Abstract
Aspergillosis is a widespread disease that has impacted the demography of the Caribbean sea fan coral, Gorgonia ventalina. The innate coral immune defenses can be measured as constitutive levels of immune proteins (peroxidase [POX], prophenoloxidase [PPO], lysozyme-like activity [LYS], exochitinase [EXOC]), antioxidant (superoxide dismutase [SOD]), and antimicrobial (antibacterial [AB] and antifungal [AF]) activity. Therefore, variations in these parameters across a geographic region could provide clues to the role of environment in disease. This study examined healthy sea fans collected in July 2005 from six offshore sites in the Florida Keys lying between 24.569°N and 25.220°N, a distance of ~145 km. Contrary to expectations, small (<15 cm) colonies did not differ significantly from large colonies (>15 cm) in the protein-based levels of activity in any of the measured parameters. However, there were significant differences in many of the parameters among sites, and Molasses Reef and Looe Key Reef were the most different in POX, PPO, SOD, and AF activity. This suggests that there are potential site-specific environmental factors that shape the immune physiology of colonies. Several proxies of environmental stress were also regressed against levels of the immune parameters. The proxies included 10 year averages of benthic community composition, 5 year averages of water quality, and historic aspergillosis disease prevalence and severity. Generality about environmental drivers was limited by assaying only six sites, but several patterns did emerge. SOD, EXOC, and AF activity were all correlated with percent bare substrate cover, suggesting that certain immune components may be activated in low coral environments. LYS and EXOC activity were positively correlated with dissolved inorganic nitrogen (DIN), one proxy of water quality. There were no relationships between any of the measured immune parameters and previous disease prevalence and severity. This study is a first step in evaluating levels of within- and between-site variation in coral immunity and investigating possible environmental drivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological immunology is the study of the factors and outcomes of variation in immune functions that influence evolution and ecology (Rolff and Siva-Jothy 2003). Most of the research has focused on costs of maintaining the host immune system and the tradeoffs on other life history strategies (Norris and Evans 2000; Schmid-Hempel 2003). However, there are very few data on how the immunity of populations varies among environments and how immune function is influenced by a previous history of disease and environmental stress (Rolff and Siva-Jothy 2003; Mucklow et al. 2004; Mydlarz et al. 2006). Coral reefs and other marine environments are being heavily impacted by outbreaks of disease (Sutherland et al. 2004; Harvell et al. 2007) and yet, how coral immunity and disease resistance vary in nature is not well understood.
The immune responses of the Caribbean sea fan coral, Gorgonia ventalina, have been relatively well studied due to the widespread disease, aspergillosis. This infection, caused by the terrestrial fungus Aspergillus sydowii, leads to lesion and tumor formation surrounded by distinct deep purple coloration on affected sea fans (Smith et al. 1996; Nagelkerken et al. 1997; Geiser et al. 1998). Aspergillosis has been observed on most reefs throughout the Caribbean, Florida, and as far North as Bermuda (Weil 2004; Mullen et al. 2006; Nugues and Nagelkerken 2006), and has had dramatic effects on the abundance, reproduction, and size structure of sea fan populations (Harvell et al. 2004; Kim and Harvell 2004; Nugues and Nagelkerken 2006). During 1997–2003, the Florida Keys in particular experienced >50% of sea fan tissue loss as the result of several large outbreaks (Kim and Harvell 2004). Disease prevalence has since declined to background levels throughout Florida, which could be attributed to a number of factors, including host resistance (Kim and Harvell 2004).
These large scale impacts of aspergillosis have prompted the study of several immune-related enzymes such as peroxidase (POX), prophenoloxidase (PPO), and chitinase. POX is an enzyme responsible for scavenging and removing excess H2O2 (Torreilles et al. 1997; Mydlarz and Harvell 2007). While this enzyme assists with numerous cellular functions in other invertebrates, in sea fans POX is induced by the pathogen A. sydowii and thus may provide increased resistance to disease (Mydlarz and Harvell 2007). Prophenoloxidase (PPO) is the inactive form of phenoloxidase that catalyzes the production of radical quinones, which are converted into melanin (Cerenius and Soderhall 2004). This enzymatic cascade has been well studied in other marine and terrestrial invertebrates and several studies have linked host survival to increased phenoloxidase activity (Siva-Jothy 2000; Newton et al. 2004). In sea fans, melanin is deposited along the axial skeleton to prevent the fungal hyphae from entering the surrounding tissue (Petes et al. 2003; Mullen et al. 2004; Mydlarz et al. 2008). Furthermore, PPO levels are elevated in aspergillotic corals compared to healthy ones (Mydlarz et al. 2008). Chitinases are enzymes that modify and degrade chitin, a major component of fungal cell walls (Kramer and Muthukrishnan 1997; Bhattacharya et al. 2007; Douglas et al. 2007). Chitinase, specifically exochitinase (EXOC), also aids in cellular defense in sea fans and is released during physical stress (Douglas et al. 2007). Sea fans also contain an array of lipid- and water-soluble antibacterial (AB) and antifungal (AF) compounds, which can be present in higher concentrations in diseased fans and induced by A. sydowii (Kim et al. 2000a, b; Dube et al. 2002; Kim and Harvell 2002; Douglas et al. 2007; Mydlarz and Harvell 2007; Ward et al. 2007). In other invertebrates and plants younger individuals or newer tissue contain higher constitutive and induced levels of immune defenses than older individuals (Meyer and Montgomery 1987; Sauve and Fournier 2005; Zerofsky et al. 2005). Similarly, higher levels of lipid-soluble AF activity have been detected in recruits than in large, mature sea fan colonies (Dube et al. 2002; Ward 2007). One hypothesis is that large sea fans are more prone to disease due to lower levels of defense, which could explain the higher prevalence of disease in larger colonies (Dube et al. 2002; Kim and Harvell 2002; Harvell et al. 2004). Furthermore, Dube et al. (2002) detected variation in lipid-based AF activity along reefs in the Florida Keys, which could contribute to an observed variation in disease prevalence.
