations could be used both directly as phytoremediation species themselves (Bani et al. 2010) and indirectly for the improvement of plant traits through selective breeding or as sources of genes for improvement of other remediation crops (Pollard et al. 2002).
Russia has a number of biodiversity centres of Brassicaceae species in the North Caucasus mountain areas. North Caucasus mountain Alyssum populations can occupy much smaller areas than in the case of plains. This is primarily due to the diversity of environmental conditions. Altitudinal zonation, exposure, markedly more pronounced erosion, diversity of edaphic conditions, including those due to differences in parent rocks, not only reduce the population area, but also create the prerequisites for the genetic variability at the population level.
In mountainous areas of the North Caucasus, many outcrops of ultramafic and mafic rocks give rise to soils with a wide range of compositions and concentrations of potentially toxic metals. However, very little information is available on metal uptake by natural North Caucasus vegetation in naturally mineralized soils. Investigations on metal-accumulating ability of plants and phylogenetic relationships among species and populations of plants growing on naturally metal-enriched soils can be important for the fundamental knowledge on metal uptake and tolerance mechanisms for phytoremediation of large areas of soils around the world. Therefore, the objectives of this study were (1) to determine the metal concentrations and their variability in metalliferous and non-metalliferous soils, (2) to assess the metal-accumulating ability of the North Caucasian Alyssum species and populations and (3) to analyse phylogenetic relationships in order to evaluate their potential for phytoremediation purpose.
Materials and methods
Site description and field sampling
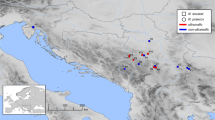
The study was conducted in four North Caucasus mountain regions of Russia: Krasnodar region (site 1), Dagestan (sites 2–4), Karachay-Cherkessia (site 5), Kabardino-Balkaria (site 6) and at altitude from 900 to 2600 m above sea level (Fig. 1). These sites with different characteristics were selected at metalliferous (naturally mineralized) and non-metalliferous localities (Table 1). Soil forming calcareous rocks of Krasnodar region (site 1) and Dagestan (site 2) areas are mostly not metalliferous. The mafic rocks of Dagestan areas (sites 3, 4) are mostly diabase, which have mineralization in Fe, Cu, Zn, and Pb sulphides (Palivoda 2008). The geological substrata of Karachay-Cherkessia and Kabardino-Balkaria areas (sites 5, 6) consist of the ultramafic rocks—serpentinous harzburgites, lherzolites, dunites, and pyroxenites, which contain minerals with high concentration of Ni, Co, and Cr (Potapenko 2004; Markin 2011). The mountain skeletal soils of studied areas are developed mainly directly on eluvium or eluvium-deluvium materials and everywhere were closely related to the bedrock. Their soil profile is not differentiated, highly stoniness, and minor amounts of fine earth. The climatic conditions of the studied areas are distinguished primarily by the degree of moisture. The climate in the western part of North Caucasus region is the wet sub boreal (site 1, 5, 6), the eastern part is more arid and continental (site 2–4).
Plants of five Alyssum species were sampled from soil derived from different rock types (Table 1). The studied species are common in the sparse grass communities of open stony and clayey habitats of the middle mountain zone of the North Caucasus. However, the number of samples depends on the occurrence in each studied site and is primarily associated with the difficult conditions of the mountainous terrain. Soils and plants were sampled in representative sub-sites (≈ 30 m2 each) from each studied site. A total five of Alyssum alyssoides, six of A. daghestanicum, three of A. gehamense, eleven of A. murale (six—in Karachay-Cherkessia and five—in Dagestan), and five samples of A. tortuosum were collected. Each sample consists of at least five adult plants of about the same size, which were randomly chosen over the entire spatial distribution of each sampling sub-site. The samples for chemical and molecular genetic analysis were collected simultaneously. The shoots of plants, which were flowering and fruiting, were analysed. Soil samples of 0–10 cm depth (below the surface layer of stone in debris areas) were taken from around the roots of all field collected plants. Combined samples consisted of three-point samples. In total, eighteen soil samples were collected.
Chemical analyses
The collected samples were transferred at ambient temperature to the laboratory and air-dried. For subsequent analysis, the soil samples were ground in a porcelain mortar and then sieved through a 1-mm screen. Soil pH was determined in a 1:2.5 soil/deionised water suspension using a glass pH electrode, Model I-160. Soil available fraction of the potentially toxic metals were extracted by shaking 3 g of air-dry soil with 30 ml 1 M ammonium acetate adjusted to pH 4.8, and Ca was extracted by shaking 1 g of air-dry soil with 40 ml 1 M ammonium acetate adjusted to pH 6.5 (Vorobyeva 1998). After shaking, the soil suspension was filtered using slow-filtering paper for the finest deposits.
