Abstract
It is well documented that climate change has a negative effect on coral reefs worldwide. Recurrent warming events, ocean acidification, and nutrient pollution are some of the hallmarks of climate change; each affects the health of coral, and together, their effects are multiplied. It is hypothesized that a healthy coral will have a strong, highly active immune system when confronted with different stressors. However, there is very little that we understand about how the coral immune system reacts to different climate change stressors. In this review, we will examine what is known about the effects of heat stress, ocean acidification, and nutrient pollution on the coral immune system. We will identify gaps in our knowledge and briefly discuss a path forward to address these gaps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The negative effects of anthropogenic climate change including higher ocean temperatures, surge in nutrient load, and increased incidences of ocean acidification on coral reef health have been well documented [1,2,3,4]. A healthy coral is host to endosymbiotic alga called Symbiodinium, bacteria, fungi, viruses, and archaea; however, recurrent, prolonged increases in these anthropogenic stressors can disrupt coral health and increase bleaching and disease outbreaks [2, 5,6,7]. However, despite this clear understanding that anthropogenic stressors disrupt the health of the coral, little is understood about the underlying cellular and molecular immune mechanisms that govern these responses, and the variation in anthropogenic stressors complicates our understanding of their effects. Heat stress, ocean acidification, and nutrient pollution are all components of climate change, yet each component potentially affects corals’ immune pathways in unique ways. This requires researchers to investigate numerous different cellular and molecular pathways in relation to a specific component of climate change.
For heat stress, the most widely studied component of climate change is its effect on corals; it is proposed that the coral immune system is repressed, which makes the coral more vulnerable to infection and diseases [8,9,10]. Although it has been challenging to directly link disease outbreaks with a specific immune pathway, researchers have made progress in identifying potential candidates for further study. For ocean acidification and nutrient pollution, most research has focused on other areas, such as skeletal density and toxicity, respectively, and only recently have studies begun to focus on the coral immune system. This review will focus on the coral immune system in relation to these different components of climate change, highlighting both what is known and also what remains to be investigated.
Coral Immune System
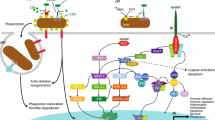
Corals possess a relatively complex innate immune system that is capable of recognizing microbes, initiating signaling responses, and mounting a downstream response to regulate the health of the coral [11,12,13,14] (Fig. 1). The coral immune system consists of many different types of receptors and signaling pathways, which lead to many different effector responses. These receptors include scavenger receptors, toll-like receptors, interleukin receptors, lectins, complement receptors, and tumor necrosis factor receptors. These different receptors activate specialized signaling pathways, which lead to the activation of inflammatory proteins, thus causing an immune reaction. For a more detailed review on the coral immune system components, see Palmer and Traylor-Knowles, 2012. In this review, we will focus on how different aspects of climate change affect these different levels of the coral immune system.
The diversity of pattern recognition receptors (PRRs), intracellular signaling pathways, and effector mechanisms that exist within the coral innate immune system. Toll-like receptors (TLRs) are a superfamily of PRRs including pro-inflammatory interleukin-1 (IL-1R) receptors that bind microbe-associated molecular patterns (MAMPs) via extracellular leucine-rich repeat domains (LRRs) and transduce signals via intracellular Toll-interleukin receptor 1 (TIR) domains [53, 94]. Lectins are another PRR class represented in corals by c-type lectins, rhamnose-binding lectins, and tachylectins that also possess extracellular LRRs for binding oligosaccharides and other MAMPs [24, 95, 96]. NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs) are cytoplasmic PRRs that bind bacterial PAMPs and viral DNA, respectively, but their functional characterization in corals is lacking [19, 61, 97]. Numerous immune signaling pathways converge upon the activation of key immune transcriptional regulator nuclear factor kappa B (NF-κB). Corals possess TLRs and NLRs with TIR domains, that may interact with gene homologs of myeloid differentiation primary response protein 88 (MyD88), IL-1R-associated kinase (IRAK), TNF receptor-associated factor 6 (TRAF), and IκB kinase (Iκκ) that cleave NF-κB inhibitors (IκBs) and allow NF-κB protein dimers to translocate into the nucleus and enhance inflammatory cytokine expression [53, 98]. TNFRs and RLRs also interact with TRAFs to activate signaling, which may also proceed through mitogen-activated protein kinase (MAPK), extracellular-signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) pathways [18, 50, 99]. The coral complement cascade contains C3, Factor B (Bf), and mannose-associated serine protease (MASP) homologs which are activated by lectin PRRs, as well as homologs of macrophage-expressed protein 1 (MPEG-1) that is putatively involved in pore formation [100]. Upon activation by detection of MAMPs, the coral phenoloxidase (PO) cascade employs multiple phenoloxidases to hydroxlate mono-phenol and diphenol substrates into polymeric melanin deposits that yield cytotoxic defenses and create barriers to infection [21, 29, 34]. Corals possess superoxide dismutases (SOD), peroxidases (PDX), and catalases (CAT) and may express these antioxidants to spatially regulate oxidative cytotoxicity [41, 52]. Coral fluorescent proteins (FPs) and non-fluorescent chromoproteins also have been shown to scavenge reactive oxygen species, suggesting a more prominent role in mitigating cellular stress [40, 101]. Antimicrobial peptides (AMPs) may also be important for directed microbial killing; however, the only AMP characterized in a stony coral to date is Damicornin from Pocillopora damicoris [70]
Effects of Heat Stress on Coral Immunity
One of the most well studied aspects of climate change is the effect of heat stress on coral reefs. Many heat stress studies have utilized genomic techniques and have discovered many previously unknown immune genes involved in the heat stress reaction [15,16,17,18,19,20,21,22]. Other studies have focused on the symbiosis between the coral host and its algal symbiont, and specifically targeted candidate immune genes that aid in recognition of the algal symbiont, or are involved in the breakdown of the symbiosis [23,24,25,26,27]. Lastly, innate immunity enzymatic pathways have proven to be critical for the coral’s response to heat stress with many studies focusing on the melanin pathway’s response to heat stress [28,29,30,31,32,33,34]. Below, we will discuss some of the most major findings in the reaction of different components of the innate immune system to heat stress (summary provided in Fig. 2).