There are several additional proteins which are considered important components of the invertebrate immune system and may play a role in sea fan immunity. Superoxide dismutase (SOD) is an antioxidant enzyme, which is responsible for scavenging harmful superoxide anions (O2 −), (Alscher et al. 2002; Gonzalez et al. 2005; Mydlarz et al. 2006), and is important for regulating oxidative stress during pathogen-induced host defenses (Krishnan et al. 2002; Kuzniak and Sklodowska 2005). In coral, SOD activity has also been used as an indicator of oxidative stress during increased temperature and UV radiation (Lesser 1997; Lesser and Farrell 2004). Lysozyme is an antibacterial enzyme responsible for breaking down the peptidoglycan cell walls of bacteria (Salton 1952; Fiolka et al. 2005). Since it is difficult to determine exactly which enzymes are involved in the degradation of bacteria’s peptidoglycan layer in these assays, most invertebrate immunology studies have referred to this process as lysozyme-like activity (LYS) (Anderson and Cook 1979; Adamo 2004). While there are numerous immune-related enzymes and antimicrobial compounds important for coral immunocompetence, little is known about within-population and between-site variation of overall coral immune response, and the factors influencing immune function (Dube et al. 2002; Kim and Harvell 2002, 2004; Fauth et al. 2006; Mydlarz et al. 2006).
Environmental stressors in marine ecosystems are believed to exacerbate diseases, but the link between environment and immune defense is not well understood (Harvell et al. 2004; Mydlarz et al. 2006). There are a variety of environmental and genetic factors that probably influence the coral innate immune response, and we adopted several of them. First, the general state of ecosystem health has been connected to environmental stress (Odum 1985; Rapport et al. 1985), and benthic community structure is typically used as a proxy of coral reef health (Porter et al. 2001; Bellwood et al. 2004; Beaver et al. 2006; Edmunds and Elahi 2007). Stressors such as hurricanes, disease, poor water quality, and rising seawater temperatures have been associated with a loss of scleractinian coral cover, diminished ecosystem health (Connell 1978; Edmunds and Elahi 2007; Dinsdale et al. 2008), and fluctuations in benthic community structure (Porter et al. 2001; Beaver et al. 2006). Nutrients and land-based runoff onto coral reefs have also lead to increased overgrowth of coral by macroalgae, sedimentation, and increased severity of fungal and bacterial diseases (Szmant 2002; Bruno et al. 2003; Fabricius 2005; Voss and Richardson 2006; Baker et al. 2007).
Historically, disease prevalence of aspergillosis similar to other aspects of ecosystem health has varied considerably across the Florida Keys. As a result, the levels of sea fan innate immune defenses may also reflect these pressures. To test this, key components of sea fan resistance were measured within-sites and across-sites. It was hypothesized that: (1) constitutive immunity is higher in smaller fans; (2) the levels of immune proteins (POX, PPO, LYS, EXOC), antioxidants (SOD) and antimicrobial activity (AB, AF) are more variable among than within-sites; (3) these differences are correlated with environmental conditions such as dissolved inorganic nitrogen and coral reef ecosystem health, measured by scleractinian coral cover, macroalgae cover, and bare substrate cover; and (4) these differences are correlated with historic disease prevalence and severity at these sites. Due to the large geographic region and the well studied nature of the Florida Keys reef tract, we believe this location provides an ideal system for a correlative study of immunity and potential environmental drivers.
Methods
Seafan collection
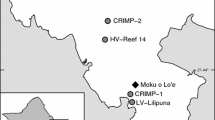
To facilitate within-site and among-site comparisons, G. ventalina 9×4 cm fragments were collected using SCUBA during a 2 week period in July 2005. Edge fragments from ten small (<15 cm) and ten large (>15 cm) colonies, a total of 20 fragments, were collected from healthy colonies at each site. These samples were collected from six sites along the Florida Keys; Carysfort (25.220°N, 80.210°W), Molasses (25.009°N, 80.376°W), Pickles (24.985°N, 80.416°W), Alligator (24.850°N, 80.617°W), Tennessee (24.745°N, 80.781°W), and Looe Key (24.569°N, 81.382°W) (Fig. 1). The sites ranged from 4 to 25 km apart, with the exception of Looe Key which was 73 km from Tennessee. These collections occurred haphazardly along the substrate near patch reefs at a depth of 3 to 6 m. Fragments were collected and flash frozen in liquid nitrogen immediately upon returning to shore, shipped to Cornell University on dry ice, and stored at −80°C.
Extract preparation
Frozen fragments were weighed individually, ground to a fine powder with a mortar and pestle in liquid nitrogen, then extracted in 0.2 M phosphate buffer, pH 7.8 with 5 mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, Missouri) for 45 min on ice. The crude protein extracts were centrifuged at 405g, the supernatants recovered and centrifuged again at 14,000g to remove lipids and cellular debris. The protein concentration of each extract was determined using the Bio-Rad DC Protein Assay Kit (Hercules, California) with a bovine serum albumin standard. Extracts were stored at −80°C between assays.
Immune assays
All colorimetric and fluorescent measurements were calculated using a Synergy HT multi-Detection microplate reader with KC4 software (Biotek Instruments, Vermont). Chemicals were purchased from Sigma-Aldrich (St Louis, Missouri) except where noted. There were no distinguishable effects of storing the extracts at −80°C on protein activity for the duration of this study. Boiled extracts were tested as negative controls and demonstrated marked inhibited activity for all immune measures (data not shown). Buffer controls with the substrate alone, and the protein extracts without substrate were included in each assay and subtracted from the final absorbance. In all assays there was negligible activity in these controls. All kinetic calculations were taken during the linear phase of the reaction.
To measure POX activity, 5 μl of the crude protein were diluted with 50 μl of 0.01 M phosphate buffer, pH 6.0 and added to 50 μl of 25 mM guaiacol in 0.01 M phosphate buffer, pH 6.0 in a 96-well plate (guaiacol final concentration 10.8 mM). The reaction was initiated with the addition of 10 μl of 20 mM hydrogen peroxide (1.7 mM final concentration) prepared in 0.01 M phosphate buffer pH 6.0 and optical density was measured over 15 min at 470 nm. POX activity was represented as the change in absorbance at 470 nm min−1 and normalized among samples to mg protein (Mydlarz and Harvell 2007).
