Abstract
Platinum Group Metals (PGMs) play a significant role in the manufacturing of catalysts, super alloys, electronics, space materials, biomedical equipments, jewellery, etc. due to their excellent electrical and thermal conductivity as well as chemical resistivity. The rising demand of PGMs in industrial applications and their limited natural resources have laid emphasis on the development of feasible and eco-friendly processes for the extraction of these metals from different sources to meet their future requirements. Present review reports commercial processes based on pyro-/hydro- and hybrid techniques to recover PGMs from various resources. The salient findings on different processes used for recovery of PGMs have been reviewed with respect to various methodologies and objectives.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Platinum group metals (PGMs) comprises of six noble metals namely Ruthenium (Ru), Rhodium (Rh), Palladium (Pd), Osmium (Os), Iridium (Ir), Platinum (Pt) which are found together in the d-block of periodic table. These transition metals possess similar physical and chemical properties. The unique properties of PGMs such as catalytic activity, chemical inertness, resistance towards corrosion, thermal as well as electrical stability make them a vital component of many industrial applications, thus, they are also called ‘Vitamin of modern industry’ [1,2,3,4]. Recent technological modernization involving advanced chemistry has commercially accelerated the use of PGMs in the field of vehicle and equipment construction, chemical industry, oil refining, medical practices, jewellery making, etc. [5]. Pt and Pd are of major commercial significance followed by Rh, Ir and Ru whereas Os has rare viable applications [6, 7]. The global demand of PGMs (Pt, Pd and Rh) is over 590 tons while their natural resources are only 66,000 tons all over the world. South Africa is the leading producer of PGMs in the world followed by Russia, Canada, Zimbabwe, USA and Colombia. Extensive deposits of PGMs are located in the norite belt of the Bushveld Igneous Complex covering the Transvaal Basin in South Africa, the Stillwater Complex in Montana, United States, the Thunder Bay District of Ontario, Canada, and the Norilsk Complex in Russia. PGMs are also found associated with base metal (Cu, Ni) sulfide minerals where their content is almost 2–10 g/t [8]. Nowadays, reefs like Merensky, Upper Group Two (UG-2) and Plat reefs are also mined due to presence of significant quantity of PGMs in them [3, 9]. They are also recovered as by-products depending on their concentration in the ore [2, 10].
It has been observed that high value of PGMs coupled with their increasing demand has fuelled its processing from low-grade resources using elaborated techniques [3]. Despite expensive multi-step processes, different innovative methods are continuously being explored to extract PGMs from primary resources. But the depletion of high grade PGMs resources due to continuous mining, has laid emphasis on their production of PGMs from secondary resources viz. automobile catalysts, e-waste, industrial waste (solid/liquid), etc. Hence, in order to explore improved possibilities for the recovery of PGMs, an attempt has been made to provide a general overview on prevailing commercial technologies. The present paper gives an overview of the commercial processes developed for the recovery of PGMs from various resources using pyro-/hydro- or hybrid techniques. The paper will be helpful for researchers in future to develop new process flow-sheet for extraction of PGMs keeping in view the drawbacks of the existing commercial process.
Processing of Primary Resources to Recover PGMs
Although extensive deposits of PGMs are available but deposits for their economical extraction are inadequate [11]. Primary ores of PGMs are broadly divided into four types: (i) Stratiform deposits (~10–1000 MT) containing 3–10 g/t PGMs; (ii) Norite intrusions (~10–1000 MT) having 1–3 g/t PGMs (iii) Ni-Cu bearing sills (~10–1000 MT) with 2–15 g/t PGMs and (iv) Placer deposits containing coarse PGMs (mainly Pt). PGMs ores are mined through conventional underground or open cut techniques followed by grinding. Further, gravity-based separation and flotation is generally used to produce a PGM-rich concentrates [12].