Summary of responses of the coral immune system to different climate change associated stressors. The red box represents the climate change stressor, yellow box represents the gene or protein response, and the purple box represents the downstream cellular or organismal response if known. Red arrows represent upregulation and blue arrows represents downregulation. Overall, the most characterized climate change stressor is heat stress; in total, there were seven different groups of genes/proteins that were found to reach to heat stress consistently throughout different studies. These include antioxidants [32, 39,40,41,42,43,44], c-type lectins [17, 18, 23], NLRs [20], complement pathway [21, 65, 66], proPO enzymatic pathway [28, 29, 32, 68], AMPs [66, 70], and TNFRs [18, 22, 78, 79]. The stressor ocean acidification had three different groups of reactive genes/proteins. These include fluorescent proteins [87, 88], c-type lectins [81,82,83], and NF-κB inhibitor [83]. Lastly, nutrient pollution had two main groups of genes/proteins. These include antioxidants [90] and components of the TNFR pathway [91,92,93]. Overall, more is understood about the reaction of the coral immune system to heat stress than any other climate change associated stressor
Antioxidants
The most well-described component of climate change’s effects on corals is heat stress. Heat stress causes bleaching, or the expulsion of Symbiodinium from the host gastrodermal cells, which compromises the health of the coral. Many studies suggest that the primary driver of bleaching is the production of reactive oxygen species (ROS) caused by impairment of photosystem II (PSII) in the thylakoid membrane of Symbiodinium chloroplasts [35,36,37]. In healthy corals, ROS is scavenged by antioxidants, which protect the coral cells and tissue structure. These antioxidants are critical for healthy immune system function [38]. If the antioxidant system is overwhelmed by ROS, then the coral’s innate immune system is repressed, and bleaching will occur [39].
Numerous studies have found that coral possess many different antioxidants, each of which are critical for the response to heat stress [32, 39,40,41,42,43,44]. These antioxidants include superoxide dismutase (SOD), catalase, fluorescent proteins, numerous peroxidases, and many other molecules [15,16,17, 40, 45,46,47]. SOD catalyzes the conversion of superoxide anions (O2 −) into O2 and H2O2, while catalase and peroxiredoxin further catalyze the conversion of H2O2 into H2O and O2 [48]. As many as five SODs and five catalase homologs have been identified from Acropora transcriptomes and have been shown to be upregulated during thermal stress [49,50,51]. Additionally, ferritin, an iron-binding antioxidant that scavenges active oxygen intermediates was found to be upregulated in the coral Acropora millepora in response to heat stress [52].
Other antioxidants that are important for immune functions are fluorescent proteins (FP). They have high H2O2 scavenging activity [40, 45] and are highly reactive during heat stress [46, 47]. In a heat stress study examining coral larvae, DsRed-type FP was found to be downregulated in response to heat [17]. Similarly in Acropora millepora adults and Montastraea faveolata adults, a green fluorescent protein (GFP) was downregulated in response to heat stress [15, 16]. An experiment examining the reactivity of FPs in different color morphs of the coral Montipora monasteriata found that coral with higher pigmentation from high light environments had a reduced amount of GFPs after exposure to a heat stress [47]. There is a clear pattern of downregulation in response to heat stress, and it is hypothesized that this downregulation is a way to protect the corals by preventing wasteful production of proteins that would not function under high temperatures [16].