PPO activity was measured by diluting 20 μl of the extract in 60 μl sterile water and monitoring the oxidation of colorless L-DOPA (3-(3, 4-Dihydroxyphenyl)-L-alanine to dopachrome (visible at 490 nm). 100 μl of 10 mM stock of L-DOPA were added (5 mM final concentration). The reaction was initiated by the addition of 20 μl of 1% SDS (0.1% final concentration) to convert PPO to the active enzyme, phenoloxidase (Decker et al. 2001), and the absorbance monitored at 490 nm for 30 min. Data are presented as change in absorbance (over 30 min) mg protein−1.
SOD activity was calculated using the SOD Assay Determination Kit-WST (Fluka, 19160) which includes Dojindo’s highly water-soluble tetrazolium salt, WST-1 (2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt), that produces a water-soluble formazan dye upon reduction with superoxide anion. Sea fan extracts were diluted fivefold for this assay. 20 μl of diluted extract were incubated with WST-1 and xanthine oxidase, which produce superoxide anion and absorbance at 450 nm. Inhibition of absorbance at 450 nm was monitored in wells containing sea fan extracts and SOD standards and compared to untreated samples. The percent inhibition was normalized by mg protein and presented as SOD activity units.
LYS activity was measured by following a decrease in absorbance at 425 nm of freeze dried Micrococcus luteus suspensions. 20 μl of extract were added to 100 μl of a 0.3% M. luteus suspension in 10 mM phosphate buffer, pH 7.4. The absorbance after an 18 h incubation at room temperature was taken, compared to the absorbance of M. luteus suspensions with buffer alone, and divided by mg protein to give percent lysis.
EXOC activity was assayed as described by Douglas et al. (2007). Sea fan extracts were diluted 2,000-fold in a sodium acetate buffer (100 mM sodium acetate, 0.1% SDS, 0.1% Triton X-100, 10 mM EDTA, 10 mM β-mercaptoethanol, pH 5.0) for a fluorogenic chitinase activity assay (modified from Tronsmo and Harman 1993). 30 μl were combined with 30 μl of 0.2 mM 4-methylumbelliferyl N-acetyl-β-D-glucosaminide in sodium acetate buffer in a 96-well microtiter plate. The plates were incubated for 30 min at 37°C and the reaction was terminated by addition of 30 μl of 0.5 M Na2CO3. Fluorescence of the liberated methylumbelliferone (excitation 365 nm, emission 460 nm) was compared with a dilution series of free methylumbelliferone in 0.5 M Na2CO3.
AB activity of the aqueous sea fan extracts was measured by comparing the inhibitory effects of the extracts on the growth rate of a pathogenic Vibrio sp. isolated from an Indo-Pacific diseased coral (courtesy of Garriet Smith). A concentrate of this bacterial strain was streaked on a marine broth agar plate and incubated at 26°C for 24 h. One distinct colony was removed and suspended in 7 ml of sterilized marine broth. The culture was incubated again in a shaker at 26°C for 24 h. To quantify the concentration of bacteria in the culture, absorbance was measured at 600 nm. The predetermined standard absorbance used for this assay was 0.2, which corresponded to ~5 × 107 cells ml−1 (S. Merkel, personal communication). A standard growth curve was conducted to identify the period of logarithmic growth, which occurred between 240 and 360 min. A dose response of sea fan extract was run to determine the appropriate concentration to obtain 50–70% growth inhibition. To determine the inhibitory effects of each extract, extracts were diluted to 2 mg protein ml−1 in 0.2 M phosphate buffer pH 7.8. 10 μl of each extract was added to 105 μl of marine broth, and 15 μl of the bacteria culture. Positive controls using 0.05 mg ml−1 of tetracycline and negative controls using 0.2 M phosphate buffer, pH 7.8 were included on each plate. The number of generations was determined for each sample with the following equation: 3.3 × log(final abs/initial abs) and the growth rate calculated. Percent inhibition was calculated with the following equation: I = (BC − BE)/BC × 100; I = percent inhibition, BC = mean growth rate of bacteria control, BE = mean growth rate of bacteria with extract.
AF activity of the aqueous sea fan extracts was measured against the Florida Keys isolate 1 (FK1) of A. sydowii following the methods modified from Thevissen et al. (2000) and Douglas et al. (2007). 90 μl of spore solution (105 spores ml−1 in half concentration yeast peptone glucose broth) or control medium were added to each well of a 96-well plate and the spores allowed to germinate for 18 h at 25°C. 10 μl of the extract were added to each well and absorbance at 595 nm measured on a plate reader over the course of 24 h. Percent inhibition of fungal growth was calculated using the following equation: I = (FC − FE)/FC × 100; I = percent inhibition, FC = mean growth rate of fungus control, FE = mean growth rate of fungus with extract.
Correlations with ecological survey data
Empirical survey data from the Coral Reef Evaluation and Monitoring Project (CREMP) from 1996 to 2005 were provided by Michael Callahan, Florida Fish and Wildlife Research Institute. The total percent hard coral cover, percent bare substrate cover, percent macroalgae cover and hard coral species richness were averaged from 1996 to 2005 for Carysfort, Molasses, Conch, Alligator, Tennessee, and Looe Key. Pickles Reef was not surveyed by the CREMP project, so to compare ecological data and immune responses the closest site, Conch Reef (24.977ºN, 80.450ºW) monitored by CREMP was substituted.
Correlations with water quality data
Water quality data were provided by Joseph Boyer, Southeast Environmental Research Center (SERC). There were no water quality measurements for Pickles Reef, so the Conch Reef station (6 km away) was substituted. Based on previous studies, which showed correlations with disease (Bruno et al. 2003; Baker et al. 2007), dissolved inorganic nitrogen (DIN) was used as a proxy. Water quality data from 2000 to 2005 were averaged.
