Abstract
Plants face many stressful conditions during their lifetimes and because of their sessile nature they have to adapt to these conditions in order to survive. One unfortunate and unavoidable consequence of all major biotic and abiotic stresses is the overproduction of reactive oxygen species (ROS). ROS are highly reactive and toxic chemical entities and can cause serious damage to cellular proteins, lipids, carbohydrates and DNA, leading to irreparable metabolic dysfunction and cell death. Plant cells and their organelles, particularly the chloroplasts, mitochondria and peroxisomes have antioxidant defence systems, composed of enzymatic and non-enzymatic components, to counter the deleterious effects of ROS and/or to perform signalling functions. It is an established fact that the timely induction of antioxidant defences is a key to protection of plant cells from oxidative damage due to stress. Enzymatic antioxidants include superoxide dismutase, catalase, peroxidases and glutathione reductase, while the major non-enzymatic antioxidants are compatible osmolytes (glycinebetaine, GB; and proline), ascorbic acid, reduced glutathione, α-tocopherol, amino acids and polyphenols. Stimulated biosynthesis and accumulation of low molecular weight compatible osmolytes is one of the most effective mechanisms evolved by plants to maintain their cellular integrity and ensure survival when exposed to multiple abiotic stresses. Glycinebetaine, an N-trimethyl derivative of glycine and a quaternary ammonium compound, is one of the most studied and efficient compatible solutes. Due to its unique structural features, it interacts both with the hydrophobic and hydrophilic domains of macromolecules, including enzymes and proteins. GB has been reported to protect plants from the antagonistic effects of a range of abiotic stresses, by maintaining the water balance between plant cells and environment, osmotic adjustment, protecting the thylakoid membrane system, protein stabilization, photosystem and photosynthetic electron transport chain protection and by modulating ROS detoxification. In recent years, GB has attained unprecedented attention due to its multifunctional roles in plants under stressful conditions. In this chapter, we summarize our understanding of ROS formation under abiotic stress and GB biosynthesis and accumulation, as an adaptive mechanism, with particular emphasis on the new insights into the biochemical and molecular mechanisms involved in GB-mediated abiotic oxidative stress tolerance in plants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Plants face many stressful conditions during their lifetimes and because of their sessile nature they have to adapt to these conditions in order to survive. In their natural environment plants are exposed to various biotic and abiotic stressors. Abiotic stressors include drought, flooding, salinity, extreme temperatures, heavy metals, nutrient deficiency, high light intensities and UV radiation, all of which can have negative impacts on plant growth, development and crop production and can reduce product quality. Fuelled by the ever-increasing human population, increased crop productivity, from available lands, coupled with minimization of crop losses due to abiotic stress has become the highest agricultural priority, in recent times (Tuteja et al. 2011). However, production of abiotic stress tolerant plants requires a comprehensive understanding of the complex mechanisms associated with how plants respond to stress. One of the most active fields of plant science research focuses on understanding the molecular, physiological and genetic responses of plants to environmental stress, coupled with the development of approaches to improve abiotic stress acclimation and tolerance (Cabello et al. 2014).
One unfortunate and inevitable consequence of abiotic stress is the induction of oxidative stress. Therefore, oxidative stress is considered as a component of all major abiotic stresses. A common feature of plants’ responses to these stressors is the overproduction of reactive oxygen species (ROS) (Petrov et al. 2015). They are formed by the incomplete reduction or excitation of molecular oxygen and are major causative factors of oxidative damage to lipid membranes and other essential macromolecules found in plants, including pigments, proteins, DNA and RNA. Overproduction of ROS can lead to irreparable metabolic dysfunction and ultimately causes cell death (Qureshi et al. 2013; Petrov et al. 2015). However, ROS also have the ability to work as signalling molecules, at lower cellular concentrations, and regulate plant development, as well as various aspects of stress tolerance (Ismail et al. 2014). Interestingly, plants have evolved cellular repair mechanisms to maintain cellular redox balance and to convert oxidized macromolecules back to their reduced states (Krishnamurthy and Rathinasabapathi 2013). These mechanisms involve the induction of plant antioxidant systems, with enzymatic and non-enzymatic components, to detoxify or scavenge ROS (Khare et al. 2015). Upon exposure of plants to abiotic stressors, metabolic shifts occur, which result simultaneously to changes in the levels and range of cellular metabolites (Chen and Murata 2011). As a result, plants accumulate common cell solutes such as carbohydrates, organic acids and inorganic ions, which contributes to enhanced stress tolerance. However, high concentrations of these common solutes can if not localized inhibit enzyme activities and so plants often accumulate these solutes in vacuoles, where their increasing concentrations do not harm cellular metabolism (Kurepin et al. 2015).
In addition to these common solutes, plants often produce compatible solutes, or compatible osmolytes, which are membrane-impermeable solutes that accumulate in the cytoplasm to very high concentrations (C ≥ 0.2 M) in response to stress (Kurepin et al. 2015). One of the best-documented and important abiotic stress-responsive mechanisms adopted by plants is the biosynthesis and accumulation of compatible osmolytes. Compatible osmolytes are found in many living organisms, ranging from bacteria to plants and animals, and show considerable chemical diversity among living organisms. As they accumulate in the cytoplasm and remain non-toxic, even at molar concentrations, in response to water deficit they are also often called osmoprotectors. The most common compatible osmolytes include amino acids (proline, glutamate, glutamine and alanine) and their derivatives (ectoine and hydroxyectoine), quaternary amines (glycinebetaine, polyamines and dimethyl sulfonioproprionate), sugars (trehalose) and polyols including sugar alcohols (mannitol, sorbitol, pinitol, glycerol and galactinol) (Khan et al. 2009; Jewell et al. 2010; Kumar and Khare 2015). These osmoprotectors have wide-spectrum functions including scavenging of ROS, balancing cell redox, acting as osmoprotectants or osmoticums, and the stabilization of cytosolic pH, proteins, enzymes and membranes, in addition to acting as a potential source of carbon and nitrogen for plants both during stress events and the subsequent recovery phases (Kumar and Khare 2015). Amongst these, one of the most efficient compatible solutes is glycinebetaine (abbreviated as GB; N,N,N-trimethylglycine), which helps to protect plants against the stress-induced oxidative damage (Wani et al. 2013). The numerous properties of GB include a antichaotropic function related to its zwitterionic nature (Papageorgiou et al. 1985), a low molecular weight, a high solubility and a low viscosity, all of which make GB one of the most efficient osmoregulators (Yancey 2005; Kurepin et al. 2015). This chapter aims to describe and discuss various aspects of GB-mediated oxidative stress tolerance in plants.