If the antioxidant response to ROS production is overwhelmed, coral cell membranes and structural components become damaged due to a buildup of ROS. This process produces distinct damage-associated molecular patterns (DAMPs) that interact with immune receptors, such as lectins, to initiate an alarm response [23]. Scavenger receptors that bind a variety of DAMPs such as low-density lipoproteins and free DNA fragments have been identified in coral transcriptomic datasets and may be the primary responders to molecular distress signals generated by ROS [53,54,55].
Lectins
A critical part of the immune reaction is the ability of immune cells to recognize foreign invading microbes. One way that this is done is by using pattern recognition receptors (PRRs). These receptors recognize microbe-associated molecular patterns (MAMPs), which are present on particular classes of bacteria [56]. One of the most studied PRRs involved in heat stress response is the lectin receptor [57]. Lectins are a ubiquitous and diverse class of PRRs that are involved in pattern recognition by binding microbe surface glycans especially oligosaccharide residues on distal regions of glycoproteins and glycolipids [57, 58]. During a thermal stress experiment on the coral Pocillopora damicornis, mannose-binding c-type lectin was found to be downregulated during thermal stress, suggesting that it has a role in maintaining a healthy symbiotic state and that heat stress disrupts this relationship [23]. This pattern of downregulation during heat stress has been found in other corals, including Acropora hyacinthus adults and Acropora millepora larvae [17, 18] implying that PRRs are critical components in maintaining healthy symbiotic relationships for many different species of coral.
Nucleotide Oligomerization Domain-like Receptors
Nucleotide oligomerization domain (NOD)-like receptors (NLRs) are NACHT domain-containing intracellular receptors that recognize bacterial peptidoglycans and transduce signals through caspase-mediated apoptosis and inflammatory signaling pathways [59, 60]. A large and diverse number of NLRs have been identified in the coral Acropora digitifera, with putative signaling pathways and potential roles in symbiosis establishment that have yet to be fully characterized [19]. This large repertoire suggests that tandem genome duplications may have occurred in Acropora; however, we do not understand the functional significance of these duplications in corals [61]. One possible function is in disease resistance, where NOD-like receptor family CARD domain containing 5 (NLRC5) was upregulated in disease-resistant corals [62]. Another possible function is in heat stress, where NOD-like receptor family CARD domain containing 3 (NLRC3) were shown to suppress the immune system by negative regulation of the NF-κB signaling pathway, which authors suggest cause bleaching [20]. With such diversity present in this gene family, it is possible that these genes have an array of functions beyond immunity.
The Complement Pathway
The complement pathway is a proteolytic cascade by which PRRs initiate intracellular signaling to enact downstream inflammatory and phagocytic immune responses [63]. The main components of the complement system consist of complement C3, Factor B (Bf), lectins, and mannose-binding lectin-associated serine protease (MASP) [63]. In the coral, Acropora millepora, the complement C3 protein has been shown to be activated in response to bacterial challenge [64]. Additionally, the identification of complement gene homologs (C3, C2/C4, MASP, Bf) from transcriptomic datasets and evidence of lectins recognizing both bacteria and symbionts suggest a role for the coral complement system in symbiosis and host defense [24,25,26,27]. Transcriptomic evidence has also suggested that the complement system may play a role in the response to heating stress [21]. In a study on bleaching in the Caribbean coral Orbicella faveolata, MASP1 was downregulated in bleached samples [65]. However, in another study on a different coral, Pocillopora damicornis, components of the complement pathway were upregulated in response to heat [66]. This variability in response could be due to the experimental design, the coral species, and their immune reactivity; however, these studies do show that the complement pathway is responding to heat stress and could be a critical pathway in the bleaching response.
proPO Enzymatic Pathway and Antimicrobial Pathways
One of the most thoroughly studied enzymatic mediators in the coral immune response is the melanization cascade, or prophenoloxidase (proPO) pathway [28,29,30,31,32,33,34]. The proPO pathway is a rapidly inducible proteolytic cascade that results in the deposition of pigmented cytotoxic melanin along the front of a wound margin, clot, or near an area of infection [67]. In corals, strong proPO enzymatic function correlates with less bleaching and disease susceptibility [28, 29, 32, 68]. ProPO enzymatic factors are activated in response to heat stress and bacterial challenge [68], and proPO enzymatic factors have diverse reactions in different species of corals in response to heat stress and pathogen stress, indicating that different species of coral can modify this system for their immune needs [32, 68].
