Abstract
Cnidarians comprise a diverse and phylogenetically basal phylum, some of which—such as scleractinian corals (Anthozoa)—are responsible for the formation and maintenance of ecosystems. Anthozoan immunology is a relatively new field, yet has great potential to inform invertebrate immunology, medicine, as well as coral reef conservation and restoration. Here we review cnidarian innate immune mechanisms in the context of invertebrate effector responses. We focus on anthozoans and discuss the blurred boundary between immune and stress responses. We conclude by high 1ighting unique aspects of coral biology and exploring the role of immunology in coral reef conservation and restoration through climate change.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Cnidaria is a diverse phylum, representing animals of dramatically different morphologies, life histories, and ecological functions but united by the presence of a specialized cell type—the cnidocyte . Cnidocytes secrete organelle-like capsules with eversible microtubules called cnidae (Daly et al. 2007). Anthozoa is the most speciose class of the phylum Cnidaria, with an estimated 7500 extant species (Daly et al. 2007), including the subclasses Hexacorallia and Octocorallia , which comprise stony corals and anemones, and soft corals and gorgonians, respectively (Won et al. 2001) (Fig. 1). Anthozoans are phylogenetically basal, both within the Metazoa as a whole and arguably within Cnidaria (Kayal et al. 2013), with Scleractinia (stony corals) appearing in the mid-Triassic (c. 250 million years ago [MYA]) (Romano and Palumbi 1996), possibly having evolved from anemones.
The first dinoflagellates , single-celled eukaryotes—“protists”—also purportedly appeared during the Triassic (Fensome 1993; Stanley 2006), and eventually, after a series of extinctions, formed an obligate endosymbiosis with a wide range of multicellular organisms, including anthozoans (Stanley 2006; Aranda et al. 2016). The majority of extant anthozoans live in this obligate endosymbiosis with members of the genus Symbiodinium (Aranda et al. 2016)—a relationship that underpins the ecological success of the class. In this intimate association, the Symbiodinium can provide over 90% of the energy requirements of the anthozoan (Muscatine and Porter 1977) and, in the case of hard coral, facilitate exoskeleton calcification, enabling the formation of tropical reef ecosystems (Fig. 2). In return, and in lieu of living in typically nutrient-poor waters, the anthozoan host protects its algal partner and uses it to recycle waste carbon and nitrogen (Jeong et al. 2012). Under stress conditions , such as increased water temperature or infection, this obligate endosymbiosis can break down, turning the coral white as the cHL: Intelectin-1orophyll-pigmented Symbiodinium leave or die, revealing the coral skeleton through the translucent host tissue. The “bleached” anthozoan (Fig. 3) host is then susceptible to starvation, disease, and death (Fig. 4). This scleractinian coral–algal association is the best studied of the relationships anthozoans have with microbiota. It is becoming increasingly apparent that the specific, variable, and diverse microbiome associated with anthozoans is crucial to their health and likely modulated, in part, by coral immune mechanisms (Bourne et al. 2016). Deciphering the immunological intricacies of coral–microbe symbioses is an ecologically important field of research and will likely provide insight into the establishment and functioning of symbioses throughout the animal kingdom. This is particularly so as, despite their phylogenetic position (Fig. 1) and apparent morphological simplicity, anthozoans are immunologically complex (Miller et al. 2007; Shinzato et al. 2011b), with large genomes and gene families that are comparable with those of the Bilateria (Augustin and Bosch 2010). Unlike many bilaterians, however, anthozoans have evaded gene loss (Miller et al. 2007), making them an interesting group for studying the evolution of immunity as well as mutualisms.
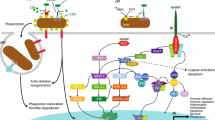
The immune system is a highly integrated suite of mechanisms and processes that enable organisms to resist infection and maintain tissue integrity to promote survival (Medzhitov 2008; Cooper 2010). Like all organisms, anthozoans possess innate immune mechanisms (Palmer and Traylor-Knowles 2012), but as invertebrates they lack the more complex adaptive arm of immunity. Innate immunity provides a non-specific and immediate response to perceived endogenous and exogenous threats in a bid to re-establish homeostasis (Beutler 2004; Medzhitov 2008). Concomitantly, anthozoans use a diverse repertoire of immune receptors (Miller et al. 2007) (Table 1), signaling pathways (Wolenski et al. 2011), and effector and “stress” responses (Palmer et al. 2008), which eliminate pathogens, seal wounds, mitigate self-harm, and defend self by maintaining homeostasis (Palmer and Traylor-Knowles 2012; Mydlarz et al. 2016).
Shifts in environmental conditions, driven by climate change and local anthropogenic disturbances, are threatening the long-term survival of many species and systems, and coral reefs are among the most threatened (Hughes et al. 2017). Unfavorable environmental conditions are negatively affecting the health of coral reefs; the ancient, co-evolved symbiotic relationship that is so important to coral health is being pushed beyond its limit, resulting in mass bleaching and die-off events (Hughes et al. 2017). Longer-lived organisms, such as scleractinian corals, are particularly vulnerable to the anthropogenically increased rates of climate change, which exceed the time needed for a population to adapt through natural selection (van Oppen et al. 2017). To conserve coral reefs through this high rate of change, the potential of genetically engineered “super corals” that can withstand environmental change, is being explored (van Oppen et al. 2017). It is increasingly apparent that immunity, as the basis of the maintenance and reestablishment of health, needs to be at the forefront of coral reef health and disease research. Anthozoan immunology offers hope that we will better understand the drivers behind coral health in order to more effectively conserve and restore the reefs systems that are of high ecological and societal value.
Anthozoan Innate Immunity
Cnidarians use a diverse suite of characteristic innate immune mechanisms to maintain and re-establish homeostasis (Palmer and Traylor-Knowles 2012). Unlike the majority of animals , which possess either a protective exoskeleton (e.g., Arthropoda) or a thick epidermal tissue layer (e.g., mammals), the protective physical and biochemical layers of an anthozoan include only a single-cell host epithelium and surface mucus layer (SML ). Once these protective barriers have been breached, and in the presence of a threat, an innate immune response occurs in the three broad immunity phases described across phyla: recognition of a threat, signaling pathways to activate appropriate response, and effector responses that eliminate the threat and mitigate self-harm (Hoffmann et al. 1999).
Anthozoans, like all invertebrates and higher organisms, use a suite of pattern recognition receptors (PRRs ) and soluble proteins to recognize a broad array of conserved microorganism-associated molecular patterns MAMPs e.g., lipopolysaccharides (Loker et al. 2004; O’Neill et al. 2013) and host-derived damage-associated molecular patterns (Medzhitov and Janeway 2000a; Beutler 2004; Palmer and Traylor-Knowles 2012). In insects and other arthropods, the binding of MAMPs and/or DAMPs to PRRs , such as the Toll-like receptor (TLR) (Medzhitov and Janeway 2000b), activates serine protease cascades (Cerenius et al. 2010) and rapid-acting transcription factors , such as nuclear factor (NF)-κB. This leads to gene transcription and ultimately protein translation, which induces immune signaling pathways and appropriate effector responses (Medzhitov and Janeway 2000a). These receptors, signaling pathways, and downstream responses are being elucidated in anthozoans (see the summary in Table 1). Here we discuss progress in cnidarian immunology, with a focus on anthozoans, in relation to the broader field of invertebrate immunology.
The Mucosal Epithelia
SMLs evolved with the Cnidaria and are present in all multicellular phyla (Bythell and Wild 2011). Similar to the mucosal surfaces of the intestinal cell epithelia of the human gut (Artis 2008), the anthozoan SML overlays single-cell epithelia and is home to an array of commensal bacteria, distinct from the microbiota of the surrounding environment (Sweet et al. 2011). While the methods of many coral mucus studies may have led to variable accounts of the SML-associated microbiota (Sweet et al. 2011), it is evident that the SML represents a physical protective barrier and a niche for many members of the coral microbiome (Kaiko and Stappenbeck 2014).
The coral SML is composed of a mixture of secreted compounds, including large glycoproteins called mucins . Mucins are released from epithelial mucocytes and form gels of varying viscosity (Jatkar et al. 2010) that are ultimately responsible for providing epithelial protection (Bythell and Wild 2011). The coral SML is dynamic, enables the transfer of gases and storage of metabolites (Bythell and Wild 2011), is used to remove sediment (Fig. 5), and also varies with the environment and over time (Brown and Bythell 2005). Importantly, the SML offers a niche for commensal coral-associated microbes that fulfill important functions including nutrient provision and antimicrobial defense (Ritchie 2006; Krediet et al. 2013). While the ability of host immune systems to regulate populations of commensal bacteria is conserved across phyla, it is unclear how innate immune mechanisms distinguish beneficial and commensal microbes from potential pathogens (Rohwer et al. 2002; Artis 2008; Bourne et al. 2016). In the case of the coral SML , innate immunity of the host epithelium must be hypo-responsive to commensal microbes, while remaining reactive against pathogens (Rakoff-Nahoum et al. 2004; Artis 2008).
Effector Responses: Activation and Signaling
Effector responses eliminate a recognized threat that may be exogenously derived—like pathogens and toxins—or endogenously derived, such as signals from stressed or malfunctioning cells (Medzhitov 2008). The effector response resulting from endogenous activation of the immune system is sometimes referred to as a “cellular stress response”, and may be triggered by changes in environmental conditions (e.g., Kültz 2005). The immediate and typically non-specific nature of innate immunity means that many effector responses are often mediated without gene transcription, and are instead reliant on serine protease cascades and redox signaling (Cerenius et al. 2010). In the following sections we discuss anthozoan effector responses and provide information on the current information on related receptors and signaling pathways (Table 1).
Immune Cells, Phagocytosis , and Wound Healing
Mobile immune cells eliminate pathogens via phagocytosis, seal wounds, and release bioactive compounds at sites of infection. Cell–cell and cell–extracellular matrix communication is key for each of these effector responses, and often involves integrins (Johnson et al. 2009). Integrins are a group of transmembrane α–β heterodimer receptors that are involved in cell migration and differentiation, fibrillar matrix formation, and signal transduction (Takada et al. 2007). Integrins have been identified within many anthozoan genomes, and show a surprising amount of complexity (Table 1) (Knack et al. 2008). For example, there are three α- and four β-integrin subunits identified in Nematostella vectensis, the starlet sea anemone (Putnam et al. 2007; Reitzel et al. 2008), and two β-subunits in the hard coral Acropora millepora (Brower et al. 1997; Miller et al. 2007). In a study on N. vectensis wound healing and regeneration, one of the highest upregulated genes during wound healing was α-integrin (DuBuc et al. 2014). α-Integrin is part of the mitogen-activated protein kinase (MAPK ) signaling pathway (Table 1), which is proposed to be one of the primary mechanisms involved in N. vectensis wound healing (DuBuc et al. 2014).
The lectin-activated complement pathway is also important for cellular immune responses. This pathway is highly conserved and promotes phagocytosis and pathogen killing by aggregating and opsonizing pathogens (Fujita et al. 2004). Lectins are a diverse family of PRRs that include ficolins and mannose-binding lectins (MBLs), which recognize specific bacterial MAMPs (Fujita et al. 2004). The primary complement pathway components include complement C3, Factor B (Bf), lectins, and MBL-associated serine protease (MASP) (Carroll 1998). A wide diversity of lectins have been found within cnidarians, and are activated, along with other complement pathway components, in response to various stimulants including pathogen challenge, initiation of symbiosis, thermal stress, and wound healing (see Table 1) (Ocampo et al. 2015). For example, in the scleractinian coral, Pseudodiploria strigose; the lectins C-type, fucolectins, D-galactoside/L rhamnose-binding lectins, galectins and tachylectins C3, Bf, and MASP; and pathway components A2M and CD109 are all activated in response to a pathogen challenge. (Ocampo et al. 2015). In Hydra, components of the lectin-activated complement pathway are upregulated during wound healing and regeneration of the head (Wenger et al. 2014). In particular, MASP is upregulated in bisected animals, and it is proposed that the lectin-activated complement pathway may promote opsonization of invading pathogens (Wenger et al. 2014). The presence and activation of the lectin-activated complement pathways in cnidarians demonstrates its key role in phagocytosis and cellular responses during an immune response to infection.