Biosynthesis of Glycinebetaine
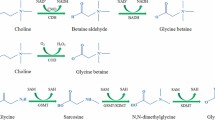
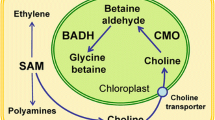
GB is a quaternary ammonium compound that occurs naturally in most biological systems ranging from prokaryotes, eukaryotic microorganisms, most animals, plants and microorganisms like cyanobacteria, algae and fungi. GB is synthesized via two pathways, using choline and glycine as respective substrates. In plants, the key enzyme for choline synthesis is phosphoethanolamine N-methyltransferase (PEAMT; EC 2.1.1.103), a cytosolic enzyme that catalyses all three of the methylation steps required to convert phospohoethanolamine to phosphocholine, the precursor to choline biosynthesis (McNeil et al. 2001). In plants choline is then transported into the chloroplast where it undergoes a two-step oxidation reaction: first choline is oxidized to betaine aldehyde, a toxic intermediate, which then is oxidized to GB. The first oxidation is catalysed by choline monooxygenase (CMO, EC 1.14.15.7), an unusual ferredoxin-dependent soluble protein with a motif characteristic of Rieske-type iron-sulphur proteins. In animals and bacteria this oxidation reaction is catalysed by choline dehydrogenase (CDH; encoded by the betA gene), but some bacteria may also use choline oxidase for the first step GB synthesis. The second oxidation step is catalysed by NAD+-dependent betaine aldehyde dehydrogenase—BADH, EC 1.2.1.8, in most organisms (Rathinasbapathi et al. 1997), although in some bacteria CDH and choline oxidase can also catalyse the second step. In higher plants, GB can also be synthesized in the chloroplast from serine via ethanolamine and betaine aldehyde (Rhodes and Hanson 1993). Although both CMO and BADH are localized in the stroma of chloroplasts, they are encoded by nuclear genes and contain transit sequences targeting them to chloroplasts. An alternate biosynthetic pathway of betaine from glycine, catalysed by two N-methyltransferase enzymes, has been reported for cyanobacterium and Arabidopsis, and it was found that the co-expression of N-methyltransferase genes caused accumulation of betaine that confers stress tolerance. Interestingly, in some naturally GB-accumulating plants, including mangrove (Hibino et al. 2001) and barley (Fujiwara et al. 2008), no CMO activity was detected in chloroplasts.
Though, GB is found in many plant species, its distribution is sporadic amongst them. For instance, many higher plants, including Arabidopsis and tomato, have been reported not to accumulate GB. While GB is a small organic metabolite and is highly soluble in water, it also contains a non-polar moiety consisting of 3-methyl groups. Owing to its unique structural features, it has the ability to interact with the hydrophobic and hydrophilic domains of macromolecules including proteins (Gupta and Huang 2014).
Transportation and Translocation of Glycinebetaine in Plants
Though, little is known about the transport of GB in plant cells, it is likely that transporters of GB are located in the plasma and chloroplast membranes, but no GB-specific transporters have been reported to date (Chen and Murata 2011). The transport of GB from the cytosol to various subcellular compartments is also poorly understood. In spinach, GB levels in the chloroplasts of unstressed spinach plants were ~0.7 μmol mg−1 against 6.6 μmol mg−1 chlorophyll in stressed plants’ chloroplasts indicating most GB accumulation in response to salt stress was found in the chloroplasts. This concentration gradient across the chloroplast envelope suggests the existence of a specific transport mechanism. Schwacke et al. (1999) demonstrated that the product of the tomato gene LeProT1, a homologue of a proline transporter in Arabidopsis, transported GB with high affinity and both proline and γ-amino butyric acid (GABA) with low affinity, when expressed in yeast. Similar results were also reported for the GABA and proline transporter, ProT2, whose gene was cloned from Arabidopsis (Breitkreuz et al. 1999). Here, the ProT2-mediated transport of GABA/proline was strongly inhibited by GB, indicating that GB had strong affinity for the transporter. These results suggest that the transporters of both proline and GABA might also transport GB. However, Ueda et al. (2001) cloned the gene for a proline transporter (HvProT) from the roots of salt-stressed barley and demonstrated that the uptake of proline by the yeast cells expressing HvProT was not inhibited by GB, suggesting that HvProT does not act as a transporter of GB.
GB translocation was studied with (14C)-labelled GB in barley (Ladyman et al. 1980), tomato, pea, soybean and turnip (Makela et al. 1996) and the results revealed the translocation of GB within 2 h from the roots to the leaves via the phloem and labelled GB was found throughout the plant within 24 h. Heat girdling of the leaf sheaths of barley plants prevented the export of [14C]-GB from the leaf blades. It appears that GB, synthesized by mature leaves during exposure of plants to abiotic stress, behaves as an inert end product, which upon re-watering of plants is translocated to the expanding leaves. Makela et al. (1996) found similar results for tomato plants.
The GB biosynthesis pathway is present in many higher plant species, including alfalfa (Medicago sativa L.; Wood et al. 1991), algarrobo (Prosopis alba Griseb.; Meloni et al. 2004), barley (Hordeum vulgare L.; Ladyman et al. 1983; Kishitani et al. 1994; Hattori et al. 2009), bean (Phaseolus vulgaris L.; Gadallah 1999), cotton (Gossypium hirsutum L.; Desingh and Kanagaraj 2007), corn (Zea mays L.; Quan et al. 2004), pea (Pisum sativum L.; Takhtajan 1980), sorghum [Sorghum bicolor (L.) Moench; Mickelbart et al. 2003], spinach (Spinacia oleracea L.; McCue and Hanson 1990), strawberry (Fragaria × ananassa Duchesne; Rajashekar et al. 1999), townsend’s cordgrass (Spartina × townsendii H. Groves and J. Groves; Storey et al. 1977) and wheat (Triticum aestivum L.; McDonnell and Jones 1988; Wang et al. 2010). However, some plant species exhibit undetectable levels of GB when exposed to abiotic stress, e.g. Arabidopsis thaliana (Hibino et al. 2002), eggplant (Solanum melongena L.; de Zwart et al. 2003), potato (Solanum tuberosum L.; de Zwart et al. 2003), tobacco (Nicotiana tabacum L.; Nuccio et al. 1998), tomato (Solanum lycopersicum L.; Park et al. 2004) and rice (Oryza sativa L.; Sakamoto and Murata 1998) are reported to have no detectable accumulation of GB in response to abiotic stress . Furthermore, in sugar beet (B. vulgaris), betaine applied exogenously to old leaves was translocated preferentially to young leaves and roots (Yamada et al. 2009). When GB was applied to individual mature leaves of tomato plants, a large fraction of the incorporated GB were translocated to meristem-containing tissues, which included flower buds and shoot apices (Park et al. 2006). Variations in the levels of GB in different plant organs indicate active and, possibly, regulated translocation from the original site of application accumulation/storage. Translocation of GB with photosynthetic assimilates to actively growing and expanding parts of plants has also been reported, indicating that the long-distance transport of GB is phloem related (Makela et al. 1996). In GB-accumulating transgenic Arabidopsis (Sulpice et al. 2003) and tomato plants (Park et al. 2004, 2007), the highest levels of GB are found in actively growing tissues, such as flowers and shoot apices, indicating that GB is efficiently translocated from source to sink tissues via the phloem.
Cellular Glycinebetaine Accumulation and Abiotic Stress Tolerance
As detailed earlier, although GB biosynthesis takes place in many plant species, it does not appear to be ubiquitous in all species. Plant species such as eggplant, potato, Arabidopsis, tomato and many cultivars of rice are reported not to accumulate the detectable amounts of GB (Kurepin et al. 2015). Therefore, genes associated with GB biosynthesis, of plant or bacterial origin, have been introduced/overexpressed in these non-accumulators of GB (Chen and Murata 2011). Such studies have resulted in a better understanding of the roles of GB in plants stress responses. Levels of accumulated GB are generally directly correlated with the plants’ tolerance to abiotic stresses (Giri 2011; Chen and Murata 2011; Wani et al. 2013). GB has been reported to accumulate intra-cellularly to high concentrations as a result of biosynthesis, uptake, or both, abiotic stresses in a variety of plants (Bhuiyan et al. 2007; Hassine et al. 2008; Hattori et al. 2009; Wang et al. 2010). However, Sarwas et al. (2006) reported that endogenous GB levels varied greatly between various cotton (Gossypium hirsutum L.) genotypes, as did the tolerance to drought stress, and the authors observed that genotypes with increased drought tolerance had higher endogenous GB levels.