In addition to the proPO enzymatic pathway, antimicrobial peptides (AMPs) have been studied in corals under heat stress [66, 69, 70]. These peptides are small (4–15 kDa) protein products of single genes that are able to kill gram negative and gram-positive bacteria and are involved in the immunomodulation of the innate immune system [71,72,73]. Currently, the most well-described antimicrobial peptide in scleractinian corals is Damicornin, discovered in Pocillopora damicornis. [66, 70]. Damicornin, along with two less described AMPs, mytimacin and LBP-BPI, were repressed when the vibrio, V. corallilyticus, was in its virulent state, a state that is activated at higher temperatures [66, 70]. Conversely, these AMPs were activated in response to the non-virulent state of this V. corallilyticus [66, 70]. The temperature activation of V. corallilyticus and the resulting repression of these AMPs is critical evidence that increased temperatures will increase the likelihood of immune suppression, and pathogen activation. Thus, as temperature perturbations increase in severity, the ability of corals to produce diverse suites of functional AMPs may dictate disease resistance and the tolerance of populations to infections.
Tumor Necrosis Factor Receptor
Another critical responder to heat stress is the tumor necrosis factor receptor (TNFR). The TNFR genes belong to a large super family of receptors and ligands known as the tumor necrosis factor ligand/receptor superfamily [74]. The mammalian TNFR-1 receptor is a type-1 transmembrane protein, but within this superfamily, there are examples of proteins that are secreted as soluble molecules and ones that are bound to the plasma membrane via glycophospolipids [75]. The TNFR gene family is involved with the maintenance, activation, and modulation of the immune system in vertebrate models, as well as apoptosis [76, 77]. Interestingly, coral possess a large diversity of these genes; the highest diversity of any known multicellular organisms [14]. Previously, it was found that coral colonies exposed to a fluctuating temperature treatment over 72 h, had a single TNFR upregulated with a threefold increase in response to heat stress [18]. In a reciprocal transplant study under field conditions, different TNFRs had elevated transcript abundances in corals that were native to a hotter, more variable environment [22]. Additionally, a recombinant human TNF-α ligand was shown to bind to coral cells in culture, increase local caspase activity, and cause apoptosis of coral host cells in vitro [78]. While the direct interaction between a coral TNF-α ligand and a coral TNFR remains to be understood, it is hypothesized that the human TNF-α ligand binding to coral TNFR caused the bleaching in cell culture via apoptotic activity [78]. This study is confounded by another study that found that multiple TNFRs were expressed only within cnidocytes (specialized stinging cells in corals) and not within the gastrodermal cells, which contain Symbiodinium, indicating that their function may not be in bleaching, but rather in the generalized heat stress response [79]. This complexity in proposed function may be due to the complexity of the gene family, and it is possible that some TNFRs initiate apoptotic outcomes while others initiate inflammatory stress response outcomes [14].
Effects of Ocean Acidification on Coral Immunity
Along with heat stress, another critical aspect of climate change is ocean acidification, which has received much less attention in terms of its effect on immunity. In ocean acidification, a decrease of carbonate ions causes a cascade of harmful, interrelated effects: a decrease in the saturation state of carbonate minerals increases the amount of CO2 uptake by corals, resulting in reduced calcification, and adversely affecting the growth and the survivability of coral reefs, while increasing bioerosion [80]. While there is information available on the effects of increased CO2 uptake on the expression of skeletal genes and skeletal growth, there have been few studies on the effects of increased CO2 on the coral’s immune system. Experiments that have yielded the most information on the immune system are transcriptomic or microarray studies (summary provided in Fig. 2).
Lectins
As mentioned previously, lectins are critical PRRs used to sense the sugar molecules found on the surface of foreign microbes [57]. Conflicting evidence is available for the role of lectins during ocean acidification. In one transcriptomic study on cold-water corals, a mannose-binding c-type lectin was upregulated in elevated CO2 conditions in comparison to the ambient conditions [81]. However, in another study examining ocean acidification in tropical reef building corals using a microarray, a c-type lectin was found to be downregulated in elevated CO2 conditions, indicating that the immune system of these corals may be compromised [82]. This is further confirmed by a study on corals found in an environment with natural CO2 seeps, where downregulation of c-type lectin was discovered [83]. The conflicting evidence from these studies could be due to the species of corals that were studied; cold-water corals could be more acclimated to higher CO2 conditions and therefore have higher immune tolerance. More studies on the role of lectins in ocean acidification in multiple coral species should help to tease apart these contradictory results.
NF-κB Signaling
The NF-κB transcription factor is a critical stress responder that activates proteins involved in inflammation and apoptosis [84]. This transcription factor is activated by many different upstream receptors and adaptors, including tumor necrosis factor receptor associated factors (Trafs) [85]. In a study examining the effects of pH and temperature changes on coral health, increased co-expression of NF-κB and several Trafs was observed [82]. It is proposed that the shifts in expression of this pathway could indicate a compromised immune system [82]. In another study, which focused on the gene expression of corals found in an environment with natural CO2 seeps, a NF-κB inhibitor and a Traf were downregulated in CO2 seep corals [83]. The downregulation of a NF-κB inhibitor could indicate that NF-κB pathway is actually activated and further hints at a potential role of the immune system in ocean acidification.