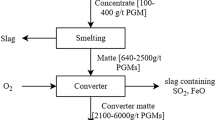
PGMs from high grade chromite ores (containing 200–2000 g/t PGM along with 0.4–2.8% Cr2O3) are conventionally recuperated by matte-smelting-refining process as shown in Fig. 1 [3, 11]. The chromite rich ores are generally processed by conventional flotation in a mill-float-mill-float (MF2) open circuit prior to recovery of PGMs, in order to prevent the accumulation of chromite fines generated during over grinding of ores. Initially, the ore is ground by crushing and ball milling in several stages or by SAG (semi-autogenous grinding) and then followed by smelting. The matte obtained undergoes hydrometallurgical treatment for removal of impurities such as Fe, Co, Ni and Cu leaving 10–50% of PGMs in the slime [13]. Emission of SO2, accumulation of highly refractory chromite spinel layers in the furnace and environmental pollution as a result of high temperature smelting are some of the major limitation of the conventional smelting and converting processes [14,15,16].
Conventional matte-smelting-refining technology to get PGM concentrates [3]
Several researchers have also reported hydrometallurgical or the combination pyro-hydrometallurgical processes as a pre-treatment step for the recovery of PGM from ores/concentrates [17,18,19,20,21,22]. Hydrometallurgical processes consisting of leaching operation for enrichment PGMs from base metal (Cu, Ni, Co, Fe) sulfide minerals have also been employed. During this process, the base metals are dissolved leaving behind the PGM concentrate for further refining. The same process has also been used in Ni and Cu-Ni refineries for the enrichment of PGMs [23]. Apart from high grade ores, hydrometallurgical processes have also been developed for the commercial extraction of PGMs from low grade refractory sulfide ores and concentrate as presented in Table 1.
Processing of Secondary Resources to Recover PGMs
PGMs are vital component of several products like mobile phones, industrial catalysts, ceramic glazes, hard disks, aircraft turbines, etc. Catalysts and electronic wastes are two imperative secondary resources containing significant amount of PGMs due to their remarkable resistance to high temperature corrosion and oxidation. Among these, PGMs are widely used as a catalyst in various chemical reactions like reduction, reformation, hydrogenation, isomerisation, conversion, etc. [41,42,43,44,45,46,47,48,49,50,51,52,53]. Automobile industry is the largest consumer of PGMs. Almost 34% Pt, 55% Pd and 95% Rh out of their total demand is used for the manufacturing of catalytic filters-neutralizers of exhaust gases in automobile industries [54,55,56,57]. Varying amount of Pt, Pd and Rh are used in auto catalysts depending upon the type of vehicle, manufacturer, country, etc. which helps in regulating the harmful emission of CO, NOx and hydrocarbons. During the catalytic conversion, Pt helps in converting hydrocarbons and CO to H2O and CO2, while Rh is highly efficient in reducing NOx to N2 whereas Pd alone can handle all three pollutants, but less efficiently compared to Pt and Rh [58, 59]. Thus, spent automobile catalysts are important supplementary source for the recycling and recovery of PGMs. Processing 2 mg of spent automobile catalysts to recover PGMs can prevent the mining of 150 kg of their ores [58]. The rise in demand of PGMs in automobile industries and strict environmental regulations make their recycling indispensable. The spent automobile catalyst contains an average of ~4 g PGMs which is quite high in comparison to primary resources of PGMs [58, 60, 61]. Moreover, the high price of Pt, Pd and Rh, makes their recovery from used catalysts profitable. Based on their chemical composition and nature, the recycling of these PGM-bearing catalysts is carried out through hydrometallurgical as well as pyrometallurgical processes. Several corporations and industries like Umicore, Belgium; Hereaus, Germany; BASF/Engelhard, USA; Johnson Matthey, UK; Nippon/Mitsubishi, Japan, etc. have already developed successful commercial processes for the recycling of PGMs from secondary resources [2].