Many halotolerant plants accumulate GB in their chloroplasts and plastids to increase tolerance against range of abiotic stresses (Chen and Murata 2008). GB-mediated enhancement of tolerance to abiotic stresses may be attributed to the ability of GB to protect the functioning of the photosynthetic apparatus, by protecting the enzymes and lipids required to maintain optimal linear electron flow through the electron transports chains embedded in the thylakoid membranes and to maintain CO2 assimilation (Sakamoto and Murata 2002; Chen and Murata 2011). Besides the earlier protective roles, GB also helps to limit stress-induced inactivation of the PSII complex, which is considered the most vulnerable component of the photosynthetic apparatus and plays a pivotal role in the photosynthetic responses of plants to abiotic stress (Murata et al. 1992; Allakhverdiev et al. 2003, 2007; Adams et al. 2013).
Due to its properties as a compatible osmolyte, GB protects cells against osmotic inactivation via increasing water retention (Sakamoto and Murata 2002; Ashraf and Foolad 2007; Kurepin et al. 2015). Since compatible osmolytes like GB remain uncharged at neutral pHs and are highly soluble in water, they are excluded from the hydration sphere of proteins and help to stabilize the proteins (Low 1985). GB stabilizes the quaternary structures of enzymes and other complex proteins, as well as maintaining the ordered state of membranes, at non-physiological temperatures and high salt concentrations (Papageorgiou and Murata 1995). The effects of GB in mitigating the detrimental effects of oxidative bursts induced by various abiotic stressors are well established. GB acts as an activator or stabilizer of some ROS-scavenging enzymes or as a repressor of ROS production, by the mechanisms largely unknown. GB is known for its strong protective roles in the reproductive organs of plants growing under abiotic stress conditions, which is considered critical for maintaining high crop yields (Chen and Murata 2008).
ROS Production Under Abiotic Stress
As described earlier, the electrons that have a high-energy state are transferred to molecular oxygen (O2) to form ROS (Mittler 2002), which comprise of singlet oxygen (1O2), super oxide anion (O2 •−), hydrogen peroxide (H2O2) and hydroxyl radical (OH•). In plants, ROS are primarily formed at low levels as by-products of several aerobic metabolic processes like photosynthesis and respiration in the organelles such as chloroplasts, mitochondria, peroxisomes, plasma membranes, endoplasmic reticulum, cell walls and the apoplastic space (Rhoads et al. 2006; Møller et al. 2007; Ahmad et al. 2010a, b; Sharma et al. 2012). During abiotic and biotic stress conditions, the generation rates of ROS are highly elevated (Ahmad et al. 2009, 2010a, b; Sharma et al. 2012; Mostofa et al. 2015a, b) leading to the onset of oxidative stress (Mittler 2002; Mittler et al. 2011; Kotchoni et al. 2006; Hossain et al. 2015).
The light-dependent electron transport chains (ETCs) in photosystems I (PS I) and II (PS II) are considered to be the main source of ROS in plant cells (Doyle et al. 2010; Khanna-Chopra 2011; O’Brien et al. 2012). ROS production by these sources is enhanced by conditions limiting CO2 fixation, such as drought, salt, extreme temperatures and high light (Sharma et al. 2012). In response to salinity and drought stress, plants decrease their stomatal conductance, to reduce excess water loss, which ultimately leads to a decrease in internal CO2 concentrations and slows down the reduction of CO2 by the Calvin cycle and induces photorespiration (Abogadallah 2010; Sanda et al. 2011). High temperatures suppress the carboxylation reaction catalysed by ribulose 1,5-bisphosphate carboxylase (RuBisCO) by reducing the specificity of the enzyme for CO2 (Kim and Portis 2004; Kaushal et al. 2011), whereas low temperatures slow the activities of the Calvin cycle enzymes, causing NADP+ depletion (Wise 1995). With respect to cadmium (Cd) stress metal ions, such as Ca2+ and Mn2+, present in the PS II centre can be replaced by Cd ions, thereby limiting photosystem reactions and leading to uncoupling of electron transport in the chloroplast (Mohanty and Mohanty 1988; Atal et al. 1991).
Mitochondria can also produce ROS in plants. Under normal aerobic conditions, electron transport and ATP syntheses are tightly coupled, but stress can lead to changes in the mitochondrial electron transport chains (ETC) that can lead to overreduction of electron transporters and the excess production of ROS (Noctor et al. 2007; Blokhina and Fagerstedt 2006). Increased ROS production as a result of ETC perturbations has been reported in plants exposed to chilling (Prasad et al. 1994a, b; Purvis et al. 1995), salinity (Hernández et al. 1993; Mittova et al. 2003), high temperatures (Schwarzlander et al. 2009), exposure to Cd (Schwarzlander et al. 2009) and phosphate deficiency (Juszczuk et al. 2001; Malusa et al. 2002). Metal ions such as Fe, Cu and Zn are essential for the proper functioning of the mitochondrial enzymes involved in the TCA cycle, ATP synthesis, electron transport and antioxidant defences (Tan et al. 2010; Nouet et al. 2011). ROS production in theendoplasmic reticulum (ER) could facilitate the transmission of toxic Cd2+ ions at the ER–mitochondria interface. Again, H2O2 could diffuse out of the ER and attack the membranes of neighbouring mitochondria, bypassing the protection conferred by mitochondrial SOD that is located in the mitochondrial matrix (Karuppanapandian et al. 2011).
Peroxisomes compartmentalize the enzymes involved in the β-oxidation of fatty acids, the C2 photorespiratory cycle and they are major sites of intracellular H2O2 production due to their essentially oxidative metabolism (del Río et al. 2006). Peroxisomes produce O2 •− as a consequence of normal metabolism (Corpas et al. 2001), with three integral peroxisomal membrane polypeptides (PMPs), with molecular masses of 18, 29 and 32 kDa embedded in the membrane, having been shown to form O2 •− (del Río et al. 2002).
In addition to the earlier sites of ROS production, plant cells have several other potential sites of ROS production. Electron transporting oxidoreductases are ubiquitous in plasma membranes and can generate ROS. Plasma membrane-bound NADPH oxidases have been proposed to play key roles in the production and accumulation of ROS in plants and are involved in responses to various abiotic stressors (Orozco-Cardenas et al. 2001; Kwak et al. 2003), including nutrient deficiency or excess of Cd, copper (Cu) and nickel (Ni) (Quartacci et al. 2001; Hao et al. 2006). Cell walls are also regarded as active sites for ROS production as cell wall-associated peroxidases and oxidases are involved in H2O2 generation. ROS generation by cell-wall-located peroxidases has been shown to occur during the hypersensitive response (HR) , triggered in cotton by the bacterium Xanthomonas campestris pv. malvacearum (Martinez et al. 1998), and potassium (K) deficiency in Arabidopsis (Kim et al. 2010; Higuchi 2006). Production of O2 •− and H2O2, was noted in the cell walls of maize roots (Liszkay et al. 2004) and OH• generation was demonstrated in vivo and in vitro in the cell walls of several other plant species (Schopfer 2001; Spiteller 2003). The apoplast is also an important site for ROS accumulation in response to abiotic stressors, such as drought, salinity, high and low temperatures, ozone and high light (Hernández et al. 2001; Zhu 2001; Miller et al. 2009; Vahisalu et al. 2010) and the cell-wall-located enzymes have been shown to be responsible for apoplastic ROS production (Apel and Hirt 2004; Heyno et al. 2011). H2O2 accumulation in the apoplast is involved in the acclimation responses of plants, such as modulation of growth rate and cell wall strengthening, to drought and salt stress (Hernández et al. 2001; Zhu 2001; Rodríguez et al. 2004).