Correlations with aspergillosis disease prevalence and severity
Historic disease prevalence and severity data were obtained from surveys conducted by Dube et al. (2002). There were no disease measurements for Pickles Reef, so the Conch Reef was substituted. In addition, no disease measurements were collected from Tennessee Reef and no suitable replacement was available. At each site, all sea fans within three haphazardly chosen 25 × 2 m transects were visually censused (Kim and Harvell 2004). From these observations, mean disease prevalence (% of individuals infected) and severity (% of colony area infected by disease, actual error based on image analysis ±2.5%, n = 18 sea fans) were determined for each site.
Statistical analysis
The data from the immune parameter assays were tested for normality (Shapiro–Wilk’s Test) and homogeneity of variances (Levene’s) before analysis. None of the immune parameters were normally distributed or homoscedastic, and were therefore transformed using the Box-Cox Y power transformation to meet parametric requirements. POX, PPO and AB could not be transformed, so non-parametric statistics using Kruskal–Wallis and Tukey–Kramer post-hoc tests were employed. The sample volume of the small sea fans was insufficient to perform the antimicrobial assays, so colony size comparisons were not conducted for AB and AF. A two-way multivariate analysis of variance (MANOVA) and Tukey–Kramer tests (site and size) was performed on the parametric immune measures. The effect of colony size comparisons at each site for each non-parametric parameter (POX, PPO) were analyzed independently using t-tests.
Regression analyses were used to test the relationship between immune measures and ecological surveys, nutrient and disease prevalence, and severity data. Once sequential Bonferroni tests (Rice 1989) were applied to these data, the relationships were not significant. However, since the goal of this research was to utilize a number of different immune measures it was difficult to achieve statistical significance with the small number of sites using a sequential Bonferroni correction. All statistical analyses were conducted using JMP Statistical Discovery Software version 6.0.2. (SAS Institute Inc., Cary, North Carolina, USA).
Results
Within-site variation in immune defense parameters
No significant differences in SOD, LYS, or EXOC activity were detected between small and large fans at the six sites (Table 1). There were also no significant differences between the size classes in levels of POX and PPO within each site (data not shown).
Among-site variation in immune defense parameters
Small and large sea fan samples were pooled to obtain 18–20 samples per site. The immune protein, anti-oxidant, and antimicrobial activity of sea fans from six Florida Keys reef sites are summarized in Table 2. The six sites varied significantly in SOD, LYS, and AF activity (Table 1). There were also significant differences in POX activity (Kruskal–Wallis chi squared = 51.3, P > 0.0001) and PPO activity (Kruskal–Wallis chi squared = 54.56, P > 0.0001) between the six sites. AB activity did not vary significantly among-sites (Kruskal–Wallis chi squared = 8.1439, P = 0.1485).
In a site-by-site comparison, both POX and PPO activity (Table 2) generally increased from North to South. PPO activity was higher at Carysfort and Looe than Molasses and Pickles (Fig. 2). SOD activity also varied significantly across the reef tract, with the highest activity at Molasses and lowest at Looe Key (Table 2, Fig. 3). AF activity varied across the sites with the highest activity at Molasses and lowest at Looe Key (Table 2, Fig. 4), with a significant positive correlation between AF activity and SOD activity (R 2 = 0.198, P = 0.0005). Therefore, there were major differences between Molasses and Looe Key in POX, PPO, SOD, and AF activities. LYS activity exhibited a different pattern with highest activity in centrally located reefs, with the highest at Tennessee Reef.
Drivers of coral immune defenses
While the number of sites sampled was small, and therefore these results should be considered preliminary, relationships between several environmental factors and levels of immunity were detected (Table 3) using an alpha of 0.05 (non-Bonferroni corrected). There were significant positive relationships between mean percent bare substrate cover, and SOD (P = 0.0381), EXOC (P = 0.0357), and AF activity (P = 0.0306) (Fig. 5). Significant positive relationships were found between DIN and LYS activity (P = 0.0106), and EXOC (P = 0.0254) activity (Table 4). While there was also a significant correlation between POX and scleractinian cover, this relationship was strongly driven by Looe Key. There were no significant correlations between any of the measured immune parameters and disease prevalence or severity.
Discussion
Contrary to the findings of Dube et al. (2002) and Ward (2007), immune defenses did not vary significantly between small and large sea fans in the current study. The large sample size provides confidence in this result (Table 1) but there are several possible explanations for it. First, the previous studies only assayed for lipid-soluble resistance between small and large individuals and did not perform protein-based assays. Since there were not enough samples to perform the protein-based AB and AF assays on the small sea fan protein extracts, it was not possible to make a direct comparison to the previous studies. However, variation in immune response such as POX and PPO activity as a function of age or size has been measured in other invertebrates (Viarengo et al. 1991; Sauve and Fournier 2005; Zerofsky et al. 2005). Second, Ward (2007) observed a significant difference in constitutive resistance as measured by antifungal activity from the edge of young sea fans and centers of mature fans. In the present study, only the growing edges of sea fans of all sizes were sampled, suggesting that the differences in immune defenses, whether lipid- or protein-based, may be more pronounced in growing versus older tissue, and not simply between small and large fans. In addition, all previous studies of sea fan disease resistance (Dube et al. 2002; Ward 2007) were normalized to tissue weight.
Due to the large variation in prevalence and severity of sea fan aspergillosis along the Florida Keys reef tract (Dube et al. 2002; Kim and Harvell 2004; Baker et al. 2007), and high variation in lipid-based resistance among sites found in previous studies, it was hypothesized that other key measures of immune function would also vary. The main goal of this study was to identify immune parameters which could be used to assess coral immune function and apply them to ecosystem health, water quality, and relevant disease data. The present study demonstrates that sea fan corals at each reef seem to have distinct levels of POX, PPO, SOD, LYS, and AF activity. This striking site variability may also explain why variation in immunity was not detected as a function of size class. Based solely on immune defense, it appears that Molasses and Looe Key Reef were the most different, especially in levels of PPO and SOD (Figs. 2, 3). While a strong inverse relationship between these two proteins was not detected, other studies have indicated that low levels of SOD activity are indicative of an organism free of physiologic stress (Bowler et al. 1992; Lesser 1997; Manduzio et al. 2004), and high levels of PPO could signify a state of heightened immunocompetence (Butt and Raftos 2007). Therefore, sea fans at Molasses, with nearly undetectable PPO and heightened SOD activity, may have been experiencing higher levels of stress and were less equipped to mount an immune response to pathogens than those at Looe Key, which had higher PPO and lower SOD. Although the effects of constitutive PPO activity on disease resistance has not been characterized in sea fans, elevated PPO activity has been detected in fungus-infected sea fan tissue (Mydlarz et al. 2008), and evidence from other invertebrate models provides further support that increased disease resistance is correlated with high constitutive levels of PPO and phenoloxidase (Nigam et al. 1997; Siva-Jothy 2000; Newton et al. 2004).