Congruent with the identification of immune cell receptors and complement pathway components within anthozoan genomes, multiple anthozoan immune cells have been identified (Palmer and Traylor-Knowles 2012). Mobile, phagocytic cells—amoebocytes —were first identified in the sea anemone Actinia equina, and were shown to have bioactive capabilities within the mesenterial filaments (Hutton and Smith 1996). Additionally, a population of cells showing phagocytic activity were identified in the sea anemone Exaiptasia pallida using fluorescent-activated cell sorting (Rosental et al. 2017). Within gorgonians, immune cells have been identified in Swiftia exserta in response to injury (Olano and Bigger 2000) and unstimulated immune cells have been located using enzymatic histochemistry, suggesting a bioactive role in immunity (Menzel and Bigger 2015). Additional investigations in the gorgonian Plexaurella fusifera have provided insights into the processes and cells involved in anthozoan wound healing (Meszaros and Bigger 1999). Amoebocytes have also been observed in response to infection in Gorgonia ventalina (Mydlarz et al. 2008; Couch et al. 2013). In this response, melanin-producing amoebocytes of G. ventalina encapsulated infected tissue (Mydlarz et al. 2008). Similarly, encapsulation has been observed in a hybrid of Sinularia maxima (Slattery et al. 2013). Within scleractinian corals several types of immune cells have also been identified, including granular amoebocytes (Vargas-Angel et al. 2007; Renegar et al. 2008), melanin-containing cells (Palmer et al. 2010), chromophore cells (Domart-Coulon et al. 2006), agranular (hyaline) cells (Palmer et al. 2011b), and fibroblast-like cells in response to injury (Palmer et al. 2011b). Similar characteristics among many scleractinian coral immune cells suggest that they may originate from a common stem cell (Palmer et al. 2011b), consistent with observations of Hydra (Bosch et al. 2010).
Tissue damage requiring wound healing is a common occurrence for many organisms. Having an open lesion leaves an organism susceptible to infection, making it imperative that wounds are rapidly and effectively sealed. In scleractinian corals, wounding occurs naturally primarily via predation (fish bites; Fig. 6), boring invertebrates , algal abrasion, fragmentation, and storm damage, and is often associated with distinct changes in tissue coloration (Fig. 7). Wound healing across the Metazoa occurs in four sequential stages using specialized cells (Galko and Krasnow 2004; Martin and Leibovich 2005), and has been described in the scleractinian coral Porites cylindrica , based on histological analysis (Palmer et al. 2011b). The wound healing process is characterized by (1) insoluble clot (plug) formation to seal the lesion, prevent fluid loss, and minimize infection (Theopold et al. 2004), via the transglutaminase and melanin synthesis pathways in invertebrates (Palmer et al. 2012); (2) infiltration and phagocytosis of cellular debris and foreign organisms; (3) proliferation and formation of granulation tissue that consists of multiple cell types, collagen, and a basic extracellular matrix, providing a platform for re-epithelialization (Galko and Krasnow 2004; Biressi et al. 2010); and (4) re-epithelialization and wound maturation, often involving apoptosis (Martin and Leibovich 2005; Biressi et al. 2010; Palmer et al. 2011b). Immune cells involved in wound healing of the hard coral P. cylindrica include melanin-containing granular cells (Fig. 8), agranular amoebocytes, fibroblast-like cells, and granular amoebocytes (Palmer et al. 2011b).
The Melanin Synthesis Pathways
Melanin synthesis pathway by products (e.g., reactive species and quinones) and deposited melanin pigment are key constituents of the invertebrate immune repertoire (Söderhäll and Cerenius 1998). They are also the first classic invertebrate immune responses to be documented within anthozoans (Couch et al. 2008; Mydlarz et al. 2008; Palmer et al. 2008; Gimenez et al. 2014; Zaragoza et al. 2014). In arthropods, melanin synthesis is initiated by PRRs that trigger the activation of serine protease cascades, leading to the cleavage of the prophenoloxidase (PPO) zymogen and resulting in the formation of active phenoloxidase (PO) enzymes (Cerenius et al. 2010). The POs then initiate rapid proteolytic cascades involved the catalysis of monophenol hydroxylation, diphenol oxidation, and autocatalytic reactions that results in melanization (Cerenius et al. 2010). PPOs and POs exist in various isoforms that represent different components of several melanin synthesis pathway types. These pathway types include tyrosinase (Cerenius et al. 2008) and laccase (Luna-Acosta et al. 2010), and likely have different functions (Sugumaran 2002; Cerenius et al. 2008; Palmer et al. 2012). For example, the tyrosinase-type melanin synthesis pathway is highly cytotoxic and therefore ideal for resisting infection (e.g., Bidla et al. 2008; Seppala and Jokela 2011), whereas the laccase-type pathway is less cytotoxic and likely has a role in cuticle formation within many invertebrates (Cerenius et al. 2008; Luna-Acosta et al. 2010).
The receptors and mechanisms involved in melanin synthesis pathway activation have not been elucidated for anthozoans, as for many other invertebrates (Takahashi et al. 2015). However, genes homologous to those associated with melanin synthesis pathways in some arthropods have been identified within the genomes or transcriptomes of several anthozoans, such as C-type lectins (Yu and Kanost 2004) (Table 1). In scleractinian corals many different types of lectins have been discovered, including C-type lectins, rhamnose-binding lectins, tachylectins, fucolectins, and galectins (Table 1). One well-studied example of the C-type lectin is “millectin”, discovered in A. millepora. Millectin can bind to both pathogens and algal symbionts (e.g., Kvennefors et al. 2008) (Table 1). However, another study found no increase in C-type lectin expression during bacterial challenge (Brown et al. 2013). The conflicting evidence for lectin reactivity suggests that the complete picture on the role of lectins in anthozoan immunity is still unknown (Palmer and Traylor-Knowles 2012).
In addition to the lectin pathway , many other pathways are linked to melanin synthesis. The TLR and Toll pathway are involved in melanin pathway activation in insects (Cerenius et al. 2010), and concomitantly TLR genes are present in anthozoans (Table 1). Similarly, tyrosinase genes, which are involved in melanin pathway activation, have been found within the genomes of the sea anemone N. vectensis and the hydrozoan Hydra magnipapillata (Esposito et al. 2012). Expression of trypsin-like serine proteases has also been documented during immune challenge in the scleractinian coral A. millepora (Weiss et al. 2013), and a laccase-3 gene and shrimp PPO-activating enzyme (PPAE) homolog found in the scleractinian coral Pocillopora damicornis (Vidal-Dupiol et al. 2014). Also, a single contig predicted to encode a PO was found within the transcriptome of the Caribbean reef-building coral P. strigosa (Ocampo et al. 2015). Crucially, as rapid proteolytic cascades control melanin synthesis, gene expression studies will likely only loosely represent the immune activity of this effector response (Cerenius et al. 2010). As such, PO activity is frequently measured enzymatically in invertebrate immunology to determine presence and regulation of melanin synthesis pathways (e.g., Cerenius et al. 2008; Haine et al. 2008; Palmer et al. 2010).
Biochemical PO and PPO activities of the tyrosinase-type pathway were first reported in two reef-building coral species, A. millepora and Porites sp. (Palmer et al. 2008) and the gorgonian sea fan G. ventalina (Mydlarz et al. 2008). Tyrosinase-type PO and/or PPO activity has since been enzymatically demonstrated within numerous scleractinian corals from the Caribbean and Indo-Pacific (Palmer et al. 2012), soft corals (Alcyonacea), anemones (Actiniarian), and zoanthids (Zoantharia) (Palmer et al. 2010, 2011c, 2012a; Mydlarz and Palmer 2011; D’Angelo et al. 2012; Anithajothi et al. 2014; Gimenez et al. 2014; Sheridan et al. 2014; Zaragoza et al. 2014; van de Water et al. 2016). Laccase-type PO activity, which potentially has a role in coral photosensing and structural support (Palmer et al. 2012), has also been biochemically demonstrated in multiple scleractinian corals from the Indo-Pacific (Palmer et al. 2012) and Caribbean (Mydlarz and Palmer 2011), and in larvae and juveniles (Palmer et al. 2012). These reports demonstrate the ubiquity, and therefore likely significance, of melanin synthesis across anthozoans.
Melanin-associated encapsulation and structural support involves the degranulation of immune cells within which the melanin synthesis pathway is active (Galko and Krasnow 2004). Examples of these immune cells in other invertebrate organisms include crystal cells of insects (Bidla et al. 2008) and hemocytes of crustaceans (Söderhäll and Smith 1986). Melanization and associated amoebocytes have been shown to form a barrier against fungal infection, and were first documented within the anthozoan sea fan G. ventalina (Petes et al. 2003; Mullen et al. 2004; Mydlarz et al. 2008). Melanin-containing cells and/or melanin deposits have also been found within a suite of healthy Indo-Pacific corals (Scleractinia and Alcyonacea) (Palmer et al. 2010) and multiple Caribbean coral species (Mydlarz and Palmer 2011), suggesting such cells may be ubiquitous among anthozoans. The increase in melanin cell density in both compromised and infected coral tissue (Palmer et al. 2008, 2009a) and their degranulation at lesions (Palmer et al. 2011b) indicates their prominent role in coral immunity (Fig. 8).
Though the cytotoxicity of melanin synthesis hasn’t been explicitly explored within corals, the upregulation of PO in injured (D’Angelo et al. 2012; Sheridan et al. 2014; van de Water et al. 2015c), pathogen challenged, and infected corals (Palmer et al. 2008, 2011a, c; Zaragoza et al. 2014) suggests pathway activities are part of an effector response, as well as immune signaling (Mydlarz and Palmer 2011; Palmer et al. 2012). However, it has also been proposed that coral PO is used in growth, rather than immunity, due to the correlation between PO activity, fluorescence, and cell proliferation (D’Angelo et al. 2012). However, given the key role of PO in immunity throughout invertebrate phyla (Cerenius et al. 2010), upregulated PO in fast-growing coral tissue likely provides additional protection to the most at-risk parts of the coral colony. Growing tissues that demonstrate proliferation of melanin-containing granular cells and increased PO are also those most likely to come into contact with potentially harmful competitors, algae, and biofilm-associated microbes, as is the case during larval settlement (Palmer et al. 2012).
Coagulation
Coagulation is the process by which a liquid, such as invertebrate hemolymph, is converted into an insoluble clot—often in the form of a gel (Theopold et al. 2004). In invertebrates, coagulation ensures the rapid re-establishment of tissue integrity upon injury by preventing fluid loss and entrapping pathogens during infection (Cerenius et al. 2010). While there are likely multiple clotting mechanisms within invertebrates, one key pathway involves transglutaminases that form a gel upon interaction with plasma proteins (reviewed by Cerenius et al. 2010). Transglutaminases have previously been identified within several different invertebrates including molluscs (e.g., Nozawa et al. 2001) and many types of arthropods (see Cerenius et al. 2010), and is often followed by melanization for clot hardening (reviewed by Theopold et al. 2004). Transglutaminase activity has only been documented within one anthozoan: the reef-building coral P. cylindrica (Palmer et al. 2012). Within P. cylindrica, transglutaminase activity increased in response to injury (Palmer 2010), confirming its role within anthozoan wound sealing.