ROS Scavenging and Detoxification by Antioxidants
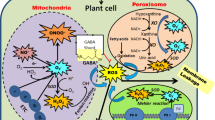
Plants possess complex antioxidant defence systems to protect cellular components from oxidative damage. These systems include enzymatic and non-enzymatic components to scavenge and detoxify ROS. The enzymatic antioxidants include multiple superoxide dismutases (SOD; EC 1.15.1.1), ascorbate peroxidase (APX; EC 1.11.1.11), monodehydroascorbate reductase (MDHAR; EC 1.6.5.4), dehydroascorbate reductase (DHAR; EC 1.8.5.1), glutathione reductase (GR; EC 1.6.4.2), catalase (CAT; EC 1.11.1.6), glutathione peroxidase (GPX; EC 1.11.1.9), glutathione S-transferase (GST; EC 2.5.1.18), peroxidase (POX, EC 1.11.1.7), guaiacol peroxidase (GPOX; EC 1.11.1.7), and the non-enzymatic antioxidants include ascorbate (AsA), glutathione (GSH), tocopherol, carotenoids, flavonoids, proline and GB (Ahmad et al. 2010a, b; Gill and Tuteja 2010; Hossain et al. 2011a, b; Sharma et al. 2012; Mostofa et al. 2015a, b). Increased levels of ROS scavenger enzymes, in response to various abiotic stress, are found in different cellular locations, e.g. chloroplasts, mitochondria, the plasma membrane and cell wall (Das et al. 2015; Hossain et al. 2015). ROS scavenging and detoxifying in the different cellular compartments represents a coordinated response (Pang and Wang 2008). The key ROS detoxification systems found in plants are shown in Fig. 5.1. Various components of the antioxidant defence systems found in plants have been manipulated, overexpressed or down-regulated to aid in our understanding of the roles antioxidant systems play in the responses of plants to stress (Das et al. 2015).
Reactive oxygen species detoxification systems in plants (modified from Hossain et al. 2014). SOD, CAT, APX, GPX and GST are the proteins responsible for eliminating ROS. Enzymes that promote the elimination of ROS via the ascorbate-glutathione cycle are APX, MDHAR, DHAR and GR. The elimination of ROS by non-enzymatic processes is carried out by carotenoid (vitamin A), α-tocopherol (vitamin E), AsA (vitamin C) and glutathione. Superoxide produced in different cell organelles is rapidly converted to H2O2 by SOD, which, in turn, is converted to H2O by APX and CAT. The oxidation of AsA caused by ROS or by APX leads to the formation of monodehydroascorbate (MDHA) and dehydroascorbate (DHA). MDHA is reduced to AsA by MDHAR with the utilization of NADPH and DHA is converted to AsA by DHAR with the utilization of GSH. GR is responsible for recycling of GSSG to GSH by the expense of NADPH. GST and GPX catalyse the GSH-dependent reduction of H2O2 and organic peroxides, including lipid peroxides to H2O or alcohols. During lipid peroxidation, carotenoid, α-tocopherol and AsA help regenerate GSSG back into GSH through vitA, vit E and vit C cycle. Abbreviations are defined in the text
The Molecular Mechanisms of ROS Quenching by Glycinebetaine in Plants Under Stress
Though, GB does not appear to scavenge the ROS directly, various studies have attributed the ability of GB to protect photosystems to ROS scavenging (Murata et al. 2007; Chen and Murata 2011; Giri 2011). Excessive light causes inactivation of PSII, a phenomenon known asphotoinhibition , which is an unavoidable process in photosynthetic organisms, due to light being the driving force of photosynthesis (Chen and Murata 2011). Under normal conditions plants can efficiently repair of PSII (Aro et al. 1993), but excessive generation of ROS in stressed plants can interfere with PSII repair, mainly via suppression of de novo protein synthesis (Takahashi and Murata 2006).
Various transgenic studies support the role of GB in ROS scavenging and detoxification . For example, Kathuria et al. (2009) concluded that GB plays a significant role in reducing the accumulation of and in the detoxification of ROS in transgenic plants overexpressing genes encoding enzymes involved in GB biosynthesis, compared to their wild-type plants. In addition, GB has been reported to lower the rate of membrane lipid peroxidation, a consequence of oxidative stress, via inducing the expression of fatty acid desaturase and lipoxygenase genes, and therefore helping to maintain membrane integrity in tomato plants subjected to low temperature stress (Karabudak et al. 2014). GB has also been reported to help maintain ROS homeostasis in wheat plants under salinity stress by up-regulating the transcription of alternative oxidase (AOX), H+/Na+ antiporter exchanger (NHX1) and salt overly sensitive 1 (SOS1) genes (Badran et al. 2015). Cruz et al. (2013) critically examined the effectiveness of application of GB to Carapa guianensis plants growing under water deficit, and they observed that GB caused a significant increase in APX activity and attenuated lipid peroxidation in stressed plants.
Enhanced Abiotic Oxidative Stress Tolerance via the Exogenous Application of Glycinebetaine
All forms of abiotic stress , such as salinity, drought, chilling, freezing, heat and heavy metals, can cause an excessive accumulation of ROS leading to irreparable dysfunction and death in plants. In this section, we will discuss the involvement of exogenous GB in modulating ROS and MG detoxification systems as a means of inducing oxidative stress tolerance.
A series of recent experiments have shown that exogenous application of GB to plants increases abiotic oxidative stress tolerance (Hossain et al. 2010, 2011a, b, 2014; Hu et al. 2012; Anjum et al. 2012; ffSorwong and Sakhonwasee 2015). Park et al. (2006) reported that exogenous application of GB induces chilling tolerance in a GB non-accumulating variety of tomato (Lycopersicon esculentum Mill. cv. Moneymaker). After 2 days of chilling treatment GB-treated plants had lower H2O2 levels and higher CAT activities than control plants. In was concluded that GB-induced chilling tolerance involves the induction of H2O2 detoxifying antioxidant defence systems. Even pre-treatment of seeds with GB can enhance chilling tolerance in hybrid maize (Zea mays L.), through the maintenance of higher water contents; reduced electrolyte leakage (EL) and higher SOD, CAT and APX activities (Farooq et al. 2008a).
The role of GB in modulating salinity-induced oxidative stress tolerance has been well documented in plants. Hoque et al. (2007) showed that exogenous GB enhances salinity-induced oxidative stress tolerance in cultured tobacco (BY-2) cells, by modulating the activities of AsA-GSH cycle enzymes. In addition, it has also been shown that exogenous GB application enhances salt tolerance by reducing protein oxidation and by modulating GST, GPX and glyoxalase system enzymes activities (Hoque et al. 2008). Nawaz and Ashraf (2010) studied the role of exogenous GB application as a modulator of salt tolerance in two maize (Zea mays L.) genotypes. Salt stress led to a decrease in photosynthetic activity, chlorophyll content and SOD activity in both of maize cultivars. Exogenous application of GB significantly enhanced the photosynthetic capacity and the activities of SOD, CAT and POD in treated plants compared to controls. These results suggested that GB-induced enhancement in antioxidant enzyme activities might help to protect chloroplasts from salt-induced oxidative damage. In a study of mung bean (Vigna radiata) seedlings under salinity stress, salt led to a robust increase in ROS and MDA levels. Exogenous application of GB significantly enhanced the activities of ROS and MG detoxification systems and reduced salt-induced oxidative damage, with lower ROS and MDA levels compared to the seedlings not treated with GB (Hossain et al. 2011a, b). Hu et al. (2012) showed that exogenous application of GB increased salinity tolerance in perennial ryegrass (Lolium perenne). Plants subjected to salt stress showed higher EL, MDA and proline contents than control plants, but exogenous application of GB reduced the EL, MDA and proline contents under salt stress. Salt stress significantly reduced the activities of the antioxidant enzymes SOD, CAT and APX. Importantly, addition of GB increased activities of ROS detoxifying enzymes. In addition, GB treatment reduced the Na+ accumulation whereas increased the K+ content of shoots, which led to a higher K+/Na+ ratio under saline conditions. These results indicated that GB-induced salt tolerance is at least in part due to higher SOD, CAT and APX activities, and improved ion homeostasis, resulting in less ROS induced damage. Recently, Hasanuzzaman et al. (2014) showed that exogenous application of GB (5 mM) to rice seedlings enhanced salinity-induced oxidative stress tolerance through the up-regulation of the ROS and MG detoxification pathways. Yildirim et al. (2015) showed that exogenous application of GB to lettuce plants could ameliorate the harmful effects of salt stress by reducing lipid peroxidation, H2O2 levels and membrane permeability. Importantly, exogenous application of GB also found to increase the gibberellic acid (GA) and salicylic acid (SA) and indole acetic acid (IAA) content under salt stress condition.