Another interesting result of this study was the positive correlation between AF and SOD activity. Ward et al. (2007) found that increased AF activity in sea fans was induced by temperature and the fungal pathogen A. sydowii, both of which can stimulate oxidative stress. AF activity may also be induced during oxidative and environmental stress. Environmental stress has been linked to reduced fitness and increased susceptibility to disease (Harvell et al. 1999), so induction of AF activity under stress could be an important survival mechanism.
It is important to note that since we measured the immune defenses of the entire coral holobiont, the variation in immunity may have derived from the coral, its endosymbiotic algae, its microbial community, or a combination of all three. Levels of immune-related enzymes such as POX, SOD, and EXOC have been measured in various invertebrates, algae, and bacteria (Hawkridge et al. 2000; Wong et al. 2004; Duo-Chuan 2006; Bhattacharya et al. 2007; Mydlarz and Harvell 2007), and many marine algae and bacteria contain a suite of antimicrobial compounds. In contrast, the PPO cascade and melanization is a well described invertebrate defense pathway and is likely a result of coral metabolic pathways. Nevertheless, since the coral’s endosymbiotic algae, and microbial community are vital and integral parts of its physiology, it is appropriate to consider the immunity of all components together.
The drivers of the variation in immune defenses observed in the present study are likely complex, and integrate many environmental and genetic variables. The genetic structure of G. ventalina across the Florida Keys and Caribbean supports the hypothesis that fans are broadcast spawners with wide dispersal (J. Andras, personal communication). Population genetic structure has been observed in several species of Caribbean coral at spatial scales similar to the range of this study (Gutierrez-Rodriguez and Lasker 2004; Baums et al. 2006; Severance and Karl 2006), however, other corals such as the gorgonian, Plexaura flexuosa, are fairly homogenous across similar reef habitats in Florida (Kim et al. 2004). We cannot directly determine the role of host genetics in the differences in immune defenses among sites, but infer that it is small relative to environmental factors, given that the host is likely a long distance disperser.
The loss of scleractinian coral cover and increase in macroalgae cover are typically used as indicators of a stressed coral reef ecosystem (Bellwood et al. 2004; Edmunds and Elahi 2007), however, the positive correlation between SOD, EXOC, and AF activity, and bare substrate (α = 0.05) may indicate that bare substrate cover corresponds more closely with immune activity in the Florida Keys (Table 4, Fig. 5). Reefs experiencing frequent or chronic stress exhibit a decline in the coral fitness, and if conditions persist this can lead to a loss of the reef framework (Nystrom et al. 2000; Bellwood et al. 2004). Others have suggested that reef ecosystems with more unoccupied and exposed bare substrate indicate an ecosystem experiencing chronic disturbance or stress and therefore, a lower rate of recolonization by sessile organisms (Turner et al. 2007).
Another environmental variable that may influence coral immune defenses is the wide variation of hydrology across the Florida Keys, which can influence water quality and flow of nutrients across the reefs (Boyer and Briceño 2005). In general, the Upper Keys region experiences water exchange with the Florida current frontal eddies and partly with Biscayne Bay, the Middle Keys receive water flow from Florida Bay, and the Lower Keys are most affected by hurricanes (Boyer and Briceño 2005). We identified one proxy of water quality which may affect coral health, DIN (Bruno et al. 2003), and there was a positive relationship between sea fan coral’s innate immune defenses (LYS, EXOC) and 10 year averages of DIN (Table 4). One possible explanation for these relationships is that DIN is assimilated by marine bacteria and fungus and could promote their growth (Olutiola and Cole 1977; Smith 1988). As a result, sea fans may have increased their constitutive LYS and EXOC activity to combat increased microbial growth. However, since there were no relationships between AB and AF activity and DIN, this explanation is certainly open to further interpretation.
The present study offers preliminary evidence of a relationship between coral innate immune defenses, coral reef ecosystem health, and ambient nutrient levels. We consider it preliminary because only six offshore sites were sampled and only once during 2005. Differences in immune activity might be greater in a cross-shelf comparison because there are significant differences in water quality and various measurements of reef health between inshore and offshore reefs (reviewed by Lirman and Fong 2007). Moreover, the different innate immune measures may vary on different time scales, so a study that samples numerous sites throughout the year might improve our understanding of the causes of these spatial differences.
No relationships were detected between sea fan innate immune defenses and historic disease prevalence or severity among the sites. These results were consistent with those of Dube et al. (2002), who also observed no significant difference between mean AF activity in lipid-soluble sea fan extracts and disease prevalence or severity among nine sites in the Florida Keys. A smaller number of sites have been surveyed for disease in recent years, so it was not possible to make comparisons with current levels of disease. However, based on the continued presence of aspergillosis in the Florida Keys, coincident disease surveys and monitoring of the immune defenses of sea fans should be conducted.
Understanding the factors influencing the innate immune defenses of corals is increasingly important as the condition of reefs worldwide continues to decline. The present study applied a number of immune parameters, widely used in other invertebrate studies, as measures of the coral immune response. It was clear that the sea fan corals varied more in their levels of immune protein, antioxidant, and antimicrobial activity among the sites in the Florida Keys reef tract than between two size classes at individual sites. Temporal sampling over a larger number of sites is needed to discern whether the between-site differences are consistent over time and thus whether resilience to disease between sites can be expected to vary. The mechanisms driving these variations are complex and likely include factors other than ecosystem condition, nutrient levels, and historic disease levels. Nevertheless, this study provides approaches to cataloguing immune function of populations of coral and shows sharp differences in levels of enzymatic and antimicrobial activities across sites.