For the analyses of the total Ni, Co, Zn, Cu and Pb concentrations in the plant samples, 2 g of oven-dried plant material was weighed into quartz cups, ashed at 450 °C in a muffle furnace, and then transferred into a desiccator for cooling. The ash was taken up in 6 ml mixture of 1.5 M HNO3 and 3.71 M HCl then filtered using slow-filtering paper in a volumetric flask, and the digest made up to 25 ml volume with deionized water.
Plant and soil extracts were analysed by atomic absorption spectrometry. Performance of the instrument was checked by analysing the reference standard material solutions: State Standard Samples GSO 7272–96, and GSO 7325–96. All analyses were carried out in duplicate.
The statistical analyses were in order to identify potential differences between plant species. Data were analysed nonparametrically using Kruskal–Wallis ANOVA by Ranks test (p < 0.05). A Bivariate Spearman correlation was used to correlate chemical element concentrations in soils and plants (p < 0.05). All statistical evaluations were performed using Statistica 7.0 software.
Molecular genetic analysis methods
DNA was extracted from dry leaves (≈ 100 mg) with CTAB protocol (Doyle & Doyle, 1987). The primers ITS-A (5′-GGAAGGAGAAGTCGTAACAAGG-3′) and ITS-B (5′-CTTTTCCTCCGCTTATTGATATG-3′) (Blattner 1999) were used for the amplification of ITS1-5.8S-ITS2 region of nuclear rDNA. Polymerase chain reaction amplifications were performed in a total volume of 20 μl containing 1 × Phire Plant PCR Master Mix F160L (Thermo Fisher Scientific) and 4 ng of template DNA according to the manufacturer’s recommended protocol: initial denaturation 98 °C 5 min; 35 cycles (denaturation 98 °C 5 s; annealing 56 °C 5 s; extension 72 °C 20 s); final extension 72 °C 1 min. Reactions were performed in a Bio-Rad T100 thermocycler (Bio-Rad Laboratories, Inc.). Automated DNA sequencing was performed on both strands directly from the ITS-A and ITS-B primers on the purified PCR products using BigDye Terminator v.3.1 chemistry and an ABI 3130 sequencer (Applied Biosystems, Norwalk, CT, USA) according to the manufacturer’s recommendations. The alignment was slightly modified manually.
The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura–Nei model (Tamura and Nei 1993). The bootstrap consensus tree inferred from 500 replicates (Felsenstein 1985). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches (Felsenstein 1985). The initial tree for the heuristic search was obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The analysis involved 44 nucleotide sequences and 641 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016). Parsimony informative nucleotide substitutions and variable sites are indicated according to the reference sample Alyssum tortuosum (EF514625). Variable sites were evaluated according to the trace data in abi format produced by sequencers. The site was considered as variable if in the forward and reverse sequence the variant peaks differ by no more than 60%. Single-letter codes: Y (T or C), R (G or A), S (G or C), M (A or C), N (A or T or G or C). Parsimony informative and variable sites were sorted by phylogenetic tree with OpenOffice 4 software (The Apache Software Foundation).
Results and discussion
Soil characterization
Soil from the sampling sites had a neutral to alkaline pH 7.23 to 7.95. Many studies have analysed metalliferous areas with moderately acid and acid soils and native plants, which can grow on these soils (Abreu et al. 2012; Bech et al. 2012a). Much less information is available on metal uptake by natural vegetation in neutral to alkaline soils with high metal burdens. However, investigation on natural vegetation simultaneously adapted to high Ca content in soils and high metal availability can be important for the fundamental knowledge on metal uptake and tolerance mechanism (Bech et al. 2012b). According to our data, extractable Ca varied fairly highly between studied soils (Table 2). Soils from Dagestan derived from calcareous and mafic rocks (site 2 and 3, respectively) presented significant differences from the other studied soils; moreover, Ca concentration in soils of site 2 reached the highest levels (31.6 g kg−1). Calcium is one of the main antagonistic elements in relation to the absorption and metabolism of many trace elements (Kabata-Pendias and Pendias 2001). This is confirmed by the results of this study in which significant relation between Ca soil concentrations and plant concentrations of Ni (r = − 0.50), Co (r = − 0.60), Zn (r = − 0.65), Cu (r = − 0.53) and Pb (r = − 0.42) were shown.