Fluorescent Proteins
Fluorescent proteins are antioxidants that are critical for scavenging peroxide molecules in corals [40]. These proteins are extremely abundant and diverse in corals [86]; however, our understanding of their functional significance is not well understood. Previously, it was discovered that the gene products of these proteins are highly downregulated in response to acute CO2 conditions in juvenile corals [87], and in studies of the compounded effects of increased CO2 and temperature, fluorescent protein gene transcripts were some of the most upregulated [88]. This difference in the pattern of expression between these two studies could be due to the compounded effects of temperature and CO2, as well as due to the diversity of the fluorescent proteins; different fluorescent proteins may react differently to the same stimulus.
Effects of Nutrient Pollution on Coral Immunity
Nutrient pollution is another critical aspect of climate change. This anthropogenic process is mainly observed at a local scale, but can have devastating impacts on coral reefs worldwide [89]. Laboratory and field studies examining the effects of nutrient pollution on coral reefs have yielded insights into possible pathways that respond to these stressors. Nutrient pollution causes eutrophication, where high amounts of nutrients, usually caused by run-off, can cause hypoxia. Coral reefs are extremely sensitive to these hypoxic conditions, and many times, these events cause local die-off of coral reefs. Here, we will discuss what is understood about the effects of nutrient pollution on specific immune factors.
Antioxidants
Antioxidants are scavengers of ROS and have protective properties for corals [39]. In response to nutrient pollution, antioxidants are very reactive. For example, a study on Acropora aspera found that SOD, catalase, peroxidase, and gluathione s-transferase were upregulated in response to nutrient stress, while peroxiredoxin and thioredoxin were downregulated [90]. Additionally, fluorescent proteins, which are also important antioxidants, were found to be downregulated in response to nutrient stress [90]. These varied outcomes for the different antioxidants may indicate that they have different roles in stress response, and that the ones that are upregulated are the primary immunity responders to this type of stress.
Tumor Necrosis Factor Receptor Pathway
In several independent studies, this pathway was found to be one of the most reactive in response to nutrient pollution [91,92,93]. In the coral Galaxea facicularis, a TNF receptor and the downstream transcription factor, NF-κB were significantly upregulated in sites with high nutrient pollution [91]. Additionally, other components of the TNFR pathway, including Trafs, were also upregulated, indicating that different levels of the signaling pathway were being activated in response to the nutrient stress, further bolstering the idea that this pathway is activated [91]. In another study, examining the response of Pocillopora damicornis to elevated ammonium levels, of the 24 Gene Ontology (GO) terms that were overrepresented, two were for tumor necrosis factor receptor superfamily member genes [92]. Authors proposed that this overrepresentation, along with some apoptotic genes, could indicate that apoptosis is being activated [92]. Lastly, in a study which examined the effects of ammonium and heat stress on the coral Pocillopora damicornis, 13 GO terms for TNFR pathway components were highly upregulated in not only the heat stress samples, but also in the ammonium-heat stress conditions [93]. All of these studies’ findings are examples of the reactivity of components of the TNFR pathway to nutrient pollution, and further investigation into this pathway could lead to critical insights into the coral’s ability to tolerate and survive nutrient pollution.
Major Gaps and Future Directions
Coral immunology is a growing field, and there are still many major mechanisms, especially in context of climate change, that are not well understood. In this review, we outlined what is currently known about the effects of different aspects of climate change on the coral immune system; however, there are still many gaps in our knowledge. We understand quite a bit about the effects of heat stress but very little about the impacts of ocean acidification. Focus on these aspects will shed light on the overall health of the coral and could potentially yield useful biomarkers. Additionally, little is understood about the effects of nutrient pollution on the coral immune system. Nutrient pollution is a complex, fluctuating stressor that has very different transcriptomic results depending on the local environment, the temperature, and the timing of the sampling. This complexity has made it a challenging stressor to study, but with more controlled laboratory experiments, a better understanding of these intricacies will be achieved.
In the future, emphasis on connecting the transcriptomic data to cellular and enzymatic data and then connecting all of this to organismal outcomes will be critical for understanding the impacts and the importance of the coral immune system in the context of climate change. Techniques such as in situ hybridization, histology, fluorescence-activated cell sorting, and differential protein expression, paired with transcriptomic studies will greatly aid in this endeavor.
Anthropogenic climate change has been devastating to the health of coral reefs [1, 2]. Coral cover has been lost, and we are in a race to quickly understand what corals can do to combat the threats of climate change before it is too late [1, 2]. To fully understand the sensitivity of the coral immune system to these threats, and to aid in the conservation of coral reefs, we need to create a more accurate and robust diagnostic system, one that combines the data from our varied disciplinary approaches. All of our data, from gene expression to protein and cellular reactions, will need to be analyzed and understood if we are to encourage the survival of these important species.
References
Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301(5635):929–33.
Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543(7645):373.
Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301(5635):955–8.