Pyrometallurgical process is usually employed to concentrate the PGMs followed by refining technology to recover them. Pyrometallurgical process including crushing, batching, granulation, smelting, separation, has become a traditional method to recover PGMs from spent catalyst [41, 58]. The spent catalyst is initially mixed with fluxes, collector and reductant during smelting and a PGM-collector alloy is obtained which further undergoes purification [41]. The choice of collector plays an important role during smelting. The selection between collector and PGMs is based on their mutual solubility, melting point and chemical properties. Metals like Cu, Ni, Pb and Fe are generally considered good collectors [1]. PGM-Pb collection is one of the oldest methods [62, 63] that has been used to process secondary resources in Inco, Johnson Matthey, Impala, etc. before 1980s. The process is simple to operate, require low smelting temperature, followed by simple refining process, and needs less investment but the major disadvantages are low recovery of Rh and generation of hazardous lead oxide. PGM-Cu collection is another method for treating the spent auto-exhaust catalyst in an electric arc furnace with addition of fluxes (SiO2, CaO, etc.), collector (CuCO3 or CuO) and reductant [64, 65]. PGMs are collected at low temperature and weak reduction atmosphere. A semi-industrial process combining pyrometallurgy and electrolytic refining for the recovery of PGMs from spent auto-exhaust catalyst using metal copper collection has been performed by the Institute for Mining and Metallurgy Bor, Serbia as shown in Fig. 2 [66]. The Nippon PGM Co. Japan works on the well-known Rose Process (Fig. 2) which is basically copper collection process. The final concentrates contain almost 30% of PGMs whereas the CuxO produced can be reused during the smelting process [1, 58].
Industrial process for the recovery of PGMs from spent automobile catalyst [1]
The Umicore operated at Hoboken, Belgium is an integrated metal smelter and refinery, which also recovers PGMs along with other metals from auto-catalysts/printed circuit boards/electronic components [67]. PGM-Fe collection process mainly involves the method of plasma arc smelting and mineral phase reconstruction. The plasma arc smelting technology was very popular during 1980s to recover PGMs from spent auto-exhaust catalyst [68,69,70]. High energy density, high temperatures and flexibility in the plasma gases are the vital advantages of this technology [71]. The Plasma arc smelting process for recovery of PGMs from the spent auto-exhaust catalyst has also been commercialized in Texasgulf, USA and Safina, Czech Republic [41, 70]. On the other hand, the short lifetime of plasma gun accessory restricted its practical industrial application. Based on the findings, a mineral phase reconstruction process was proposed to recover PGMs from spent auto-exhaust catalyst [72]. The Johnson Matthey process involves smelting of crushed catalyst with flux materials in a crucible containing molten collector metal (Fe or Cu), using a plasma torch [62]. The operation is carried out at temperatures between 1500–1650 °C where the alloy of collector metal is tapped off and ~95% PGMs are recovered by conventional refining methods. From the above studies, it can be concluded that metal smelting collection process is appropriate for processing various secondary materials containing PGMs. The affinity of PGM particles towards the collector metal is an important factor behind the success of this process whereas other factors like fluxes, collector, smelting equipment, operating system, etc. should also be considered. From industrial point of view, PGM-Cu collection technology has wide applications due to high efficiency, low smelting temperature, less pollution and easy industrialization.
Hydrometallurgical processing for the recovery of PGMs mainly involves dissolution using suitable acidic and alkaline solutions in the presence of additives like O2, I2, Br2, Cl2, H2O2, etc. [7, 73,74,75,76,77,78,79,80,81,82]. The spent catalyst containing PGMs is pre-treated before hydrometallurgical processing. The PGMs present in the catalysts are encapsulated by specific substances which lead to decrease in their leaching efficiency. Thus, pre-treatment steps such as fine grinding, roasting, reduction, pressure leaching, etc. are necessary prior to leaching of PGMs. Several researchers have reported various pre-treatment methods (oxidization roasting, reduction roasting, pre-leaching, etc.) to destroy organic substances on the surface of spent catalysts or change the supporter forms which hinders the leaching of PGMs present in spent catalysts [41, 58]. After pre-treatment, leaching of the spent catalysts are carried out for maximum dissolution of PGMs. HCl is the most common complexing agent, while HNO3, Cl2, or H2O2 can be used as oxidant [41, 83]. Aqua regia, commonly used leachant for dissolution precious metals, but not all PGMs can be dissolved with it. Several researchers have studied the use of aqua regia [84,85,86,87,88,89,90] to recover Pt, Pd, Rh, etc. from different spent catalysts on the commercial scale. Although leaching rate of PGMs is high in aqua regia but keeping in view environmental aspects, the process has some major drawbacks due to the generation of NOx, Cl2 and acid fumes during leaching. From the leach solution obtained, purification and separation of PGMs could be achieved by the method of cementation, solvent extraction, ion exchange, etc. An alternative method is to leach the ceramic material of the catalyst with NaOH or hot H2SO4 under pressure where PGMs (Pt, Pd, Rh) are insoluble and remains in the residue. But owing to relatively low yield of PGMs and generation of huge quantities of wastewater, the process is infeasible. The general flow-sheet for extraction of PGMs from spent catalyst using hydrometallurgical technique is shown in Fig. 3 [1].
