Abstract
In the past several decades, many technologies to recover platinum group metals (PGMs) and rhenium (Re) from electronic waste and spent catalysts have been developed and published. The reasons for the rising interest in this area are: (1) The abundance of these elements in the earth’s crust is < 10−3 ppm (~ 6.6 × 104 tons worldwide); (2) global demand for PGMs is > 590 tons; (3) electronics and catalyst industries consume > 90 pct of precious metals (about 65 pct of Pd, 45 pct of Pt and 84 pct of Rh are used in catalytic converters); (4) properties of PGMs and Re (resistance to corrosion and oxidation, high melting temperatures, electrical conductivity and catalytic activity) are of great commercial interest. Even though several comprehensive reviews on the recovery of precious metals from spent catalysts have recently been published, several developments were not focused by the scientific community. The reviews divide the technologies into hydro- and pyrometallurgical ones. However, the variety of different approaches requires a more detailed classification. This article is an overview of the recently reported works and a comparison of different technologies in terms of extraction efficiency, environmental friendliness, and capital and operational expenditures. A new electrochemical method, which is now under development, is also presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Platinum group metals incorporate six noble metals, namely platinum (Pt), palladium (Pd), iridium (Ir), osmium (Os), rhodium (Rh) and ruthenium (Ru). All the metals in this group have similar physical and chemical properties. PGMs are chemically inert, highly resistant against corrosion, electrically and thermally stable, and have high mechanical strength.[1,2,3] Their properties are highly desirable, and > 90 pct of overall PGMs are used by the electricity and catalyst industries. PGMs are limited natural resources with only 66,000 tons available worldwide. Only 2 to 10 g/ton of PGM content is present in natural ores. The recovery of PGM as a co-product or by-product depends on their percentage in the ore. Recent advances in the technologies for the extraction and recovery of PGMs have enhanced their application in the chemical industry, oil refining, medical practices, vehicle and equipment construction, jewelry making, etc.[4,5]
Production of 73 pct of primary PGMs in the world is associated with the Bushveld Complex of South Africa, while the rest of the PGM reserves are mainly concentrated in Russia, Zimbabwe, the USA and Canada.[6] The PGM ores are mainly in the form of sulfide and arsenide materials such as PtAs2, Pt(AsS)2, PtS, (Pt, Pd)S, (Pt, Pd, Ni)S, Pd3Sb and RuS2 along with elemental ruthenium.[7] Fernandez [8] presented comprehensive data related to the amounts of PGMs in different forms. PGMs are recovered from high-grade concentration ores (200 to 2000 g/ton of PGM and 0.3 to 2.5 pct Cr2O3) by the matte-smelting-refining technique. Initially, the PGM ores are crushed and ball-milled into fine particles. This ore in the form of particles is treated in gravity separators and transferred to flotation cells to produce a sulfide-rich PGM concentrate, where reagents such as dithiophosphate and xanthate collectors are used for the flotation process at 7.5 to 9 pH.[9] Smelting is performed on the sulfide-rich PGM concentrates to remove feldspar, plagioclase, pyroxene and biotite gangue materials. The matte is treated in converters where the slag containing SO2 and FeO is removed. The converter matte is transferred to a base metal refinery where Cu, Ni and other metals are removed. The PGM concentrate with > 65 pct PGM is refined in a precious metal refinery, and high-quality PGMs are recovered (see Figure 1).
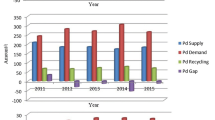
The demand for PGMs is increasing because of their various applications in industries. Figure 2 shows the demand and recycling of PGM in 2019. The demand for Pt, Pd and Rh is around 660.305 tons, but recycling only provides 189.02 tons. This shows that future acquirement of these metals will depend on recycling of secondary sources because of their lack of availability as natural resources. Concentrations of PGMs are higher in spent catalysts than in ore deposits. For example, an automotive catalytic converter contains about 2000 g/t of PGMs in a ceramic block while the average PGM concentration is < 10 g/ton in most of PGM ores.[10]
Total supply and demand for PGMs in 2019 (in tons)[104]. 1: Total supply, 2: gross demand, 3: total recycling, 4: total net demand, 5: movement in stocks. *No available data
Spent catalysts are a source of PGM recovery. The catalysts used in the refining and petrochemical industries have Al2O3-based carriers with 0.05 to 1 pct noble metals (PGM and rhenium) as the active components. The catalyst loses its catalytic properties after a certain time (3 to 5 years). Technology to regenerate and reactivate the metal-fouled catalysts is not available at present, and they are discarded as solid waste.[11,12] Spent refinery catalysts comprise only 4 pct of total refinery waste but nonetheless are considered the most hazardous waste generated in the petroleum industry.[13,14]
The catalyst industry heavily depends on PGMs, which act as active components. The oil-refining, automobile emission purification and chemical engineering industries are the main consumers of PGMs in the form of catalysts.[15] An immense amount of research has been focused on replacing PGMs with fewer waste materials but no significant results have been attained. About 45 pct of platinum (99.82 tons), 65 pct of palladium (186.5 tons) and 84 pct of rhodium (24.07 tons) were used in catalytic converters in 2018. The recycling of PGMs from spent catalysts is difficult as they are used for different purposes and in varying proportions from 200 ppm to 100 pct in the entire material of the catalyst. Table I provides information on the catalysts used in the automobile and chemical industries.
Effective recovery of PGMs and extraction from spent catalysts are environmentally and economically desirable. Three factors influence the recyclability of spent catalysts: (1) the composition of the spent catalyst, (2) the intrinsic metal value and (3) the associated lifecycle structure and the application segment, including the turnaround speed (catalyst life), recycling chain and business model.
In this article, the methods implemented to recover the PGMs from the spent catalyst are discussed. The advantages and disadvantages associated with the various recycling methods will be presented. This topic is particularly important because in the future industries will rely on recycling processes to obtain PGMs. Recycling should be efficient and environmentally friendly. The recovery of PGMs from spent catalysts is important because of:
-
the simple catalyst composition with carriers being Al2O3, activated carbon, cordierite and so on;
-
the high PGM proportion of about several kilograms per ton.
Therefore, the recovery of PGMs from the spent catalysts should be an economically advantageous, small-scale, simple and environmentally friendly process. Techniques such as non-cyanide leaching, smelting in the furnace, mild leaching and physical separation pre-treatment cause less pollution and have been developed in the last 2 decades. Nevertheless, even with many advantages, they have negative effects on the environment and recovery process.[16,17]
Techniques Used for the PGM Recovery from Spent Catalysts
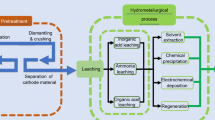
The supply of spent catalysts to the recycling process is a crucial step as it is a decisive factor in the recovery of the PGMs. The spent catalyst should undergo (1) dismantling, (2) size reduction and (3) physical separation before the start of the main process.[18] The selection of the recycling technique mainly depends on three factors: (1) type of catalyst supporter, (2) PGM (loading) content and (3) presence of other base metals. Finally, recycling of spent catalysts to extract PGMs can be divided into five basic steps: sampling/homogenization, pre-concentration, dissolution, enrichment and purification. Several recovery techniques have been introduced over the past 20 years and a few of them are already being employed at the industrial level.[19,20] Some of the techniques are presented and briefly discussed below.
Hydrometallurgy
In the hydrometallurgical process, the spent catalysts are dissolved in the aqua regia, cyanide or strong acids such as HNO3, HCl and H2SO4. The leaching efficiency is enhanced by adding oxidizing agents such as O2, H2O2, Cl2 and I2. Before the leaching process, the PGMs should be reduced to the metallic state as they turn into the inert state because of sulfuration or oxidation. In this process, Pd is leached easily while Rh cannot be leached for > 90 pct. Cyanide leaching is a more efficient recovery process, but as this method is performed at high temperatures, cyanide is highly toxic.
Hydrochloric acid + oxidant system leaching
In this process, PGMs are leached in HCl medium with oxidizing agents such as Cl2, H2O2, HNO3 and NaClO3, etc.[21,22,23] The PGMs form soluble chloro-complexes when reacted with HCL and the oxidizing agent, according to the following reactions:
The above reactions (1) through (3) show the formation of the complex PGM ions when the HNO3 oxidizing agent is used, and similar ions are obtained when Cl2 is used as an oxidizing agent, as can be seen from the reactions[24]:
Decomposition of hydrochloric acid when oxidizing agents are added occurs according to reactions (7)–(10). The obtained Cl2 reacts with the PGMs, leading to the formation of soluble PGM chloro-complexes and reduces the processing time.