Pandolfi JM, et al. Are U.S. coral reefs on the slippery slope to slime? Science. 2005;307:1725–6.
Miller J, et al. Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs. 2009;28(4):925–37.
Miller J, et al. Coral bleaching and disease combine to cause extensive mortality on reefs in US Virgin Islands. Coral Reefs. 2006;25(3):418–8.
Muller EM, et al. Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs. 2008;27(1):191–5.
Harvell CD, et al. Emerging marine diseases—climate links and anthropogenic factors. Science. 1999;285(5433):1505–10.
Harvell CD, et al. Ecology—climate warming and disease risks for terrestrial and marine biota. Science. 2002;296(5576):2158–62.
Harvell D, et al. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20(1):172–95.
Palmer CV, Traylor-Knowles N. Towards an integrated network of coral immune mechanisms. Proc Biol Sci. 2012;279(1745):4106–14.
Sutherland KP, Porter JW, Torres C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser. 2004;266:273–302.
Bosch TC. Cnidarian-microbe interactions and the origin of innate immunity in metazoans. Annu Rev Microbiol. 2013;67:499–518.
Quistad S, Traylor-Knowles N. Precambrian origins of the TNFR superfamily. Cell Death Discovery. 2016:2(16058).
DeSalvo MK, et al. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol Ecol. 2008;17(17):3952–71.
Smith-Keune C, Dove S. Gene expression of a green fluorescent protein homolog as a host-specific biomarker of heat stress within a reef-building coral. Mar Biotechnol (NY). 2008;10(2):166–80.
Rodriguez-Lanetty M, Harii S, Hoegh-Guldberg O. Early molecular response of coral larvae to hyperthermal stress. Mol Ecol. 2009;18:5101–14.
Barshis DJ, et al. A genomic basis for coral resilience to climate change. Proc Natl Acad Sci U S A. 2013; https://doi.org/10.1073/pnas.1210224110.
Shinzato C, et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476(7360):320–3.
Zhou Z, et al. Suppression of NF-kappaB signal pathway by NLRC3-like protein in stony coral Acropora aculeus under heat stress. Fish Shellfish Immunol. 2017;67:322–30.
van de Water, J.A.J.M., et al., Comparative immune responses of corals to stressors associated with offshore reef-based tourist platforms. Conservation Physiology, 2015. 3.
Palumbi SR, et al. Mechanisms of reef coral resistance to future climate change. Science. 2014;344(6186):895–8.
Vidal-Dupiol, J., Adjeroud, M., Roger, E., Foure, L., Duval, D., Mone, Y., Ferrier-Pages, C., Tambutte, E., Tambutte, S., Zoccola, D., Allemand, D., Mitta, G., Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiology, 2009. 9(14).
Zhou Z, et al. Dual recognition activity of a rhamnose-binding lectin to pathogenic bacteria and zooxanthellae in stony coral Pocillopora damicornis. Developmental & Comparative Immunology. 2017;70:88–93.
Poole AZ, Kitchen SA, Weis VM. The Role of Complement in Cnidarian-Dinoflagellate Symbiosis and Immune Challenge in the Sea Anemone Aiptasia pallida. Frontiers in Microbiology. 2016;7
Miller DJ, et al. The innate immune repertoire in cnidaria—ancestral complexity and stochastic gene loss. Genome Biol. 2007;8(4):R59.
Kvennefors EC, et al. Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Dev Comp Immunol. 2010;34(11):1219–29.
Mydlarz LD, Harvell CD. Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp Biochem Physiol A Mol Integr Physiol. 2007;146(1):54–62.
Mydlarz LD, Palmer CV. The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp Biochem Physiol A Mol Integr Physiol. 2011;159(4):372–8.
Mydlarz LD, McGinty ES, Harvell CD. What are the physiological and immunological responses of coral to climate warming and disease? J Exp Biol. 2010;213(6):934–45.
Palmer CV. Biological mechanisms of Scleractinian immunity. Newcastle University and James Cook University. 2010;
Palmer CV, et al. Patterns of coral ecological immunology: variation in the responses of Caribbean corals to elevated temperature and a pathogen elicitor. J Exp Biol. 2011;214(Pt 24):4240–9.
Palmer CV, Mydlarz LD, Willis BL. Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proc Biol Sci. 2008;275(1652):2687–93.
Palmer CV, Bythell JC, Willis BL. Enzyme activity demonstrates multiple pathways of innate immunity in Indo-Pacific anthozoans. Proceedings of the Royal Society B-Biological Sciences. 2012;279(1743):3879–87.
Tchernov D, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci U S A. 2004;101(37):13531–5.
Tolleter D, et al. Coral bleaching independent of photosynthetic activity. Curr Biol. 2013;23(18):1782–6.
Bieri, T., et al., Relative contributions of various cellular mechanisms to loss of algae during cnidarian bleaching. Plos One, 2016. 11(4).