Antimicrobial Activity
Cnidarian antimicrobial peptides (AMPs) are cationic and hydrophobic, targeting the cell walls of microorganisms, and often providing broad-spectrum defense (Destoumieux-Garzón et al. 2016). Across the Metazoa, AMPs are typically located within granular immune cells and in association with epithelial tissue layers (Zasloff 2002). AMP transcription is initiated by the activation of the TLR pathway and subsequently the transcription factor NF-κB complexes with other adaptor proteins (Anderson 2000). AMPs are then used to disrupt microbial cell membranes (Shai 2002) and inhibit bacterial metabolic processes (Brogden 2005; Vidal-Dupiol et al. 2011b). A wide variety of invertebrates have been shown to possess a diverse suite of AMPs (e.g., Lemaitre and Hoffmann 2007; Otero-Gonzalez et al. 2010; Destoumieux-Garzón et al. 2016). Within Cnidaria, AMPs have been identified within Scyphozoa jellyfish, Aurelia aurita (aurelin) (Ovchinnikova et al. 2006), the Hydrozoa Hydra (arminin) (Miller et al. 2007; Augustin and Bosch 2010; Franzenburg et al. 2013), and Anthozoa, as reviewed by Mydlarz et al. (2016). The AMP, Damicornin, has been isolated from the scleractinian coral P. damicornis and demonstrated activity in response to Gram-positive bacteria and fungi (Vidal-Dupiol et al. 2011b). Two other AMPs have been bioinformatically characterized from P. damicornis—a mytimacin-like protein that binds to lipopolysaccharides and a bactericidal/permeability-increasing protein (LBP-BPI) (Vidal-Dupiol et al. 2014). The antimicrobial compound Homarine has previously been shown to demonstrate antifouling and predator deterrent functions in other invertebrates and was subsequently found to be a critical AMP for gorgonian Leptogorgia virgulata (Shapo et al. 2007). In several Hydra species the AMP, arminin, has been shown to have a distinct, species-specific function in dictating which bacterial communities can associate with specific polyps of Hydra (Franzenburg et al. 2013). This specificity is maintained even when different species of Hydra are co-cultured, suggesting that host immunity determines the composition of the bacterial community (Franzenburg et al. 2013). The diversity and the continual discovery of these bioactive compounds has promise for discovering novel AMPs that could have important medical applications.
As members of Cnidaria, anthozoans represent some of the most poisonous known organisms, producing toxic, bioactive compounds for defense and predation (Parisi et al. 2014). These bioactive chemicals are a key area of bioprospecting due to their potential for human medicine for their anti-inflammatory, cytotoxic and antinociception activities (reviewed by Cooper et al. 2014). Production of secondary metabolites enables this taxonomic group to be one of the most effective sessile benthic colonizers (Harvell et al. 1993; Changyun et al. 2008; Kelman et al. 2009). Between 2008 and 2014, 244 diterpenoids, a class of compounds with antimicrobial activity, were isolated from Gorgonian corals (Changyun et al. 2008), and while the biological function has not been assigned to each compound, this provides a glimpse of the potential complexity of immune, defense, and microbial interactions that are continually occurring on a coral reef. Compounds such as diterpenoids, sesquiterpenoids, and sterols are used for chemical defense and allelopathy, the use of chemicals to influence competitors’ biology, by soft corals and gorgonians, providing protection against predation (Van Alstyne et al. 1994) (reviewed in Changyun et al. 2008). Similarly, the antimicrobial activity of scleractinian and gorgonian extracts has been widely reported (e.g., Harvell et al. 1993; Kim et al. 2000; Gochfeld and Aeby 2008; Palmer et al. 2011c), though the nature of the chemical compounds and mechanisms employed are not always clear. A homogenate of coral tissues is often used to measure antimicrobial activity, and this contributes to activities being highly variable. This is particularly notable in immune challenge experiments, where some experiments result in increased bacterial growth while others demonstrate effective antimicrobial activity (e.g., Gochfeld and Aeby 2008; Palmer et al. 2011c). However, there are cases where extracted compounds have clear antimicrobial activity, for example diterpenoids extracted from the soft coral Sinularia flexibilis (Aceret et al. 1998). Similarly, diterpenoids and sterols are also involved in S. flexibilis allelopathy (Fang et al. 2005) (reviewed in Changyun et al. 2008). Scleractinian corals are highly dependent on allelopathy (Koh 1997; Gochfeld and Aeby 2008; Kelman et al. 2009; Slattery and Gochfeld 2012), with high competition for space from other corals, algae and biofilms (Chadwick and Morrow 2011).
Apoptosis
Apoptosis is a tightly regulated form of cell death that occurs during normal development, stress, injury, and infection (Brentnall et al. 2013). It is linked to the endogenous activation of innate immunity in response to signals generated by damaged or malfunctioning cells, and typically occurs when stress and redox imbalance exceeds the tolerance limits of the cell (Medzhitov 2008). The intrinsic apoptosis pathway is activated and regulated by proteolytic enzymes called caspases (Brentnall et al. 2013). Extrinsic apoptosis is triggered by cell surface receptors in the presence of specific ligands, such as those on the membrane of cytotoxic cells, and is primarily mediated by the highly conserved tumor necrosis factor (TNF) receptor (TNFR) ligand superfamily (Quistad et al. 2014; Quistad and Traylor-Knowles 2016).
Within anthozoans, both caspases (Moya et al. 2016) and members of the TNF superfamily have been identified (Quistad et al. 2014; Quistad and Traylor-Knowles 2016) and they display more diversity than within other organisms (Quistad and Traylor-Knowles 2016), demonstrating the functional conservation of apoptotic pathways within the Metazoa. As for all animals (Jacobson et al. 1997), apoptosis plays a role in wound maturation in coral, by eliminating excess cells produced during the proliferation phase of wound healing (Palmer et al. 2011b) and disease mediation (e.g., Ainsworth et al. 2015; Lawrence et al. 2015). However, apoptosis in coral also occurs in response to changing environmental conditions, such as with reduced water pH (Kvitt et al. 2015) and has been most intensively studied in relation to thermal bleaching (e.g. Hawkins et al. 2013). Apoptosis is activated in the host through stimulation by the reactive species nitric oxide during the process of temperature-induced breakdown in anthozoan-algal mutualisms, known as bleaching (Hawkins et al. 2013). Apoptosis enables the release of Symbiodinium from the host endodermal cell and interacts with autophagy to expel the redundant symbiont (Dunn et al. 2007; Tchernov et al. 2011). Concomitantly, the TNFR signaling pathway, which can initiate either inflammation via NF-κB or apoptosis (Aggarwal 2003), is activated in response to thermal stress (Barshis et al. 2013; Palumbi et al. 2014; Rose et al. 2015).
Reactive Species
Reactive species are essential molecules derived from oxygen or nitrogen, or other molecules, that are more reactive than the element from which they were derived, which in some cases, such as oxygen, is itself toxic (Halliwell and Gutteridge 2015). Reactive species are involved in cellular reduction–oxidation (redox) reactions that occur under normal processes of metabolism, cell signaling, development, and immunity (Bartosz 2009). Examples of reactive species include superoxide anion radical, hydrogen peroxide, hydroxyl radical, nitric oxide radical, peroxynitrite, and electronically excited states such as singlet molecular oxygen that vary reactivity (Halliwell and Gutteridge 2015; Sies 2015). These cytotoxic and abundant molecules are continually kept in check by suites of antioxidant compounds and enzymes, so as to prevent damage to biomolecules and cells (Halliwell and Gutteridge 2015). Changes in local abiotic conditions, such as chronic or acute changes in temperature, pH, or salinity, induce the production of cellular reactive species (Tomanek 2015), potentially leading to a state of oxidative stress in which antioxidants are no longer able to maintain redox homeostasis. Oxidative stress occurs when the production of toxic reactive species overwhelms a system’s ability to eliminate them with antioxidant molecules and enzymes (Halliwell and Gutteridge 2015; Tomanek 2015). This situation can lead to extensive damage; due to their transmissibility across membranes, reactive species have the potential to adversely affect all parts of the cell—from DNA to lipids to membranes—leading to disease and potentially necrosis (Halliwell and Gutteridge 2015). Oxidative stress, as a result of increased reactive species produced by photosystem II of the algal endosymbiont of coral, is a key factor in coral bleaching—the breakdown of symbiosis with Symbiodinium spp. (Lesser 1997; Gardner et al. 2017). However, during bleaching the coral host also increases reactive species, creating an unfavorable environment for the symbionts, leading to their death and/or expulsion (Weis 2008). This is one example of how anthozoan stress and immune responses are inextricably linked with the cytotoxicity of reactive species.
Cytotoxic reactive species can also be used to a host’s advantage by being produced deliberately during immune responses to kill pathogens. These may be as “by-products” of immune pathways, such as the melanin synthesis pathway (Cerenius and Söderhäll 2004), or by oxidase enzymes during phagocytosis, known as a respiratory burst (Berton et al. 2015). Respiratory bursts have been described within gorgonians (Mydlarz and Jacobs 2006) with the resultant reactive species released into the local environment (Shaked and Armoza-Zvuloni 2013). Additionally, in order to prevent self-harm, the increased production of reactive species and/or a measurable immune response is often coupled with an increase in antioxidants (Bartosz 2009), including within anthozoans (e.g., Mydlarz and Harvell 2007).
Antioxidants
The potential damage caused by oxidative stress means that the stakes are high when increasing reactive species production. In order to mitigate or minimize self-harm, a suite of antioxidants are always present and upregulated with increases in reactive species, such as during abiotic stress events or an immune response. Many compounds have antioxidant capacity, including pigments such as melanin (Meredith et al. 2006) and carotenoids (Cornet et al. 2007), but enzymatic antioxidants, such as superoxide dismutase, catalase and glutathione (peroxidases), and thioredoxin-dependent systems, are crucial in maintaining redox homeostasis (Williams et al. 2013).
Anthozoans possess many different types of enzymatic antioxidants, including peroxidases (Downs et al. 2002; Mydlarz and Harvell 2007), superoxide dismutase (Diaz et al. 2016), and catalase activity (hydrogen peroxide-scavenging) (Hawkridge et al. 2000; Mydlarz and Palmer 2011; Palmer et al. 2012). Consistent with a damage mitigation role during abiotic stress, coral antioxidant activity varies with shifts in environmental conditions and particularly during coral bleaching (e.g., Downs et al. 2002; Merle et al. 2007; Jin et al. 2016). Similarly, corals upregulate antioxidants in response to both injury and infection (Mydlarz et al. 2010; Mydlarz and Palmer 2011; Palmer et al. 2011c; Palmer 2010), which indicates the necessity of redox stabilization during an immune response.
Heat Shock Proteins
Heat shock proteins (HSPs ) are ubiquitous soluble, constitutively expressed proteins responsible for a suite of cellular housekeeping functions that are essential to organism survival. HSPs fall into ten family categories, present in all metazoans. HSPs are molecular chaperones, assisting in protein folding and preventing denaturing and, though first identified in relation to heat shock in Drosophila, are not restricted to roles in thermal stress mitigation (Srivastava 2002). HSPs help ensure homeostasis, and are therefore involved in both abiotic stress and immune responses (Srivastava 2002; Tenor and Aballay 2007). HSPs can generate reactive species and activate melanin synthesis (Baruah et al. 2014), and, similar to many immune factors, transcription is related to the Toll family of immune receptors (Tenor and Aballay 2007). Anthozoans possess many types of HSPs (Table 1).
The first anthozoan HSPs were found within the scleractinian Montastraea faveolata, the sea anemone E. pallida (Black et al. 1995), and the scleractinian Goniopora djiboutiensis (Sharp et al. 1997). Subsequently, a HSP found in the scleractinian coral Stylophora pistillata (SP HSP70) was used to monitor coral stress responses (Tom et al. 1999). Congruently, coral HSPs are upregulated during environmental change events, including thermal stress and bleaching (Barshis et al. 2013; Ross 2014; Traylor-Knowles et al. 2017b), pH change (Moya et al. 2015), and in response to disease (Seveso et al. 2015). HSPs have also been proposed to confer resistance of corals to heat stress (Palumbi et al. 2014). Additionally, HSPs have also been shown to be involved in the stress response to laboratory bacteria challenge experiments (e.g., Brown and Rodriguez-Lanetty 2015). Specifically, HSP70 was discovered to be involved in promoting a primitive form of the defense response in the sea anemone E. pallida (Brown and Rodriguez-Lanetty 2015). Brown and Rodriguez-Lanetty further propose that this short-term priming could confer immune strength during seasonal times of pathogen exposure. While the traditional studies of HSPs have focused on their role in general stress response and thermal stress response, more evidence now suggests that they may have a broader role in the immune response in anthozoans.