Abbreviations
- POX:
-
Peroxidase
- PPO:
-
Prophenoloxidase
- SOD:
-
Superoxide dismutase
- LYS:
-
Lysozyme-like
- EXOC:
-
Exochitinase
- AB:
-
Antibacterial
- AF:
-
Antifungal
References
Adamo SA (2004) Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J Insect Physiol 50:209–216. doi:https://doi.org/10.1016/j.jinsphys.2003.11.011
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341. doi:https://doi.org/10.1093/jexbot/53.372.1331
Anderson RS, Cook ML (1979) Induction of lysozyme-like activity in the hemolymph and hemocytes of an insect, Spodotera eridania. J Invertebr Pathol 33:197–203. doi:https://doi.org/10.1016/0022-2011(79)90153-8
Baker DM, MacAvoy SE, Kim K (2007) Environmental drivers of coral disease: the relationship between water quality, δ15 N, and aspergillosis of Caribbean sea fan corals. Mar Ecol Prog Ser 343:123–130. doi:https://doi.org/10.3354/meps06937
Baums IB, Miller MW, Hellberg ME (2006) Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecol Monogr 76:503–519
Beaver C, Brooke S, Callahan M, Johnson D, Kidney J, Kupfner S et al. (2006) Coral reef evaluation and monitoring project 2005 executive summary EPA steering committee meeting June 2006. Florida fish and wildlife conservation commission
Bellwood DR, Hughes TP, Folke C, Nystrom M (2004) Confronting the coral reef crisis. Nature 429:827–833. doi:https://doi.org/10.1038/nature02691
Bhattacharya D, Nagpure A, Gupta RK (2007) Bacterial chitinases: Properties and potential. Crit Rev Biotechnol 27:21–28. doi:https://doi.org/10.1080/07388550601168223
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116. doi:https://doi.org/10.1146/annurev.pp.43.060192.000503
Boyer JN, Briceño HO (2005) FY2005 annual report of the water quality monitoring project for the water quality protection program of the Florida Keys national marine sanctuary. Southeast environmental research center, Technical Report #T-327
Bruno JF, Petes LE, Harvell CD, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases. Ecol Lett 6:1056–1061. doi:https://doi.org/10.1046/j.1461-0248.2003.00544.x
Butt D, Raftos D (2007) Immunosuppression in Sydney rock oysters (Saccostrea glomerata) and QX disease in the Hawkesbury river, Sydney. Mar Freshw Res 58:213–221. doi:https://doi.org/10.1071/MF06080
Cerenius L, Soderhall K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126. doi:https://doi.org/10.1111/j.0105-2896.2004.00116.x
Connell JH (1978) Diversity in tropical rain forests and coral reefs—high diversity of trees and corals is maintained only in a non-equilibrium state. Science 199:1302–1310. doi:https://doi.org/10.1126/science.199.4335.1302
Decker H, Ryan M, Jaenicke E, Terwilliger N (2001) SDS-induced phenoloxidase activity of hemocyanins from Limulus polyphemus, Eurypelma californicum, and Cancer magister. J Biol Chem 276:17796–17799. doi:https://doi.org/10.1074/jbc.M010436200
Dinsdale EA, Pantos O, Smirga SS, Edwards RA, Angly F, Wegley L et al (2008) Microbial ecology of four coral atolls in the northern Line Islands. PLoS One 3:e1584. doi:https://doi.org/10.1371/journal.pone.0001584
Douglas NL, Mullen KM, Talmage SC, Harvell CD (2007) Exploring the role of chitinolytic enzymes in the sea fan coral, Gorgonia ventalina. Mar Biol (Berl) 150:1137–1144. doi:https://doi.org/10.1007/s00227-006-0444-8
Dube D, Kim K, Alker AP, Harvell CD (2002) Size, structure, and geographic variation in chemical resistance of sea fan corals Gorgonia ventalina to a fungal pathogen. Mar Ecol Prog Ser 231:139–150. doi:https://doi.org/10.3354/meps231139
Duo-Chuan L (2006) Review of fungal chitinases. Mycopathologia 161:345–360. doi:https://doi.org/10.1007/s11046-006-0024-y
Edmunds PJ, Elahi R (2007) The demographics of a 15 year decline in cover of the Caribbean reef coral Montastraea annularis. Ecol Monogr 77:3–18. doi:https://doi.org/10.1890/05-1081
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146. doi:https://doi.org/10.1016/j.marpolbul.2004.11.028
Fauth JE, Dustan P, Pante E, Banks K, Vargas-Angel B, Downs CA (2006) Final report: Southeast Florida coral biomarker local action study. Office of coastal and aquatic managed areas, of the Florida department of environmental protection
Fiolka MJ, Ptaszynska AA, Czarniawski W (2005) Antibacterial and antifungal lysozyme-type activity in Cameraria ohridella pupae. J Invertebr Pathol 90:1–9. doi:https://doi.org/10.1016/j.jip.2005.06.015
Geiser DM, Taylor JW, Ritchie KB, Smith GW (1998) Cause of sea fan death in the West Indies. Nature 394:137–138. doi:https://doi.org/10.1038/28079
Gonzalez M, Romestand B, Fievet J, Huvet A, Lebart MC, Gueguen Y et al (2005) Evidence in oyster of a plasma extracellular superoxide dismutase which binds LPS. Biochem Biophys Res Commun 338:1089–1097. doi:https://doi.org/10.1016/j.bbrc.2005.10.075
Gutierrez-Rodriguez C, Lasker HR (2004) Microsatellite variation reveals high levels of genetic variability and population structure in the gorgonian coral Pseudopterogorgia elisabethae across the Bahamas. Mol Ecol 13:2211–2221
Harvell CD, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith GW et al (2007) Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography (Wash DC) 20:58–81
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ et al (1999) Review: marine ecology—Emerging marine diseases—Climate links and anthropogenic factors. Science 285:1505–1510. doi:https://doi.org/10.1126/science.285.5433.1505
Harvell CD, Aronson R, Baron N, Connell J, Dobson A, Ellner S et al (2004) The rising tide of ocean diseases: unsolved problems and research priorities. Front Ecol Environ 2:375–382