Hydrometallurgical processing of PGMs [1]
The chemical and metallurgical industries have used the method of cyanide leaching to recover PGMs [13, 91] due their ability to form stable complexes in alkaline medium. PGMs extraction by cyanidation show poor kinetics at room temperature and atmospheric pressure, thus, effective leaching of the PGMs is carried out at high temperature and pressure. In cyanide leaching process, the rate of reaction rate is proposed to be controlled by a surface chemical reaction, which is similar to gold cyanidation mechanism [92]. Cyanide leaching of PGMs requires special equipment as well as proper management of the toxic waste generated which may lead to severe environmental problem. Thus, the industrial application of this process is difficult and it is still in exploratory stage. Several processes for the commercially extracting PGMs from secondaries have been successfully developed. Platinum Lake Technology Inc., Canada [93] has successfully developed a hydrometallurgical process for the recovery PGMs (95% Pt and 98% Pd) from spent automotive catalysts. Nippon PGM Co. has reported the production of PGMs from different resources on commercial-scale [94]. Heraeus, Germany has reported the recovery of PGMs from spent materials using hydrometallurgical processes consisting of leaching in HCl in presence of oxidant followed by selective precipitation and ion exchange [2]. BASF Catalysts LLC, USA also developed a novel process for the recovery of PGMs from membrane electrode assemblies (MEAs) eradicating the release of HF (highly toxic gas) generated during the current combustion recycling process [2]. Studies have also been carried out at the Mining and Materials Processing Institute of Japan to recycle PGMs from the residue of automobile catalyst with high leaching efficiency [7]. Hydrometallurgical route offers a faster rate of metal recovery at low capital costs. In addition, the energy consumption is low compared to pyrometallurgical process, which requires high temperatures to melt the raw material. Moreover, the wastewater generated at the industrial scale could be treated in effluent treatment plant and possibilities could be explored for further recovery of value added products.
Conclusion
Based on the review it can be concluded that the recovery and recycling of PGMs from both primary and secondary resources are essential due to their rising demand in various industrial applications. Due to significant conflict between availability of natural resources of PGMs and their increasing demands, it is necessary to exploit indigenous resources of PGMs. Several pyrometallurgical and hydrometallurgical processes for recovery of PGMs are already available but development of modern and productive technologies to utilize indigenous resources as well as improvement in prevailing technologies, will be helpful to meet the future demand of PGMs in various applications. More emphasis should be laid on the recycling of PGMs from secondary sources (spent automobile catalysts, e-waste, industrial wastes, etc.) in order to economise the natural resources and to minimise the environmental pollution in connection to production of PGMs. Thus, R & D efforts should be made to develop hybrid processes consisting of physical beneficiation/pyro-/hydro-/electro metallurgy for efficient recovery of PGMs from various resources.