Fluorescent Proteins
A striking feature of many anthozoans is their multiple fluorescent proteins (FPs ) (Alieva et al. 2008) (Fig. 9). FPs consist of a chromophore that spontaneously forms in the presence of oxygen and which is housed within a hydrophobic core in a robust ß-barrel structure (Sample et al. 2009). The barrel restrains the vibrations of the excited chromophore and prevents radiation-less loss of energy (Sample et al. 2009; Seward and Bagshaw 2009). FPs utilize their protein microenvironments to modulate and refine their photophysical characteristics, enabling them to absorb light at different wavelengths (Sample et al. 2009). FPs function by absorbing a photon of light at a low wavelength, for example within the blue or UV spectra, which then shifts the chromophore to an excited state through pronotation (Sample et al. 2009). Energy is emitted as light of a longer, red-shifted wavelength as the chromophore returns to its ground state. In the excited state, FPs produce reactive species, which, if not moderated, can lead to photobleaching of FPs and impact the local microenvironment through oxidative stress (Halliwell and Gutteridge 2015). The protein scaffold of the FP is a molecular sink for reactive species, which thus protects the chromophore and surrounding microenvironment (Sample et al. 2009). Across different FPs there are multiple types of chromophore, which contribute to the spectral diversity found in FPs across taxa (Bogdanov et al. 2009). Cnidarian FP diversity is used commercially as markers of gene and protein expression, and has revolutionized modern biomedicine (Sample et al. 2009).
All organisms, from microbes to large mammals, use pigments for signaling, crypsis, or mitigation of oxidative stress. In the animal kingdom, the type and intensity of coloration is often indicative of immune competence (e.g., Nolan et al. 2006). However, while color production in the ocean is common (Widder 2010), the biological roles of the higHL: Intelectin-1y conserved scleractinian FPs remains debated (Takashashi-Kariyazono et al. 2016). The function of FPs in scleractinians is postulated to be primarily for the maintenance of the endosymbiotic relationship with Symbiodinium (Gittins et al. 2015). Symbiodinium is of great importance to the overall health of scleractinian coral, so it is beneficial for the scleractinian coral host to protect them. FPs alter the local light environment of the scleractinian host, and therefore may facilitate photosynthesis (Smith et al. 2017), provide protection during high light conditions (Salih et al. 1998), and act as a photo-attractant (Hollingsworth et al. 2005). However, the presence of FPs in anthozoans that lack Symbiodinium (azooxanthellate) (Wiedenmann et al. 2004) suggests that they have additional or alternative roles within cnidarian biology.
The visible increase of fluorescence in scleractinians with compromised tissue (Fig. 7) (Palmer et al. 2008; Palmer et al. 2009a) and its coincidence with elevated PO activity (Palmer et al. 2008; D’Angelo et al. 2012; Palmer et al. 2012; Palmer 2010) suggests that FPs may have a role in immunity. Concomitantly, and consistent with the general description of the biochemistry of FPs (Sample et al. 2009), scleractinian FPs scavenge hydrogen peroxide (Palmer et al. 2009b), indicating that FPs are potentially very useful for managing oxidative stress potential during immune responses and periods of environmental stress (Seneca et al. 2010; Roth and Deheyn 2013; van de Water et al. 2016).
Ecological Immunity : Focus on Scleractinian Corals
Ecological immunology theory postulates that variations among and within constituent immunity and immune responses are due to energetic trade-offs among costly life history traits like reproduction, growth, and maintenance/immunity (Sheldon and Verhulst 1996; Sadd and Schmid-Hempel 2009). Scleractinian corals, from both the Indo-Pacific (Palmer et al. 2010, 2012) and Caribbean (Mydlarz and Palmer 2011; Palmer et al. 2011c), demonstrate high intra-taxon variation in baseline levels of immunity, which for the Indo-Pacific scleractinians predicts susceptibility to both disease and bleaching at the family level (Palmer et al. 2010). These family-level differences in immunity corresponded to life history strategies; for example, fast-growing scleractinians with high reproductive output (e.g., Acroporiidae) are among those with the lowest constituent immunity (Palmer et al. 2010). Similarly, a short-term temporal study of three Indo-Pacific scleractinians found that constituent PO activity varied among species and with water temperature fluctuations (van de Water et al. 2016), indicating that immune function is influenced by the environment and life history of the scleractinian.
One of the first signs of coral distress is the increased production of mucus (Brown and Bythell 2005) (Fig. 5). The increased production of mucus requires large energetic investments, which leads to the depletion of metabolic reserves, resulting in an immune-compromised state (Sheridan et al. 2014). Similarly, following a thermal bleaching event, involving the loss of energy-providing Symbiodinium, disease is often the cause of scleractinian death (Fig. 4) (Brandt and McManus 2009). The breakdown of this mutualism results in a starvation state and an energy deficit, leaving the scleractinian host ill-equipped to resist infection, as for other invertebrates (e.g., Siva-Jothy and Thompson 2002). Similarly, infection is energetically costly as resources are invested in resisting, or tolerating, the disease (Mayack and Naug 2009).
The effects of paying the high energetic costs to promote health have been observed in scleractinian corals when comparing thermally bleached and diseased colonies to healthy ones (Palmer et al. 2011a). Both the thermally bleached and diseased colonies of the scleractinian A. hyacinthus had lower levels of PO and peroxidase activity than healthy controls (Palmer et al. 2011a). The exception being at the disease lesion edge, where immune response was equivalent to constituent levels of immunity in healthy controls (Palmer et al. 2011a). As with other invertebrates, the ability of a coral host to deliver an optimal immune response and maintain healthy constituent immune levels depends on the availability of energy (Sheridan et al. 2014). Energy availability and trade-offs therefore likely contribute to the intra-species variation in immunity observed within scleractinians (Wright et al. 2017; Palmer 2010). It is increasingly apparent that the environmental context needs to be considered with measurements of immunity (van de Water et al. 2015a; Wright et al. 2017). It is also evident that innate immune responses and responses to environmental change (“stress responses”) are intertwined. Immunity therefore has the potential to be used as an effective indicator of coral health (Palmer et al. 2010; Traylor-Knowles and Palumbi 2014; Jin et al. 2016). Thus, the expansion of coral ecological immunology is important for analyzing the influence of more frequent climate extremes on scleractinian corals as well as the ecosystems that they support.
Immunity, Climate Change, and Conservation of Scleractinian Corals
In this Anthropocene era, humans have placed themselves in an arms race against an aggravated natural world. Scientists and conservationists are racing to understand complex systems, develop technologies and conserve organisms and ecosystems before human-induced climate change shifts them beyond repair in this sixth mass extinction event. Coral reefs have declined globally and at an accelerating rate since the 1980’s e.g. (Bruno and Selig 2007; Hughes et al. 2017). Now, even with dramatic reductions in global carbon production, the persistence of coral reefs as biodiverse, economically significant systems (Graham et al. 2014) rests largely on our ability to conserve and effectively restore them through increasingly severe conditions. Cnidarian immunity will be critical in determining the long-term success of scleractinian species and coral reef communities (Palmer and Traylor-Knowles 2012; Mydlarz et al. 2016).
Anthozoans demonstrate a “stress response” to environmental perturbations, which is often investigated as distinct from immunity. With warming waters, increased hurricane activity, increased pathogen load, and ocean acidification, climate change stands to significantly undermine cnidarian immunity and therefore coral reef health. Scleractinian immune mechanisms have the potential to mitigate many of these assaults; however, studies investigating how these factors influence constituent immunity and immune responses are somewhat limited (e.g., Palmer et al. 2011c; van de Water et al. 2015c; Traylor-Knowles et al. 2017a). As such, cnidarian immunology has not yet been encorporated into the fields of coral conservation and restoration, though they stand to be highly informative.
In the face of climate change, our inefficacy in conserving and protecting wild coral reef systems is concerning. For example, the Great Barrier Reef, one of the world’s best-protected marine systems, has undergone catastrophic coral loss in the past 2 years (Hughes et al. 2017). While the term “restoration” refers to the act of returning something to its original condition, in the context of climate change it is well-acknowledged that reefs of the future will look quite different to the reefs of the past (Graham et al. 2014). With this in mind, starting in the 1990s, coral restoration projects using coral out-planting (coral gardening) have been under taken to promote biodiversity conservation and ecosystem function of scleractinians (Rinkevich 1995). Coral gardens are now abundant globally (Rinkevich 2014) and are a main method for restoring reef habitats. Coral gardening involves propagating small fragments of specific scleractinian genotypes on tree-like structures in mid-water nurseries, monitoring them to understand their disease and stress tolerances, and then out-planting them to prepared reef areas (Rinkevich 2014). Rapid coral colony growth through micro-fragmentation methods are promising; however, the physiological consequences of high fragmentation are as yet unknown (Forsman et al. 2015) and, as growth is high in terms of energetic cost, may come at the cost of impaired immunity (van der Most et al. 2011). However, numerous coral gardening restorations projects have been deemed successful, and arguably offer a viable mechanism for mediating the effects of climate change through ecological engineering (Schopmeyer 2012; Rinkevich 2014).
An alternative method to coral gardening restoration is larval seeding, where scleractinian coral larvae are reared ex situ and introduced in high densities to denuded reefs. However, the long-term benefits are still not well-understood (Edwards et al. 2015). Additionally, assisted evolution, via the use of selective breeding, epigenetics and microbiome manipulation, has recently begun to be used to create corals that are able to tolerate more extreme climate conditions (van Oppen et al. 2017). Assisted evolution has the potential to be integrated into ongoing coral gardening restoration efforts, and it is argued that the rate of coral loss means this is essential. van Oppen et al. (2017) have proposed targeting genes that underpin the “ubiquitous minimal cellular stress response” (Kültz 2005) and highlight coral stress and thermal resilience studies (e.g., Barshis et al. 2013). It is increasingly clear that cellular stress responses cannot be decoupled from immunity (Baruah et al. 2014; Pinsino and Matranga 2015), and therefore including specific immune-related genes as targets for assisted evolution and restoration will likely be of benefit.
Conclusion
In this chapter we have summarized the rapidly expanding field of cnidarian immunology, touching on the identified immune mechanisms, their roles in immune responses, and the relevance of cnidarian immunology to understanding ecological patterns in health and disease, and in improving restoration efforts. There are many gaps in our knowledge, including understanding the gene versus proteolytic regulation of responses to immune challenge and adverse conditions, as well as the identification of additional anthozoan-specific immune mechanisms. Many mechanisms still remain to be more extensively explored within anthozoans, including endocrine-like signaling (Song et al. 2015; Tarrant 2015) as well as locating sites of immune compound production, storage, and use. Lastly, cnidarian immunology will greatly benefit from more understanding of how the immune pathways fit together and influence each other. Cnidarian immunology is a burgeoning field that stands to inform conservation efforts, and biomedicine, as well as the field of comparative immunology.