The extraction of PGMs highly depends upon its dissolution and the formation of chloride complexes, which are stable at pH < 1, whereas at high pH values, the chloride complexes are hydrolyzed, forming hydroxy complexes.[25] To avoid slow leaching, PGMs should be pre-treated as their surface is covered with many organic substances. Processes such as pre-leaching, reduction roasting and oxidation roasting are suggested.[26]
Shams et al.[27] conducted a study where 280 g of spent dehydrogenation catalyst was decoked at 400 °C to 450 °C and then crushed to the particular size required. Leaching of the particles was then done with a mixture of 3850 g of concentrated hydrochloric acid (5 mol L−1) and nitric acid (HNO3—0.2 vol pct) solution, which was used as a leaching agent in a 5000-cc container. The recovery process is followed by the ion exchange of the metallic complexes with the help of a strong base ion exchange resin (AMBERTJET 4200 Cl, industrial grade). The replacement of chloride anions by hydroxyl group ions is carried out to treat the strong basic anionic resin by using a sodium hydroxide solution. It was observed that the PGM recovery increased with the increase of HCl concentration in the solution. At 9 mol L−1 HCl concentration, the results were excellent. This process is temperature-sensitive with the best extraction obtained at 100 °C. This shows that the recovery of PGMs from spent catalysts using HCl requires less severe leaching reactions. The process is also not time-efficient (slow) as studies show that after 24 hours of leaching no further recovery of PGMs is possible. Angelidis et al.[28] developed a laboratory-scale hydrometallurgical process for the recovery of Pt and Rh from spent catalysts. The process constitutes oxidation leaching (NaClO, HCL and AlCl3), reduction leaching (H2SO4 and N2H6SO4) and final leaching with HCl followed by neutralization of the metal. About 94 pct of Pt and 89 pct of Rh were recovered. Similar results were obtained in a process where CeO2 was used as an additive to increase the recovery rate of the PGMs, although while separating the PGMs from the residue, a significant amount of Ce was leached along with the PGMs.[29]
Matjie et al.[30] reported that spent catalysts were first calcined at around 800 °C to remove the wax in them to heighten the extraction efficiency during the leaching process. The calcined spent catalysts containing 7.67 pct Pt were then dissolved in 1000 mL aqua regia (3HCl:1HNO3) at 90 °C for 240 minutes at atmospheric pressure to attain chloroplatinic acid species. Then, filtration was carried out to separate the metal from the insoluble aqua regia residue. XRD was performed on aqua regia residues to determine the Pt content, and only traces of Pt were seen, showing that almost all the Pt present in the calcined catalyst was dissolved (see Figure 3).
PFD of the aqua-regia leaching process[30]
HCl leaching was performed on spent activated carbon-supported Pd catalysts with hydrogen peroxide as a leaching agent for Pd recovery. The leaching process was carried out for 90 minutes at 90 °C. A maximum leaching rate of 99 pct was attained for Pd when the solution mixture contained 10 vol pct HCl and 5 vol pct H2O2. An increase in temperature and time improved the leaching efficiency. The optimum conditions to recover Pd were when the solution with 10 vol pct HCl and 5 vol pct H2O2 was used at 90 °C for 180 minutes.[31] Paiva et al.[32] investigated the hydrometallurgical process to extract Pd from spent alumina catalysts, where HCl solution was used for the leaching. The main task of this investigation was to achieve a promising recovery of Pd with minimal Al contamination. H2O2 was used as an oxidizing agent, which improved the Pd leaching in HCl to > 90 pct; the ideal conditions were (2.0 mol L−1) HCl and (1.0 mol L−1) H2O2 at 25 °C with 10 to 15 minutes reaction time . Al contamination can be decreased by reducing the acidity. A mixture of HCl, H2O2 and additives such as NH4Cl or MgCl2 was tested, and a high Pd leaching rate was obtained for long periods (30 to 60 minutes) with low Al contamination.
Kasuya et al.[33] introduced a novel method for the recovery of the Pt in which the PGMs are dissolved in HCl using complex oxides. Platinates were produced by calcining the mixture of Pt/Al2O3 and alkali metal salts. The formation reaction of platinates includes O2 and CO2 (Eq. 11, where M = Li and Na). At 600 °C, Pt is converted into Li2PtO3 when reacted with Li2CO3. This supports the fact that alkali metal salts enhance the oxidation of Pt.
The platinates obtained are leached in HCl (12 mol L−1) at 180 °C for 120 minutes, and the solubility of Pt was nearly 100 pct with proper dissolution conditions. The PGM recovery through the leaching process from the spent catalyst is widely adopted because of its simple technique. The disadvantage of the process is the low recovery of PGMs, especially rhodium; it is also a difficult process to extract PGMs from low-grade spent catalysts.
A microwave (MW)-assisted PGM extraction process was performed by Spooren and Atia.[34] The spent catalyst of about 1 g was initially milled and mixed with a sulfate salt (7.5 g NaHSO4·H2O, 8.37 g KHSO4 or 0.5 g H2SO4 solution) and oxidation agent (2.5 g NaClO3, 5 mL NaOCl or 5 mL H2O2). They were well mixed with a pestle and mortar, transferred to the ceramic crucible and MW roasted at 750 W for 30 minutes. The roasted mixture was leached using 100 mL of deionized water for 30 minutes at 60 °C. The slurry was cooled at room temperature, and the solid residue was separated and dried at 105 °C. The remaining leachate was analyzed to determine the presence of PGMs (see Figure 4).
PFD of the microwave-assisted extraction process[34]
It was observed that the leachability rate of PGMs improved significantly when using 1 M HCl instead of de-ionized water. The ideal parameters suggested for the recovery of PGMs according to the studies were as follows:
-
the salt: spent catalyst ratio of 5
-
NaClO3:NaHSO4·H2O molar ratio of 0.05
-
the liquid-to-salt ratio of 10 and HCl = 1 M
At the above conditions, the leachabilities of Pd, Pt and Rh of 96 ± 1 pct, 85 ± 5 pct and > 96 pct were achieved, respectively. The concentrations of Pd, Pt and Rh in 0.34 g of solid residue were measured to be 97 ± 18 mg/kg, 207 ± 66 mg/kg and < 20 mg/kg, respectively. It can be highlighted that when leaching the mixture without NaClO3, the leaching efficiency of Pd and Rh was low, and it was 0 pct for Pt. Although using KHSO4 salt resulted in a slightly higher leachability rate of Pt, using NaHSO4 was considered economical.
In References 35, 36 novel phosphonium (P8,8,8,12Cl)-based ionic liquid was used to separate the PGMs from the automobile leach liquor. Initially, 570 g of the spent catalyst was mechanically crushed followed by ball milling for 30 to 300 minutes until the particle size of 75 µm was attained. The leaching process was performed using HCl with 5 mol L−1 concentration for 2 days at 70 °C. The P8,8,8,12Cl was added to the leachate and centrifuged at 8000 rpm for 5 minutes. The Pd was extracted (reaction 12) within 5 minutes while the extraction of Rh from the raffinate to P8,8,8,12Cl was performed after the removal of Pd and Pt. The process is highly efficient for the extraction of PGMs. Scrubbing of Fe is performed using 1.2 M Na2SO3 while stripping of PGM (back extraction) from the complex ion is performed using the required solutions accordingly (see Figure 5).
Cyanide solution leaching
Industries have tried to recover PGMs by using cyanide-leaching technology for decades.[37,38,39] The following equations are the dissolution reactions occurring when using cyanide solution:
The decomposition of cyanide occurring while reacting with the oxidizing agent is as follows:
Shams et al.[40] reported a process where the spent catalyst was first crushed into small particles; its size was determined by the mesh size and sieved to determine the dependency of the particle size with the recovery of PGMs. The spent catalysts are leached with 1 wt pct of NaCN at 160 °C to 180 °C at a pressure between 618 and 1002 kPa for an hour. The weight ratio between the sodium cyanide solution and the spent catalyst is 2:1. To prevent sintering, the spent catalyst was decoked at a temperature range of 450 °C to 480 °C. Pt in the form of anion Pt[CN] 2–4 is replaced by anion Cl– in an ion exchange reaction with the result being N+(CH3)3]2 Pt[CN] 2–4 (styrene divinylbenzene copolymer) formation. It was observed that decoking of the coked catalyst is not required at high-temperature cyanide leaching. With the decrease in the particle size of the catalyst, the Pt recovery increases. At a 2:1 weight ratio of NaCN and catalyst and a temperature between 140 °C and 180 °C, optimum results were attained.
Chen et al.[39] investigated the recovery of PGMs from spent catalysts with NaCN leaching. The recovery of Pt was 96 pct, Pd 98 pct and Rh 92 pct with an oxygen pressure of 1.5 MPa, NaCN 0.1275 mol L−1, and NaCN-to-catalyst ratio of 4:1 at around 160 °C. The cyanide leaching order was Pt > Pd > Rh because of the complex ions’ metal bonding strength. The cyanide leaching process is highly toxic at high temperatures and still difficult to implement at the industrial level. The optimization of the method is still being considered.
