Abstract
As an important secondary resource with abundant platinum group metals (PGMs), spent catalysts demand recycling for both economic and environmental benefits. This article reviews the main pyrometallurgical processes for PGM recovery from spent catalysts. Existing processes, including smelting, vaporization, and sintering processes, are discussed based in part on a review of the physiochemical characteristics of PGMs in spent catalysts. The smelting technology, which produces a PGM-containing alloy, is significantly influenced by the addition of various collectors, such as lead, copper, iron, matte, or printed circuit board (PCB), considering their chemical affinities for PGMs. The vaporization process can recover PGMs in vapor form at low temperatures (250–700°C), but it suffers high corrosion and potential environmental and health risks as a result of involvement of the hazardous gases, mainly Cl2 and CO. The sintering process serves as a reforming means for recycling of the spent catalysts by in situ reduction of their oxidized PGMs components. Among these processes, the smelting process seems more promising although its overall performance can be further improved by seeking a suitable target-oriented collector and flux, together with proper pretreatment and process intensification using an external field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Platinum (Pt), palladium (Pd), rhodium (Rh), ruthenium (Ru), iridium (Ir), and osmium (Os) are called the platinum group metals (PGMs). As the “Vitamin of modern industry” and strategic materials vital for economic development,1 these elements have been widely used in a variety of applications, including electronic devices, catalytic converters, thermocouples, fuel cells, corrosion-resistant materials, jewelry, etc.,2 mainly attributed to their unique physical and chemical properties, such as catalytic activity, chemical inertness, corrosion resistance, thermoelectric stability, and magnificent color.1
PGMs are scarce as a result of their low abundance in the Earth’s crust. Their primary sources are limited with global reserves of only 66,000 tons.1 They are geographically concentrated in certain locations, including the norite belt of the Bushveld Igneous Complex covering the Transvaal Basin in South Africa (the world’s largest producer of platinum), the Stillwater Complex in Montana, United States, the Thunder Bay District of Ontario, Canada, and the Norilsk Complex in Russia.1 , 3 Because of their low concentration (2–10 g/t) and close associations with base metal sulfide minerals, they are often recovered as by-products or co-products of copper or nickel smelting.3
As a result of depletion of rich natural PGMs deposits, the production of the metals from secondary resources, such as spent catalysts, has become an important issue. Figure 1 shows the global supply, demand, and recycling amounts of PGMs from 2011 to 2015.4 It shows that the major PGMs, including Pt, Pd, and Rh, were basically in short supply (“Gap” < 0) in the period of time. Considering the limited and stagnant supply of PGMs,5 there will be a noticeable increase in the supply risk associated with PGMs in the near future.
Spent catalysts are one of the most important secondary resources of PGMs, primarily generated as a result of the application to automobile catalytic converters (also known as the “mobile PGMs mine” accounting for more than 60% of the use of PGMs) for control of automobile exhaust emission.6 , 7 Although the catalysts have very low PGM loadings (approximately 1 wt.%), the PGMs represent the main cost of the product. Moreover, because the spent catalysts often contain coke, vanadium, nickel, and other heavy metals that may cause ground and water pollution and affect animal and plant life, they are classified as a hazardous waste with restricted disposal in landfills. Apparently, it is crucial to recover PGMs from spent catalysts from the perspective of economics and environmental protection.
In the last decade (2007–2016), many efforts have been devoted to recycling of spent catalysts, which has been well documented in the literature. According to the database of Thomson Scientific’s Web of Science, the number of papers published in the field of recycling spent catalysts increased sharply from 18/year to 60/year. In other words, there exists increasing and intensive attention on the recovery of PGMs from the waste.
Generally speaking, there is no universally acceptable procedure for classification of the available methods of PGM recovery from spent catalysts. Yet, the dominant tendency is toward classifying them as hydrometallurgical and pyrometallurgical methods.8 The spent materials are initially segregated, crushed, ground, and then processed by pyro/hydrometallurgical processes to recover the PGMs.