Hawkridge JM, Pipe RK, Brown BE (2000) Localisation of antioxidant enzymes in the cnidarians Anemonia viridis and Goniopora stokesi. Mar Biol (Berl) 137:1–9. doi:https://doi.org/10.1007/s002270000324
Kim E, Lasker HR, Coffroth MA, Kim K (2004) Morphological and genetic variation across reef habitats in a broadcast-spawning octocoral. Hydrobiologia 530-31:423–432. doi:https://doi.org/10.1007/s10750-004-2646-8
Kim K, Harvell CD (2002) Aspergillosis of sea fan corals: disease dynamics in the Florida Keys. In: Porter JW, Porter KG (eds) The everglades, Florida bay, and coral reefs of the Florida Keys: an ecosystem sourcebook. CRC Press, Boca Raton, pp 813–824
Kim K, Harvell CD (2004) The rise and fall of a 6 year coral-fungal epizootic. Am Nat 164:S52–S63. doi:https://doi.org/10.1086/424609
Kim K, Kim PD, Alker AP, Harvell CD (2000a) Chemical resistance of gorgonian corals against fungal infections. Mar Biol (Berl) 137:393–401. doi:https://doi.org/10.1007/s002270000333
Kim K, Harvell CD, Kim PD, Smith GW, Merkel SM (2000b) Fungal disease resistance of Caribbean sea fan corals (Gorgonia spp.). Mar Biol (Berl) 136:259–267. doi:https://doi.org/10.1007/s002270050684
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27:887–900. doi:https://doi.org/10.1016/S0965-1748(97)00078-7
Krishnan N, Chattopadhyay S, Kundu JK, Chaudhuri A (2002) Superoxide dismutase activity in hemocytes and hemolymph of Bombyx mori following bacterial infection. Curr Sci 83:321–325
Kuzniak E, Sklodowska M (2005) Fungal pathogen-induced changes in the antioxidant systems of leaf peroxisomes from infected tomato plants. Planta 222:192–200. doi:https://doi.org/10.1007/s00425-005-1514-8
Lesser MP (1997) Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16:187–192. doi:https://doi.org/10.1007/s003380050073
Lesser MP, Farrell JH (2004) Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23:367–377. doi:https://doi.org/10.1007/s00338-004-0392-z
Lirman D, Fong P (2007) Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida reef tract. Mar Pollut Bull 54:779–791. doi:https://doi.org/10.1016/j.marpolbul.2006.12.014
Manduzio H, Monsinjon T, Galap C, Leboulenger F, Rocher W (2004) Seasonal variations in antioxidant defenses in blue mussels Mytilus edulis collected from a polluted area: major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquat Toxicol 70:83–93. doi:https://doi.org/10.1016/j.aquatox.2004.07.003
Meyer GA, Montgomery ME (1987) Relationship between leaf age and the food quality of cottonwood foliage for the gypsy moth, Lymanthria dispar. Oecologia 72:527–532. doi:https://doi.org/10.1007/BF00378978
Mucklow PT, Vizoso DB, Jensen KH, Refardt D, Ebert D (2004) Variation in phenoloxidase activity and its relation to parasite resistance within and between populations of Daphnia magna. Proc R Soc Lond B Biol Sci 271:1175–1183. doi:https://doi.org/10.1098/rspb.2004.2707
Mullen K, Peters EC, Harvell CD (2004) Coral resistance to disease. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 377–399
Mullen KM, Harvell CD, Alker AP, Dube D, Jordán-Dahlgren E, Ward JR et al (2006) Host range and resistance to aspergillosis in three sea fan species from the Yucatan. Mar Biol (Berl) 149:1355–1364. doi:https://doi.org/10.1007/s00227-006-0275-7
Mydlarz LD, Harvell CD (2007) Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp Biochem Physiol A Mol Integr Physiol 146:54–62. doi:https://doi.org/10.1016/j.cbpa.2006.09.005
Mydlarz LD, Jones LE, Harvell CD (2006) Innate immunity environmental drivers and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst 37:251–288. doi:https://doi.org/10.1146/annurev.ecolsys.37.091305.110103
Mydlarz LD, Holthouse SF, Peters EC, Harvell CD (2008) Cellular responses in sea fan corals: granular amoebocytes react to pathogen and climate stressors. PLoS One 3:e1811
Nagelkerken I, Buchan K, Smith GW, Bonair K, Bush P, Garzon-Ferreira J et al (1997) Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar Ecol Prog Ser 160:255–263. doi:https://doi.org/10.3354/meps160255
Newton K, Peters R, Raftos D (2004) Phenoloxidase and QX disease resistance in Sydney rock oysters (Saccostrea glomerata). Dev Comp Immunol 28:565–569. doi:https://doi.org/10.1016/j.dci.2003.10.004
Nigam Y, Maudlin I, Welburn S, Ratcliffe NA (1997) Detection of phenoloxidase activity in the hemolymph of tsetse flies, refractory and susceptible to infection with Trypanosoma brucei rhodesiense. J Invertebr Pathol 69:279–281. doi:https://doi.org/10.1006/jipa.1996.4652
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26. doi:https://doi.org/10.1093/beheco/11.1.19
Nugues MM, Nagelkerken I (2006) Status of aspergillosis and sea fan populations in Curacao 10 years after the 1995 Caribbean epizootic. Rev Biol Trop 54:153–160
Nystrom M, Folke C, Moberg F (2000) Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol Evol 15:413–417. doi:https://doi.org/10.1016/S0169-5347(00)01948-0
Odum EP (1985) Trends expected in stressed ecosystems. Bioscience 35:419–422. doi:https://doi.org/10.2307/1310021
Olutiola PO, Cole OO (1977) Some environmental and nutritional factors affecting growth and sporulation of Aspergillus sydowii. Physiol Plants 39:239–242
Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM (2003) Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser 264:167–171. doi:https://doi.org/10.3354/meps264167