Potentially toxic metal concentrations (available fraction extracted with ammonium acetate) in soils, in which Alyssum plants grew, are shown in Table 2. In this study, we used data on available fraction of metals; since this represents the most mobile and readily plant-available fraction, which corresponds to the best correlation values between the trace element concentration in soils and plants (Kumar et al. 2011; Santos et al. 2012). For example, Abreu et al. (2012) reported that Pb tolerance of Cistus salviifolius plants, which were growing on soils presenting a wide range of Pb concentration, is not related to the total concentration but to the available fraction of metal.
Soils from Karachay-Cherkessia and Kabardino-Balkaria (sites 5, 6) presented greater concentrations of Ni and Co in the available fraction than other studied soils. High levels of these elements in soils may generate from ultramafic parent rocks, which are associated with Ni and Co minerals. A large number of chemical analyses have been made for soils over ultramafic rocks. They show that most of these soils have the available concentrations of Ni and Co at much higher levels than other soil types (Proctor and Nagy 1992; Oze et al. 2008). In this study, the mean level of Ni in the soils derived from ultramafic rocks ranged from 9.33 to 15.9 mg kg−1 and Co from 0.70 to 0.86 mg kg−1. For the soils derived from mafic and calcareous rocks the corresponding values were much lower (0.41–0.67 mg kg−1 and below the detection limit, respectively). Thus, soils from two ultramafic areas (sites 5, 6) presented concentration of Ni that exceeded the Russian maximum permissible value (MPV) for acetate ammonium-extractable fraction of this element in the soils (5.0 mg kg −1, MPV 2006).
The Dagestan soils of site 3 were extreme in their high available concentration of Zn and Pb (97.7 mg kg−1 and 36.4 mg kg−1, respectively), exceeding MPV values (23 mg kg−1 and 6.0 mg kg−1, respectively), while other studied soils had significantly lower concentrations of Zn and Pb (Table 2). High concentrations of these metals in the site 3 due to the parent material of these soils that was Zn and Pb-enriched diabasic eluvium. Ghaderian et al. (2007) have been reported by the high soil concentrations of Pb (20 mg kg−1) and Zn (30 mg kg−1) in the available fraction in the Irankouh area (Central Iran), which is a vast mountainous region with naturally mineralized soils. Copper concentration in the skeletal soil derived from Cu enriched Dagestan mafic rocks (site 4) was relatively low, but considerably (3–8 times) higher in this soil than at other sites (Table 2).
A high proportion of total metals are an immobile fraction not immediately bioavailable for plants and therefore are not directly toxic (Pollard et al. 2002; McLaughlin et al. 2008). However potentially toxic metals in the available fraction may be directly absorbed by plants from the soils. Thus, the extreme concentration of Ni and Pb–Zn in the available fraction in soils of sites 1, 5 and site 3, respectively, suggest high toxicity.
Metal accumulation in plants
According to our results, Ca in Alyssum plants was very high (Table 3) and was not statistically correlated with Ca in soils (r = 0.29). Alyssum species have an effective Ca absorption system, regardless of the element concentration in the soil, as reported by Tumi et al. (2012) for A. murale, which contained up to 35,350 mg Ca kg−1 in the leaves when growing on serpentine soils in Serbia with low Ca level (310 mg kg−1). Several studies have reported exceptional concentrations of Ca in leaves A. murale, predominantly in the CaCO3 nodules on the surface of the trichomes (Tappero et al. 2007; Broadhurst and Chaney 2016).
Our data clearly demonstrates that concentrations of chemical elements in available fraction of the soils (Table 2) significantly affected the concentrations of Ni (r = 0.63), Zn (r = 0.48), Co (r = 0.86), but not significantly on Cu (r = 0.33) and Pb (r = 0.31) concentrations in the shoots of Alyssum plants (Table 3). Alyssum daghestanicum from non-metallicolous soils derived from calcareous rocks (site 2) with low potentially toxic metal contents (Table 2) had shoot concentrations of Zn and Cu significantly lower than other studied plants (Table 3). Alyssum gehamense and A. murale population from metallicolous Karachay-Cherkessian soil had the highest concentrations of Zn and Ni–Co, respectively, which could be a consequence of these high metal concentrations in the geological material and soil (Table 2).