The hydrometallurgical process leaches precious metals from solid wastes using a strong alkaline solution (e.g., NaOH9 and cyanides such as NaCN10) or acidic solution (e.g., aqua regia,11 , 12 HCl,13 H2SO4,14 and NaClO3 15). The precious metals are subsequently concentrated from the leach solution by methods such as precipitation, cementation, and solvent extraction. Although the hydrometallurgical process, featured by low-energy consumption, seems effective in separating PGMs from spent catalysts because of the selectivity of chemical agents on the metals, it has several evident disadvantages. For instance, when PGMs in spent catalysts are leached in alkaline solution that contains toxic and readily decomposed agents such as NaCN, some gaseous compounds (e.g., NO) can be formed, posing a threat to the environment.3 Meanwhile, the decomposition of PGMs-bearing cyanide complexes in this technique often requires severe conditions, including high temperature and pressure, e.g., 250–270°C at 2758–6206 kPa.16 For leaching of PGMs from spent catalyst in acidic solution, some expensive agents such as HNO3, H2O2 and other high-concentration mineral acids are often used for improvement of extraction of the metals, especially Rh.8 To avoid high reagent consumption, severe pollution and instability of PGM extraction resulting from the complex composition of the catalyst substrate, repeated leaching, or a preparation step, including thermal reduction (for reduction of oxidized PGMs above 250°C),8 calcining (for elimination of coke and water above 400°C),5 and roasting (for fusion of catalyst carrier by adding alkalis, alkali metal, or alkali earth metal carbonates or hydrosulfates at 450–800°C for 1–5 h),1 , 3 are necessary. Overall, the repeating adjustment of the initial acidities of the feed solutions and multisteps of pretreatment unit operations cause large quantities of liquid and solid wastes, making the process time consuming, costly, and sometimes toxic.
In pyrometallurgical processes, the spent catalysts can be melted with the addition of flux components and metal collectors (or related additives) at high temperatures to form a PGM-containing alloy from which the precious metals are recovered through appropriate refining techniques. The catalysts may also be vaporized for recovery of PGMs or reduced in situ as a reforming means for their recycling. Because of the simple operation, short flow sheet, and large-scale treatment potential, the pyrometallurgical processes are extensively used in industry for extracting precious metals from spent catalysts.1 , 17
Currently there is no detailed overview focusing on the pyrometallurgical recycling of spent catalysts. The aim of this article is to offer an in-depth review of recovering PGMs from the catalysts via the established pyrometallurgical processes. Based on the analysis of the physical and chemical properties of the spent catalysts, it compares and evaluates various methods, including the smelting process, chlorination process, and sintering process. It is anticipated to provide a useful guide for treatment of spent catalysts for claiming sufficient economic and environmental benefits.
Physical and Chemical Properties of PGM-Containing Spent Catalysts
The PGMs in catalysts work as active sites for catalytic reactions, such as oxidation of carbon monoxide and hydrocarbon, and various nitrous oxides. The PGM particles of several nanometer thicknesses usually adhere to a monolithic ceramic carrier (substrate) with a honeycomb structure (as a PGM support),8 on which porous oxides such as alumina (Al2O3), ceria (CeO2), cordierite (2MgO·2Al2O3·5SiO2), or silica (SiO2) are coated, as summarized in Table I.1 , 5 , 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 These porous oxides have a role in dispersing the PGMs and in maintaining their large surface area.
Catalysts are deactivated because of fouling, poisoning, thermal degradation/sintering, or all three. The most common form of catalyst fouling is a result of the deposition of coke, whereas poisoning of catalysts occurs because of strong chemical bonds between feed components, such as sulfur, or catalytic reaction products with active sites on the catalyst surface. The thermal degradation after a certain period of usage at high temperature is associated with the inward migration of PGMs located on the surface and their subsequent migration into the supported oxides, which enhance the refractoriness of the spent catalysts. Also, high-temperature sintering causes an increase of metallic particle diameters (PGM metallic particles with diameters less than 2.5 nm for a freshly prepared auto-catalyst),8 irreversible in thermodynamics, and thereby a decrease of specific surface areas of the particles.
From the analysis, it is evident that the recyclability of the spent catalysts primarily depends on three factors: (I) the content of PGMs, (II) the properties of PGMs carriers in spent catalysts (especially the presence of refractory materials, such as cordierite, α-alumina, and large quantities of washcoat additives such as CeO2, ZrO2, and BaO),1 and (III) the amount of impurities accumulated on the catalysts (e.g., Pb, Fe, Cu, Cr, S, Zn, P, etc.), depending on the applications of catalysts.54 As shown in Table I, the amount of precious metals contained in spent auto-catalysts varies significantly from about several grams per ton to 100 wt.%. Platinum is the most extensively applied active metal, followed by palladium and rhodium. The spent catalysts have a simple composition, but its refractory oxide carriers, constituted by alumina, cordierite, activated carbon, or all three, have to be destroyed or removed for efficient recovery of PGMs.