The protective roles of GB have also reported in plants subjected to drought stress. Farooq et al. (2008b) showed that exogenous application of GB enhanced drought tolerance in fine grain aromatic rice (Oryza sativa L.). Drought stress greatly reduced rice growth while GB application improved plant growth both under well watered and drought conditions. Importantly, foliar application of GB under drought stress significantly altered the level of ROS and MDA and increased the activities of SOD, APX and CAT. Farooq et al. (2008b) concluded that GB-induced increased antioxidant production reduced the oxidative damage in plants under drought stress. Anjum et al. (2012) showed that exogenous application of GB modulates drought stress tolerance in two maize cultivars contrasting of their drought stress tolerance. Prolonged drought stress increased lipid peroxidation whereas GB treatment significantly reduced oxidative damage, as indicated by lower MDA levels. The activities of POD, SOD and CAT increased initially but subsequently declined with continued drought stress. Importantly, GB-treated plants maintained higher levels of ROS detoxifying enzymes that would have contributed to greater stress tolerance and improved growth and yields. Recently, Molla et al. (2014) showed that exogenous application of GB reduces drought-induced oxidative stress in lentil (Lens culinaris) seedlings. Drought stress led to a significant increase in oxidative stress, as indicated by higher H2O2 and increased glutathione disulphide levels (GSSG). Exogenous application of GB (15 mM) to drought stressed plants resulted in a significant increase in the GSH content and the activities of the enzymes GST and Gly I, with a simultaneous reduction in GSSG and H2O2 levels. Molla et al. (2014) also suggested that exogenous GB enhances drought stress tolerance by limiting H2O2 accumulation and by increasing the activities of the antioxidant and glyoxalase systems. Additionally, Hossain et al. (2014) showed that exogenous application of GB to mustard (Brassica juncea) modulated drought-induced oxidative stress tolerance as indicated by higher ROS and glyoxalase pathway enzymes along with the lower level of H2O2 and lipid peroxidation (Hossain et al. 2014).
The roles of exogenous GB in Cd stress tolerance were studied by Islam et al. (2009a) in cultured tobacco BY-2 cells under Cd stress (100 μM Cd). Cd stress caused growth inhibition and oxidative damage, as indicated by higher MDA levels. Addition of GB caused an increase in endogenous GB, CAT activity, decreased MDA levels and lower Cd accumulation. In a second study, Islam et al. (2009b) showed that exogenous application of GB application also increased the activities of APX, DHAR, MDHAR and GR, which helped to protect key cellular components from Cd-induced oxidative damage (Islam et al. 2009b). Consequently, we (Hossain et al. 2010) showed that exogenous application of GB increased Cd tolerance in mung bean (Vigna radiata L.) seedlings. Imposition of short-term (24 h) Cd stress (1 mM) led to a significant increase in H2O2 and MDA levels in mung bean leaf tissues in comparison to control plants. Inactivation or insufficient up-regulation of MG and ROS detoxifying enzymes such as APX, MDHAR, DHAR, GPX, GST, CAT, Gly I and Gly II and AsA and GSH contents was found in seedlings subjected to Cd stress. Surprisingly, 5 mM GB application favourably modulated the ROS and MG detoxifying enzyme activities and the glutathione redox state, making the plants more tolerant to Cd stress-induced oxidative damage.
Duman et al. (2011) studied the effects of exogenous GB application on the responses of duckweed (Lemna gibba L.) to Cd exposure. Duckweed samples were subjected to various concentrations of Cd for 6 days in the absence or presence of GB. Treatment with GB had no significant influence on Cd accumulation, but GB had a significant influence on endogenous proline accumulation, ROS detoxifying enzyme activities, the level of lipid peroxidation and photosynthetic activity. They concluded that GB has a defensive role in plants exposed to Cd, reducing both ROS and MDA levels. Cao et al. (2013) showed that exogenous application of GB induces oxidative stress tolerance in rice seedlings exposed to Cd. Rice seedlings, pre-treated with 100 μM GB and then exposed to Cd for 5 days, had greater root lengths, fresh and dry weights, higher chlorophyll contents and less ROS-induced damage, as shown by lower MDA levels, and higher SOD activities, in stem tissues, compared to control plants not pre-treated with GB. Recently, Ali et al. (2015) showed the exogenous application of GB enhanced chromium tolerance in wheat (Triticum aestivum L.). Cr stress significantly inhibited growth, chlorophyll and protein contents, and increased antioxidant enzyme activities. Foliar application of GB (0.1 mM) under Cr stress reduces Cr accumulation in grains and modulated the activities of APX and CAT in root and shoot tissues. Increased antioxidant enzyme activities with GB application under Cr stress might be one of the possible mechanisms of GB-induced metal tolerance in plants. Additionally, Lou et al. (2015) showed that GB application induces Cd stress on perennial ryegrass (Lolium perenne). Cd stress resulted in a decrease in turf quality, shoot growth, transpiration rates and Chl contents, with significant increases in EL, MDA content, SOD, CAT, POD activities, and oxalic and tartaric acid levels. Exogenous applications of GB (20 mM) reversed the adverse impacts of Cd stress. Their findings suggested that GB could alleviate the detrimental effects of Cd on perennial ryegrass and that amelioration was mainly related to elevation of SOD, CAT and POD activities and higher stress responsive gene expression.
Sorwong and Sakhonwasee (2015) showed that GB can enhance heat stress tolerance in marigold cultivars. Heat stress caused photoinhibition and lower CO2 assimilation, stomatal conductances and transpiration rates in heat-treated marigold plants compared to control plants grown at a constant 25 °C. Significant increase in H2O2, lipid peroxidation and cell death in all cultivars were observed under heat stress. Foliar application of GB significantly reduced the levels of H2O2, superoxide and MDA. Sorwong and Sakhonwasee (2015) conduced that the mechanisms of GB-induced heat stress tolerance involved protection of the photosynthetic machinery, increased gas exchange and ROS detoxification.
From the above reports it has become clear that GB plays a pivotal role in keeping ROS levels, induced by various abiotic stressors, under control by regulating the activities of enzymes involved in ROS scavenging and detoxification, and also by regulating the glyoxalase system. However, more in-depth studies might also reveal subtler regulatory roles for GB in modulating abiotic stress tolerance.
Glycinebetaine-Accumulating Transgenic Plants and Abiotic Oxidative Stress Tolerance
The cloning of various genes (codA and BADH) encoding enzymes that catalyse the biosynthesis of GB has been reported, and many lines of transgenic plants have been produced expressing GB biosynthetic genes with enhanced abiotic and abiotic oxidative stress tolerance in plants (Yang et al. 2007; Ahmad et al. 2010a, b; Zhang et al. 2011; Fan et al. 2012; Li et al. 2014a, b; Di et al. 2015).