Bioleaching
In bioleaching, the microorganisms with their metabolites are used to extract metals from the spent catalyst. This process is eco-friendly and cost-efficient compared to other leaching techniques.[41] The bioleaching method can be used in two ways: a direct (one- or two-step process) or indirect two-step process. In the one-step process (direct), fermentation and leaching take place at the same time, which means that the metal leaching and microorganism cultivation happen at the same time. In the two-step (direct) process, the cultivation takes place before the leaching even though both processes take place in the same container. In an indirect process, the leaching process takes place in the spent medium (microorganism-free medium obtained through fermentation).[42,43]
Malekian et al.[44] conducted an experiment on Pt recovery through bioleaching, (Aspergillus niger produced oxalic acid solution) in which direct (one-step, two-step) and indirect (spent medium bioleaching with and without pH control) processes were performed. The production of oxalic acid and Pt recovery were higher in the pH-controlled process. The optimal conditions were: pH 0.5, 1 wt pct pulp and bioleaching temperature of 70 °C, which resulted in 37 pct recovery of Pt. By replacing HNO3 with HCl, the pH value was adjusted resulting in the increase of Pt recovery to 41 pct. The bioleaching process is extensively used at the industrial scale to recover metals such as Ni, V and Mo but is still under study for PGM recovery. The recovery of the PGMs using bioleaching is low compared with the conventional methods, but still the former is environmentally friendly and energy-efficient while the latter is a hazardous and energy-consuming process. Improvements can be made to increase the PGM recovery percentage by optimizing the process accordingly.
Supercritical fluid oxidation
The use of supercritical fluids (SF) is an alternative method of PGM extraction, which is eco-friendly, low energy and easily recyclable, has good sensitivity and is facile. SFs are used for different types of reactions as unique solvents.[45] It is known that creating an SF is simple: by moving the substance beyond its critical point (critical temperature, critical pressure) with increasing temperature and pressure, supercritical fluids are obtained. Due to its relatively low critical temperature and pressure (Tc = 31.1 °C, Pc = 7.38 MPa), CO2 is considered the best substance for making supercritical fluid. CO2 is low in cost, reactivity, and toxicity and is also widely available. Supercritical CO2 can be used to extract nonpolar and slightly polar substances such as aldehydes, alkanes, terpenes, esters and alcohols.[46]
By using the modifiers (polar: acetone, methanol; nonpolar: propane, octane), the solubility of polar and nonpolar solids in SF CO2 can be enhanced. In the SF solution, low concentrations (5 vol pct) of modifiers are added. As SF CO2 possesses low polarizability, the addition of modifiers improves this property, and the same has been described in other papers.[47,48] Faisal et al.[49] conducted an experiment to determine the extraction of PGMs using supercritical CO2 in combination with tributyl phosphate; no extraction of PGMs was observed. HNO3 ligand was introduced into the system, and > 96 pct of Pd extraction was attained. The present process was not suitable for the recovery of Pt and Rh (as < 3 pct of these metals is recovered). The optimal conditions for the extraction process are when it is performed at 60 °C, 20 MPa for 60 minutes of static extraction time. Supercritical water was used for the recovery of PGMs from the homogeneous precious metal catalysts by Collard et al.[50] At 500 °C to 600 °C with 30 MPa and oxygen injection (0 to 15 pct), > 95 pct leaching rates for Pt, Pd and Rh from an organic catalyst was attained. The process has been effective but is limited to only certain types of catalysts.
Supported liquid membranes
The possibility of utilizing thin layers of organic solutions (supported liquid membranes, SLM), immobilized on microporous inert supports interposed between two aqueous solutions (feed and strip), for selectively removing metal ions (A) from a mixture (AB+…) was first proposed > 55 years ago.[51] The schematic representation of the process is shown in Figure 6. In some processes, the addition (C) is added to the strip to form the insoluble compound (AC).
The general problems of using this technique are connected with the membrane instability due to the pressure difference, the carrier and membrane dissolution, the presence of osmotic pressure and other issues described in Reference 52. Since it was first proposed, supported liquid membrane technology has been used for the separation of a wide range of metal ions.[53]
Fontas et al.[54,55] described the new system combining two hollow-fiber-supported liquid membranes for the separation and concentration of platinum, palladium and rhodium from spent automotive catalytic converters. Selective separation of these metals was achieved when the feed solution (to which a small amount of thiocyanate had been added) was first introduced into the HFLM system containing Cyanex 471 (triisobuthylphosphine sulfide) as the extractant and then passed through the second liquid membrane system consisting of Aliquat 336 (tricaprylmethylammonium chloride) as the carrier. Only an insignificant amount of aluminum was co-transported. In a later work,[55] palladium separation and concentration were achieved in a single step when using the liquid membrane system in hollow fiber geometry. Its efficiency has been demonstrated working with real solutions of PGMs obtained from the leaching of automotive catalysts, where palladium was efficiently separated and concentrated, while no platinum extraction was observed. More developments of the supported liquid membranes technique are described elsewhere.[56]
Pyrometallurgy
In the pyrometallurgical process, the spent catalyst is transformed physically and chemically by performing a thermal treatment. Calcination, chlorination and PGM smelting collection are considered the three kinds of pyrometallurgical technologies.[57,58] These technologies can be performed at a higher rate compared to the hydrometallurgical processes, as in the latter case there is no geometrical significance for the scrap materials used in the extraction of PGMs. In this process, a large amount of energy is consumed and leads to the emission of SO2, which is an environmental hazard.[59]
Sintering process
The sintering process was performed by Bronshtein et al.[60] to extract PGMs with high efficiency by mixing the spent catalyst with metal chloride salts including (for Pd extraction)/excluding (for Pt extraction) fumed silica powder. Two procedures were followed: in procedure 1, CaCl2 was mixed with the crushed catalyst materials under dry conditions; in procedure 2, the spent catalyst was immersed in an aqueous calcium chloride solution followed by drying. An optimal chloride salt-to-catalyst ratio was 6.7:2.5 for procedure 2. During the experiment, continuous airflow was provided into the reactor. In procedure 1, the sintering process was performed for 120 minutes, and Pt extraction efficiency did not exceed 63 ± 3 pct for temperatures between 500 °C to 1000 °C. Calcium hypochlorite addition (20 pct w.r.t. catalyst wt pct) to the dry mixture improved the extraction efficiency to 74 ± 4 pct. In procedure 2, the extraction efficiency of Pt was up to 80 ± 4 pct; the addition of ammonium chloride and sodium chloride salts as a supplement to the mixture to enhance the Pt extraction did not show any positive effect. At 1100 °C, Pt was 100 pct extracted from the catalyst mixture using procedure 2.
Pd extraction using procedures 1 and 2 was poor (< 3 pct); fumed silica paste was used as an additive, and a significant improvement in the extraction efficiency was observed (76 ± 4 pct) when sintered between 1000 °C to 1100 °C. The addition of silica paste leads to a significant negative Gibbs energy for the reaction to form CaSiO3 from CaO and SiO2 (Eq. 18) (ΔG0 = − 220 KJ/mol at T = 1100 °C) and without silica paste addition, ΔG0 = − 45 KJ/mol.
The primary advantage of the process is that it does not use strong acids or bases, corrosive gases or hazardous chemicals. It produces nontoxic silicate waste as a by-product, which is minimal.
Chlorination process
In this process, PGMs from the spent catalysts are transformed into their respective chlorides at high temperatures.[61] PGMs are then separated based on the difference between the metal chlorides, by adsorption on an activated carbon bed or by repulp washing.[38] Murray et al.[62] in his patent mentioned that the PGMs in the spent catalyst are converted into chlorides in a chlorine gas/fluid field at 600 °C to 1200 °C. The separation is then carried out by absorption of PGM chlorides by ammonium chloride liquor or water. The recovery of Pd, Pt and Rh was up to 80 to 90 pct, 80 to 90 pct and 85 to 90 pct, respectively.
Horike et al.[63] proposed a chlorination process for efficient Pt recovery. CuCl2 was used as a chlorine solution for the chlorination of Pt at 400 °C to 600 °C. Due to high chemical stability, pure Pt was insoluble in HCl (aq); scrap containing Pt was then alloyed with Mg, physically mixed with CuCl2 at 500 °C and then dissolved in HCl (aq). The solubility of Pt in the HCl(aq) improved after the chlorination process. The recovery of Pt using this process can be environmentally friendly.
Xu et al.[64] proposed a method where the spent catalyst was pre-treated by crushing, roasting and reducing. Then, NaCl was added to it to perform chlorination at 600 °C to 700 °C. The mixture was then dealt with using hot water and steam, resulting in a PGM chloride salt solution. The recovery of Pd, Pt and Rh was up to 90 pct. This process is not adaptable to the industrial scale as it results in the emission of toxic gases, has a high equipment requirement and is performed at high temperatures.