References
Aceret TL, Coll JC, Uchio Y, Sammarco PW (1998) Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 120:121–126
Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3:745
Ainsworth T, Knack B, Ukani L, Seneca F, Weiss Y, Leggat W (2015) In situ hybridisation detects pro-apoptotic gene expression of a Bcl-2 family member in white syndrome-affected coral. Dis Aquat Org 117:155–163
Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, Beltran-Ramirez V, Miller DJ, Wiedenmann J, Salih A, Matz MV (2008) Diversity and evolution of coral fluorescent proteins. PLoS One 3:e2680
Anderson KV (2000) Toll signaling pathways in the innate immune response. Curr Opin Immunol 12:13–19
Anderson DA, Walz ME, Weil E, Tonellato P, Smith MC (2016) RNA-Seq of the Caribbean reef-building coral Orbicella faveolata (Scleractinia-Merulinidae) under bleaching and disease stress expands models of coral innate immunity. PeerJ 4:e1616
Anithajothi R, Duraikannu K, Umagowsalya G, Ramakritinan C (2014) The presence of biomarker enzymes of selected scleractinian corals of Palk Bay, southeast coast of India. Biomed Res Int 2014:1–6
Aranda M, Li Y, Liew YJ, Baumgarten S, Simakov O, Wilson MC, Piel J, Ashoor H, Bougouffa S, Bajic VB, Ryu T, Ravasi T, Bayer T, Micklem G, Kim H, Bhak J, LaJeunesse TC, Voolstra CR (2016) Genomes of coral dinoflagellate symbionts higHL: Intelectin-1ight evolutionary adaptations conducive to a symbiotic lifestyle. Sci Rep 6:39734
Artis D (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8:411
Augustin R, Bosch TC (2010) Cnidarian immunity: a tale of two barriers. Adv Exp Med Biol 708:1–16
Barshis DJ, Stillman JH, Gates RD, Toonen RJ, Smith LW, Birkelands C (2010) Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Mol Ecol 19:1705–1720
Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci U S A 110:1387–1392
Bartosz G (2009) Reactive oxygen species: destroyers or messengers? Biochem Pharmacol 77:1303–1315
Baruah K, Norouzitallab P, Linayati L, Sorgeloos P, Bossier P (2014) Reactive oxygen species generated by a heat shock protein (Hsp) inducing product contributes to Hsp70 production and Hsp70-mediated protective immunity in Artemia franciscana against pathogenic vibrios. Dev Comp Immunol 46:470–479
Baumgarten S, Simakov O, Esherick LY, Leiw YJ, Lehnert EM, Michell CT, Li Y, Hambleton EA, Guse A, Oates ME, HGough J, Weis VM, Aranda M, Pringe JR, Voolstra CR (2015) The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc Natl Acad Sci U S A 22:11893–11898
Berton G, Castaldi M, Cassatella M, Nauseef W (2015) Celebrating the 50th anniversary of the seminal discovery that the phagocyte respiratory burst enzyme is an NADPH oxidase. J Leukoc Biol 97:1–2
Beutler B (2004) Innate immunity: an overview. Mol Immunol 40:845–859
Bidla G, Hauling T, Dushay MS, Theopold U (2008) Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innate Immun 1:301–308
Biressi A, Zou T, Dupont S, DaHL: Intelectin-1berg C, Di Benedetto C, Bonasoro F, Thorndyke M, Carnevali MDC (2010) Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology 129:1–19
Black RE, Bloom L (1984) Heat shock proteins in aurelia (Cnidaria, Scyphozoa). J Exp Zool 230:303–307
Black NA, Voellmy R, Szmant AM (1995) Heat shock protein induction in Montastraea faveolata and Aiptasia pallida exposed to elevated temperatures. Biol Bull 188:234–240
Bogdanov AM, Mishin AS, Yampolsky IV, Belousov VV, Chudakov DM, Subach FV, Verkhusha VV, Lukyanov S, Lukyanov KA (2009) Green fluorescent proteins are light-induced electron donors. Nat Chem Biol 5:459–461
Bosch T, Praetzel G (1991) The heat shock response in hydra: immunological relationship of hsp60, the major heat shock protein of Hydra vulgaris, to the ubiquitous hsp70 family. Hydrobiologia 216/217:513–517
Bosch T, Krylow SM, Bode HR, Steele RE (1988) Thermotolerance and synthesis of heat shock proteins: these responses are present in Hydra attenuata but absent in Hydra oligactis. PNAS 85:7927–7931
Bosch TCG, Anton-Erxleben F, Hemmrich G, Khalturin K (2010) The hydra polyp: nothing but an active stem cell community. Develop Growth Differ 52:15–25
Bourne DG, Morrow KM, Webster NS (2016) Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu Rev Microbiol 70:317–340
Brandt ME, McManus JW (2009) Disease incidence is related to bleaching extent in reef-building corals. Ecology 90:2859–2867
Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH (2013) Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 14:32
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238
Brower D, Brower S, Hayward D, Ball E (1997) Molecular evolution of integrins: genes encoding integrin beta subunits from a coral and a sponge. Proc Natl Acad Sci U S A 94:9182–9187
Brown BE, Bythell JC (2005) Perspectives on mucus secretion in reef corals. Mar Ecol Prog Ser 296:291–309
Brown T, Rodriguez-Lanetty M (2015) Defending against pathogens–immunological priming and its molecular basis in a sea anemone, cnidarian. Sci Rep 5:17425
Brown T, Bourne D, Rodriguez-Lanetty M (2013) Transcriptional activation of c3 and hsp70 as part of the immune response of Acropora millepora to bacterial challenges. PLoS One 8:e67246
Bruno JF, Selig ER (2007) Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One 2:e711
Burge CA, Mouchka ME, Harvell CD, Roberts S (2013) Immune response of the Caribbean sea fan, Gorgonia ventalina, exposed to an Aplanochytrium parasite as revealed by transcriptome sequencing. Front Physiol 4:180
Bythell JC, Wild C (2011) Biology and ecology of coral mucus release. J Exp Mar Biol Ecol 408:88–93
Carpenter LW, Patterson MR, Bromage ES (2010) Water flow influences the spatiotemporal distribution of heat shock protein 70 within colonies of the scleractinian coral Montastrea annularis (Ellis and Solander,1786) following heat stress: implications for coral bleaching. J Exp Mar Biol Ecol 387:52–59
Carroll MC (1998) The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol 16:545–568
Cerenius L, Söderhäll K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126
Cerenius L, Lee BL, Söderhäll K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Cerenius L, Kawabata SI, Lee BL, Nonaka M, Soderhall K (2010) Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem Sci 35:575–583
Chadwick NE, Morrow KM (2011) Competition among sessile organisms on coral reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 347–371
Changyun W, Haiyan L, Changlun S, Yanan W, Liang L, Huashi G (2008) Chemical defensive substances of soft corals and gorgonians. Acta Ecol Sin 28:2320–2328
Choresh O, Ron EZ, Loya Y (2001) The 60-kDa heat shock protein (HSP60) of the sea anemone Anemonia viridis: a potential early warning system for environmental changes. Mar Biotechnol 3:501–508
Chow AM, Ferrier-Pages C, Khalouei S, Reynaud S, Brown IR (2009) Increased light intensity induces heat shock protein Hsp60 in coral species. Cell Stress Chaperones 14:469–476
Cooper EL (2010) Evolution of immune systems from self/not self to danger to Artificial Immune Systems (AIS). Phys Life Rev 7:55–78
Cooper EL, Hirabayashi K, Strychar KB, Sammarco PW (2014) Corals and their potential applications to integrative medicine. Evid Based Complement Alternat Med 2014:9
Cornet S, Biard C, Moret Y (2007) Is there a role for antioxidant carotenoids in limiting self-harming immune response in invertebrates? Biol Lett 3:284–288
Couch CS, Mydlarz LD, Harvell CD, Douglas NL (2008) Variation in measures of immunocompetence of sea fan coral, Gorgonia ventalina, in the Florida Keys. Mar Biol 155:281
Couch CS, Weil E, Harvell CD (2013) Temporal dynamics and plasticity in the cellular immune response of the sea fan coral, Gorgonia ventalina. Mar Biol 160:2449–2460
Daly M, Brugler MR, Cartwright P, Collins AG, Dawson MN, Fautin DG, France SC, McFadden CS, Opresko DM, Rodriguez E (2007) The phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 182:127–182
D'Angelo C, Smith EG, Oswald F, Burt J, Tchernov D, Wiedenmann J (2012) Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by Green Fluorescent Protein (GFP)-like pigments. Coral Reefs. https://doi.org/10.1007/s00338-012-0926-8
Daniels C, Baumgarten S, Yum L, MIchell C, Bayer T, Arif C, Roder C, Weil E, Voolstra C (2015) Metatranscriptome analysis of the reef-building coral Orbicella faveolata indicates holobiont response to coral disease. Frontiers in Marine. Science 2:62
Desalvo MK, Voolstra CR, Sunagawa S, Schwarz JA, Stillman JH, Coffroth MA, Szmant AM, Medina M (2008) Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol Ecol 17:3952–3971
Destoumieux-Garzón D, Rosa RD, Schmitt P, Barreto C, Vidal-Dupiol J, Mitta G, Gueguen Y, Bachere E (2016) Antimicrobial peptides in marine invertebrate health and disease. Philos Trans R Soc B 371:20150300
Detournay O, Schnitzler CE, Poole AZ, Weis V (2012) Regulation of cnidarian–dinoflagellate mutualisms: evidence that activation of a host TGFβ innate immune pathway promotes tolerance of the symbiont. Dev Comp Immunol 38:525–537
Diaz JM, Hansel CM, Apprill A, Brighi C, Zhang T, Weber L, McNally S, Xun L (2016) Species-specific control of external superoxide levels by the coral holobiont during a natural bleaching event. Nat Commun 7:13801
Dishaw LJ, Smith SL, Bigger CH (2005) Characterization of a C3-like cDNA in a coral: phylogenetic implications. Immunogenetics 57:535–548
Domart-Coulon IJ, Traylor-Knowles N, Peters E, Elbert D, Downs CA, Price K, Stubbs J, McLaugHL: Intelectin-1in S, Cox E, Aeby G, Brown PR, Ostrander GK (2006) Comprehensive characterization of skeletal tissue growth anomalies of the finger coral Porites compressa. Coral Reefs 25:531–543
Downs CA, Mueller EM, Phillips S, Fauth JE, Woodley CM (2000) A molecular biomarker system for assessing the health of coral (Montastraea faveolata) during heat stress. Mar Biotechnol 2:533–544
Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM (2002) Oxidative stress and seasonal coral bleaching. Free Radic Biol Med 33:533–543
Downs CA, Fauth JE, Robinson CE, Curry R, Lanzendorf B, Halas JC, Halas J, Woodley CM (2005) Cellular diagnostics and coral health: declining coral health in the Florida Keys. Mar Pollut Bull 51:558–569
Drake JL, Massa T, Haramatya L, Zelzionb E, Bhattacharya D (2013) Falkowaski PG proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. PNAS 110:3788–3793
DuBuc TQ, Traylor-Knowles N, Martindale MQ (2014) Initiating a regenerative response; cellular and molecular features of wound healing in the cnidarian Nematostella vectensis. BMC Biol 12:24
Dunn SR, Schnitzler CE, Weis VM (2007) Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proc R Soc B Biol Sci 274:3079–3085
Edwards AJ, Guest JR, Heyward AJ, Villanueva RD, Baria MV, Bollozos IS, Golbuu Y (2015) Direct seeding of mass-cultured coral larvae is not an effective option for reef rehabilitation. Mar Ecol Prog Ser 525:105–116
Esposito R, D'Aniello S, Squarzoni P, Pezzotti MR, Ristoratore F, Spagnuolo A (2012) New insights into the evolution of Metazoan tyrosinase gene family. PLoS One 7:e35731
Fang L-S, Huang S-P, Lin K-L (1997) High temperature induces the synthesis of heat-shock proteins and the elevation of intracellular calcium in the coral Acropora grandis. Coral Reefs 16:127–131
Fang F, Yan T, Liu Q (2005) Application of chemical ecology in controlling marine fouling organisms. Ying Yong Sheng Tai Xue Bao 16:1997–2002
Fensome RA (1993) A classification of living and fossil dinoflagellates. Micropaleontol Spec Publica 7:351
Forsman ZH, Page CA, Toonen RJ, Vaughan D (2015) Growing coral larger and faster: micro-colony-fusion as a strategy for accelerating coral cover. PeerJ 3:e1313
Franzenburg S, Fraunea S, Kunzel S, Baines JF, SDomazet-Loso T, Bosch T (2012) MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers. Proc Natl Acad Sci U S A 109:19374–11979