References
Dong H, Zhao J, Chen J, Wu Y, Li B (2015) Recovery of platinum group metals from spent catalysts: a review. Int J Miner Process 145:108–113
Jha MK, Lee JC, Kim MS, Jeong J, Kim BS, Kumar V (2013) Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: a review. Hydrometallurgy 133:23–32
Mpinga CN, Eksteen JJ, Aldrich C, Dyer L (2015) Direct leach approaches to platinum group metal (PGM) ores and concentrates: a review. Miner Eng 78:93–113
Afolabi AS, Nkobane MP, Abdulkareem AS (2012) Development of PGMs and chrome extraction circuit from UG-2 ore. In: Proceedings of the world congress on engineering 3
Kononova ON, Melnikov AM, Borisova TV (2012) Simultaneous sorption recovery of platinum and rhodium from sulfate–chloride solutions. Hydrometallurgy 117–118:101–107
Fact Sheet (2012) Platinum group metals. Polinares working paper 35 March 2012
Harjanto S, Cao YC, Shibayama A, Naitoh I, Nanami T, Kasahara K, Okumura Y, Liu KJ, Fujita T (2006) Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater Trans 47(1):129–135
Kyriakakis G (2005) Extraction of gold from platinum group metal (PGM) ores. Dev Miner Process 15:897–917
Cramer LA (2001) The extractive metallurgy of South Africa’s platinum ores. J S Afr Inst Min Metall
Liu SJ (2013) Metallurgy of platinum group metals. Central South University Press, Changsha
Cole S, Joe Ferron C (2002) A review of the beneficiation and extractive metallurgy of the platinum group elements, highlighting recent process innovations. SGS minerals services technical paper-03
Vermaak CF (1995) The platinum-group metals: a global perspective. Mintek, Randburg, South Africa
Chen J, Huang K (2006) A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 82:164–171
Eksteen JJ (2011) A mechanistic model to predict matte temperatures during the smelting of UG2-rich blends of platinum group metal concentrate. Miner Eng 24(7):676–687
Eksteen JJ, Bezuidenhout GA, Van Beek B (2011) Cracking a hard nut: an overview of Lonmin’s operations directed at smelting of UG2-rich concentrate blends. J South Afr Inst Min Metall 111(10):681–690
Ritchie S, Eksteen JJ (2011) Investigating the effect of slag bath conditions on the existence of ‘‘mushy’’ zones in PGM smelting furnaces using computational fluid dynamics. Miner Eng 24(7):66–675
Li Y, Kawashima N, Li J, Chandra AP, Gerson AR (2013) A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv Colloid Interface Sci 197–198:1–32
Li J, Miller JD (2006) A review of gold leaching in acid thiourea solutions. Miner Process Extr Metall Rev 27:177–214
Wang S (2005) Copper leaching from chalcopyrite concentrates. JOM
Xiao Z, Laplante AR (2004) Characterizing and recovering the platinum group Minerals-a review. Miner Eng 17:961–979
Fleming CA (2002) Platsol™ process provides a viable alternative to smelting. SGS minerals services technical paper 2002–01
Maurice D, Hawk JA (1999) Ferric chloride leaching of a mechanically activated pentlandite-chalcopyrite concentrate. Hydrometallurgy 52:289–312
Kerfoot DGE (1986) Review of hydrometallurgical nickel refining operations: Responses to questionnaire. In: Ozberk E, Marcusson SW (eds), Nickel metallurgy, Volume I, extraction and refining of nickel, 25th annual conference of metallurgists, CIM, March, p 426441
Dreisinger D (2006) Copper leaching from primary sulfides: options for biological and chemical extraction of copper. Hydrometallurgy 83:10–20
Gok O, Anderson CG (2013) Dissolution of low-grade chalcopyrite concentrate in acidified nitrite electrolyte. Hydrometallurgy 134–135:40–46
Nazari G, Dixon DG, Dreisinger DB (2012) The role of galena associated with silver-enhanced pyrite in the kinetics of chalcopyrite leaching during the Galvanox™ process. Hydrometallurgy 113–114:177–184