The highest mean concentration of Zn (1640 ± 100 mg kg−1) was in the shoots of A. gehamense from the Dagestan soils derived from mafic rocks (site 3). Total Zn shoot concentration in the other studied plants ranges between 10 and 31 mg kg−1. These values do not excessively exceed the metabolic needs (< 10 mg kg−1) common of non-accumulator plants. Among all the plant species studied, none was close to the minimum concentration criteria for being considered a Zn hyperaccumulator plant. However, A. gehamense had an ability to accumulate Zn in their shoots. Shoots of A. gehamense also showed higher Pb concentrations (Table 3). However, the Pb bioaccumulation factor for this plant was low—0.43. According to the study by Szczygłowska et al. (2011), Brassica species could accumulate lower amounts of Pb than other metals. Moreover, Pb is neither essential nor beneficial to plant nutrition; it is usually quite immobile and poorly accumulated in upper plant parts.
The concentrations of Cu in the shoots of plants found in the present study were in the range of 1.84 to 3.96 mg kg−1. The highest value was recorded in the shoots of A. murale (site 5). The relatively low shoot Cu accumulation can be attributed to both low concentrations of this metal in the available fraction in studied soils (Table 2) and taxon-specific features in the accumulation of this element. Ghasemi et al. (2015) have discussed the tolerance, uptake, accumulation and interactions of Ni and Cu in a range of Alyssum species from the Mediterranean region and have concluded that no tolerance to high concentrations of Cu was observed in any of the species tested. However, in the presence of Ni, an increased Cu concentration was observed in both roots and shoots of the Ni hyperaccumulator plants, but not in the non-accumulators. According to Broadhurst and Chaney (2016) Ni hyperaccumulators, including A. murale, are very specific to Ni and do non-selectively accumulate/hyperaccumulate other transition metals such as Fe, Cr, or Cu.
Among studied plant species, only A. murale includes both Ni-accumulating and non-accumulating populations growing on soils derived from ultramafic and non-ultramafic rocks, respectively. The plants from the nickel-rich Karachay-Cherkessia soils (site 5) had Ni concentrations in the shoots within ranges 4280–12,130 mg kg−1. This is in agreement with other published values for Ni hyperaccumulation in this and some other European A. murale populations (Bani et al. 2010; Tang et al. 2012). Alyssum murale emerging as Ni hyperaccumulator from this study would be the first reported from the North Caucasus.
The shoot Co concentration of Ni-accumulating population in this study was 33.1 mg kg−1 with average values for other studied species from below the detection limit to 1.52 mg kg−1 (Table 3). The bioconcentration factor shown in Table 4, based on ammonium acetate extractable soil concentration, revealed the expected accumulation of Ni and Co in the shoots of population A. murale from Karachay-Cherkessia was greater than in plants of other species. The fairly high bioaccumulation of Ni in the Dagestan A. murale population that grows in the soils with low Ni concentration seems to suggest that it also has an effective Ni absorption system. The ability of A. murale to accumulate Ni and Co has been studied in detail under controlled-environmental conditions, particularly in relation to tolerance to these respective metals (Tappero et al. 2007). These findings suggest exceptionally high uptakes of Ni and Co, but specialized biochemical processes linked with Ni high tolerance in A. murale do not confer high tolerance to Co. Malik et al. (2000) reported that A. murale hyperaccumulated Co from a low Ni sandy soil to which Co salt had been added. Nevertheless, in a greenhouse experiment with two Ni hyperaccumulator A. murale and A. corsicum grown on non-serpentine Ni-contaminated soils, showed that both species hyperaccumulated Ni, but not Co (Li et al. 2003).
The mean Ni concentrations of A. murale population from Dagestan were much lower than the Karachay-Cherkessia (Table 3). Thus, Ni concentrations of studied A. murale populations ranged from 20 to 12,000 mg kg−1. According to this result, A. murale are classed as ‘facultative’ Ni hyperaccumulator. Analyses of the A. murale populations, which are found both on and off the soils derived from ultramafic rocks (Reeves et al. 1997) showed a range of Ni concentrations from < 30 mg kg−1 to > 1000 mg kg−1, according to the substrata. Kazakou et al. (2010) considered A. lesbiacum is as a ‘micro edaphic’ endemic Ni hyperaccumulator, since Ni hyperaccumulation varies considerably among different natural populations, depending on soil Ni concentration. Leaf analysis of the widespread Rinorea bengalensis, for example, includes 23 specimens showing Ni concentrations of 1000–17,750 mg kg−1 and another 77 specimens with 1–300 mg kg−1 according to sampling sites (from soils derived from ultramafic and non-ultramafic rocks, respectively), and this species is hence a typical facultative hyperaccumulator (van der Ent et al. 2013).