Franzenburg S, Walter J, Künzel S, Wang J, Baines JF, Bosch TC, Fraune S (2013) Distinct antimicrobial peptide expression determines host species-specific bacterial associations. Proc Natl Acad Sci U S A 110:E3730–E3738
Fuess LE, Weil E, Grinshpon RD, Mydlarz LD (2017) Life or death: disease-tolerant coral species activate autophagy following immune challenge. Proc R Soc B 284:20170771
Fujita T, Matsushita M, Endo Y (2004) The lectin-complement pathway - its role in innate immunity and evolution. Immunol Rev 198:185–202
Galko MJ, Krasnow MA (2004) Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol 2:1114–1126
Gardner SG, Raina J-B, Ralph PJ, Petrou K (2017) Reactive Oxygen Species (ROS) and dimethylated sulphur compounds in coral explants under acute thermal stress. J Exp Biol 220:1787
Gimenez A, Haran N, Pereira N, Acuña F (2014) First report of phenoloxidase and peroxidase activities in two intertidal sea anemone species of Argentina. Invertebr Surviv J 11:192–196
Gittins JR, D'Angelo C, Oswald F, Edwards RJ, Wiedenmann J (2015) Fluorescent protein-mediated colour polymorphism in reef corals: multicopy genes extend the adaptation/acclimatization potential to variable light environments. Mol Ecol 24:453–465
Gochfeld DJ, Aeby GS (2008) Antibacterial chemical defenses in Hawaiian corals provide possible protection from disease. Mar Ecol Prog Ser 362:119–128
Goldstone JV (2008) Environmental sensing and response genes in cnidaria: the chemical defensome in the sea anemone Nematostella vectensis. Cell Biol Toxicol 24:483–502
Graham NA, Cinner JE, Norström AV, Nyström M (2014) Coral reefs as novel ecosystems: embracing new futures. Curr Opin Environ Sustain 7:9–14
Haguenauer A, Zuberer F, Ledoux J-B, Aurelle D (2013) Adaptive abilities of the Mediterranean red coral Corallium rubrum in a heterogeneous and changing environment: from population to functional genetics. J Exp Mar Biol Ecol 449:349–357
Haine ER, Pollitt LC, Moret Y, Siva-Jothy MT, Rolff J (2008) Temporal patterns in immune responses to a range of microbial insults (Tenebrio molitor). J Insect Physiol 54:1090–1097
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. OUP, Oxford, USA
Hamada M, Shoguchi E, Shinzato C, Kawashima T, Miller DJ, Satoh N (2013) The complex NOD-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations. Mol Biol Evol 30:167–176
Harvell CD, Fenical W, Roussis V, Ruesink JL, Griggs CC, Greene CH (1993) Local and geographic variation in the defensive chemistry of a West Indian gorgonian coral (Briareum asbestinum). Mar Ecol Prog Ser 93:165–173
Hashimoto K, Shibuno T, Murayama-Kayano E, Tanaka H, Kayano T (2004) Isolation and characterization of stress-responsive genes from the scleractinian coral Pocillopora damicornis. Coral Reefs 23:485–491
Hawkins TD, Bradley BJ, Davy SK (2013) Nitric oxide mediates coral bleaching through an apoptotic-like cell death pathway: evidence from a model sea anemone-dinoflagellate symbiosis. FASEB J 27:4790–4798
Hawkridge JM, Pipe RK, Brown BE (2000) Localization of antioxidant enzymes in the cnidarians Anemonia viridis and Goniopora stokesi. Mar Biol 137:1–9
Hayes ML, Eytan RI, Hellberg ME (2010) High amino acid diversity and positive selection at a putative coral immunity gene (tachylectin-2). BMC Evol Biol 10:150
Hemmrich G, Miller DJ, Bosch TCG (2007) The evolution of immunity: a low-life perspective. Trends Immunol 28:449–454
Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB (1999) Phylogenetic perspectives in innate immunity. Science 284:1818–1318
Hollingsworth LL, Kinzie RA, Lewis TD, Krupp DA, Leong JAC (2005) Phototaxis of motile zooxanthellae to green light may facilitate symbiont capture by coral larvae. Coral Reefs 24:523–523
Huang C, Morlighem J-ERL, Cai J, Liao Q, Perez CD, Braga Gomes P, Guo M, Radis-Baptista G, Ming-Yuen Lee S (2017) Identification of long non-coding RNAs in two anthozoan species and their possible implications for coral bleaching. Sci Rep 7:5333
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hutton DMC, Smith VJ (1996) Antibacterial properties of isolated amoebocytes from the sea anemone Actinia equina. Biol Bull 191:441–451
Iguchi A, Shinzato C, Foret S, Miller D (2011) Identification of fast-evolving genes in the scleractinian coral acropora using comparative EST analysis. PLoS One 6:e20140
Jacobson M, Weil M, Raff M (1997) Programmed cell death in animal development. Cell 88:347–354
Jatkar AA, Brown BE, Bythell JC, Guppy R, Morris NJ, Pearson JP (2010) Coral mucus: the properties of its constituent mucins. Biomacromolecules 11:883–888
Jeong HJ, Yoo YD, Kang NS, Lim AS, Seong KA, Lee SY, Lee MJ, Lee KH, Kim HS, Shin W, Nam SW, Yih W, Lee K (2012) Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium. Proc Natl Acad Sci U S A 109:12604–12609
Jin YK, Lundgren P, Lutz A, Raina J-B, Howells EJ, Paley AS, Willis BL, van Oppen MJH (2016) Genetic markers for antioxidant capacity in a reef-building coral. Sci Adv 2:e1500842
Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D (2009) Integrins during evolution: evolutionary trees and model organisms. BBA-Biomembranes 1788:779–789
Kaiko GE, Stappenbeck TS (2014) Host–microbe interactions shaping the gastrointestinal environment. Trends Immunol 35:538–548
Kayal E, Roure B, Philippe H, Collins AG, Lavrov DV (2013) Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evol Biol 13:5–5
Kelman D, Kashman Y, Hill RT, Rosenberg E, Loya Y (2009) Chemical warfare in the sea: the search for antibiotics from Red Sea corals and sponges. Pure Appl Chem 81:1113–1121
Kenkel C, Aglyamova G, Alamaru A et al (2011) Development of gene expression markers of acute heat-light stress in reefbuilding corals of the genus Porites. PLoS One 6:e26914
Kenkel CD, Meyer E, Matz MV (2013) Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol Ecol 22:4322–4334
Kvennefors ECE, Leggat W, Kerr ATD, Hoegh-Guldberg O, Barnes AC (2010) Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Dev Comp Immunol 34(11):1219–1229
Kim K, Kim PD, Alker AP, Harvell CD (2000) Chemical resistance of gorgonian corals against fungal infections. Mar Biol 137:393–401
Kingsley RJ, Afif E, Cox BC, Kothari S, Kriechbaum K, Kuchinsky K, Neill AT, Puri AF, Kish VM (2003) Expression of heat shock and cold shock proteins in the Gorgonian Leptogorgia virgulata. J Exp Zool 296A:98–107
Knack BA, Iguchi A, Shinzato C, Hayward DC, Ball EE, Miller DJ (2008) Unexpected diversity of cnidarian integrins: expression during coral gastrulation. BMC Evol Biol 8:136
Koh EGL (1997) Do scleractinian corals engage in chemical warfare against microbes? J Chem Ecol 23:379–398
Krediet CJ, Ritchie KB, Paul VJ, Teplitski M (2013) Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc R Soc B Biol Sci 280:20122328
Kültz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
Kvennefors E, Leggat W, Hoegh-Guldberg O, Degnan B, Barnes A (2008) An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Dev Comp Immunol 32:1582–1592
Kvitt H, Kramarsky-Winter E, Maor-Landaw K, Zandbank K, Kushmaro A, Rosenfeld H, Fine M, Tchernov D (2015) Breakdown of coral colonial form under reduced pH conditions is initiated in polyps and mediated through apoptosis. Proc Natl Acad Sci U S A 112:2082–2086
Lawrence S, Davy J, Wilson W, Hoegh-Guldberg O, Davy S (2015) Porites white patch syndrome: associated viruses and disease physiology. Coral Reefs 34:249–257
Leggat W, Seneca F, Wasmund K, Ukani L, Yellowlees D et al (2011) Differential responses of the coral host and their algal symbiont to thermal stress. PLoS One 6:e26687
Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743
Lesser MP (1997) Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16:187–192
Libro S, Vollmer SV (2016) Genetic signature of resistance to white band disease in the Caribbean Staghorn coral Acropora cervicornis. PLoS One 11:e0146636
Libro S, Kaluziak ST, Vollmer SV (2013) RNA-seq profiles of immune related genes in the Staghorn coral Acropora cervicornis infected with white band disease. PLoS One 8:e81821
Loker ES, Adema CM, Zhang SM, Kepler TB (2004) Invertebrate immune systems – not homogeneous, not simple, not well understood. Immunol Rev 198:10–24
Luna-Acosta A, Rosenfeld E, Amari M, Fruitier-Arnaudin I, Bustamante P, Thomas-Guyon H (2010) First evidence of laccase activity in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol 4:719–716
Martin P, Leibovich SJ (2005) Inflammatory cells during wound, repair: the good, the bad and the ugly. Trends Cell Biol 15:599–607
Mayack C, Naug D (2009) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J Invertebr Pathol 100:185–188
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428
Medzhitov R, Janeway CA (2000a) Innate immune recognition: mechanisms and pathways. Immunol Rev 173:89–97
Medzhitov R, Janeway JC (2000b) The toll receptor family and microbial recognition. Trends Microbiol 8:452–456
Menzel LP, Bigger CH (2015) Identification of unstimulated constitutive immunocytes, by enzyme histochemistry, in the coenenchyme of the octocoral Swiftia exserta. Biol Bull 229:199–208
Meredith P, Powell BJ, Riesz J, Nighswander-Rempel SP, Pederson MR, Moore EG (2006) Towards structure-property-function relationships for eumelanin. Soft Matter 2:37–44
Merle PL, Sabourault C, Richier S, Allemand D, Furla P (2007) Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic Biol Med 42:236–246
Meszaros A, Bigger C (1999) Qualitative and quantitative study of wound healing processes in the coelenterate, Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences. J Invertebr Pathol 73:321–331
Meyer E, Aglyamova GV, Wang S, Buchanan-Carter J, Abrego D, Colbourne JK, Willis BL, Matz MV (2009) Sequencing and de novo analysis of a coral larval transcriptome using 454 GSFlx. BMC Genomics 10:219
Miller D, Hemmrich G, Ball E, Hayward D, Khalturin K, Funayama N, Agata K, Bosch T (2007) The innate immune repertoire in Cnidaria – ancestral complexity and stochastic gene loss. Genome Biol 8:R59
Moya A, Huisman L, Foret S, Gattuso JP, Hayward DC, Ball E, Miller DJ (2015) Rapid acclimation of juvenile corals to CO2-mediated acidification by upregulation of heat shock protein and Bcl-2 genes. Mol Ecol 24:438–452
Moya A, Sakamaki K, Mason BM, Huisman L, Forêt S, Weiss Y, Bull TE, Tomii K, Imai K, Hayward DC, Ball EE, Miller DJ (2016) Functional conservation of the apoptotic machinery from coral to man: the diverse and complex Bcl-2 and caspase repertoires of Acropora millepora. BMC Genomics 17:62
Mullen K, Peters EC, Harvell CD (2004) Coral resistance to disease. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 377–399
Muscatine L, Porter JW (1977) Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27:454–460
Mydlarz LD, Harvell CD (2007) Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp Biochem Physiol A Mol Integr Physiol 146:54–62
Mydlarz LD, Jacobs RS (2006) An inducible release of reactive oxygen radicals in four species of gorgonian corals. Mar Freshw Behav Physiol 39:143–152
Mydlarz LD, Palmer CV (2011) The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp Biochem Physiol A Mol Integr Physiol 159:372–378
Mydlarz LD, Holthouse SF, Peters EC, Harvell CD (2008) Cellular responses in sea fan corals: granular amoebocytes react to pathogen and climate stressors. PLoS One 3:e1811
Mydlarz LD, McGinty ES, Harvell CD (2010) What are the physiological and immunological responses of coral to climate warming and disease? J Exp Biol 213:934–945
Mydlarz LD, Fuess LE, Mann WT, Pinzón CJH, Gochfeld DJ (2016) Cnidarian immunity: from genomes to phenomes. In: Goffredo S, Dubinsky Z (eds) The Cnidaria, past, present and future. Springer, Cham, pp 441–466