Metal smelting collection
In this process, the spent catalysts are mixed with the flux, collector and reducing agent and then smelted in a high-temperature electric arc, plasma furnace or induction furnace at around 1000 °C (Figure 7). PGMs are easily converted into treatable complex ions and later refined to recover the PGMs. The pre-treatment processes such as the dismantling of non-metallic components, calcination or reduction are performed before the smelting, which is done at high temperatures with the addition of collectors (with high specific gravity) such as lead, iron and copper. The PGMs form an alloy with the collectors; at the same time catalysts and carriers are melted in the presence of flux to form a slug with low viscosity. Then, a separation procedure is performed in which the PGM-enriched alloy is separated from the slag and the purification process is carried out. High PGM recovery rates are expected from this process.[65]
Lead collection is the oldest method used to recover PGMs from the spent catalyst.[66] In this process, the spent catalyst is crushed and then added to a mixture containing PbO, anhydrous borax, sodium carbonate and potassium bitartrate; it is then smelted for 2 hours at 1100 °C. The PbO is reduced to Pb, forming an alloy with the PGMs while SO2 from the catalyst is oxidized to form a slag, namely borosilicate, which floats on top of the molten lead layer. The borosilicate slag is removed, and molten Pb containing PGMs is left for purification. This process is easy to operate, and Pt recovery is highly efficient but also has disadvantages: the Rh recovery is < 80 pct, and toxic PbO gas is emitted.
The copper collection process is recommended for spent catalysts containing cordierite carriers.[67,68,69] The copper collectors are CuCO3, CuO or Cu with the flux being SiO2 and CaO; the reducing agent is usually powder coke. The collector, flux, reducing agent and spent catalyst mixture are placed in an induction furnace and smelted at 1400 °C. PGMs are collected at low temperature under ambient atmosphere. The ceramic carriers of the PGMs form a slag on the top of the molten copper, which can be poured out easily. The PGM containing copper is cast into anodes, and electrolysis is performed. A slime containing 25 pct PGMs is obtained after electrolysis; 99 pct of PGMs can be recovered overall through this process.
Using iron as a collector is economically beneficial. Fe shows a strong chemical affinity to PGMs.[70] The crushed spent catalyst mixed with the flux (CaO), Fe powder and reducing agent (powder coke) is smelted in a plasma arc furnace at 1500 °C to 2000 °C. The PGM-enriched Fe alloy and the carrier slag can be easily separated because of their large density differences (density ratio = 2:1). The recovery rates of Pd, Pt and Rh were > 98 pct, 98 pct and 97 pct, respectively.[71]
The matte collection is a relatively new method used for the recovery of PGM from spent catalysts.[72,73] When the spent catalyst is smelted, a matte type substance is formed, which acts as a collector of PGMs. PGM-enriched matte is obtained at 1000 °C to 1450 °C by adding Ni or NiS and the flux (Na2B4O7, Na2CO3, CaO). You et al.[74] reported that nickel and sulfur can be used as collectors in the smelting of spent catalysts in the presence of borax and sodium carbonate for 30 minutes at 1050 °C. The recoveries of Pd, Pt and Rh were 93, 90 and 88 pct, respectively, for this process.
A novel Pd recovery method was proposed by Zhang et al.[75] where eutectic copper was used to capture Pd from spent automobile catalysts. The recovery of Pd is difficult compared to the rest of the PGMs, as the element has an inert behavior.[76] Cu is used as a capture agent, where Pd forms an alloy with Cu at a temperature > 1250 °C. The studies were conducted at temperatures between 1200 °C and 1400 °C. The spent catalyst mainly was comprised of cordierite (2MgO:2Al2O3:5SiO2), aluminum oxide and 1650 mg/kg Pd. The spent catalyst sample was transferred to a crucible along with CuO, 5 wt pct C, 30 wt pct CaO, 20 wt pct SiO2, 10 wt pct borax and 8 wt pct Na2CO3, where borax and Na2CO3 enhance the melting of the slag. The CaO-SiO2-Al2O3 glass phase, which plays a key role in the formation of Cu-Pd alloy, is expected to form. At 1200 °C, the recovery of Pd was 0 pct as the spent catalyst was not in the molten form. At 1250 °C, the Pd recovery reached 90 pct and was up to 100 pct when the operating temperature reached 1400 °C. The authors suggested the optimal temperature to be 1350 °C where the Pd recovery rate was 97 pct.
The metal smelting collection process can be implemented on a wide range of secondary materials, especially low-grade and refractory spent catalysts. Factors such as the collector materials, flux, reducing agents and operational conductions affect the efficiency of the process. Copper collection technology has relatively low cost, high efficiency and low smelting temperature. The collector material can be re-used. Therefore, this method can be easily adapted to an industrial scale. It is also worth mentioning that adding metal collectors to the briquettes increases the PGM yield and significantly decreases the dust-gas emission.[77]
Physical Separation
In the spent catalyst, the catalyst carriers are honeycomb-structured items with porous catalyst layers on the surface. PGMs are only present on these porous layers where a simple energy-efficient and inexpensive process like physical separation can be implemented to separate the PGMs or PGM-containing layer from the carrier would be significant to eliminate the toxic gases usually released from the regular recycling process. By pulverizing the spent catalyst, a magnetic separation technique can be employed to physically separate the PGMs from the catalyst carriers. Magnetic separation can be performed only on old catalysts with nickel content. Techniques such as selective grinding followed by size separation[78,79] and selective grinding followed by quenching/heat treatment[80] can enhance the PGM-containing catalyst layer concentration. Taninouchi et al.[81] developed a new process (Figure 8) using a magnetic separation technique to recover PGMs from spent catalysts.
Flowchart illustrating the physical concentration technique[81]
In this process, Ni deposition on the PGMs is done using an electroless-plating bath along with a complexing agent and reduction agent, which are glycine and sodium hypophosphite, respectively. The composition of the electroless plating bath is (NiSO4•6H2O = 35.6 ci/g.L−1, glycine = 22.0 ci/g.L−1, NaHPO2. H2O = 24.1 ci/g L−1 and PbCl2 = 0.004 ci/g L−1). The pH value was adjusted to 7.7 ± 0.4 at room temperature (25 °C) using NaOH(aq.). The catalyst sample is immersed in the plating solution (40 mL) at room temperature. The container with the catalyst sample and plating solution is then heated to 70 °C for 15 minutes. The ferromagnetic nickel was plated on the surface of the porous catalyst layer successfully without additional steps such as sensitization or activation. Crushing and pulverization were performed on catalyst samples. Neodymium magnet (Nd-Fe-B alloy) was used for the magnetic separation process under dry conditions. Results of the separation process showed that this process can be further developed and can be adapted to the industrial scale.
The recovery of PGM by treating the spent catalyst with FeClx (x = 2, 3) was tested by Taninouchi et al.[82,83] The idea was to convert Pt into the Pt-Fe alloy, which exhibits ferromagnetic behavior. The FeCl2 vapor reacted with Pt samples under the coexistence of Fe metal at 927 °C for 60 minutes, resulting in the formation of the γ2-FePt ferromagnetic phase. Fe was transported from the metallic Fe to Pt sample because of the disproportionation of FeCl2 vapor, and the gaseous phase FeClx acted as a mediator between the metals. When the γ2-FePt alloy was exposed to FeCl3, Fe was removed from the alloy resulting in the loss of the magnetic property. It can be stated that Pt can be only treated with FeCl2 but not with FeCl3. Further tests were conducted on the rest of the PGMs, and the results were the same. Pd and Rh formed alloys with Fe showing a strong ferromagnetic character. Carriers such as Al2O3 did not react with the FeCl2 but La2O3 and CeO2 were converted into their respective oxychlorides. Magnetic separation was effective on the PGMs after the FeCl2 vapor treatment. These processes showed promising results and have the potential to be used at a higher level. The process can be performed at a low cost with a quick pace and is environmentally friendly.
Electrochemical Techniques
High-temperature electrochemical treatment can be applied to extract precious metals from spent catalysts. In general, the electrochemical methods are easy to arrange and can be performed with low waste emissions and low energy and reactant consumption. The major disadvantage is that these methods are not universal. They can be applied for the treatment of catalyst based on the γ-Al2O3 carrier with a minor content of other oxides (SiO2, Fe2O3, MgO, TiO2, CeO2, etc.); however, automotive exhaust gas convertors, the catalysts with the highest annual amount, can barely be successfully treated by the electrochemical process because of their complexity. They generally have cordierite (2MgO·2Al2O3·5SiO2) as a substrate coated with a γ-Al2O3 wash coat where precious metals (basically Pt, Pd, Rh) are deposited. Each of the oxide components requires separate treatment and adjustment of the technology.
Two-step method
Belov et al.[84,85,86] proposed an electrochemical method for the extraction of Pd from spent Al2O3-based catalysts, which was carried out in several stages, based on the Hall–Heroult process for primary aluminum production and the three-layer refining process for high-purity aluminum production (shown in Figure 9). These stages are:
Schematic representation of the commercial Hall–Heroult cell and the aluminum refinery cell[87]
-
1.
Calcination of the catalysts at 800 °C under air (or oxygen) atmosphere to remove organic impurities and partially transform PGMs into oxides;
-
2.