Pyrometallurgical Recovery Methods
Pyrometallurgical processes are a primary method to recover PGMs from spent catalyst.3 In general, they can be divided into three groups: smelting, chlorination, and sintering processes.
Smelting Process
The smelting process is the mostly commonly employed approach for concentrating PGMs in many companies with advanced metallurgical and refining technologies, such as Umicore, Johnson Matthey, and Badische Anilin-und-Soda-Fabrik.1 Spent catalyst is usually mixed with flux, collector, and reducing agent and then smelted in a high-temperature plasma furnace, electric arc furnace, or inductive furnace at a high temperature (usually above 1000°C).55 PGMs are enriched in the metal phase or converted into easily treated compounds, which can be refined later to recover PGMs. The process may involve initial pretreatment steps (e.g., dismantling/incineration of nonmetallic components, calcination, or reduction) before high-temperature smelting in which a suitable collector, in many cases, a high specific gravity base metal (lead, copper, nickel, etc.), collects the PGMs, forming a PGM-enriched alloy. Meanwhile, the catalyst carriers, such as alumina, mullite, or cordierite, are melted in the presence of suitable fluxes to obtain a low-viscosity liquid slag. After its separation from slag, the PGM-containing alloy is sent for PGM purification. In the choice of collector, the mutual solubility, melting points, and chemical properties of collector and PGMs are important. Generally, lead, copper, iron, matte, and other materials containing these metals (such as printed circuit boards, abbreviated as PCBs) are good collectors for smelting of spent catalysts. The other metals, such as calcium, magnesium, and zinc, may also be used but they usually give unsatisfactory overall recoveries of PGMs (<80%).55,56,57
Lead Collection
Lead collection is the oldest method for handling secondary resources including spent catalysts.58 The spent catalyst is first grounded and mixed with litharge (e.g., PbO), sodium carbonate, anhydrous borax, and potassium bitartrate or other additives for high-temperature fusion and smelting at about 1100°C for 1–2 h.59 During the treatment, the PbO is reduced to molten lead in which the PGMs are extracted, whereas the SiO2 in the spent catalyst is oxidized to a borosilicate slag that floats on top of the molten lead layer. After decanting the hot melt, a small lead button containing the PGMs is obtained and used for cupellation in a porous magnesia cupel at around 1000°C. After volatilization of part of the lead and oxidation-collection of the other part in the cupel, metal beads containing the PGMs will remain for further purification. The lead collection has the advantages of simple operation, low smelting temperature, simple subsequent refining, and small investment.60 Its disadvantages, however, are low recovery of Rh (70–80% for Rh versus 90–95% for Pt and Pd) and PbO-induced health and environmental risks.1 , 59
Copper Collection
Copper collection is appropriate for treating various spent catalysts, especially the cordierite-type catalyst, in an electric arc furnace or inductive furnace by smelting them with flux (SiO2, CaO, etc.), copper collector (CuCO3, CuO, Cu, etc.) and reducing agent (often powdery coke) at a low temperature (1450–1600°C) under a weak reducing atmosphere or natural atmosphere.61 For improving the industrial smelting process, such as the well-known Rose process that produces PGMs after oxidizing blowing of copper alloy generated in smelting,55 , 62 the properties of copper source materials are crucial. It was revealed that the sources should have a grain diameter of 0.1–10 mm for optimal smelting.63 Another promising copper collection process consists of crushing, milling, homogenization, pelletizing, drying, melting, electrolysis, and refining, as shown in Fig. 2.64 In this process, after enrichment of PGMs into the molten copper, the ceramic carriers of PGMs in spent catalysts create a slag with less than 0.2 wt.% cu that is then poured out of the furnace for recycling of valuable components (e.g., Al2O3). The PGM-containing molten copper is casted in the form of an anode with about 1 wt.% PGMs in steel molds for electrolysis. After electrorefining, a slime containing approximately 20–25 wt.% PGMs is obtained on the anode, whereas high-purity copper (99.99%) is captured on the cathode and can be reused for smelting. The overall recovery of PGMs by copper collection may approach 99%.64 , 65
Iron Collection
Iron is a low-cost collector with strong chemical affinity for PGMs to form a solid solution.66 In iron collection, crushed spent catalysts mixed with iron ore (or metallic iron powder), reducing agent (e.g., coke), and flux (e.g., CaO) are melted in a plasma arc furnace at a temperature of 1500–2000°C or in an electric arc furnace at a temperature of 1450°C, as shown in Fig. 3.1 , 55 The former technology, involving use of a plasma torch (e.g., the Johnson Matthey process), is characterized by high-energy density, high temperature, and good flexibility in the plasma gases despite the short lifetime of the plasma gun accessory and refractory ceramics. It is the most extensively used process for recovery of PGMs from the spent auto-exhaust catalyst at 1500–1650°C, enriching PGMs in the molten metallic iron phase (5–10 wt.% PGMs).58 Because of the large difference between densities of the obtained Fe-PGMs alloy and slag (6.0–7.0 g/cm3 versus 3.0–3.5 g/cm3),66 the alloy and slag are separated easily. Furthermore, as a result of the active chemical property of iron, recovering the PGMs from the alloy through dissolution will be facilitated for a high overall PGMs recovery (approximately 95% from a charge with less than 0.1 wt.% PGMs),58 despite possible adverse effect of impurities of the alloy (e.g., Si and C). The latter technology uses an electric arc furnace for molten iron collection at a lower temperature than that in the plasma smelting technology. It was reported that more than 99% of Pt, 99% of Pd, and 97% of Rh could be recovered after smelting by this process.1 From the perspective of efficiency of PGMs recovery, the electric arc furnace smelting seems more promising for production of Fe-PGMs alloy, provided the smelting temperature is further reduced by optimizing the composition of slag with a low melting point.