Nakamura M, Morita M, Kurihara H, Mitarai S (2012) Expression of HSP70, HSP90 and HSF1 in the reef coral Acropora digitifera under prospective acidified conditions over the next several decades. Biol Open 1:75–81
Neubauer EF, Poole AZ, Weis V, Davy SK (2016) The scavenger receptor repertoire in six cnidarian species and its putative role in cnidarian-dinoflagellate symbiosis. PeerJ 4:e2692
Nolan PM, Dobson FS, Dresp B, Jouventin P (2006) Immunocompetence is signalled by ornamental colour in king penguins, Aptenodytes patagonicus. Evol Ecol Res 8:1325–1332
Nozawa H, Cho SY, Seki N (2001) Purification and characterization of transglutaminase from squid gill. Fish Sci 67:912–919
Ocampo ID, Zárate-Potes A, Pizarro V, Rojas CA, Vera NE, Cadavid LF (2015) The immunotranscriptome of the Caribbean reef-building coral Pseudodiploria strigosa. Immunogenetics 67:515–530
Olano CT, Bigger CH (2000) Phagocytic activities of the gorgonian coral Swiftia exserta. J Invertebr Pathol 76:176–184
Olsen K, Ritson-Williams R, Ochrietor JD, Paul VJ, Ross C (2013) Detecting hyperthermal stress in larvae of the hermatypic coral Porites astreoides: the suitability of using biomarkers of oxidative stress versus heat-shock protein transcriptional expression. Mar Biol 160:2609–2618
O'Neill LAJ, Golenbock D, Bowie AG (2013) The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13:453–460
Otero-Gonzalez AJ, Magalhaes BS, Garcia-Villarino M, Lopez-Abarrategui C, Sousa DA, Dias SC, Franco OL (2010) Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J 24:1320–1334
Ovchinnikova TV, Balandin SV, Aleshina GM, Tagaev AA, Leonova YF, Krasnodembsky ED, Men’shenin AV, Kokryakov VN (2006) Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem Biophys Res Commun 348:514–523
Palmer CV (2010) Biological mechanisms of Scleractinian immunity [Doctoral Thesis] James Cook University, Australia, Newcastle University, UK
Palmer CV, Traylor-Knowles N (2012) Towards an integrated network of coral immune mechanisms. Proc R Soc B 279:4106–4114
Palmer C, Mydlarz L, Willis B (2008) Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proc R Soc B 275:2687–2693
Palmer CV, Roth MS, Gates RD (2009a) Red fluorescent protein responsible for pigmentation in trematode-infected Porites compressa tissues. Biol Bull 215:68–74
Palmer CV, Modi CK, Mydlarz LD (2009b) Coral fluorescent proteins as antioxidants. PLoS One 4:e7298
Palmer CV, Bythell JC, Willis BL (2010) Immunity parameters of reef corals underpin bleaching and disease susceptibility. Fed Am Soc Exp Biol 24:1935–1946
Palmer CV, Bythell JC, Willis BL (2011a) A comparative study of phenoloxidase activity in diseased and bleached colonies of the coral Acropora millepora. Dev Comp Immunol 10:1098–1101
Palmer CV, Traylor-Knowles NG, Willis BL, Bythell JC (2011b) Corals use similar immune cells and wound-healing processes as those of higher organisms. PLoS One 6:e23992
Palmer CV, McGinty ES, Cummings D, Bartels E, Mydlarz LD (2011c) Patterns of coral ecological immunology: variation in the responses of Caribbean corals to elevated temperature and a pathogen elicitor. J Exp Biol 15:4240–4249
Palmer C, Bythell JC, Willis B (2012a) Enzyme activity demonstrates multiple pathways of innate immunity in Indo-Pacific anthozoans. Proc R Soc Lond B Biol Sci:rspb20112487
Palmer CV, Graham E, Baird AH (2012b) Immunity through early development of coral larvae. Dev Comp Immunol 38:395–399
Palmer CV, Bythell JC, Willis BL (2012c) Enzyme activity demonstrates multiple pathways of innate immunity in Indo-Pacific corals. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2011.2487
Palmer CV, Bythell JC, Willis BL (2012d) Enzyme activity demonstrates multiple pathways of innate immunity in Indo-Pacific anthozoans. Proc R Soc B 279:3879–3887
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Science 344:895–898
Parisi M, Trapani M, Cammarata M (2014) Granulocytes of sea anemone Actinia equina (Linnaeus, 1758) body fluid contain and release cytolysins forming plaques of lysis. ISJ 11:63
Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM (2003) Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser 264:167–171
Pey A, Zamoum T, Allemand D, Furla P, Merle P-L (2011) Depth-dependent thermotolerance of the symbiotic Mediterranean gorgonian Eunicella singularis: evidence from cellular stress markers. J Exp Mar Biol Ecol 404:73–78
Pinsino A, Matranga V (2015) Sea urchin immune cells as sentinels of environmental stress. Dev Comp Immunol 49:198–205
Pinzón JH, Kamel B, Burge CA, Harvell CD, Medina M, Weil E, Mydlarz LD (2015) Whole transcriptome analysis reveals changes in expression of immune-related genes during and after bleaching in a reef-building coral. R Soc Open Sci 2:140214
Polato NR, Voolstra CR, Schnetzer J, Desalvo MK, Randall CJ (2010) Location-specific responses to thermal stress in larvae of the reef-building coral Montastraea faveolata. PLoS One 5(6):e11221
Poole AZ, Weis VM (2014) TIR-domain-containing protein repertoire of nine anthozoan species reveals coral–specific expansions and uncharacterized proteins. Dev Comp Immunol 46:480–488
Poole AZ, Kitchen SA, Weis V (2016) The role of complement in Cnidarian-dinoflagellate symbiosis and immune challenge in the sea anemone Aiptasia pallida. Front Microbiol 7:519
Portune KJ, Voolstra CR, Medina M, Szmant A (2010) Development and heat stress-induced transcriptomic changes during embryogenesis of the scleractinian coral Acropora palmata. Mar Genomics 3:51–62
Puill-Stephan E, Seneca FO, Miller DJ, van Oppen MJH, Willis BL (2012) Expression of putative immune response genes during early ontogeny in the coral Acropora millepora. PLoS One 7:e39099
Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94
Putnam HM, Mayfield AB, Fan TY, Chen CS, Gates RD (2013) The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near future increases in temperature and pCO2. Mar Biol 160:2157–2173
Quistad S, Traylor-Knowles N (2016) Precambrian origins of the TNFR superfamily. Cell Death Dis 2:16058
Quistad SD, Stotland A, Barott KL, Smurthwaite CA, Hilton BJ, Grasis JA, Wolkowicz R, Rohwer FL (2014) Evolution of TNF-induced apoptosis reveals 550 My of functional conservation. Proc Natl Acad Sci U S A 111:9567–9572
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118:229–241
Reitzel AM, Sullivan JC, Traylor-Knowles N, Finnerty JR (2008) Genomic survey of candidate stress-response genes in the esturine anemone Nematostella vectensis. Biol Bull 214:233–254
Renegar D-EA, Blackwelder P, Miller J, Gochfeld D, Moulding AL (2008) Ultrastructural and histological analysis of Dark Spot Syndrome in Siderastrea siderea and Agaricia agaricites
Richier S, Rodriguez-Lanetty M, Schnitzler CE, Weis V (2008) Response of the symbiotic cnidarian Anthopleura elegantissima transcriptome to temperature and UV increase. Comp Biochem Physiol Part D 3:283–289
Rinkevich B (1995) Restoration strategies for coral reefs damaged by recreational activities: the use of sexual and asexual recruits. Restor Ecol 3:241–251
Rinkevich B (2014) Rebuilding coral reefs: does active reef restoration lead to sustainable reefs? Curr Opin Environ Sustain 7:28–36
Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Rodriguez-Lanetty M, Harii S, Hoegh-Guldberg O (2009) Early molecular response of coral larvae to hyperthermal stress. Mol Ecol 18:5101–5114
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10
Romano SL, Palumbi SR (1996) Evolution of Scleractinian corals inferred from molecular systematics. Science 271:640–642
Rose NH, Seneca FO, Palumbi SR (2015) Gene networks in the wild: identifying transcriptional modules that mediate coral resistance to experimental heat stress. Genome Biol Evol 8:243–252
Rosental B, Kozhekbaeva Z, Fernhoff N, Tsai JM, Traylor-Knowles N (2017) Coral cell separation and isolation by Fluorescence-Activated Cell Sorting (FACS). BMC Cell Biol 18:30
Ross C (2014) Nitric oxide and heat shock protein 90 co-regulate temperature-induced bleaching in the soft coral Eunicea fusca. Coral Reefs 33:513–522
Roth MS, Deheyn DD (2013) Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci Rep 3:1421
Rough L, Downs CA, Richmond RH, Ostrander GK (2006) Alteration of normal cellular profiles in the scleractinian coral Pocillopora damicornis following laboratory exposure to fuel oil. Environ Toxicol Chem 25:3181–3187
Sadd BM, Schmid-Hempel P (2009) Principles of ecological immunology. Evol Appl 2:113–121
Salih A, Hoegh-Guldberg O, Cox G (1998) Photoprotection of symbiotic Dinoflagellates by fluorescent pigments in reef corals. In: Greenwood JG, Hall NJ (eds) Proceedings of the Australian Coral Reef Society 75th Anniversary Conference, Heron Island October 1997. University of Queensland, Brisbane, pp 217–230
Sample V, Newman RH, Zhang J (2009) The structure and function of fluorescent proteins. Chem Soc Rev 38:2852–2864
Schnitzler CE, Weis VM (2010) Coral larvae exhibit few measurable transcriptional changes during the onset of coral-dinoflagellate endosymbiosis. Mar Genomics 3:107–116
Schopmeyer SA (2012) In situ coral nurseries serve as genetic repositories for coral reef restoration after an extreme cold-water event. Restor Ecol 20:696–703. -2012 v.2020 no.2016
Schwarz JA, Brokstein PB, Voolstra C, Terry A, Miller D, Szmant A, Coffroth MA, Medina M (2008) Coral life history and symbiosis: functional genomic resources for two reef building Caribbean corals, Acropora palmata and Montastraea faveolata. BMC Genomics 97:1471–2164
Seneca FO, Palumbi SR (2015) The role of transcriptome resilience in resistance of corals to bleaching. Mol Ecol 24:1467–1484
Seneca FO, Forêt S, Ball EE, Smith-Keune C, Miller DJ, Oppen MJH (2010) Patterns of gene expression in a scleractinian coral undergoing natural bleaching. Mar Biotechnol 12:594–604
Seppala O, Jokela J (2011) Immune defence under extreme ambient temperature. Biol Lett 7:119–122
Seveso D, Montano S, Giovanni S, Orlandi I, Vai M, Galli P (2012) Up-regulation of Hsp60 in response to skeleton eroding band disease but not by algal overgrowth in the scleractinian coral Acropora muricata. Mar Environ Res 78:34–e39
Seveso D, Montano S, Strona G, Orlandi I, Galli P, Vai M (2013) Over-expression of higHL: Intelectin-1y conserved mitochondrial 70-kDa heat-shock protein in the sea anemone Anemonia viridis. Mar Environ Res 90:96e103
Seveso D, Montano S, Reggente MAL, Orlandi I, Galli P, Vai M (2015) Modulation of Hsp60 in response to coral brown band disease. Dis Aquat Org 115:15–23
Seward HE, Bagshaw CR (2009) The photochemistry of fluorescent proteins: implications for their biological applications. Chem Soc Rev 38:2842–2851
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236–248
Shaked Y, Armoza-Zvuloni R (2013) Dynamics of hydrogen peroxide in a coral reef: sources and sinks. J Geophys Res Biogeo 118:1793–1801
Shapo JL, Moeller PD, Galloway SB (2007) Antimicrobial activity in the common seawhip, Leptogorgia virgulata (Cnidaria: Gorgonaceae). Comp Biochem Physiol B: Biochem Mol Biol 148:65–73
Sharp VA, Miller D, Bythell JC (1994) Expression of low molecular weight HSP 70 related polypeptides from the symbiotic sea anemone Anemonia viridis Forskall in response to heat shock. J Exp Mar Biol Ecol 179:179–193
Sharp VA, Brown BE, Miller D (1997) Heat shock protein (hsp 70) expression in the tropical reef coral Goniopora djiboutiensis. J Therm Biol 22:11–19
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Sheridan C, Grosjean P, Leblud J, Palmer C, Kushmaro A, Eeckhaut I (2014) Sedimentation rapidly induces an immune response and depletes energy stores in a hard coral. Coral Reefs 33:1067–1076
Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M (2011) Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476:320
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183
Siva-Jothy MT, Thompson JJW (2002) Short-term nutrient deprivation affects immune function. Physiol Entomol 27:206–212
Slattery M, Gochfeld DJ (2012) Chemically mediated competition and host–pathogen interactions among marine organisms handbook of marine natural products. Springer, Berlin, pp 823–859
Slattery M, Renegar D, Gochfeld D (2013) Direct and indirect effects of a new disease of alcyonacean soft corals. Coral Reefs 32:879–889
Smith EG, D'angelo C, Sharon Y, Tchernov D, Wiedenmann J (2017) Acclimatization of symbiotic corals to mesophotic light environments through wavelength transformation by fluorescent protein pigments. Proc R Soc B 284:20170320
Söderhäll K, Cerenius L (1998) Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol 10:23–28
Söderhäll K, Smith VJ (eds) (1986) The prophenoloxidase activating system: the biochemistry of its activation and role in arthropod cellular immunity, with special reference to crustaceans. Springer, Berlin
Song L, Wang L, Zhang H, Wang M (2015) The immune system and its modulation mechanism in scallop. Fish Shellfish Immunol 46:65–78
Srivastava P (2002) Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2:185
Stanley GD (2006) Photosymbiosis and the evolution of modern coral reefs. Science 312:857–858
Stewart AK, Pavasovic A, Hock DH, Prentis PJ (2017) Transcriptomic investigation of wound healing and regeneration in the cnidarian Calliactis polypus. Sci Rep 7:41458
Sugumaran M (2002) Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res 15:2–9
Sullivan JC, Kalaitzidis D, DGilmore TD, Finnerty JR (2007) Rel homology domain-containing transcription factors in the cnidairan Nematostella vectensis. Dev Genes Evol 217:63–72
Sullivan JC, Wolenski FS, Reitzel AM, French CE, Traylor-Knowles N, Gilmore TD et al (2009) Two alleles of NF-κB in the sea anemone Nematostella vectensis are widely dispersed in nature and encode proteins with distinct activities. PLoS One 10:e7311
Sunagawa S, Wilson EC, Thaler M, Smith ML, Caruso C, Pringe JR, Weis V, Medina M, Schwarz J (2009) Generation and analysis of transcriptomic resources for a model system on the rise: the sea anemone Aiptasia pallida and its dinoflagellate endosymbiont. BMC Genomics 10:258
Sweet MJ, Croquer A, Bythell JC (2011) Bacterial assemblages differ between compartments within the coral holobiont. Coral Reefs 30:39–52
Takada Y, Ye X, Simon S (2007) The integrins. Genome Biol 8:215
Takahashi D, Garcia BL, Kanost MR (2015) Initiating protease with modular domains interacts with β-glucan recognition protein to trigger innate immune response in insects. Proc Natl Acad Sci U S A 112:13856–13861
Takahashi-Kariyazono S, Gojobori J, Satta Y, Sakai K, Terai Y (2016) Acropora digitifera encodes the largest known family of fluorescent proteins that has persisted during the evolution of Acropora species. Genome Biol Evol 8:3271–3283
Tarrant AM (2015) Endocrine‐like signaling in corals. In: Woodley CM, Downs CA, Bruckner A, Porter J, Galloway SB (eds) Diseases of coral. John Wiley and Sons, Inc, p 138–149
Tchernov D, Kvitt H, Haramaty L, Bibby TS, Gorbunov MY, Rosenfeld H, Falkowski PG (2011) Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proc Natl Acad Sci U S A 108:9905–9909
Teixeira T, Diniz M, Calado R, Rosa R (2013) Coral physiological adaptations to air exposure: heat shock and oxidative stress responses in Veretillum cynomorium. J Exp Mar Biol Ecol 439:35–41
Tenor JL, Aballay A (2007) A conserved toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep 9:103
Theopold U, Schmidt O, Soderhall K, Dushay MS (2004) Coagulation in arthropods: defence, wound closure and healing. Trends Immunol 25:289–294
Tom M, Douek J, Yankelevich I, Bosch TC, Rinkevich B (1999) Molecular characterization of the first heat shock protein 70 from a reef coral. Biochem Biophys Res Commun 262:103–108
Tomanek L (2015) Proteomic responses to environmentally induced oxidative stress. J Exp Biol 218:1867–1879
Traylor-Knowles N, Palumbi SR (2014) Translational environmental biology: cell biology informing conservation. Trends Cell Biol 24:265–267
Traylor-Knowles N, Granger BR, Lubinski TJ, Parikh JR, Garamszegi S, Xia Y, Marto JA, Kaufman L, Finnerty JR (2011) Production of a reference transcriptome and transcriptomic database (PocilloporaBase) for the cauliflower coral, Pocillopora damicornis. BMC Genomics 12:585
Traylor-Knowles N, Rose NH, Palumbi SR (2017a) The cell specificity of gene expression in the response to heat stress in corals. J Exp Biol 220:1837–1845
Traylor-Knowles N, Rose NH, Sheets EA, Palumbi SR (2017b) Early transcriptional responses during heat stress in the coral Acropora hyacinthus. Biol Bull 232:91–100
Van Alstyne KL, Wylie CR, Paul VJ (1994) Antipredator defenses in tropical Pacific soft corals (Coelenterata: Alcyonacea) II. The relative importance of chemical and structural defenses in three species of Sinularia. J Exp Mar Biol Ecol 178:17–34
van de Water JA, Leggat W, Bourne DG, van Oppen MJ, Willis BL, Ainsworth TD (2015a) Elevated seawater temperatures have a limited impact on the coral immune response following physical damage. Hydrobiologia 759:201–214
van de Water JAJM, Lamb JB, van Oppen M, Willis B, Bourne DG (2015b) Comparative immune responses of corals to stressors associated with offshore reef-based tourist platforms. Conserv Physiol 3:cov032
van de Water JAJM, Ainsworth TD, Leggat W, Bourne DG, Willis BL, van Oppen MJH (2015c) The coral immune response facilitates protection against microbes during tissue regeneration. Mol Ecol 24:3390–3404
van de Water JAJM, Lamb JB, Heron SF, van Oppen MJH, Willis BL (2016) Temporal patterns in innate immunity parameters in reef-building corals and linkages with local climatic conditions. Ecosphere 7:e01505. -n/a
van der Burg CA, Prentis PJ, Surm JM, Pavasovic A (2016) Insights into the innate immunome of actiniarians using a comparative genomic approach. BMC Genomics 17:850
van der Most PJ, de Jong B, Parmentier HK, Verhulst S (2011) Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct Ecol 25:74–80
van Oppen MJH, Gates RD, Blackall LL, Cantin N, Chakravarti LJ, Chan WY, Cormick C, Crean A, Damjanovic K, Epstein H, Harrison PL, Jones TA, Miller M, Pears RJ, Peplow LM, Raftos DA, Schaffelke B, Stewart K, Torda G, Wachenfeld D, Weeks AR, Putnam HM (2017) Shifting paradigms in restoration of the world’s coral reefs. Glob Chang Biol 23:3437–3448
Vargas-Angel B, Peters EC, Kramarsky-Winter E, Gilliam DS, Dodge RE (2007) Cellular reactions to sedimentation and temperature stress in the Caribbean coral Montastraea cavernosa. J Invertebr Pathol 95:140–145
Venn AA, Quinn J, Jones RJ, Bodnar A (2009) P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquat Toxicol 93:188–195
Vidal-Dupiol J, Adjeroud M, Roger E, Foure L, Duval D, Mone Y, Ferrier-Pages C, Tambutte E, Tambutte S, Zoccola D, Allemand D, Mitta G (2009) Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiol 9:14
Vidal-Dupiol J, Ladriere O, Meistertzheim AL, Foure L, Adjeroud M, Mitta G (2011a) Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus. J Exp Biol 214:1533–1545
Vidal-Dupiol J, Ladriere O, Destoumieux-Garzon D, Sautiere PE, Meistertzheim AL, Tambutte E, Tambutte S, Duval D, Foure L, Adjeroud M (2011b) Innate immune responses of a scleractinian coral to vibriosis. J Biol Chem 286:22688–22698
Vidal-Dupiol J, Dheilly NM, Rondon R, Grunau C, Cosseau C, Smith KM, Freitag M, Adjeroud M, Mitta G (2014) Thermal stress triggers broad Pocillopora damicornis transcriptomic remodeling, while Vibrio coralliilyticus infection induces a more targeted Immuno-suppression response. PLoS One 9:e107672
Voolstra C, Schnetzert J, Peshkin L, Randall CJ, Szmant A, Medina M (2009) Effects of temperature on gene expression in embryos of the coral Montastraea faveolata. BMC Genomics 10:627
Voolstra CR, Sunagawa S, Matz MV, Bayer T, Aranda M, Buschiazzo E, DeSalvo MK, Lindquist E, Szmant AM, Coffroth MA, Medina M (2011) Rapid evolution of coral proteins responsible for interaction with the environment. PLoS One 6:e20392
Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211:3059
Weiss Y, Forêt S, Hayward DC, Ainsworth T, King R, Ball EE, Miller DJ (2013) The acute transcriptional response of the coral Acropora millepora to immune challenge: expression of GiMAP/IAN genes links the innate immune responses of corals with those of mammals and plants. BMC Genomics 14:400
Wenger Y, Buzgariu W, Reiter S, Galliot B (2014) Injury-induced immune responses in hydra. Semin Immunol 26:277–294
Widder EA (2010) Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science 328:704–708
Wiedenmann J, Ivanchenko S, Oswald F, Nienhaus GU (2004) Identification of GFP-like proteins in nonbioluminescent, azooxanthellate anthozoa opens new perspectives for bioprospecting. Mar Biotechnol 6:270–277
Wiens M, Ammar MSA, Nawar AH, Koziol C, Hassanein HMA, Eisinger M, Mullera IM, Mullera WEG (2000) Induction of heat-shock (stress) protein gene expression by selected natural and anthropogenic disturbances in the octocoral Dendronephthya klunzingeri. J Exp Mar Biol Ecol 245:265–276
Williams DL, Bonilla M, Gladyshev VN, Salinas G (2013) Thioredoxin glutathione reductase-dependent redox networks in Platyhelminth parasites. Antioxid Redox Signal 19:735–745
Wolenski FS, Garbati MR, Lubinski TJ, Traylor-Knowles N, Dresselhaus E, Stefanik DJ, Goucher H, Finnerty JR, Gilmore TD (2011) Characterization of the core elements of the NF-κB signaling pathway of the sea anemone Nematostella vectensis. Mol Cell Biol 31:1076–1087
Wolenski FS, Bradham CA, Finnerty JR, Gilmore TD (2013) NF-κB is required for cnidocyte development in the sea anemone Nematostella vectensis. Dev Biol 373:205–215
Won J, Rho B, Song J (2001) A phylogenetic study of the Anthozoa (phylum Cnidaria) based on morphological and molecular characters. Coral Reefs 20:39–50
Wood-Charlson E, Hollingsworth L, Krupp D, Weis V (2006) Lectin/glycan interactions play a role in recognition in coral/dinoflagellate symbiosis. Cell Microbiol 8:1985–1993
Wood-Charlson EM, Weis VM (2009) The diversity of C-type lectins in the genome of a basal metazoan, Nematostella vectensis. Dev Comp Immunol 33(8):881–889
Wright RM, Kenkel CD, Dunn CE, Shilling EN, Bay LK, Matz MV (2017) Intraspecific differences in molecular stress responses and coral pathobiome contribute to mortality under bacterial challenge in Acropora millepora. Sci Rep 7:2609
Yu X-Q, Kanost MR (2004) Immulectin-2, a pattern recognition receptor that stimulates hemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev Comp Immunol 28:891–900
Zaragoza WJ, Krediet CJ, Meyer JL, Canas G, Ritchie KB, Teplitski M (2014) Outcomes of infections of sea anemone aiptasia pallida with Vibrio spp. pathogenic to corals. Microb Ecol 68:388–396
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhou Z et al (2017) Dual recognition activity of a rhamnose-binding lectin to pathogenic bacteria and zooxanthellae in stony coral Pocillopora damicornis. Dev Comp Immunol 70:88–93
Zou J, Chang M, Nie P, Secombes CJ (2009) Origin and evolution of the RIG-I like RNA helicase gene family. BMC Evol Biol 9:85
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Palmer, C.V., Traylor-Knowles, N.G. (2018). Cnidaria: Anthozoans in the Hot Seat. In: Cooper, E. (eds) Advances in Comparative Immunology. Springer, Cham. https://doi.org/10.1007/978-3-319-76768-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-76768-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76767-3
Online ISBN: 978-3-319-76768-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)