Dissolution of the catalysts in the molten fluoride system (Na3AlF6-AlF3-CaF2) and electrodeposition of aluminum and PGM on the cathode alloy with the simultaneous evolution of carbon dioxide on the carbon anode according to the reaction:
$$ {\text{Al}}_{2} {\text{O}}_{3} \left( {\text{dis}} \right) + 1.5{\text{C}}\left( {\text{s}} \right) \Rightarrow 2{\text{Al}}\left( {\text{l}} \right) + 1.5{\text{CO}}_{2} \left( {\text{g}} \right) $$(19) -
3.
Electrolytic refining of the Al-Cu-PGM alloy (Cu is added to heighten density) with electrodeposition of high-purity aluminum at the cathode and concentration of PGMs in the anode alloy.
This process requires high (950 °C to 970 °C) temperature and should be operated within a narrow window of catalyst concentrations to prevent sludge formation and the emission of perfluorocarbons during low- or high-voltage anode effects, which in case of slow dissolution can be a difficult problem to overcome.
Despite the mentioned considerations, several experimental facts confirm the perspectivity of the electrochemical extraction method[84,85,86]:
-
with the introduction of palladium oxide into molten cryolite, palladium is sufficiently fully reduced and dissolved in aluminum, while the addition of finely dispersed palladium to the melt leads to its appreciable distribution between aluminum and cryolite (in the latter, palladium forms a suspension);
-
palladium from the catalysts is dissolved and almost completely concentrated in primary aluminum (98 to 99 pct);
-
replacing smelter grade alumina with the catalyst in the Hall–Heroult process has virtually no effect on the performance of the aluminum production;
-
the presence of palladium in the anode alloy in the three-layer refining process has almost no effect on the current efficiency of the aluminum refining;
-
a high palladium extraction degree (> 98 pct) and the simultaneous production of high-purity aluminum (A97, A99) were achieved.
One-step method
The cost of noble metal extraction from spent catalysts can be significantly reduced by using a one-step electrochemical method. Moreover, the temperature of the process can be lowered to 700 °C to 850 °C. by replacing sodium cryolite with low-temperature fluoride or chloride melts with considerable solubility of Al2O3. A promising option, the molten 1.3KF-AlF3 system has been studied elsewhere.[87,88,89] Dividing the cell into two parts (half-cells) by a supported vertical or unsupported horizontal liquid Al membrane (bipolar electrode) can be a beneficial solution for one-step extraction. The first half-cell acts as the aluminum reduction cell; the second one plays the role of the aluminum refinery cell. The schematic representation of the cell is shown in Figure 10.
Concept of the one-step electrometallurgical process: (1) KF–AlF3 electrolyte for catalyst decomposition; (2) Al-based bipolar electrode; (3) wettable substrate TiB2 or W—optional; (4) NaCl-KCl-AlF3 (+ optional BaCl2) electrolyte for removing Al from the bipolar electrode; (5) oxygen-evolving anode; (6) cathode for high-purity aluminum evolution
This process is patented[90] and now is under the laboratory-scale studies[91] with the spent Pt(+ Re)/γAl2O3 catalysts from the petrochemical industry with 0.35 and 0.2 wt pct Re (optional). The catalysts were calcined under air atmosphere in the electrical furnace at 800 °C for 30 minutes with manual stirring, ground and treated with mechanical activation for 10 seconds for better performance in terms of dissolution rate. The 24 hours experiment showed that Pt extraction reaches 99 pct. This value was obtained by comparing the Pt concentration in the bipolar electrode and high-purity aluminum. The detailed description of these results is a subject of further publications. The spent catalyst used by the authors for the recovery of Pt is shown in Figure 11.
While Pt is successfully concentrated in the alloy, Re requires additional effort to be extracted as it is oxidized in a wide range of temperatures. The boiling temperatures of rhenium oxides are < 620 °C, which makes it difficult to extract it to the alloy. This is another drawback of this process when the catalyst contains Re.
The catalysts based on cordierite can also be dissolved in molten cryolite. In that case, silicon (and iron) will be collected in the alloy along with precious metals. Magnesium will be collected in the refinery electrolyte in the form of MgCl2 because of the significant differences between the electrode potentials of Al and Mg. The same is true for cerium, which will also be collected in the electrolyte in an oxidized form.
The complexity of cordierite (and another mineral support such as zeolites) makes the possibility of using electrochemical technology for the treatment of spent catalysts other than Al2O3-based ones questionable. Major changes should be made to adjust this technology for different types of catalysts.
Comparative Analysis of the Technologies
The comparison between different approaches for the extraction of metals from spent catalysts is presented in Table II.
The methods can be united into several groups according to the diagram presented in Figure 12; however, this classification is rather suppositive as combined approaches are often used in practice.
The comparison shows that among the huge variety of technologies, several have prospects of further development. Different products in the processes require appropriate treatment to extract valuable metals and purify them. Common significant drawbacks of the methods often are the high cost of equipment and consumables, the high specific energy consumption and the huge amount of waste.
Separation Process
In this process, individual PGMs, which are in their chlorocomplex form, are separated from the leaching solution. Solvent extraction, ion exchange and precipitation methods are usually implemented, where the extractants are added to the synthetic solution for individual PGM separation. Extractants, ion exchange resins and precipitants play a vital role in the separation behavior of Pt and Pd metals. In this section, the three separation methods mentioned above will be discussed in brief along with the effects of extractants/ion exchange resin/precipitants on the separation behavior of PGMs from the leach liquor. (Note: these separation methods are only used in hydrometallurgical processes)
Solvent Extraction (SX) Method
In the SX method, extractants are added to the pretreated leaching liquor where ideal concentrations of PGMs are available for the separation. The three types of extractants added according to the requirements are basic (amine), neutral and acidic extractants. According to the literature study conducted in Nguyen et al.,[92] Pt and Pd can be completely extracted by selective stripping from the leaching solution by using amines, although complications with the formation of the third phase and difficulties in stripping leading to the compulsion in adjusting the stripping solution might occur. By using neutral extractants, selective extraction of Pd can be carried out (Table III).
The separation behavior of Pt(IV) and Ir(III) in three different leaching solutions (HCl/HNO3/H2SO4) at varying concentrations between 0.1 and 6 mol/L using Cyanex 471X extractant was investigated.[93] Cyanex 471X was diluted with toluene, and the concentration of the extractant in this organic solution was 0.1 mol/L. The extraction percentage of Pt(IV) was about 80 pct at low HCl concentrations of 0.1 to 0.5 mol/L, and a sudden decrease in the percentage to 50 pct was recorded at 2.0 mol/L HCl and remained constant on a further increase of the HCl concentration. The same phenomena were seen while using HNO3 and H2SO4 solutions as the initial recovery rate was 55 and 67 pct, respectively, but with the increase in their concentrations the recovery rate decreased. The reason reported was that Pt(IV) could not form an extractable sulfate complex. In contrast to Pt(IV), Ir(III) was easily extracted in HCl solution as it could form extractable chlorocomplexes. Ir(III) from HNO3 and H2SO4 solutions had a high recovery rate at 0.1 mol/L concentration, which was low when the respective concentrations reached 6.0 mol/L.
Ion-Exchange Method
In the ion-exchange method, the ion exchange resin is added to the leaching solution, sometimes combined with a synthetic solution loaded with PGMs in their complex form. Ion exchangers play a vital role in the separation process, and some of the details on the ion exchangers are mentioned in Table IV. PGMs are adsorbed on the resin surface. The elution process is carried out on loaded organic resins by adding eluents such as Na2CO3, NaCl and a mixture of thiourea and HCl to strip the PGMs. Separation of Pt and Rh from the HCl solution with varying concentrations of 0.1 to 5 M using the IX technique was investigated in Reference 94. Three different ion exchangers were used, namely AGMP-1, AG1-x2 and AG1-x8. The adsorption phenomena of Pt and Rh were similar in all the anion exchangers. Pt showed a better adsorption percentage even in 5M HCl solution while the percentage was < 20 pct in the case of Rh in concentrations from 0.1 to 5 M. It is worth noting that an increase in the concentration of exchangers in the solution increased the adsorption percentage of both Pt and Rh. The Langmuir isotherm was followed by resins for Pt adsorption. The adsorption percentage of Rh increases with an increase in the HCl concentration, while the percentage of Pt decreases. AG1-x8 was a better exchanger compared to the other two, as AG1-x8 had better Pt loading capacity and also a high separation factor between Rh and Pt. Further continuous column experiments showed that Pt and Rh can be completely separated using a 0.1 HCl solution mixture. Na2CO3, NaCl and a mixture of thiourea and HCl were used as eluting agents, and thiourea was more effective in eluting Pt from the loaded organic resin. The process is effective and can be adapted to the commercial level. The resin used in this process can be regenerated after the desorption of the PGMs.