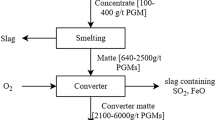
Matte Collection
Matte collection is a newer method for treatment of spent catalyst although it has been applied to exploitation and processing of metal-bearing sulfide ores for a long time.67,68,69 The matte formed during smelting of spent catalyst serves as a collector because of the close affinity of matte for PGMs. PGMs are enriched in the matte phase at a lower temperature (1000–1450°C) by adding metal (e.g., Ni) and sulfur or metal sulfide (e.g., NiS and Ni3S2) with flux (e.g., CaO, Na2CO3, Na2B4O7 or their mixture) for smelting.70 , 71 A recent study reported smelting of spent catalysts using nickel and sulfur as collectors in the presence of sodium carbonate and borax at 1050°C for 30 min.71 It was shown that the recoveries of platinum, palladium, and rhodium reached 90%, 93%, and 88%, respectively. For this method, the smelting atmosphere is important as oxidation of nickel will cause difficult refining (e.g., dissolution in HCl) after separation of PGM-containing matte from slag. Meanwhile, the silicon acidity of slag, defined as the mass ratio of oxygen in SiO2 to that in Al2O3 and MgO, should be controlled appropriately for optimal smelting. This is because a low acidity of slag will enhance desulfurization reactions, forming Na2S that has the potential to enrich partial PGMs in slag, causing metal losses. Conversely, with too high silicon acidity, the viscosity of slag will increase, thereby inhibiting separation of matte and slag.
PCB Collection
In PCB collection, the metal components such as copper, tin, and iron contained in obsolete PCBs are used as the “metal collector” for PGMs, whereas spent catalysts are employed as a slag formative to extract precious metals simultaneously such as gold, platinum, and palladium from obsolete PCBs and spent catalysts by smelting without any additional collector.56 , 72 The obsolete PCBs and catalysts are grounded, incinerated, and melted after adding flux and a reducing agent that reduces the oxides in PCBs, such as CuO, SnO2, and FeO, to metal collectors. Figure 4 shows the flow chart of the process that consists of two major steps: incineration and melting. Initially, the PCBs and spent catalysts are incinerated at 600°C for a given period of time (30–90 min) to remove their organic components in air.73 The mixture then undergoes a reductive melting at a lower temperature (1250–1700°C) by designing a suitable composition of the slag through optimizing the mixing ratio of incinerated PCBs and catalysts (e.g., 7:3) based on phase diagram analysis. With the slag composition of 16.8 wt.% Al2O3, 28.2 wt.% CaO, 45.0 wt.% SiO2, and 10.0 wt.% MgO, the recovery percentages were 99.0% for Au, 98.5% for Pt, 98.0% for Pd, more than 88.0% for Cu and Sn, and less than 50% for Fe and Pb.73 In other words, Au, Pt, and Pd can be predominantly collected in the Cu-Sn alloy phase, whereas Fe and Pb are distributed in the alloy and slag phases. The process is simpler than conventional processes, and it has an additional benefit of recovering other valuable metals, e.g., Cu, Sn, and Fe, from obsolete PCBs. Nevertheless, it is currently restrained by the complicated composition of raw materials in recycling.