Nazari G, Dixon DG, Dreisinger DB (2012) The role of silver-enhanced pyrite in enhancing the electrical conductivity of sulfur product layer during chalcopyrite leaching in the Galvanox™ process. Hydrometallurgy 111–112:35–45
Nazari G, Dixon DG, Dreisinger DB (2012) The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the Galvanox™ process. Hydrometallurgy 113–114:122–130
Kowalczuk P, Chmielewski T (2008) Search for a new technology producing copper from chalcopyrite. Scientific papers of the institute of mining, No 122, conferences No 51, 7th PhD studies scientific conference, interdisciplinary topics in mining and geology, Ofic Wyd PWr, Wroclaw, pp 94–100
Ellis S, Mines KCG, Kalgoorlie WA (2008) Ultra fine grinding- a practical alternative to oxidative treatment of refractory gold ores
Turner DW, Hourn M (2013) Albion process simplicity in leaching: copper applications. Xstrata technology
Nazari GT (2012) Enhancing the kinetics of pyrite catalyzed leaching of chalcopyrite. Ph.D thesis, University of British Columbia
Dixon DG, Tshilombo AF (2005) Leaching process for copper concentrates. United States Patent Application 2005(0269208):A1
Dreisinger D (2012) Hydrometallurgical extraction of base, rare, and precious metals from complex and low grade resources. In: XXVI International Mineral Processing Congress (IMPC) 2012 Proceedings/New Delhi, India/24–28 September 2012
Milbourne J, Tomlinson M, Gormely L (2003) Use of hydrometallurgy in direct processing of base metal/PGM concentrates. Hydrometallurgy 625
Lundström M, Aromaa J, Forsen O, Hyvarinen O, Barker MH (2005) Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy 77:89–95
Lundström M, Aromaa J, Forsen O (2009) Redox potential characteristics of cupric chloride solutions. Hydrometallurgy 95:285–289
Senanayake G (2009) A review of chloride assisted copper sulfide leaching by oxygenated sulfuric acid and mechanistic considerations. Hydrometallurgy 98:21–32
Turkmen Y, Kaya E (2009) Acidified ferric chloride leaching of a chalcopyrite concentrate. J Ore Dressing 11(22)
Liddell KS, Adams MD (2012) Kell hydrometallurgical process for extraction of platinum group metals and base metals from flotation concentrates. J South Afr Inst Min Metall 112
Aberasturi DJD, Pinedo R, Larramendi IRD, Larramendi JIRD, Rojo T (2011) Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic convertors. Miner Eng 24:505–513
Matjie RH, Scurrel MS, Bunt J (2005) The selective dissolution of alumina, cobalt and platinum from a calcined spent catalyst using different lixiviants. Miner Eng 18:801–810
Pinheiro AA, Lima TS, Campos PC, Afonso JC (2004) Recovery of platinum from spent catalysts in a fluoride-containing medium. Hydrometallurgy 74:77–84
Avci AK, Trimm DL, Erhan Aksoylu A, İlsen Önsan Z (2004) Hydrogen production by steam reforming of n-butane over supported Ni and Pt–Ni catalysts. Appl Catal A 258:235–240
Ghosh SK, Mandal M, Kundu S, Nath S, Pal T (2004) Bimetallic Pt–Ni nanoparticles can catalyze reduction of aromatic nitro compounds by sodium borohydride in aqueous solution. Appl Catal A 268:61–66
Malyala RV, Rode CV, Arai M, Hegde SG, Chaudhari RV (2000) Activity, selectivity and stability of Ni and bimetallic Ni-Pt supported on zeolite Y catalysts for hydrogenation of acetophenone and its substituted derivatives. Appl Catal A 193:71–86
Mahmoud MHH (2003) Leaching platinum-group metals in sulfuric acid/chloride solution. J Miner Met Mater Soc 55(4):37–40
Epron F, Gauthard F, Barbier J (2002) Influence of oxidizing and reducing treatments on the metal-metal interactions and on the activity for nitrate reduction of a Pt-Cu bimetallic catalyst. Appl Catal A 237:253–261
Saberi MA, Mao RLV, Martin M, Mak AWH (2001) Effect of Zn loading of the Pt-Zn-Hy trifunctional catalysts on the hydroisomerization of n-heptane. Appl Catal A 214:229–236
Grzelczuk S, Popowicz M, Berak JM, Schimmelpfennig Z, Gora M, Staszak S (1985) PL 131079 B1. Instytut Chemii Przemyslowej, Poland