In Reference 95 the separation behavior of Pt, Pd and Rh in 2.35 M HCl solution was investigated where ion exchanger resins, namely XUS 43600 (thiouronium functional group), Lewatit MonoPlus (M+) MP 600 (quaternary ammonium functional group) and Purolite S985 (polyamine functional group), were used. It was reported that Pt and Pd show a high adsorption percentage in XUS 43600 while Rh had a poor adsorption behavior for all three resins. Sodium thiocyanate (2 mol/L), thiourea (1 mol/L) in sodium hydroxide (2 mol/L), thiourea (1 mol/L) in hydrochloric acid (2 mol/L), and hydrochloric acid (2 mol/L) were used as an eluting agent. Desorption of Pt and Pd from the loaded resins was better using acidic thiourea. Both [94] and [95] state that the adsorption of Rh on the resins and desorption from the loaded resins using the eluting agent were difficult. Alternative methods need to be used for the recovery of Rh.
Precipitation Method
In this method, a suitable precipitating agent is added to the leaching solution containing PGM ions, where the PGMs interact with the agents and form insoluble substances and are individually separated thereafter. The further step involves filtration where their pure metallic forms can be obtained. In Reference 96 selective separation of Rh from the HCl solution containing Pt (IV), Pd (II) and Rh (III) (1 mmol/L) metals was performed using the precipitation method. 4-Hexylaniline and 4-butylaniline were used as precipitants in high HCl-concentrated solution for Rh separation. Centrifugation was used to separate the metal precipitates from the mixture. The findings showed that while using the 4-hexylaniline precipitant, > 90 pct of Pt and Pd were precipitated in 1 to 2 M HCl concentration although with a sudden decrease in the precipitation percentage with a further increase in HCl concentration. In contrast, 85 pct of Rh was precipitated from the solution at high HCl concentrations (3 to 8 M). Using 4-butylaniline led to a poor precipitation percentage of Pt and Pd throughout the (1 to 8 M) HCl concentration, while Rh was successfully precipitated with an increase in HCl concentration. The color of the Rh precipitate was pinkish, and a unique ion pair complex composed of [RhCl6]3− /chloride/anilinium ions in a 1:3:6 ratio was found. The process shows promising results for the selective separation of Rh, and the authors claim that the process could influence the PGM recovery process at an industrial scale.
High extraction of Pt from HCl/H2O2 leach liquor (9 M HCl and 0.8 vol pct H2O2) was attained using the precipitation technique in Reference 97 The suitable precipitation concentration of Pt species in the leaching solution is 0.2 g/L, which was attained by heating the solution at 190 °C to make the excess liquid evaporate. NH4Cl (290 g/L) was added to the solution with simultaneous stirring at 40 °C. The yellowish Pt precipitate in a complex form of (NH4)2[PtCl6] was obtained. The later steps involved washing the precipitate with NH4Cl solution (140 g/L) followed by calcination. The Pt recovery rate was 96 pct, and the purity was around 99.6 pct. This method is simple, energy-efficient and promising with no harmful gas emissions.
Industrial Operations
On an industrial level, many advancements and optimizations have been made in extracting PGMs from spent catalysts. Commercial processes for PGM recovery from the spent catalyst and used electronic products have been successfully implemented by industries such as Engelhard (USA), BASF (USA), Hereaus (Germany), Johnson Matthey (UK), Mitsubishi (Japan), Umicore (Belgium) and Nippon (Japan). Table V shows the processes already in use commercially.
A new novel semi-industrial process combining pyrometallurgical treatment and electrolytic refining has been proposed by the Institute for Mining and Metallurgy Bor.[98] They claim that the process is economical, environmentally friendly and highly efficient. A novel membrane electrode assembly process has been developed by BASF Catalysts LLC (USA) to eliminate the emission of highly toxic gas (HF) in the present recycling processes.[99] Cyanide leaching has been extensively used by the industries, although special attention needs to be given to the harmful by-products released by this process. A novel three-liquid-phase extraction has shown promising results at the laboratory scale and can open a new path in developing an environmentally friendly extraction technique.[100,101] In a novel combination of pyro- and hydrometallurgical processes, the catalytic converter carrier and Pt-Cu alloy samples acquired from the pyrometallurgical method were treated with aqua-regia (with and without fluoric acid). Solution and solid wastes were by-products of this process, making it undesirable, although it has been stated that economic and environmental benefits can be excepted with an adequate combination of pyro- and hydrometallurgical methods and can be adopted at a commercial scale.[102] Biotechnological recovery in which a metal ion-reducing bacterium (Shewanella algae) reduces and deposits PGMs ions [Pt(IV), Pd(II), and Rh(III)] into metal nanoparticles at neutral pH and room temperature within 60 minutes using the electron donor formate was successfully developed by Saitoh et al.[103] The adoption of this technology would be revolutionary and environmentally friendly. Currently, much research is being conducted to find the optimal way to recover PGMs from spent catalysts, and much has been achieved at the laboratory scale. Some of the research is transferable to the industrial level.
Conclusion
Platinum group metals are widely used in various industrial applications. Demand for PGMs is inevitable because of their unique properties and because natural PGM resources are limited, which demands recycling PGMs in spent catalysts. The recycling process satisfies demand and consumes the leftover catalysts in an environmentally friendly way. This article gives an overview of the processes used for recycling of spent catalysts. The processes are classified into hydrometallurgy, pyrometallurgy, physical magnetic separation and electrochemical, which all have advantages and disadvantages. When using the hydrometallurgical process, by-products such as toxic water wastes are released, and recovery of Rh is poor compared with the pyrometallurgical process. The bioleaching process is eco-friendly but the PGM recovery efficiency should be improved. The pyrometallurgical process requires huge amounts of energy and investments but the process is environmentally friendly compared to the hydrometallurgical process. The process can be economically beneficial when used at the large-scale industrial level. The physical magnetic separation process is relatively new and novel, but promising results have been attained. Nonetheless, the process is still in its beginning stages. A new one-step electrometallurgical process with Pt recovery up to 99 pct was proposed by the authors of this article. The process is still under study, and major results are expected to be obtained soon. Much work has been done on different recycling processes, and there is still room for further improvement.
Future Scope
Hydrometallurgical techniques are highly efficient for Pd extraction and have received much more attention than other ones in recent years; however, > 90 pct Rh cannot be leached. Strong solvents (aqua regia, cyanide, H2SO4, etc.) are used to dissolve the spent catalysts in the leaching process. PGMs should be reduced to the metallic state before this. Cyanide leaching is the more efficient recovery process, but it requires high temperatures, and cyanide is highly toxic. Pyrometallurgical techniques have been well studied and applied in industry. The advantage is that calcination, chlorination and PGM smelting collection (infiltration) can be performed at a higher rate compared to hydrometallurgical processes. However, the pyrometallurgical process consumes a large amount of energy. The emission of SO2 is also a significant drawback. Physical separation techniques are simpler, energy-efficient and inexpensive. They also eliminate the toxic gases usually released when using the hydro- and pyrometallurgical methods. Ni deposition on PGMs is done by using an electroless-plating bath, complexing and reducing agents. High-temperature electrochemical methods are much less studied. They are easy to set up and can be performed with low waste emissions and with low energy and reactant consumption. However, they are not universal as they can only be applied to the γ-Al2O3—based catalysts. Complex cordierite-based (or similar) automotive exhaust gas converters can barely be successfully treated by the electrochemical process. Optimization of this technology is needed for each of the oxide components. Nevertheless, the one-step electrochemical method appears to be a promising solution. However, it requires further research for implementation on an industrial scale.
References
Richards, J.: Journal of Cleaner Production, 2006, vol. 14, pp. 324–333.
Mpinga, C.N., Eksteen, J.J., Aldrich, C., Dyer, L.: Minerals Engineering, 2015, vol. 78, pp. 93–113.
Suoranta, T., Zugazua, O., Niemela, M., Peramaki, P.: Hydrometallurgy, 2015, vol. 154, pp. 56–62.
Marcilly, C.: Journal of Catalysis, 2003, vol. 216, pp. 47–62.
Kononova, O.N., Melnikov, A.M., Borisova, T.V.: Hydrometallurgy, 2012, vol. 117–118, pp. 101–107.
Jewell, S., Kimball, S.M.: Mineral Commodity Summaries 2015. U.S. Geological Survey. Reston, Virginia: CreateSpace Independent Publishing Platform, 2015, vol. 196, pp. 120–21.
Xiao, Z., Laplante, A.R.: Minerals Engineering, 2004, vol. 17, pp. 961–979.
Fernandez, V.: International Review of Financial Analysis, 2017, vol. 52, pp. 333–347.
Safarzadeh, M.S., Horton, M., Van Rythoven, A.D.: Mineral Processing and Extractive Metallurgy Review, 2018, vol. 1, pp. 1–17.
Angelidis, T.N., Sklavounos, S.A.: Applied Catalysis A: General, 1995, vol. 1, pp. 121–132.
Marafi, M., Stanislaus, A.: Resources Conservation and Recycling, 2008, vol. 6, pp. 859–873.
Marafi, M., Stanislaus, A.: Resources Conservation and Recycling, 2008, vol. 1–2, pp. 1–26.