Knight JA. Review: free radicals, antioxidants, and the immune system. Ann Clin Lab Sci. 2000;30(2):145–58.
Lesser MP. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs. 1997;16(3):187–92.
Palmer CV, Modi CK, Mydlarz LD. Coral fluorescent proteins as antioxidants. PLoS One. 2009;4(10):e7298.
Krueger T, et al. Differential coral bleaching-contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2015;190:15–25.
Armoza-Zvuloni R, Shaked Y. Release of hydrogen peroxide and antioxidants by the coral Stylophora pistillata to its external milieu. Biogeosciences. 2014;11(17):4587–98.
Flores-Ramirez LA, Linan-Cabello MA. Relationships among thermal stress, bleaching and oxidative damage in the hermatypic coral, Pocillopora capitata. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2007;146(1–2):194–202.
Griffin SP, Bhagooli R, Weil E. Evaluation of thermal acclimation capacity in corals with different thermal histories based on catalase concentrations and antioxidant potentials. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2006;144(2):155–62.
Palmer CV, Roth MS, Gates RD. Red Fluorescent Protein Responsible for Pigmentation in Trematode-Infected Porites compressa. Biol Bull. 2009;216:68–74.
Roth MS, Deheyn DD. Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci Rep. 2013;3
Dove S, et al. Response of holosymbiont pigments from the scleractinian coral Montipora monasteriata to short-term heat stress. Limnol Oceanogr. 2006;51(2):1149–58.
Sheng YW, et al. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114(7):3854–918.
Meyer E, Weis VM. Study of cnidarian-algal symbiosis in the “omics” age. Biol Bull. 2012;223(1):44–65.
Seneca FO, et al. Patterns of gene expression in a scleractinian coral undergoing natural bleaching. Mar Biotechnol (NY). 2010;12(5):594–604.
Louis YD, et al. Gene expression biomarkers of heat stress in scleractinian corals: promises and limitations. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2017;191:63–77.
Csaszar NBM, Seneca FO, van Oppen MJH. Variation in antioxidant gene expression in the scleractinian coral Acropora millepora under laboratory thermal stress. Mar Ecol Prog Ser. 2009;392:93–102.
Schwarz JA, et al. Coral life history and symbiosis: functional genomic resources for two reef building Caribbean corals, Acropora palmata and Montastraea faveolata. BMC Genomics. 2008;9:97.
Dunn SR. Immunorecognition and immunoreceptors in the Cnidaria. Isj-Invertebrate Survival Journal. 2009;6(1):7–14.
Ocampo ID, et al. The immunotranscriptome of the Caribbean reef-building coral Pseudodiploria strigosa. Immunogenetics. 2015;67(9):515–30.
Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14(7):668–75.
Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2(5):346–53.
Sharon N, Lis H. Lectins—cell-agglutinating and sugar-specific proteins. Science. 1972;177(4053):949.
Franchi L, et al. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–28.
Proell, M., et al., The Nod-Like Receptor (NLR) Family: A Tale of Similarities and Differences. Plos One, 2008. 3(4).
Hamada M, et al. The complex NOD-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations. Mol Biol Evol. 2013;30(1):167–76.
Libro, S. and S.V. Vollmer, Genetic Signature of Resistance to White Band Disease in the Caribbean Staghorn Coral Acropora cervicornis. Plos One, 2016. 11(1).
Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–68.
Brown T, Bourne D, Rodriguez-Lanetty M. Transcriptional activation of c3 and hsp70 as part of the immune response of Acropora millepora to bacterial challenges. PLoS One. 2013:8(7).
Pinzon JH, et al. Whole transcriptome analysis reveals changes in expression of immune-related genes during and after bleaching in a reef-building coral. R Soc Open Sci. 2015;2(4):140214.
Vidal-Dupiol, J., et al., Thermal Stress Triggers Broad Pocillopora damicornis Transcriptomic remodeling, while Vibrio coralliilyticus infection induces a more targeted immuno-suppression response. Plos One, 2014. 9(9).
Cerenius L, Lee BL, Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29(6):263–71.
Palmer CV, Bythell JC, Willis BL. Levels of immunity parameters underpin bleaching and disease susceptibility of reef corals. FASEB J. 2010;24(6):1935–46.
Banin E, et al. Proline-rich peptide from the coral pathogen Vibrio shiloi that inhibits photosynthesis of zooxanthellae. Appl Environ Microbiol. 2001;67(4):1536–41.
Vidal-Dupiol J, et al. Innate immune responses of a scleractinian coral to vibriosis. J Biol Chem. 2011;286(25):22688–98.
Destoumieux-Garzon D, et al. Antimicrobial peptides in marine invertebrate health and disease. Philosophical Transactions of the Royal Society B-Biological Sciences. 2016:371(1695).
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–95.