Jeliyaskova M, Sariev I, Koralska S, Aneva S, Pankova M, Yancheva M (1982) Recovery of platinum from spent catalysts. Chem. Techniek 34(12):651–653
Kluksdhal HE (1971) New bimetallic catalyst with high activity for petroleum naphtas reforming. US Patent: 3558477
McCoy CS, Munk P (1971) Symposium on catalytic reforming. 68th National meeting of AIChE, Houston, Tex., paper 42a
Buslaeva TM (1999) Platinum group metals and their role in contemporary society. Sorosovskiy Obrazovatelny Zhurnal 11:45–4
Taran OP, Polyanskaya EM, Ogorodnikova OL, Descorme C, Besson M (2010) Ruthenium carbon-based catalysts for catalytic wet air oxidation of phenol. J Siberian Fed Univ Chem 3(3):245–252
Zolotov YA, Varshal GM, Ivanov VM (2003) Analytical Chemistry of Platinum Group Metals. Editorial URSS, Moscow
Kim CH, Woo SI, Jeon SH (2000) Recovery of platinum-group metals from recycled automotive catalytic converters by carbochlorination. Ind Eng Chem Res 39:1185–1192
Fornalczyk A, Saternus M (2009) Removal of platinum group metals from the used auto catalytic converter. Metalurgija 48(2):133–136
Votsmeier M, Kreuzer T, Gieshoff J, Lepperhoff G (2009) Automobile exhaust control. In: Ullmann’s encyclopedia of industrial chemistry, Wiley-VCH, Weinheim, Germany
Rumpold R, Antrekowitsch J (2012) Recycling of platinum group metals from automotive catalysts by an acidic leaching process. In: The Southern African Institute of mining and metallurgy platinum, pp 695–714
Chevalier P (2004) PGM, Canadian minerals yearbook, pp 41.1–41.16
Benson M, Bennett CR, Harry JE, Patel MK, Cross M (2000) The recovery mechanism of platinum group metals from catalytic convertors in spent automotive exhaust systems. Resour Conserv Recy 31:1–7
Keyworth B (1982) The role of pyrometallurgy in the recovery of precious metals from secondary materials. In: 6th international precious metals institute, California, pp 509–538
Ezawa N (1993). Process of recovering platinum group metal. US Patent 5252305
Hoffmann JE (1988) Recovering platinum-group metals from auto catalyst. JOM 40(6):40–44
Ivanović SZ, Trujuć VK, Gorgievski MD, Mišić LD, Božić DS (2011) Removal of platinum group metals from the spent automobile catalyst by the pyrometallurgical process. In: 15th international research/expert conference “Trends in the development of machinery and associated technology”, pp 12–18
Hagelüken C (2004) Umicore precious refining-the power of integration. Precious Metals Market Report. Umicore AG & Co.KG, Belgium, pp 10–11
Mishra RK, Reddy RG (1986) Pyrometallurgical processing and recovery of precious metals from auto-exhaust catalyst using plasma arc smelting. In: 10th International Precious Metals Institute, Florida, pp 217–231
Saville J (1985) Recovery of PGM’s by plasma arc smelting. In: 9th International precious metals institute conference, New York, pp 157–167
Zhao HZ (1998) Analysis of phases in metallurgical products of plasma treating PGMs secondary resources. Chin J Nonferr Met 8(2):314–317
Burkhard R, Hoffelner W, Eschenbach RC (1994) Recycling of metals from waste with thermal plasma. Resour Conserv Recycl 10(2):11–16
Wang YH, Wu XF, Tong WF, Zhao C, Zan LH, Fan XX, Li BY, Li N (2009) A method for extraction of Pt, Pd and Rh from automotive catalyst by mineral phase restruction. Chinese Patent, 200910094112.7
Angelidis TN (2001) Development of a laboratory scale hydrometallurgical procedure for the recovery of Pt and Rh from spent automotive catalysts. Top Catal 1–4:419–423
Bolinski L, Distin PA (1992) Platinum group metals recovery from recycled auto catalyst by aqueous processing. Publ Australas Inst Min Metall 9/92
Duyvesteyn S, Liu H, Duyvesteyn WPC (1994) Recovery of platinum group metals from oxide ore-TML process. In: Proceedings of international symposium on hydrometallurgy. Chapman & Hall, London, pp 887–912