Yang et al. (2007) reported that transgenic tobacco plants overexpressing a BADH gene showed enhanced heat stress tolerance. The activities of antioxidant enzymes (APX, MDHAR, DHAR, GR and CAT) all decreased in response to heat stress in wild-type (WT) plants, but in the transgenic plants the activity of many of these enzymes increased significantly or remained unchanged, and the levels of AsA and GSH were higher in the transgenic plants. These findings suggest that over-accumulation of GB in transgenic plants could lower ROS levels, which contributes to heat stress tolerance. Ahmad et al. (2010a, b) found that overexpression of multiple genes (codA, SOD and APX) in potato plants enhanced stress tolerance, as compared to plants overexpressing only a SOD or APX gene. Transgenic plants expressing multiple genes showed higher methyl viologen (MV)-induced oxidative stress tolerance, as compared to the single gene transgenic plants. Additionally, plants overexpressing three genes showed higher SOD, APX and CAT activities as compared to wild type or APX or SOD-expressing plants, under salt or drought stress. The synergistic effects of GB, SOD and APX appeared to help the transgenic plants grow and develop better under conditions of abiotic stress.
Zhang et al. (2011) showed that transgenic cotton (Gossypium hirsutum L.) plants overexpressing a betA gene (a gene for GB synthesis) showed enhanced salinity tolerance, as indicated by higher rates of photosynthesis, better osmotic adjustment, higher relative water contents, and lower levels of lipid peroxidation and ion leakage. Fan et al. (2012) showed that transgenic sweet potato (Ipomoea batatas) plants overexpressing a GB biosynthetic gene (BADH) had enhanced abiotic stress tolerance. Transgenic plants maintained higher photosynthetic activities, and lower H2O2 and MDA levels under salt, chilling and MV-induced oxidative stress. Transgenic plants also showed higher SOD gene expression and enzyme activities. Higher expression levels of CAT, APX, MDHAR, DHAR, GR, GPX and POD genes were also observed in plants under salt, drought and MV stress. Fan et al. (2012) concluded that better abiotic stress tolerance in transgenic plants was in part due to improved ROS scavenging. Similarly Li et al. (2014a) showed that transgenic tomato (Lycopersicon esculentum cv. ‘Moneymaker’) plants overexpressing a BADH gene exhibited higher heat stress (42 °C) tolerance as compared to WT plants. Transgenic plants showed higher photosynthetic activities, and lower levels of H2O2 and superoxide and lipid peroxidation as compared to WT plants. In addition, transgenic plants showed higher antioxidant enzyme activities under stressful conditions. In a second study, Li et al. (2014b) showed that transgenic alfalfa (Medicago sativa L. cv. Xinjiang Daye) plants overexpressing a codA gene showed enhanced tolerance to abiotic stress. Transgenic plants showed better tolerance to MV-induced oxidative stress and better salinity-induced oxidative stress tolerance, as indicated by higher Chl contents and lower MDA levels as compared to the WT plants. From the above reports it is evident that transgenic plants overexpressing GB biosynthetic genes have enhanced abiotic stress tolerance and oxidative stress tolerance.
Conclusion and Future Perspectives
Extensive work in recent past has confirmed that GB is an important compatible osmolyte with multiple functions in plant growth and survival, both under normal as well as stressful conditions. Plants accumulate GB in their tissues in response to and to counteract the deleterious effects of abiotic stresses and usually higher GB levels are not only correlated with, but also attributable to better plant stress tolerance. Besides protecting vital enzymes and membranes, GB can also mitigate ROS-mediated oxidative damage to plant cells and help to maintain the cellular redox balance, as well as controlling potential oxidative bursts.
Due to its wide spectrum of functions, GB biosynthetic pathway genes have been used to generate transgenic plants that accumulate GB and exhibit enhanced tolerance to various abiotic stresses, including secondary oxidative bursts. Recent scientific advancements have supported the role of GB in the prevention of excess ROS generation and oxidative stress in plants cells, when coupled with increased levels of ROS-scavenging enzymes. However, further research focused more on identifying genes associates with GB biosynthesis and explore the advantages of chloroplast engineering over its genome counterpart is required. Recent findings also suggested GB-mediated up-regulation of gene cascades, some of them with ROS scavenging roles, demonstrates a possible interaction between oxidative stress, gene expression and the accumulation of GB under condition of abiotic stress. The possibility of gene stacking and co-expressing various genes, with known antioxidant potential, with genes associated with GB biosynthesis needs to be explored to produce plants with enhanced oxidative stress tolerance. Further work is also needed to establish whether the transcript changes are direct targets of GB or is just produced by transgenic plants via metabolic adjustment.
References
Abogadallah GM (2010) Antioxidative defence under salt stress. Plant Signal Behav 5:369–375
Adams W, Muller O, Cohu C, Demmig-Adams B (2013) May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth Res 117:31–44
Ahmad P, Jaleel CA, Salem MA, Nabi G (2009) Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Bot Res Int 2:11–20
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010a) Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175
Ahmad R, Kim YH, Kim MD, Kwon SY, Cho K, Lee HS, Kwak SS (2010b) Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloroplasts provides synergistically enhanced protection against various abiotic stresses. Physiol Plant 138:520–533
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22:10669–10678
Allakhverdiev SI, Hayashi H, Nishiyama Y, Ivanov AG, Aliev JA, Klimov VV, Murata N, Carpentier R (2003) Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J Plant Physiol 160:41–49
Allakhverdiev SI, Los DA, Mohanty P, Nishiyama Y, Murata N (2007) Glycinebetaine alleviates the inhibitory effect of moderate heat stress on the repair of photosystem II during photoinhibition. Biochim Biophys Acta 1767:1363–1371
Anjum SA, Saleem MF, Wang LC, Bilal MF, Saeed A (2012) Protective role of glycinebetaine in maize against drought-induced lipid peroxidation by enhancing capacity of antioxidative system. Aust J Crop Sci 6:576–583
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aro E-M, Virgin I, Anderson B (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Ashraf M, Foolad MR (2007) Roles of glycinebetaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Atal N, Sardini PP, Mohanty P (1991) Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentration of cadmium: analysis of electron transport activities and changes in fluorescence yield. Plant Cell Physiol 32:943–951
Badran EG, Abogadallah GM, Nada RM, Alla MMN (2015) Role of glycine in improving the ionic and ROS homeostasis during NaCl stress in wheat. Protoplasma 252:835–844
Bhuiyan NH, Hamada A, Yamada N, Rai V, Hibino T, Takabe T (2007) Regulation of betaine synthesis by precursor supply and choline monooxygenase expression in Amaranthus tricolor. J Exp Bot 58:4203–4212
Blokhina O, Fagerstedt K (2006) Oxidative stress and antioxidant defenses in plants. In: Singh KK (ed) Oxidative stress, disease and cancer. Imperial College Press, London, pp 151–199
Breitkreuz KE, Shelp BJ, Fischer WN, Schwacke R, Rentsch D (1999) Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett 450:280–284
Cabello JV, Lodeyro AF, Zurbriggen MD (2014) Novel perspectives for the engineering of abiotic stress tolerance in plants. Curr Opin Biotechnol 26:62–70
Cao F, Ibrahim W, Cai Y, Wu F (2013) Alleviating effects of exogenous glutathione, glyicinebetaine, brassionsteroids and salicylic acid on cadmium toxicity in rice seedlings (Oryza sativa). Agrotechnology 2:107
Chen TH, Murata N (2008) Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci 13:499–505
Chen THH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Corpas FJ, Barroso JB, del Río LA (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6:145–150