Marafi, M., Stanislaus, A.: Journal of Hazardous Materials, 2003, vol. 101, pp. 123–132.
Mishra, D., Kim, D.J., Ralph, D.E., Ahn, J.G., Rhee, Y.H.: Hydrometallurgy, 2007, vol. 88, pp. 202–209.
Deng, J., Ren, P., Deng, D., Yu, L., Yang, F., Bao, X.: Energy and Environmental Science, 2014, vol. 7, pp. 1919–1923.
Zhang, L.G., Xu, Z.M.: Journal of Cleaner Production, 2016, vol. 127, pp. 19–36.
Akcil, A., Vegliò, F., Ferella, F., Okudan, M.D., Tuncuk, A.: Waste Management, 2015, vol. 45, pp. 258–271.
Hagelüken, C.: Chemistry Today, 2006, vol. 24, pp. 14–17.
Syed, S., “Silver recovery aqueous techniques from diverse sources: hydrometallurgy in recycling”, Waste Management, 2016, vol. 50, pp. 234–256.
Işıldar, A., Rene, E.R., van Hullebusch, E.D., Lens, P.N.: Resources, Conservation & Recycling, 2018, vol. 135, pp. 296–312.
Ghodrat, M., Rhamdhani, M.A., Brooks, G., Masood, S., Corder, G.: Journal of Cleaner Production, 2016, vol. 126, pp. 178–190.
Sun, P.P., Lee, M.S.: Hydrometallurgy, 2011, vol. 110, pp. 91–98.
Marinho, R.S., da Silva, C.N., Afonso, J.C., da Cunha, J.W.: Journal of Hazardous Materials, 2011, vol. 192, pp. 1155–1160.
Green, G., Smit, D.M.C., Maumela, H., Coetzer, G.: The Journal of The South African Institute of Mining and Metallurgy, 2004, vol. 104, pp. 323–331.
Angelidis, T.N., Skouraki, E.: Applied Catalysis A: General, 1996, vol. 142, pp. 387–395.
Fornalczyk, A., Saternus, M.: Metalurgija, 2009, vol. 48, pp. 133–136.
Shams, K., Goodarzi, F.: Journal of Hazardous Materials, 2006, vol. B131, pp. 229–237.
Angelidis, T.N.: Topics in Catalysis, 2001, vol. 16–17, pp. 419–423.
Trinh, H.B., Lee, J., Srivastava, R.R., Kim, S.: Journal of Hazardous Materials, 2019, https://doi.org/10.1016/j.jhazmat.2019.120772 (In press)
Matjie, R.H., Scurrell, M.S., Bunt, J.: Materials Engineering, 2005, vol. 18, pp. 801–810.
Sarioğlan, S.: Platinum Metals Review, 2013, vol. 57, pp. 289–296.
Paiva, A.P., Ortet, O., Carvalho, G.I., Nogueira, C.A.: Hydrometallurgy, 2017, vol. 171, pp. 394–401.
Kasuya, R., Miki, T., Morikawa, H., Tai, Y.: Minerals Engineering, 2016, vol. 87, pp. 25–31.
Spooren, J., Atia, T.A.,: Minerals Engineering, 2020, vol. 46, pp. 106153.
Firmansyah, M.L., Kubota, F., Yoshida, W., Goto, M.: Industrial and Engineering Chemistry Research, 2019, vol. 58, pp. 3845–3852.
Firmansyah, M.L., Kubota, F., Goto, M.: Journal of Chemical Engineering of Japan, 2019, vol. 52, pp. 835-842.
Atkinson G. B.: 1992. US Patent. 5160711.
Allen, R.J., Foller, P.C., Giallombardo, J.: 1992, U.S. Patent 5,102,632.
Chen, J., Huang, K.: Hydrometallurgy, 2006, vol. 82, pp. 164–171.
Shams, K., Beiggy, M.R., Shirazi, A.G.: Applied Catalysis A: General, 2004, vol. 258, pp. 227–234.
Zhuang, W.Q., Fitts, J.P., Ajo–Franklin, C.M., Maes, S., Alvarez–Cohen, L., Hennebel, T.: Current Opinion in Biotechnology, 2015, vol. 33, pp. 327–335.
Asghari, I., Mousavi, S.M., Amiri, F., Tavassoli, S.: Journal of Industrial and Engineering Chemistry, 2013, vol. 19, pp. 1069–1081.
Santhiya, D., Ting, Y.P.: Journal of Biotechnology, 2005, vol. 116, pp. 171–184.
Malekian, H., Salehi, M., Biria, D.: Waste Management, 2019, vol. 85, pp. 264–271.
Eckert, C.A., Knutson, B.L., Debenedetti, P.G.: Nature, 1996, vol. 383, pp. 313–318.
Phelps, C.L., Smart, N.G., Wai, C.M.: Journal of Chemical Education, 1996, vol. 73, pp. 1163–1168.
White, G.L., Lira, C.T.: Fluid Phase Equilibrium, 1992, vol. 78, pp. 269–284.
Vitu, S., Privat, R., Jaubert, J.N., & Mutelet, F.: The Journal of Supercritical Fluids, 2008, vol. 45, pp. 1–26.
Faisal, M., Atsuta, Y., Daimon, H., Fujie, K.: Asia–Pacific J. Chem. Eng. 2008, vol. 3, pp. 364–367.
Collard, S., Gidner, A., Harrison, B., Stenmark, L.: 2006, U.S. Patent 7,122,167.
Danesi, P.R.: Sepaeparation Science and Technology, 1984, vol. 19, pp. 857–894.
Kemperman, A. J. B., Bargeman, D., Van Den Boomgaard, Th., Strathmann, H.: Separation Science and Technology, 1996, vol. 31, pp. 2733–2762.
de Gyves, J., San Miguel, R.E.: Industrial and Engineering Chemistry Research, 1999, vol. 38, pp. 2182–2202.
Fontas, C., Salvado, V., Hidalgo, M.: Industrial and Engineering Chemistry Research, 2002, vol. 41, pp.1616–1620.
Fontas, C., Salvado, V., Hidalgo, M.: Journal of Membrane Science, 2003, vol. 223, pp. 39–48.
Amini, M., Rahbar–Kelishami, A., Alipour, M., Vahidi, O.: Journal of Membrane Science and Research, 2018, vol. 4, pp. 121–135.
Jadhav, U.U., Hocheng, H.: Journal of Achievements in Materials and Manufacturing Engineering, 2012, vol. 54, pp. 159–167.
Parkinson, G., Ishio, S.: Chemical Engineering, 1987, vol. 94, pp. 25–31.
Havlik, T., Orac, D., Petranikova, M., Miskufova, A., Kukurugya, F., Takacova, Z.: Journal of Hazardous Materials, 2010, vol. 183, pp. 866–873.
Bronshtein, I., Feldman, Y., Shilstein, S., Wachtel, E., Lubomirsky, I., Kaplan, V.: Journal of Sustainable Metallurgy, 2018, vol. 4, pp. 103–114.
Kim, C.H., Woo, S.I., Jeon, S.H.: Industrial & Engineering Chemistry Research, 2000, vol. 39, pp. 1185–1192.
Murray M.J.: 1962, US Patent, 3021209.
Horike, C., Morita, K., Okabe, T.H.: Metallurgical and Materials Transactions B, 2012, vol. 43, pp. 1300–1307.
Xu, S.Q., Xu, L.: China Nat. Res. Recycl. 1998, vol. 10, pp. 7–11.
Kayanuma, Y., Okabe, T.H., Maeda, M.: Metallurgical and Materials Transactions B, 2004, vol. 35, pp. 817–824.
Benson,M., Bennett, C.R., Harry, J.E., Patel, M.K., Cross, M.: Resources, Conservation and Recycling, 2000, vol. 31, pp. 1–7.
Ezawa N.: 1993, US patent 5252305.
Hoffmann, J.E.: JOM, 1988, vol. 6, pp. 40–44.
Kolliopoulos, G., Balomenos, E., Giannopoulou, I., Yakoumis, I., Panias, D.: Open Access Library Journal, 2014, vol. 1, pp. e736.
Mishra, R.K., Reddy, R.G.: 10th International Precious Metals Institute, Florida, 1986, pp. 217–31.
He, X., Li, Y., Wu, X., Zhao, Y., Wang, H., Liu, W.: Precious Metals, 2016, vol. 37, pp. 1–5.
Van Schalkwyk, R.J., Eksteen, J.J., Akdogan, G.: Hydrometallurgy, 2013, vol. 136, pp. 36–45.
Eksteen, J.J.: Minerals Engineering, 2011, vol. 24, pp. 676–687.
You, G., Fang, W., Li, Q., Ma, Y., Yang, X., Yang, H.: Metallurgical Analysis, 2016, vol. 36, pp. 7–11.
Zhang, L., Song Q., Liu, Y., Xu, Z.: Journal of Cleaner Production, 2019, vol. 239, 1180933.