Han KN, Meng X (1996) Redox leaching of ores and spent catalysts using halide-containing solutions for recovery of platinum-group metals and rhenium. US Patent: 5542957
Meng X, Han KN (1995) Recovery of platinum and palladium from spent automobile catalytic converters by leaching with solutions containing halogen salts, ammonium and oxidants. In: Queneau PB, Peterson RD (eds), Minerals, 3rd ed. International symposium of recycling of metals engineering materials, metals and materials society, Warrendale, PA, pp 501–513
Mishra RK (1988) Recovery of platinum group metals from automobile catalytic converters-a review. The Minerals, Metals & Materials Society: Precious Metals 89:483–501
Mishra RK (1993) A review of platinum group metals recovery from automobile catalytic converters. In: Mishra RK (ed) Proceedings of International Precious Metals Ins Conf, Newport, RI: Precious Metals, pp 449–474
Yoo S (1998) Metal recovery and rejuvenation of metal-loaded spent catalysts. Catal Today 44:27–46
Eugenia G, Petru GS, Constanta G, Florica Z, Sergiu P, Dumitru G, Nedelcu A, Costel S, Dino S, Florica MB, Nicolae SV (1983) Recovery of platinum and other components from spent catalysts of the platinum. Romanian Patent, RO 71056 B 19801205
Ezawa N (1989) Recovery of platinum-group metals from spent catalysts. Japanese Patent: JP 01108390 A2 19890425
Barakat MA, Mahmoud MHH (2004) Recovery of platinum from spent catalyst. Hydrometallurgy 72:179–184
Baghalha M (2012) The leaching kinetics of an oxide gold ore with iodide/iodine solutions. Hydrometallurgy 113–114:42–50
Jafarifar D, Daryanavard MR, Sheibani S (2005) Ultrafast microwave-assisted leaching for recovery of platinum from spent catalyst. Hydrometallurgy 78:166–171
Schreier G, Edtmaier C (2003) Separation of Ir, Pd and Rh from secondary Pt scrap by precipitation and calcinations. Hydrometallurgy 68:69–75
Horner BT, McGrath RB (1994) European patent: 94–300378 (Johnson Matthey PLC)
Perte E, Ghiara C, Marc M, Ceuca O, Crucin O (1988) Romania patent: 94014 B1(Institutul de Chimie)
Muraki M, Mitsui Y (1986) Method for collecting platinum and palladium from platinum catalyst. Japanese patent: 61110731 A2 (Nippon Magnetic Dressing)
Grzelczuk S, Popowicz M, Berak JM, Schimmelpfennig Z, Gora M, Staszak S (1985) PL 131079 B1. Instytut Chemii Przemyslowej, Poland
Atkinson GB, Kuczynski RJ, Desmond DP (1992) Cyanide leachingmethod for recovering platinum group metals from a catalytic converter catalyst. U S Patent 5(160):711
Wadsworth ME, Zhu X, Thompson JS, Pereira CJ (2000) Gold dissolution and activation in cyanide solution: kinetics and mechanism. Hydrometallurgy 57:1–11
Ryder JM, Dymock K (1990) Platinum lake technology process for recovery of precious metals from scrap automotive catalysts. In: Proceedings of recycling of metalliferous materials conference. The Institution of Mining and Metallurgy, Birmingham, England, pp 255–258
Suzuki S, Ogino M, Matsumoto T (2007) Recovery of platinum group metals at Nippon PGM Co., Ltd. J MMIJ 123:734–736
Acknowledgements
The authors are thankful to the Director, CSIR-National Metallurgical Laboratory, Jamshedpur for the permission to publish this paper. One of the authors Ms. Rekha Panda would like to extend her sincere gratitude to CSIR, New Delhi (Grant: 31/10 (64)/2017-EMR-I) for providing Senior Research Fellowship to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Panda, R., Jha, M.K., Pathak, D.D. (2018). Commercial Processes for the Extraction of Platinum Group Metals (PGMs). In: Kim, H., et al. Rare Metal Technology 2018. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72350-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-72350-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72349-5
Online ISBN: 978-3-319-72350-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)