Porter JW, Dustan P, Jaap WC, Patterson KL, Kosmynin V, Meier OW et al (2001) Patterns of spread of coral disease in the Florida Keys. Hydrobiologia 460:1–24. doi:https://doi.org/10.1023/A:1013177617800
Rapport DJ, Regier HA, Hutchinson TC (1985) Ecosystem behavior under stress. Am Nat 125:617–640. doi:https://doi.org/10.1086/284368
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225. doi:https://doi.org/10.2307/2409177
Rolff J, Siva-Jothy MT (2003) Invertebrate ecological immunology. Science 301:472–475. doi:https://doi.org/10.1126/science.1080623
Salton MRJ (1952) Cell wall of Micrococcus lysodeikticus as the substrate of lysozyme. Nature 170:746–747. doi:https://doi.org/10.1038/170746a0
Sauve S, Fournier M (2005) Age-specific immunocompetence of the earthworm Eisenia andrei: exposure to methylmercury chloride. Ecotoxicol Environ Saf 60:67–72. doi:https://doi.org/10.1016/j.ecoenv.2003.12.022
Schmid-Hempel P (2003) Variation in immune defense as a question of evolutionary ecology. Proc R Soc Lond B Biol Sci 270:357–366. doi:https://doi.org/10.1098/rspb.2002.2265
Severance EG, Karl SA (2006) Contrasting population genetic structures of sympatric, mass-spawning Caribbean corals. Mar Biol 150:57–68
Siva-Jothy MT (2000) A mechanistic link between parasite resistance and expression of a sexually selected trait in a damselfly. Proc R Soc Lond B Biol Sci 267:2523–2527. doi:https://doi.org/10.1098/rspb.2000.1315
Smith GW (1988) Influence of microbial deamination on ammonium pools in marine waters. Sci Total Environ 75:319–324. doi:https://doi.org/10.1016/0048-9697(88)90043-5
Smith GW, Ives LD, Nagelkerken IA, Ritchie KB (1996) Caribbean sea-fan mortalities. Nature 383:487–487. doi:https://doi.org/10.1038/383487a0
Sutherland KP, Porter JW, Torres C (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser 266:273–302. doi:https://doi.org/10.3354/meps266273
Szmant AM (2002) Nutrient enrichment on coral reefs: is it a major cause of coral reef decline? Estuaries 25:743–766
Thevissen K, Osborn RW, Acland DP, Broekaert WF (2000) Specific binding sites for an antifungal plant defensin from dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol Plant Microbe Interact 13:54–61. doi:https://doi.org/10.1094/MPMI.2000.13.1.54
Torreilles J, Guerin MC, Roch P (1997) Peroxidase-release associated with phagocytosis in Mytilus galloprovincialis hemocytes. Dev Comp Immunol 21:267–275. doi:https://doi.org/10.1016/S0145-305X(96)00034-1
Tronsmo A, Harman GE (1993) Detection and quantification of N-acetyl-beta-D-glucosaminidase, chitobiosidase, and endochitinase in solutions and on gels. Anal Biochem 208:74–79. doi:https://doi.org/10.1006/abio.1993.1010
Turner DJ, Kildea TN, Westphalen G (2007) Examining the health of subtidal reef environments in South Australia, Part 2: status of selected South Australian reefs based on the results of the 2005 surveys. South Australian Reef and Development Institute (Aquatic Sciences), SARDI Publication Number RD03/0252-6, Adelaide
Viarengo A, Canesi L, Pertica M, Livingstone DR, Orunesu M (1991) Age-related lipid peroxidation in the digestive gland of mussels—the role of the antioxidant defense systems. Experientia 47:454–457. doi:https://doi.org/10.1007/BF01959942
Voss JD, Richardson LL (2006) Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 25:569–576. doi:https://doi.org/10.1007/s00338-006-0131-8
Ward JR (2007) Within-colony variation in inducibility of coral disease resistance. J Exp Mar Biol Ecol 352:371–377. doi:https://doi.org/10.1016/j.jembe.2007.08.014
Ward JR, Kim K, Harvell CD (2007) Temperature affects coral disease resistance and pathogen growth. Mar Ecol Prog Ser 329:115–121. doi:https://doi.org/10.3354/meps329115
Weil E (2004) Coral reef diseases in the wider Caribbean. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, pp 35–68
Wong HC, Shen CT, Chang CN, Lee YS, Oliver JD (2004) Biochemical and virulence characterization of viable but nonculturable cells of Vibrio parahaemolyticus. J Food Prot 67:2430–2435
Zerofsky M, Harel E, Silverman N, Tatar M (2005) Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4:103–108. doi:https://doi.org/10.1111/j.1474-9728.2005.00147.x
Acknowledgments
Research was supported by the NSF/NIH Ecology of Infectious Disease Program (NSF OCE-0326705), and Coral Disease Working Group of the GEF-World Bank CRTR Program. CREMP ecological survey data were provided by Michael Callahan at the Florida Fish and Wildlife Research Institute. Water data were provided by the SERC-FIU Water Quality Monitoring Network which is supported by SFWMD/SERC Cooperative Agreements #4600000352 as well as EPA Agreement #X994621-94-0. We thank D. Baker and K. Kim for field support and A. Kessler for experimental support. We are grateful to E. Bartels at Mote Tropical Marine Laboratory in Summerland Key, Florida and L. Anderson at Keys Marine Lab, Long Key, Florida for collections and dive support. We would also like to thank G. Smith for supplying the bacteria isolate used for the AB assay. Statistical assistance was provided by Cornell University Statistical Consulting Unit and J. Simonis. Samples were collected under Florida Keys National Marine Sanctuary Permit # 2004-092.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle.
Rights and permissions
About this article
Cite this article
Couch, C.S., Mydlarz, L.D., Harvell, C.D. et al. Variation in measures of immunocompetence of sea fan coral, Gorgonia ventalina, in the Florida Keys. Mar Biol 155, 281–292 (2008). https://doi.org/10.1007/s00227-008-1024-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1024-x