Volatilization Processes
In gas-phase volatilization, precious metals from spent catalysts are volatilized by selective chlorination (chlorinated with chlorine or chlorides) and condensed in a cooler zone. Most volatilization reactions are carried out at temperatures of 250–700°C.18 The PGMs are converted into their corresponding chlorides and then separated by taking advantage of the difference in vapor pressures between the metal chlorides or by repulp washing or adsorption on an activated carbon bed. A previous study explored the possibility of volatilization of spent catalyst at 600–1200°C in a chlorine gas fluid field and adsorption of PGM chlorides by water or ammonium chloride liquor. It showed that the recoveries of Pt, Pd, and Rh were 80–90%, 80–90%, and 85–90%, respectively.74 After suitable pretreatment, such as crushing, burning, and reduction, the spent catalyst may also be chlorinated by NaCl at 600–700°C. The product was dealt with by the steam and hot water from which the PGM chloride salt solution was obtained with overall recoveries of Pt, Pd, and Rh between 85% and 90%.75 Recent studies showed that use of carbon monoxide as a reducing agent in the volatilization will make the reactions thermodynamically favorable. The combined usage of chlorine and carbon monoxide extracted Pt (as Pt(CO)Cl2 vapor) and Rh from the catalysts with recoveries of 95.9% and 92.9%, respectively.18
The volatilization process makes use of different vapor pressures of metal chlorides to separate target PGMs selectively from the catalyst carrier. Although it is highly tunable and capable of producing high-purity metals, it is often accompanied by strong corrosion of furnace and associated facilities, which enhance the cost of production. The use of hazardous gases CO and Cl2 may also lead to health and environmental risks that further restrict its industrial application.
Sintering Process
The sintering process in the presence of plasma recovers PGMs from the spent catalyst by in situ reduction of its oxidized PGMs components (e.g., PtO2). When the PtO2 catalyst was sintered at >1200°C for 2–3 h in a constant flow of N2 as the central plasma gas, the organic tar with the intermolecular and intramolecular water on the surface of the spent catalyst was decomposed and converted to syngas, which reduced oxide of platinum to its metallic form.76 It was indicated that the plasma-sintered catalyst showed the similar catalytic efficacy with respect to the fresh one. Basically, the mechanism of the plasma sintering process involves two-stage reactions. In the first stage, electrification of N2 and decomposition of H2O take place to form active species such as N2 + and N intermediates, and OH radicals and H radicals, respectively. The active species react with each other, creating new active intermediates, such as NH and O radicals. In the second stage, the interaction of the active species produces H2 and H2O in parallel- or chain-type reactions. The net reaction products are believed to be syngas containing H radical and H2 by which the platinum oxide is reduced. Compared with smelting and volatilization processes, the sintering method is a less polluting process. The most important parameters are the sintering temperature and atmosphere over spent catalyst. Increasing reaction temperature in the presence of water is expected to accelerate the entire process.
Discussion
Compared with the smelting process, which plays a dominant role in pyrometallurgical recovery of PGMs from spent catalysts, the application of volatilization and sintering processes is still limited. This is because the volatilization process has evident potential health and environmental risks caused by the gases used, whereas the sintering process serves merely a means for reforming of the spent catalysts whose effectiveness strongly relies on many factors, such as sintering temperature, catalyst composition and structure, and carrier morphology. The smelting process has a good overall recovery of the metals despite higher energy consumption, possible adverse environmental impacts caused by the collector used (e.g., lead), and potential losses of PGMs because of the operation and equipment deficiency. Specifically, the copper collection technology has a broad application prospect because of the advantages of low pollution, high efficiency, moderate melting temperature, reuse of collector (copper), and easy industrialization. The matte collection technology is also promising because of its low smelting temperature and is particularly attractive for nickel smelting factories using sulfide ores as raw materials.