In general, the limits of excessive and toxic Ni and Zn levels for most plant species range from 10 to 100 mg kg−1 and from 100 to 400 mg kg−1, respectively (Kabata-Pendias and Pendias 2001). Great Ni concentrations in the shoots of A. murale and Zn in A. gehamense are not accompanied by any toxicity symptoms and growth reduction. These plants are adapted to extreme concentrations of these metals in the soil and have a mechanism that allows them to bind the absorbed metals in inert compounds, avoiding damage to their own metabolism (Brooks, 1998; Olko et al. 2008).
In our earlier work (Drozdova et al. 2017) metallicolous population of A. murale from Karachay-Cherkessia under conditions of controlled-environment of sand culture, was studied. The tolerance of the seedlings of this species to Ni was examined by adding Ni sulphate to a nutrient solution with which the plants were watered. The experiment demonstrated that A. murale has an exceptional facility for uptake of and high tolerance to Ni. The plants in the high Ni growth medium accumulated the same aboveground biomass as the control plants, which was possible due to their preservation of a high level of hydration of the tissues, the content of photosynthetic pigments, and macronutrients. The experiment confirmed characteristic traits of A. murale such as hyper-tolerance to ions Ni, hyperaccumulation of this metal in the shoot, high bioconcentration factors and high shoot/root metal translocation. Thus, the ability to Ni accumulate and tolerance might be a constitutive property of this species.
Molecular phylogenetic analysis
A comparative molecular genetic analysis of the North Caucasian populations of Alyssum murale has shown that this species is a highly polymorphic. It is distributed over three clades with characteristic nucleotide substitutions (Fig. 2). The polymorphism of this species is well-known (Mengoni et al. 2003), but the causes of this phenomenon are still not fully understood. According to our data, the hyperaccumulating population from Karachay-Cherkessia (D2, D8, D9) and the non-hyperaccumulating population from Dagestan (D5, D7, D10, D11) differ in five nucleotide substitutions (sites 54, 59, 60, 214, 611) and 16 polymorphic sites (sites 96, 109, 126, 133, 198, 205, 211, 212, 215, 236, 248, 257, 465, 537, 570 and 61) (Fig. 2). Besides, two hyperaccumulator A. murale specimens AY237936 and AY237937 (Mengoni et al. 2003) form a separate clade with 12 substitutions in ITS1 (sites 54–163) characteristic for the clade A. campestre–A. daghestanicum. The comparison of six samples from the A. daghestanicum shows that all the samples belonging to the same clade, differing significantly from all the studied A. murale samples and had variable sites that do not coincide with of A. murale the variable sites (Fig. 2). It is likely that on Dagestan soils with low Ni status, A. murale is not hyperaccumulator because of a genetic difference or, more generally, it is not hyperaccumulator because of the low availability of Ni in question. However, at the intra-population level it was seen that the more variable soil Ni concentrations are, the more genetically variable plant populations are (Galardi et al. 2007a). These data may suggest that Ni concentration variability of soil is the main factor shaping A. bertolonii genetic diversity. Earlier experimental works with different populations of metal-accumulating species originating from different soil types showed intra-specific variation in metal-accumulating ability in Thlaspi goesingense (Baker et al. 1994; Assunção et al. 2004), and A. bertolonii (Galardi et al. 2007b). The distinct intra-specific variation of this character provides opportunities for further genetic and physiological dissection of the hyperaccumulation trait.
Comparative riborype analysis of Alyssum populations from sampling sites of the North Caucasus region. The NCBI Genebank samples are indicated by corresponding accession number; the samples from this study are indicated as “D”. Sites: 1—Krasnodar region, 2–4—Dagestan, 5—Karachay-Cherkessia, 6—Kabardino-Balkaria
The genus Alyssum is a rather complex taxonomic formation with separate kinship groups. One is the monophyletic Odontarrhena section, which encompasses all the Ni accumulators, e.g. A. murale (Mengoni et al. 2003; Cecchi et al. 2010). According to our data, the Odontarrhena section also includes the A. gehamense, which is capable of high Zn accumulation (Table 3). Previously, the ability to accumulate high concentrations of other metals, in addition to Ni, in this section was not noted (Tappero et al. 2007; Verbruggen et al. 2009). More investigations of A. gehamense, which accumulate high metal concentrations, but at the same time not being a hyperaccumulator, can provide new molecular data of metal accumulation mechanisms in plants.