Wei, X., Liu, C., Cao, H., Ning, P., Jin, W., Yang, Z., Wang, H., Sun, Z.: Journal of Cleaner Production, 2019, vol. 239, pp. 1180312.
Kirichenko, A.S., Seregin, A.N., Volkov, A.I.: Metallurgist, 2014, vol. 58, pp. 250-255.
Kim, W., Kim, B., Choi, D., Oki, T., Kim, S.: Journal of Hazardous Materials, 2010, vol. 183, pp. 29–34.
Liu, G., Ichinose, T., Tokumaru, A., Owada, S.: Materials Transactions, 2014, vol. 55, pp. 978–985.
Liu, G., Tokumaru, A., Owada, S.: Resources Processing, 2013, vol. 60, pp. 28–35.
Taninouchi, Y., Watanabe, T., Okabe, T.H.: Materials Transactions, 2017, vol. 58, pp. 410–419.
Taninouchi, Y., Okabe, T.H.: Materials Transactions, 2018, vol. 59, pp. 88–97.
Taninouchi, Y., Okabe, T.H.: Metallurgical and Materials Transactions B, 2018, vol. 49B, pp. 1781–1793.
Belov, S.F., Igumnov, M.S., Lovchinovsky, I.Yu. [In Russ]: Tsetnye metally, 1997, vol. 5, pp. 46–48.
Belov, S.F., Igumnov, M.S., Lovchinovsky, I.Yu. [In Russ]: Izvestiya VUZov. Tsvetnaya metallurgiya, 1985, vol. 4, pp. 63–67.
Belov, S.F., Igumnov, M.S., Lovchinovsky, I.Yu. [In Russ]: Tsvetnye metally, 1997, vol. 5, pp. 39–41.
Padamata, S.K.; Yasinskiy, A.S., Polyakov, P.V.: Metallurgical research & technology, 2019, vol. 116, pp. 410.
Yasinskiy, A.S., Suzdaltsev, A.V., Polyakov, P.V. Padamata, S.K. Yushkova, O.V.: Ceramics International, 2020 vol. 46 (8) Part B, pp. 11539–11548.
Padamata, S.K., Yasinskiy, A.S., Polyakov, P.V.: New Journal of Chemistry, 2020 vol. 44, pp. 5152-5164.
Yasinskiy, A.S., Polyakov, P.V., Popov, Yu.N., Polyakov, A.A., Padamata, S.K.: 2018. RU Patent. 2689475.
Yasinskiy, A.S., Padamata, S.K., Polyakov, P.V., Varyukhin, D.Yu.: Non-ferrous metals, 2019, vol. 2, pp. 23–30.
Nguyen, T.H., Sonu, C.H., Lee, M.S.: Journal of Industrial and Engineering Chemistry, 2015, vol. 32, pp. 238-245.
Gupta, B., Singh, I., Mahandra, H.: Separation and Purification Technology, 2014, vol. 132, pp. 102–109.
Sun, P.P., Lee, J.Y., Lee, M.S.: Hydrometallurgy, 2012, vol. 113–114, pp. 200–204.
Nikoloski, A.N., Ang, K., Li, D.: Hydrometallurgy, 2015, vol. 152, pp. 20–32.
Matsumoto, K., Yamakawa, S., Sezaki, Y., Katagiri, H., Jikei M.: ACS Omega, 2019, vol. 4, pp. 1868−1873.
Yousif, A.M.: Journal of Chemistry, 2019, vol. 2019, 2318157.
Ivanović, S.Z., Trujuć, V.K., Gorgievski, M.D., Mišić, L.D., Božić, D.S.: 15th international research/expert conference “Trends in the development of machinery and associated technology”, 2011, pp. 12–18.
Panda, R., Jha, M.K., Pathak, D.D.: Kim H. et al. (eds) Rare Metal Technology, TMS 2018, 2018, pp. 119–30.
Zhang, C., Huang, K., Yu, P., Liu, H.: Separation and Purification Technology, 2012, vol. 87, pp. 127–134.
Zhang, C., Huang, K., Yu, P., Liu, H.: Separation and Purification Technology, 2013, vol. 108, pp. 166–173.
Fornalczyk, A., Saternus, M.: Acta Metallurgica Sinica (English Letters), 2013, vol. 26, pp. 247-256.
Saitoh, N., Nomura, T., Konishi, Y.: Kim H. et al. (eds) Rare Metal Technology. TMS 2017, 2017, pp. 129–35
Johnson Matthey, PGM Market Report February 2020.
Myrzabekov, B.E., Bayeshov, A.B., Makhanbetov, A.B., Mishra, B., Baigenzhenov, O.S.: Metallurgical and Materials Transactions B, 2018, 49, 23–27.
Giridhar, P., Venkatesan, K.A., Reddy, B.P., Srinivasan T. G., Vasudeva Rao, P. R.: Radiochimica Acta, 2006, 94, 131–136.
Tumanova, N.Kh, Kochetova, S.A., Savchuk A.V.: ECS Transactions, 2009, 16 (49) 453-459.
Leclerc, N., Legeai, S., Balva, M., Hazotte, C., Comel, J., Lapicque, F., Billy, E., Meux, E.: Metals, 2018, 8, 556.
Sharma, R., Gyergyek, S., Andersen, S.M.: ChemSusChem, 2018, 11, 3742–3750.
Song, Y., Tsuchida, Y., Matsumiya, M., Tsunashima, K.: Hydrometallurgy, 2018, 181, 164–168.
Liu, C., Sun, S.C., Zhu, X.P., Tu, G.F., Zhang, J.Y.: IOP Conf. Ser.: Mater. Sci. Eng., 2019, 479 012058.
Fornalczyk, A., Golak, S., Saternus, M.: Mathematical Problems in Engineering, 2013, Article ID 461085, https://doi.org/10.1155/2013/461085.
Haigang, D., Jiachun, Z., Jialin, C., Yuedong, W., Bojie, L.: International Journal of Mineral Processing, 2015, vol. 145, pp. 108–113.
Swain, B., Jeong, J., Kim, S.K., Lee, J.C.: Hydrometallurgy, 2010, vol. 104, pp. 1–7.
Sun, P.P., Lee, M.S.: Hydrometallurgy, 2011, vol. 109, pp. 181–184.
Yin, C.Y., Nikoloski, A.N., Wang, M.W.: Minerals Engineering, 2013, vol. 45, pp.18–21.
Mirza, M.Y.: Talania, 1980, vol. 27, pp. 101–106.
Paiva, A.P., Carvalho, G.I., Costa, M.C., Costa, A.M.R., Nogueira, C.: Solvent Extraction and Ion Exchnage, 2014, vol. 32, pp. 78–94.
Pan, L., Zhang, Z.: Minerals Engineering, 2009, vol. 22, pp. 1271–1276.
Gupta, B., Singh, I.: Hydrometallurgy, 2013, vol. 134, pp. 11–18.
Paiva, P.A.: Davis B. et al. (eds), TMS 2018, 2018, pp. 2063-2073.
Cieszynska, A., Wisniewski, M.: Separation and Purification Technology, 2011, vol. 80, pp. 385–389.
Lee, J.Y., Raju, B., Kumar, B.N., Kumar, J.R., Park, H.K., Reddy, B.R.: Separation and Purification Technology, 2010, vol. 73, pp. 213–218.
Bandekar, S.V., Dhadke, P.M.: Separation and Purification Technology, 1998, vol. 13, 129–135.
Shen, Y.F., Xue, W.Y.: Separation and Purification Technology, 2007, vol. 56. pp. 278–283.
Nguyen, T.H., Sonu, C.H., Lee, M.S.: Hydrometallurgy, 2016, vol. 164, pp. 71-77.
Reddy, B.R., Raju, B., Lee, J.Y., Park, H.K.: Journal of Hazardous Materials, 2010, vol. 180, pp. 253–258.
Rane, M.V., Venugopal, V.: Hydrometallurgy, 2006, vol. 84, pp. 54–59.
Blokhin, A.A., Abovskii, N.D., Murashkin, Y.V.: Russian Journal of Applied Chemistry, 2007, vol. 80, pp. 1058–1062.
Hubicki, Z., Leszczyńska, M., Łodyga, B., Łodyga, A.: Desalination, 2007, vol. 207 (1–3), pp. 80–86.
Hubicki, Z., Wołowicz, A.: Journal of Hazardous Materials, 2009, vol.164, pp. 1414–1419.
Jha, M.K., Lee, J.C., Kim, M.S., Jeong. J., Kim, B.S., Kumar, V.: Hydrometallurgy, 2013, vol. 133, pp. 23–32.
Acknowledgments
The reported study was funded by RFBR according to research Project No. 18–29–24122.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted September 12, 2019.
Rights and permissions
About this article
Cite this article
Padamata, S.K., Yasinskiy, A.S., Polyakov, P.V. et al. Recovery of Noble Metals from Spent Catalysts: A Review. Metall Mater Trans B 51, 2413–2435 (2020). https://doi.org/10.1007/s11663-020-01913-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01913-w