To improve the smelting process further, at first, it is crucial to select a suitable collector of PGMs for a given type of spent catalyst because the affinity of the collector for PGM particles is a key factor in improving the performance of smelting for energy conservation and maximal PGMs recovery. Second, the type and dosage of flux in the smelting should be optimized as flux not only controls the slag viscosity, and thus the efficiency of alloy–slag separation, but also enriches contaminants into the slag, improving economic and environmental benefits of the smelting process simultaneously. Third, appropriate pretreatment, such as thermal reduction or calcining for removal of contaminants, may contribute to the process as it reduces energy consumption and environmental pollution. Moreover, use of external fields such as microwaves seems helpful in enhancing smelting because of its unique advantages, including volumetric heating, selective heating, and environmental friendliness.77 , 78 In fact, microwave energy has been used in dissolution of PGMs from spent catalyst79 or as a pretreatment means (e.g., roasting that destroys the coatings) for separation and purification of the metals.80
Conclusion
As a result of the significant conflicts of the limited natural PGM resources and the increasing demand for PGMs, it is necessary to recover the precious metals from spent catalysts for both economic and environmental benefits. This article has reviewed the main pyrometallurgical processes for recovery of PGMs from spent catalysts, including smelting, vaporization, and sintering processes based on the exploration of their physicochemical properties. The smelting technology performed at higher temperatures (above 1000°C) has been extensively used for recovery of PGMs in an alloy form from spent catalyst in industry with the addition of various collectors, such as lead, copper, iron, matte, or PCB. The vaporization process provides an approach for recovering PGMs at a much lower temperature (250–700°C) although high corrosion and potential environmental and health risks are brought by the gases, such as Cl2 and CO, involved in the process. Unlike the smelting and volatilization processes that aim at separating PGMs from the spent catalysts, the sintering process serves as a reforming means for recycling of the spent catalysts containing the PGMs by plasma-induced reduction of the oxidized PGMs components at high temperatures (above 1200°C). Among these processes, the smelting process seems more promising with a higher potential in industrial production. Nevertheless, it is currently restrained by several challenges from the perspectives of energy conservation, environmental protection, and PGM recovery. Future work should be devoted to recovery of PGMs from diverse spent catalysts via smelting in a more economical and environmentally friendly way by selecting a suitable collector and flux, together with proper pretreatment and process intensification in the presence of an external field.
References
H. Dong, J. Zhao, J. Chen, Y. Wu, and B. Li, Int. J. Miner. Process. 145, 108 (2015).
P. Liu, G. Liu, D. Chen, S. Cheng, and N. Tang, Trans. Nonferrous Met. Soc. China 19, 1509 (2009).
M.K. Jha, J.C. Lee, M.S. Kim, J. Jeong, B.S. Kim, and V. Kumar, Hydrometallurgy 133, 23 (2013).
J. Matthey, PGM Market Report 1 (2016).
R. Kasuya, T. Miki, H. Morikawa, and Y. Tai, Miner. Eng. 87, 25 (2016).
J. Kašpar, O. Fornasiero, and N. Hickey, Catal. Today 77, 419 (2003).
S. Wang, A. Chen, Z. Zhang, and J. Peng, Environ. Prog. Sustain. Energy 33, 913 (2014).
S. Chen, S. Shen, Y. Cheng, H. Wang, B. Lv, and F. Wang, Hydrometallurgy 144–145, 69 (2014).
F. Wang, T. Zhang, Z. Zhang, and L. Zhang, Sep. Purif. Technol. 152, 108 (2015).
Z. Naghavi, S.M. Ghoreishi, A. Rahimi, and H. Hadadzadeh, Int. J. Chem. Reactor Eng. 14, 143 (2016).
M. Baghalha, H.K. Gh, and H.R. Mortaheb, Hydrometallurgy 95, 247 (2009).
R.S. Marinho, J.C. Afonso, and J.W.S.D. da Cunha, J. Hazard. Mater. 179, 488 (2010).
J.Y. Lee, B. Raju, B.N. Kumar, J.R. Kumar, H.K. Park, and B.R. Reddy, Sep. Purif. Technol. 73, 213 (2010).
A.S. Kirichenko, A.N. Seregin, and A.I. Volkov, Metallurgist 58, 250 (2014).
S. Harjanto, Y. Cao, A. Shibayama, I. Naitoh, T. Nanami, K. Kasahara, Y. Okumura, K. Liu, and T. Fujita, Metall. Mater. Trans. 47, 129 (2006).
K. Shams, M.R. Beiggy, and A.G. Shirazi, Appl. Catal. A 258, 227 (2004).
M. Saternus and A. Fornalczyk, Metabk 52, 267 (2013).
C.H. Kim, S.I. Woo, and S.H. Jeon, Ind. Eng. Chem. Res. 39, 1185 (2000).