Conclusion
Our results indicated a high heterogeneity of all studied potentially toxic metal concentrations of North Caucasian soils and plants according to the type and degree of the substrata mineralized. There were close positive relationship between the concentrations of Zn, Ni and Co in the plants and in the soils in the available fraction, but not for Pb and Cu. The highest concentration of Ni- and Co and Zn had plants, as metallicolous population of Alyssum murale (up to 12,100 mg kg−1) and A. gehamense (up to 1700 mg kg−1), naturally growing on soils derived from ultramafic and mafic rocks, respectively. The concentrations of these metals in plant species, as A. dagestanicum, from non-metallicolous soils derived from calcareous rocks, was lowest.
Intra-specific variation in Ni-accumulating ability in A. murale was detected for Karachay-Cherkessia and Dagestan populations originating from different soil types. Nickel concentrations among these populations ranged in average from 20 to 6800 mg kg−1. Hence, this species is a typical ‘facultative’ hyperaccumulator. The inter-population variations in Ni accumulation patterns in A. murale give support to the suggestion that the selection of metal hyperaccumulator species with enhanced phytoremediation efficiency should be considered at the population level. One of the potential directions of future research is a search for correlations between the amounts of potentially toxic metals, that can accumulate and one or another Alyssum population, and its position in the phylogenetic system.
References
Abreu, M. M., Santos, E. S., Magalhães, M. C. F., & Fernandes, E. (2012). Trace elements tolerance, accumulation and translocation in Cistus populifolius, Cistus salviifolius and their hybrid growing in polymetallic contaminated mine areas. Journal of Geochemical Exploration, 123, 52–60.
Adamidis, G. C., Aloupi, M., Kazakou, E., & Dimitrakopoulos, P. G. (2014). Intra-specific variation in Ni tolerance, accumulation and translocation patterns in the Ni-hyperaccumulator Alyssum lesbiacum. Chemosphere. https://doi.org/10.1016/j.chemosphere.2013.09.106.
Assunção, A. G. L., Costa Martins, P. D., Folter, S. D., Vooijs, R., Schat, H., & Aarts, M. G. M. (2004). Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant, Cell and Environment. https://doi.org/10.1111/j.1365-3040.2001.00666.x.
Baker, A. J. M., & Brooks, R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery, 1, 81–126.
Baker, A. J. M., McGrath, S. P., Sidoli, C. M. D., & Reeves, R. D. (1994). The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resources, Conservation and Recycling. https://doi.org/10.1016/0921-3449(94)90077-9.
Bani, A., Echevarria, G., Sulçe, S., & Morel, J. L. (2015). Improving the agronomy of Alyssum murale for extensive phytomining: A five-year field study. International Journal of Phytoremediation, 17(2), 117–127.
Bani, A., Pavlova, D., Echevarria, G., Mullaj, A., Reeves, R. D., Morel, J. L., et al. (2010). Nickel hyperaccumulation by the population of Alyssum and Thlaspi (Brassicaceae) from the soils derived from ultramafic rocks of the Balkans. Botanica Serbica, 34(1), 3–14.
Bech, J., Duran, P., Roca, N., Poma, W., Sánchez, I., Barceló, J., et al. (2012a). Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. spontaneous species from mine spoils in Peru and their potential use in phytoremediation. Journal of Geochemical Exploration, 123, 109–113.
Bech, J., Roca, N., Barceló, J., Duran, P., Tume, P., & Poschenreider, C. (2012b). Soil and plant contamination by lead mining in Bellmunt (Western Mediterranean Area). Journal of Geochemical Exploration, 113, 94–99.
Blattner, F. R. (1999). Direct amplification of the entire ITS region from poorly preserved plant material using recombinant PCR. BioTechniques, 27, 1180–1186.
Broadhurst, C. L., & Chaney, R. L. (2016). Growth and metal accumulation of an Alyssum murale nickel hyperaccumulator ecotype co-cropped with Alyssum montanum and Perennial ryegrass in serpentine soil. Frontiers in plant science, 7, 451.
Brooks, R. R. (1998). Plants that hyperaccumulate heavy metals: Their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. Wallingford: CAB International.