Krediet, C.J., et al., Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proceedings of the Royal Society B-Biological Sciences, 2013. 280(1755).
Collette Y, et al. A co-evolution perspective of the TNFSF and TNFRSF families in the immune system. Trends Immunol. 2003;24(7):387–94.
Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20(10):1589–98.
Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66(8):1403–8.
MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14(6):477–92.
Quistad SD, et al. Evolution of TNF-induced apoptosis reveals 550 My of functional conservation. Proc Natl Acad Sci U S A. 2014;111(26):9567–72.
Traylor-Knowles N, Rose NH, Palumbi SR. The cell specificity of gene expression in the response to heat stress in corals. J Exp Biol. 2017;220(10):1837–45.
Kleypas JA, et al. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science. 1999;284(5411):118–20.
Carreiro-Silva M, et al. Molecular mechanisms underlying the physiological responses of the cold-water coral Desmophyllum dianthus to ocean acidification. Coral Reefs. 2014;33(2):465–76.
Kaniewska P, et al. Major cellular and physiological impacts of ocean acidification on a reef building coral. PLoS One. 2012;7(4):e34659.
Kenkel CD, et al. Functional genomic analysis of corals from natural CO2 -seeps reveals core molecular responses involved in acclimatization to ocean acidification. Glob Chang Biol. 2017;
Gilmore TD. Introduction to NF-kappa B: players, pathways, perspectives. Oncogene. 2006;25(51):6680–4.
Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene. 2001;20(44):6482–91.
Alieva NO, et al. Diversity and evolution of coral fluorescent proteins. PLoS One. 2008;3(7):e2680.
Moya A, et al. Rapid acclimation of juvenile corals to CO2-mediated acidification by upregulation of heat shock protein and Bcl-2 genes. Mol Ecol. 2015;24(2):438–52.
Kaniewska, P., et al., Transcriptomic Changes in Coral Holobionts Provide Insights into Physiological Challenges of Future Climate and Ocean Change. Plos One, 2015. 10(10).
Koop K, et al. ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar Pollut Bull. 2001;42(2):91–120.
Rosic N, et al. Early transcriptional changes in the reef-building coral Acropora aspera in response to thermal and nutrient stress. Bmc Genomics. 2014;15
Lin, Z.Y., et al., Transcriptome profiling of Galaxea fascicularis and its endosymbiont Symbiodinium reveals chronic eutrophication tolerance pathways and metabolic mutualism between partners. Scientific Reports, 2017. 7.
Yuan C, et al. Effects of elevated ammonium on the transcriptome of the stony coral Pocillopora damicornis. Mar Pollut Bull. 2017;114(1):46–52.
Zhou Z, et al. Elevated ammonium reduces the negative effect of heat stress on the stony coral Pocillopora damicornis. Mar Pollut Bull. 2017;118(1–2):319–27.
Libro, S., S.T. Kaluziak, and S.V. Vollmer, RNA-seq Profiles of Immune Related Genes in the Staghorn Coral Acropora cervicornis Infected with White Band Disease. PLoS One, 2013. 8(11).
Kvennefors EC, et al. An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Dev Comp Immunol. 2008;32(12):1582–92.
Hayes ML, Eytan RI, Hellberg ME. High amino acid diversity and positive selection at a putative coral immunity gene (tachylectin-2). BMC Evol Biol. 2010;10:150.
Anderson, D.A., et al., RNA-Seq of the Caribbean reef-building coral Orbicella faveolata (Scleractinia-Merulinidae) under bleaching and disease stress expands models of coral innate immunity. Peerj, 2016. 4.
Oliver, E., et al., Coral-associated bacterial extracts inhibit cellular NF-κB pathway. Cogent Environmental Science, 2017. 3(1).
Mayfield AB, et al. Evaluating the temporal stability of stress-activated protein kinase and cytoskeleton gene expression in the Pacific reef corals Pocillopora damicornis and Seriatopora hystrix. J Exp Mar Biol Ecol. 2010;395(1–2):215–22.
Poole AZ, Weis VM. TIR-domain-containing protein repertoire of nine anthozoan species reveals coral-specific expansions and uncharacterized proteins. Dev Comp Immunol. 2014;46(2):480–8.
Dove S, Hoegh-Guldberg O, Lesser M. All-protein chromophores isolated from corals, quench superoxide radicals. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2006;143(4):S132–2.
Acknowledgements
Authors would like to thank Dr. Chris Langdon for the invitation to write this review, the reviewers for their helpful suggestions, and April Mann for assistance in editing of revisions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
This article is part of the Topical Collection on Corals and Climate Change
Rights and permissions
About this article
Cite this article
Traylor-Knowles, N., Connelly, M.T. What Is Currently Known About the Effects of Climate Change on the Coral Immune Response. Curr Clim Change Rep 3, 252–260 (2017). https://doi.org/10.1007/s40641-017-0077-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40641-017-0077-7