J. Oi-Uchisawa, S. Wang, T. Nanba, A. Ohi, and A. Obuchi, Appl. Catal. B 44, 207 (2003).
G. Horányi and E.M. Rizmayer, J. Electroanal. Chem. Interf. Electrochem. 140, 347 (1982).
J. Pérez-Ramíreza, F. Kapteijn, K. Schöffel, and J.A. Moulijn, Appl. Catal. B 44, 117 (2003).
Z. Liu, X.Y. Ling, X. Su, and J.Y. Lee, J. Phys. Chem. C 108, 8234 (2004).
A. Bonakdarpour, T.R. Dahn, R.T. Atanasoski, M.K. Debe, and J.R. Dahna, Electrochem. Solid State Lett. 11, 208 (2008).
V.R. Choudhary, Y.V. Ingole, C. Samanta, and P. Jana, Ind. Eng. Chem. Res. 46, 8566 (2007).
R. Horn, G. Mestl, M. Thiede, F.C. Jentoft, P.M. Schmidt, M. Bewersdorf, R. Weberd, and R. Schlögla, Phys. Chem. Chem. Phys. 18, 4514 (2004).
P.L. Hagans, X. Guo, I. Chorkendorff, A. Winkler, H. Siddiqui, and J.T. Yates Jr., Surf. Sci. 203, 1 (1988).
F. Menegazzo, T. Fantinel, M. Signoretto, and F. Pinna, Catal. Commun. 8, 876 (2007).
N. Pernicone, M. Cerboni, G. Prelazzi, F. Pinna, and G. Fagherazzi, Catal. Today 44, 129 (1998).
P.J. Debouttière, Y. Coppel, A. Denicourt-Nowicki, A. Roucoux, B. Chaudret, and K. Philippot, Eur. J. Inorg. Chem. 2012, 1229 (2012).
Y.F. Han, Z. Zhong, K. Ramesh, F. Chen, L. Chen, T. White, Q. Tay, S.N. Yaakub, and Z. Wang, J. Phys. Chem. C 111, 8410 (2007).
S. Cao, G. Chen, X. Hu, and P.L. Yue, Catal. Today 88, 37 (2003).
Y.C. Kim, N.C. Park, J.S. Shin, S.R. Lee, Y.J. Lee, and D.J. Moon, Catal. Today 87, 153 (2003).
B.K. Kim, S.J. Lee, J.Y. Kim, K.Y. Ji, Y.J. Yoon, M.Y. Kim, S.H. Park, and J.S. Yoo, J. Electron. Mater. 37, 527 (2008).
Y. Zheng, F. Xiao, X. Wu, and Q. Zhang, Precious Met. 26, 30 (2005).
S. Han, X. He, X. Wu, H. Wang, X. Yan, J. Wang, F. Zhao, and H. Gu, Precious Metall. 32, 68 (2011).
B.P. Luther, S.D. Wolter, and S.E. Mohney, Sens. Actuator B 56, 164 (1999).
S.J. Ippolito, S. Kandasamy, and K. Kalantar, Sens. Actuator B 108, 154 (2005).
D. Schanke, S. Vada, E.A. Blekkan, A.M. Hilmen, A. Hoff, and A. Holmen, J. Catal. 156, 85 (1995).
X. He, J. Guo, H. Wang, Y. Li, X. Wu, Y. Zhao, K. Li, S. Han, W. Tan, and W. Liu, Precious Met. 34, 82 (2012).
B.A. Steinhoff, S.R. Fix, and S.S. Stahl, J. Am. Chem. Soc. 124, 766 (2002).
X. Zhang and Y. Ning, Precious Met. 34, 88 (2014).
J. Adámek, P. Bartuška, P. Lejček, and B. Baretzky, Scr. Mater. 40, 485 (1999).
T. Mallat and A. Baiker, Cheminform 35, 3037 (2004).
K. Mori, T. Hara, T. Mizugaki, K. Ebitani, and K. Kaneda, J. Am. Chem. Soc. 126, 10657 (2004).
N. Luo, X. Fu, F. Cao, T. Xiao, and P.P. Edwards, Fuel 87, 3483 (2008).
A.J. Byrd, K.K. Pant, and R.B. Gupta, Fuel 87, 2956 (2008).
M.F. Mark and W.F. Maier, J. Catal. 164, 122 (1996).
F. Garin, P. Legare, and G. Maire, J. Catal. 77, 323 (1982).
M. Benaissa, G.C. Le Roux, X. Joulia, R.V. Chaudhari, and H. Delmas, Ind. Eng. Chem. Res. 35, 2091 (1996).
J.K. Lee and H.K. Rhee, J. Catal. 177, 208 (1998).