Cruz FJR, Castro GLS, Silva Junior DD, Festucci-Buselli RA, Pinheiro HA (2013) Exogenous glycinebetaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild water-stressed Carapa guianensis plants. Photosynthetica 51:102–108
Das P, Nutan KK, Singla-Pareek SL, Pareek A (2015) Oxidative environment and redox homeostasis in plants: dissecting out significant contribution of major cellular organelles. Front Environ Sci 2:70
de Zwart FJ, Slow S, Payne RJ, Lever M, George PM, Gerrard JA, Chambers ST (2003) Glycine betaine and glycine betaine analogues in common foods. Food Chem 83:197–204
del Río LA, Corpas FJ, Sandalio LM, Palma JM, Gomez M, Barroso JB (2002) Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot 53:1255–1272
del Río LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol 141:330–335
Desingh R, Kanagaraj G (2007) Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. Gen Appl Plant Physiol 33:221–234
Di H, Tian Y, Zu H, Meng X, Zeng X, Wang Z (2015) Enhanced salinity tolerance in transgenic maize plants expressing a BADH gene from Atriplex micrantha. Euphytica 2015:1515
Doyle SM, Diamond M, McCabe PF (2010) Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J Exp Bot 61:473–482
Duman F, Aksoy A, Aydin Z, Temizgul R (2011) Effects of exogenous glycinebetaine and trehalose on cadmium accumulation and biological responses of an aquatic plant (Lemna gibba L.). Water Air Soil Pollut 217:545–556
Fan W, Zhang M, Zhang H, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7:e37344
Farooq M, Aziz T, Hussain M, Rehman H, Jabran K, Khan MB (2008a) Glycinebetaine improves chilling tolerance in hybrid maize. J Agron Crop Sci 194:152–160
Farooq M, Basra S, Wahid A, Cheema Z, Cheema M, Khaliq A (2008b) Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 194:325–333
Fujiwara T, Hori K, Ozaki K, Yokota Y, Mitsuya S, Ichiyanagi T, Hattori T, Takabe T (2008) Enzymatic characterization of peroxisomal and cytosolic betaine aldehyde dehydrogenases in barley. Physiol Plant 134:22–30
Gadallah MAA (1999) Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol Plant 42:249–257
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giri J (2011) Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav 6:1746–1751
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014:1–18
Hao F, Wang X, Chen J (2006) Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci 170:151–158
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycinebetaine mediated upregulation of antioxidant defence and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res Int 2014:1–17
Hassine AB, Ghanem ME, Bouzid S, Lutts S (2008) An inland and a coastal population of the Mediterranean xerohalophyte species Atriplex halimus L. differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress. J Exp Bot 59:1315–1326
Hattori T, Mitsuya S, Fujiwara T, Jagendorf AT, Takabe T (2009) Tissue specificity of glycinebetaine synthesis in barley. Plant Sci 176:112–118
Hernández JA, Corpas FJ, Gómez M, del Río LA, Sevilla F (1993) Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89:103–110
Hernández JA, Ferrer MA, Jiménez A, Barceló AR, Sevilla F (2001) Antioxidant systems and O2 •−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127:817–831
Heyno E, Mary V, Schopfer P, Krieger-Liszkay A (2011) Oxygen activation at the plasma membrane: relation between superoxide and hydroxyl radical production by isolated membranes. Planta 234:35–45
Hibino T, Waditee R, Araki E, Ishikawa H, Aoki K, Tanaka Y, Takabe T (2002) Functional characterization of choline monooxygenase, an enzyme for betaine synthesis in plants. J Biol Chem 277:41352–41360
Hibino T, Meng YL, Kawamitsu Y, Uehara N, Matsuda N, Tanaka Y, Ishikawa H, Baba S, Takabe T, Wada K, Ishii T (2001) Molecular cloning and functional characterization of two kinds of betaine-aldehyde dehydrogenase in betaine-accumulating mangrove Avicennia marina (Forsk.) Vierh. Plant Mol Biol 45:353–363
Higuchi T (2006) Look back over the studies of lignin biochemistry. J Wood Sci 52:2–8
Hoque MA, Banu MNA, Okuma E, Amako K, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J Plant Physiol 164:1457–1468
Hoque MA, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y (2008) Proline and glycinebetaine enhance antioxidant defence and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol 165:813–824
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plant 16:259–272
Hossain MA, Hasanuzzaman M, Fujita M (2011a) Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Front Agric China 5:1–14
Hossain MA, Teixeira da Silva JA, Fujita M (2011b) Glyoxalase system and reactive oxygen species detoxification system in plant abiotic stress response and tolerance: an intimate relationship. In: Shanker AK, Venkateswarlu B (eds) Abiotic Stress/Book 1. INTECH Open Access Publisher, Rijeka, pp 235–266
Hossain MA, Mostofa MG, Burritt DJ, Fujita M (2014) Modulation of reactive oxygen species and methylglyoxal detoxification systems by exogenous glycinebetaine and proline improves drought tolerance in mustard (Brassica juncea L.). Int J Plant Biol Res 2:2014
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide-priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420
Hu L, Hu T, Zhang X, Pang H, Fu J (2012) Exogenous glycinebetaine ameliorates the adverse effect of salt stress on perennial ryegrass. J Am Soc Hort Sci 137:38–46
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2009a) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166:1587–1597
Islam MM, Hoque MA, Okuma E, Jannat R, Banu MNA, Jahan MS, Nakamura Y, Murata Y (2009b) Proline and glycinebetaine confer cadmium tolerance on tobacco Bright Yellow-2 cells by increasing ascorbate-glutathione cycle enzyme activities. Biosci Biotechnol Biochem 73:2320–2323
Ismail A, Takeda S, Nick P (2014) Life and death under salt stress: same players, different timing? J Exp Bot 65:2963–2979
Jewell MC, Campbell BC, Godwin ID (2010) Transgenic plants for abiotic stress resistance. In: Kole C, Michler C, Abbott AG, Hall TC (eds) Transgenic crop plants. Springer, Berlin, pp 67–132
Juszczuk IM, Wagner AM, Rychter AM (2001) Regulation of alternative oxidase activity during phosphate deficiency in bean roots (Phaseolus vulgaris). Physiol Plant 113:185–192
Karabudak T, Bor M, Özdemir F, Türkan İ (2014) Glycinebetaine protects tomato (Solanum lycopersicum) plants at low temperature by inducing fatty acid desaturase7 and lipoxygenase gene expression. Mol Biol Rep 41:1401–1410
Karuppanapandian T, Moon JC, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725
Kathuria H, Giri J, Nataraja KN, Murata N, Udayakumar M, Tyaqi AK (2009) Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol J 7:512–526
Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, Nayyar H (2011) Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol Mol Biol Plant 17:203–213
Khan MS, Yu X, Kikuchi A, Asahina M, Watanabe K (2009) Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol 26:125–134
Khanna-Chopra R (2011) Leaf senescence and abiotic stresses share reactive oxygen species mediated chloroplast degradation. Protoplasma 249:469–481
Khare T, Kumar V, Kishor PBK (2015) Na+ and Cl− ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 252:1149–1165
Kim K, Portis J (2004) Oxygen-dependent H2O2 production by Rubisco. FEBS Lett 57:124–128
Kim MJ, Ciani S, Schachtman DP (2010) A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol Plant 3:420–427
Kishitani S, Watanabe K, Yasuda S, Arakawa K, Takabe T (1994) Accumulation of glycinebetaine during cold acclimation and freezing tolerance in leaves of winter and spring barley plants. Plant Cell Environ 17:89–95
Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D (2006) Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ 29:1033–1048
Krishnamurthy A, Rathinasabapathi B (2013) Oxidative stress tolerance in plants: novel interplay between auxin and reactive oxygen species signaling. Plant Signal Behav 8:e25761
Kumar V, Khare T (2015) Individual and additive effects of Na+ and Cl− ions on rice under salinity stress. Arch Agron Soil Sci 61:381–395
Kurepin LV, Ivanov AG, Zaman M, Pharis RP, Allakhverdiev SI, Hurry V, Hüner NPA (2015) Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth Res 126(2):221–235. doi:10.1007/s11120-015-0125-x
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD andAtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Ladyman JAR, Hitz WD, Hanson AD (1980) Translocation and metabolism of glycinebetaine in barley plants in relation to water stress. Planta 150:191–196