Cecchi, L., Gabbrielli, R., Arnetoli, M., et al. (2010). Evolutionary lineages of nickel hyperaccumulation and systematics in European Alysseae (Brassicaceae): evidence from nrDNA sequence data. Annals of Botany. https://doi.org/10.1093/aob/mcq162.
Deng, J., Liao, B., Ye, M., & Deng, D. (2007). The effects of heavy metal pollution on genetic diversity in zinc/cadmium hyperaccumulator Sedum alfredii populations. Plant and Soil, 297(1), 83–92.
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, 19(1), 11–15.
Drozdova, I., Alekseeva-Popova, N., Kalimova, I., Belyaeva, A., & Smirnova, N. (2017). The accumulating ability and nickel tolerance of Brassicaceae species of the North Caucasus in Connection with the problem of phytoremediation. Journal of Geochemical Exploration, Part B, Remediation of Polluted Soils. Part 2, 182, 235–241.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x.
Galardi, F., Corrales, I., Mengoni, A., Pucci, S., Barletti, L., Barzanti, R., et al. (2007a). Intra-specific differences in nickel tolerance and accumulation in the Ni-hyperaccumulator Alyssum bertolonii. Environmental and Experimental Botany, 60(3), 377–384.
Galardi, F., Mengoni, A., Pucci, S., Barletti, L., Massi, L., Barzanti, R., et al. (2007b). Intra-specific differences in mineral element composition in the Ni-hyperaccumulator Alyssum bertolonii: A survey of populations in nature. Environmental and Experimental Botany, 60(1), 50–56.
Ghaderian, S. M., Hemmat, G. R., Reeves, R. D., & Baker, A. J. M. (2007). Accumulation of lead and zinc by plants colonizing a metal mining area in Central Iran. Journal of Applied Botany and Food Quality, 81, 145–150.
Ghasemi, R., Ghaderian, S. M., & Ebrazeh, S. (2015). Nickel stimulates copper uptake by nickel-hyperaccumulator plants in the genus Alyssum. Australian Journal of Botany, 63(2), 56–64.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants. Boca Raton: CRC Press.
Kazakou, E., Adamidis, G. C., Baker, A. J. M., Reeves, R. D., Godino, M., & Dimitrakopoulos, P. G. (2010). Species adaptation in serpentine soils in Lesbos Island (Greece): Metal hyperaccumulation and tolerance. Plant and Soil, 332, 369–385.
Krämer, U. (2010). Metal hyperaccumulation in plants. Annual Review of Plant Biology, 61, 517–534.
Kumar, B., Kumar, S., Mishra, M., Singh, S. K., Prakash, D., Sharma, C. S., et al. (2011). Geochemical fractionation of some heavy metals in soils in the vicinity of Sukinda mining Area, Orissa. Advances in Applied Science Research, 2(5), 263–272.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874.
Li, Y.-M., Chaney, R. L., Brewer, E. P., Angle, J. S., & Nelkin, J. (2003). Phytoextraction of Nickel and Cobalt by hyperaccumulator Alyssum population grown on Nickel-contaminated soils. Environmental Science and Technology, 37(7), 1463–1468.
Malik, M., Chaney, R. L., Brewer, E. P., Li, Y. M., & Angle, J. S. (2000). Phytoextraction of soil cobalt using hyperaccumulator plants. International Journal of Phytoremediation, 2(4), 319–329.
Markin, M. Y. (2011). Minerageny of the Malkinsky ultrabasic massif. Rostov-on-Don: Ph.D. dissertation.
McLaughlin, M. J., Zarcinas, B. A., Stevens, D. P., & Cook, N. (2008). Soil testing for heavy metals. Communications in Soil Science and Plant Analysis. https://doi.org/10.1080/00103620009370531.
Mengoni, A., Baker, A. J. M., Bazzicalupo, M., et al. (2003). Evolutionary dynamics of nickel hyperaccumulation in Alyssum revealed by ITS nrDNA analysis. New Phytologist, 159(3), 691–699.
Olko, A., Abratowska, A., Zyłkowska, J., Wierzbicka, M., & Tukiendorf, A. (2008). Armeria maritima from a calamine heap-initial studies on physiologic-metabolic adaptations to metal-enriched soil. Ecotoxicology and Environmental Safety, 69, 209–218.
Osmani, M., & Bani, A. (2018). Heavy metals concentration of dumping site soils and their accumulation in Alyssum Murale growing in selected dumping sites in Albania. Thalassia Salentina, 39, 83–98.