M. Mitsios, D. Guillaume, P. Galtier, and D. Schweich, Ind. Eng. Chem. Res. 48, 3284 (2009).
E. Blomsma, J.A. Martens, and P.A. Jacobs, J. Catal. 165, 241 (1997).
S. Kato, K. Nakagawa, N. Ikenaga, and T. Suzuki, Catal. Lett. 73, 175 (2001).
T.N. Angelidis, Top. Catal. 16–17, 419 (2001).
A. Fornalczyk and M. Saternus, Acta Metall. Sin. 26, 247 (2013).
J. Willner, A. Fornalczyk, J. Cebulski, and K. Janiszewski, Arch. Metall. Mater. 59, 801 (2014).
Y. Kayanuma, T.H. Okabe, and M. Maeda, Metall. Mater. Trans. B 35, 817 (2004).
M. Benson, C.R. Bennett, J.E. Harry, M.K. Patel, and M. Cross, Resour. Conserv. Recycl. 31, 1 (2000).
S. Compernolle, D. Wambeke, I.D. Raedt, K. Kimpe, and F. Vanhaecke, J. Anal. At. Spectrom. 26, 1679 (2011).
R. Juvonen, T. Lakomaa, and L. Soikkeli, Talanta 58, 595 (2002).
G. Kolliopoulos, E. Balomenos, I. Giannopoulou, I. Yakoumis, and D. Panias, Open Acccess Library J. 1, e736 (2014).
A. Fornalczyk and M. Saternus, Metallurgija 48, 133 (2009).
K. Yamada, M. Ogino, N. Ezawa, and H. Inoue, US Patent 7815706 B2 (2010).
S.Z. Ivanović, V.K. Trujuć, M.D. Gorgievski, L.D. Mišić, and D.S. Božić, 15th International Research/Expert Conference, 701 (2011).
A. Fornalczyk and M. Saternus, Metabk 52, 219 (2013).
X. He, Y. Li, X. Wu, Y. Zhao, H. Wang, and W. Liu, Precious Met. 37, 1 (2016).
R.T. Jones and I.J. Geldenhuys, Miner. Eng. 24, 495 (2011).
J.J. Eksteen, Miner. Eng. 24, 676 (2011).
R.F. Van Schalkwyk, J.J. Eksteen, and G. Akdogan, Hydrometallurgy 136, 36 (2013).
F. Zhang, Y. Zhang, C. Pan, X. Guo, L. Wang, Z. Xie, and Y. Chen, China Patent 201510797358.6.
G. You, W. Fang, Q. Li, Y. Ma, X. Yang, and H. Yang, Metall. Anal. 36, 7 (2016).
B.S. Kim, J.C. Lee, J. Jeong, D.H. Yang, D. Shin, and K.I. Lee, Metall. Mater. Trans. 54, 1045 (2013).
B.S. Kim, J.C. Lee, S.P. Seo, Y.K. Park, and H.Y. Sohn, JOM 56, 55 (2004).
M.J. Murray, US Patent 3021209 (1962).
S. Xu and L. Xu, China Nat. Res. Recycl. 10, 7 (1998).
K.C. Chiang, K.L. Chen, C.Y. Chen, J.J. Huang, Y.H. Shen, M.Y. Yeh, and F.F. Wong, J. Taiwan Inst. Chem. E. 42, 158 (2011).
Z. Peng and J.Y. Hwang, Int. Mater. Rev. 60, 30 (2015).
X. Lin, Z. Peng, J. Yan, Z. Li, J.Y. Hwang, Y. Zhang, G. Li, and T. Jiang, J. Clean. Prod. 149, 1079 (2017).
T. Suoranta, O. Zugazua, M. Niemelä, and P. Perämäki, Hydrometallurgy 154, 56 (2015).
A. Chen, S. Wang, L. Zhang, and J. Peng, Int. J. Miner. Process. 143, 18 (2015).
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China under Grant 51504297, the Science and Technology Major Project of Gansu Province, China, under Grant 1602FKDC007, the Innovation-Driven Program of Central South University under Grant 2016CXS021, the Shenghua Lieying Program of Central South University under Grant 502035001, the Natural Science Foundation of Hunan Province, China, under Grant 2017JJ3383, the Fundamental Research Funds for the Central Universities of Central South University under Grant 502211738, and the Open-End Fund for the Valuable and Precision Instruments of Central South University under Grant CSUZC201706.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, Z., Li, Z., Lin, X. et al. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. JOM 69, 1553–1562 (2017). https://doi.org/10.1007/s11837-017-2450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2450-3