Oze, C. J., Skinner, C., Schroth, A. W., & Coleman, R. G. (2008). Growing up green on serpentine soils: Biogeochemistry of serpentine vegetation in the Central Coast Range of California. Applied Geochemistry, 23(12), 3391–3403.
Palivoda, N. K. (2008). Predicted assessment of the reserves of complex ores and cobalt mineralization on Borchinskii site of Khnov-Borchinskii ore field (Dagestan). Proceedings of the Institute of Geology of the Dagestan Scientific Center of the Russian Academy of Sciences, 36(52), 47–54.
Pollard, A. J., Powell, K. D., Harper, F. A., & Smith, J. A. C. (2002). The genetic basis of metal hyperaccumulation in plants. Critical Reviews of Plant Sciences, 21(6), 539–566.
Pollard, A. J., Reeves, R. D., & Baker, A. J. M. (2014). Facultative hyperaccumulation of heavy metals and metalloids. Plant Science, 217–218, 8–17.
Potapenko, Y. (2004). Geology of Karachay-Cherkesia. Karachaevsk: Karachaevo-Cherkessk. University.
Proctor, J. & Nagy, L. (1992). Ultramafic rocks and their vegetation: an overview. In: A. J. M. Baker, J. Proctor & R. D. Reeves (Eds.), The vegetation on ultramafic (serpentine) soils. A word view (pp. 469–494).
Reeves, R. D., Baker, A. J. M., Jaffe, T., Erskine, P. D., Echevarria, G., & van der Ent, A. (2018). A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytologist, 218, 407–411.
Reeves, R. D, Baker, A. J. M., & Kelepertsis, A. (1997). The distribution and biogeochemistry of some serpentine plants of Greece. In: T. JaVrй, R. D. Reeves & T. Becquer (Eds.), Écologie des milieux sur roches ultramafiques et sur sols métallifères (pp. 205–207), ORSTOM, Nouméa, Documents Scientifiques et Techniques III/2.
Saad, R. F., Kobaissi, A., Machinet, G., Villemin, G., Echevarria, G., & Benizri, E. (2018). Crop rotation associating a legume and the nickel hyperaccumulator Alyssum murale improves the structure and bio functioning of an ultramafic soil. Ecological Research, 33(4), 799–810.
Santos, E. S., Abreu, M. M., Nabais, C., & Magalhães, M. C. F. (2012). Trace elements distribution in soils developed on gossan mine wastes and Cistus ladanifer L. tolerance and bioaccumulation. Journal of geochemical exploration, 123, 45–51.
Szczygłowska, M., Piekarska, A., Konieczka, P., & Namiesnik, J. (2011). Use of Brassica plants in phytoremediation and biofumigation processes. International Journal of Molecular Sciences, 12, 7760–7771.
Tamura, K., & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512–526.
Tang, Y., Deng, T., Wu, Q., et al. (2012). Designing cropping systems for metal-contaminated site: A review. Pedosphere, 22(4), 470–488.
Tappero, R., Peltier, E., Gräfe, M., Heidel, K., Ginder-Vogel, M., Livi, K. J., et al. (2007). Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytologist, 175(4), 641–654.
Tumi, A. F., Mihailović, N., Gajić, B. A., Niketić, M., & Tomović, G. (2012). Comparative study of hyperaccumulation of nickel by Alyssum murale s.l. populations from the ultramafics of Serbia. Polish Journal of Environmental Studies, 21(6), 1855–1866.
Van der Ent, A., Baker, A. J. M., Reeves, R. D., Pollard, A. J., & Schat, H. (2013). Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant and Soil, 362, 319–334.
Verbruggen, N., Hermans, C., & Schat, H. (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist, 181(4), 759–776.
Vorobyeva, L. A. (1998). Chemical analysis of soil. Moscow: Moscow State University.
Acknowledgements
This work was carried out as part of the State Order (Subject No. AAAA-A19-119030690058-2). The authors thank the Centre of collective usage of Komarov Botanical Institute RAS for the opportunity to conduct sequencing. The authors thank V. Dorofeyev for his help in identification of plants, and both L. Chagarova and I. Idrisov for their advice on the geology of the studied areas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drozdova, I., Machs, E., Kalimova, I. et al. Accumulation of potentially toxic elements by plants of North Caucasian Alyssum species and their molecular phylogenetic analysis. Environ Geochem Health 43, 1617–1628 (2021). https://doi.org/10.1007/s10653-020-00674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00674-4