Ladyman JAR, Ditz KM, Grumet R, Hanson AD (1983) Genetic variation for glycinebetaine accumulation by cultivated and wild barley in relation to water stress. Crop Sci 23:465–469
Li M, Li Z, Li S, Guo S, Meng Q, Li G, Yang X (2014a) Genetic engineering of glycinebetaine biosynthesis reduces heat-enhanced photoinhibition by enhancing antioxidative defense and alleviating lipid peroxidation in tomato. Plant Mol Biol Rep 32:42–51
Li H, Wang ZX, Ke Q, Ji CY, Jeong JC, Lee HS, Lim YP, Xu B, Deng XP, Kwak SS (2014b) Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiol Biochem 85:31–40
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2 •−, H2O2, and OH•) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Lou Y, Yang Y, Hy L, Liu H, Xu Q (2015) Exogenous glycinebetaine alleviates the detrimental effect of Cd stress on perennial ryegrass. Ecotoxicology 24:1330–1340
Low PS (1985) Molecular basis of the biological compatibility of nature’s osmolytes. In: Gilles R, Gilles-Baillien M (eds) Transport processes, iono- and osmoregulation. Springer, Berlin, pp 469–477
Makela P, Peltonen-Sainio P, Jokinen K, Pehu E, Setala H, Hinkkanen R, Somersalo S (1996) Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Sci 121:221–230
Malusa E, Laurenti E, Juszczuk I, Ferrari RP, Rychter AM (2002) Free radical production in roots of Phaseolus vulgaris subjected to phosphate deficiency stress. Plant Physiol Biochem 40:963–967
Martinez JL, Montillet E, Bresson E, Agnel JP, Dai GH, Daniel JF, Geiger JP (1998) Apoplastic peroxidase generates superoxide anions in cells of cotton cotyledons undergoing the hypersensitive reaction to Xanthomonas campestris pv. malvacearum race 18. Mol. Plant Microbe Interact 11:1038–1047
McCue KF, Hanson AD (1990) Drought and salt tolerance: towards understanding and application. Trends Biotechnol 8:358–362
McDonnell E, Jones RGW (1988) Glycinebetaine biosynthesis and accumulation in unstressed and salt-stressed wheat. J Exp Bot 39:421–430
McNeil SD, Nuccio ML, Ziemak MJ, Hanson AD (2001) Enhanced synthesis of choline and glycinebetaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc Natl Acad Sci U S A 98:10001–10005
Meloni DA, Gulotta MR, Martinez CA, Oliva MA (2004) The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz J Plant Physiol 16:39–46
Mickelbart MV, Peel G, Joly RJ, Rhodes D, Ejeta G, Goldsbrough PB (2003) Development and characterization of near-isogenic lines of sorghum segregating for glycinebetaine accumulation. Physiol Plant 118:253–261
Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2:ra45
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856
Mohanty N, Mohanty P (1988) Cation effects on primary processes of photosynthesis. In: Singh R, Sawheny SK (eds) Advances in frontier areas of plant biochemistry. Prentice-Hall, Delhi, pp 1–18
Molla MR, Ali MR, Hasanuzzaman M, Al-Mamun MH, Ahmed A, Nazim-ud-Dowla MAN, Rohman MM (2014) Exogenousproline and betaine-induced upregulation of glutathione transferase and glyoxalase I in lentil (Lens culinaris) under drought stress. Not Bot Horti Agrobo 42:73–80
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Mostofa MG, Hossain MA, Fujita M, Tran LS (2015a) Trehalose pretreatment reduces copper-induced oxidative damage and improves resistance to copper toxicity in rice (Oryza sativa L.) seedlings. Sci Rep 5:11433
Mostofa MG, Hossain MA, Fujita M (2015a) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252:461–475
Murata N, Mohanty PS, Hayashi H, Papageorgiou GC (1992) Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett 296:187–189
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Nawaz K, Ashraf M (2010) Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J Agron Crop Sci 196:28–37
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134
Nouet C, Motte P, Hanikenne M (2011) Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci 16:395–404
Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD (1998) The endogenous choline supply limits glycinebetaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J 16:487–496
O’Brien JA, Daudi A, Butt VS, Bolwell GP (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779
Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Pang CH, Wang BS (2008) Oxidative stress and salt tolerance in plants. In: Lüttge U, Beyschlag W, Murata J (eds) Progress in Botany, vol 69. Springer, Berlin, pp 231–245
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycinebetaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth Res 44:243–252
Papageorgiou GC, Fujimura Y, Murata N (1985) On the mechanism of betaine protection of photosynthetic structures in high salt environment. In: Current research in photosynthesis. Proceedings of the 8th congress of photosynthesis research, Stockholm, pp 957–960
Park EJ, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen THH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40:474–487
Park EJ, Jeknic Z, Chen THH (2006) Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol 47:706–714
Park EJ, Jeknic Z, Pino MT, Murata N, Chen THH (2007) Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ 30:994–1005
Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015) ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 6:69
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994a) Evidence for chilling- induced oxidative stress in maize seedlings and a regulatory role for hydrogen-peroxide. Plant Cell 6:65–74
Prasad TK, Anderson MD, Stewart CR (1994b) Acclimation, hydrogen-peroxide, and abscisic-acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol 105:619–627
Purvis AC, Shewfelt RL, Gegogeine JW (1995) Superoxide production by mitochondria isolated from green bell pepper fruit. Physiol Plant 94:743–749
Quan R, Shang M, Zhang H, Zhao Y, Zhang J (2004) Engineering of enhanced glycinebetaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2:477–486
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot 52:77–84
Qureshi MI, Abdin MZ, Ahmad J, Iqbal M (2013) Effect of long-term salinity on cellular antioxidants, compatible solute and fatty acid profile of sweet annie (Artemisia annua L.). Phytochemistry 95:215–223
Rajashekar CB, Zhou H, Marcum KB, Prakash O (1999) Glycinebetaine accumulation and induction of cold tolerance in strawberry (Fragaria × ananassa Duch.) plants. Plant Sci 148:175–183
Rathinasbapathi B, Burnet M, Russell BL, Gage DA, Liao PC, Nye GJ, Scott P, Golbeck JH, Hanson AD (1997) Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycinebetaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci U S A 94:3454–3458
Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN (2006) Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol 141:357–366
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Rodríguez AA, Córdoba AR, Ortega L, Taleisnik E (2004) Decreased reactive oxygen species concentration in the elongation zone contributes to the reduction in maize leaf growth under salinity. J Exp Bot 55:1383–1390
Sakamoto A, Murata AN (1998) Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol 38:1011–1019
Sakamoto A, Murata N (2002) The role of glycinebetaine in the protection of plants from stress: clue from transgenic plants. Plant Cell Environ 25:163–171
Sanda S, Yoshida K, Kuwano M, Kawamura T, Munekage YN, Akashi K, Yokota A (2011) Responses of the photosynthetic electron transport system to excess light energy caused by water deficit in wild watermelon. Physiol Plant 142:247–264
Sarwas MKS, Ullah I, Rahman MU, Ashraf MY, Zafar Y (2006) Glycinebetaine accumulation and its relation to yield and yield components in cotton genotypes grown under water deficit conditions. Pak J Bot 38:1449–1456
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28:679–688
Schwacke R, Grallath S, Breitkreuz KE, Stransky E, Stransky H, Frommer WB, Rentsch D (1999) LeProT1, a transporter for proline, glycinebetaine, and gamma-amino butyric acid in tomato pollen. Plant Cell 11:377–392
Schwarzlander M, Fricker MD, Sweetlove LJ (2009) Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim Biophys Acta 1787:468–475
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Sorwong A, Sakhonwasee S (2015) Foliar application of glycinebetaine mitigates the effect of heat stress in three marigold (Tagetes erecta) cultivars. Hort J 84:161–171
Spiteller G (2003) The relationship between changes in the cell wall, lipid peroxidation, proliferation, senescence and cell death. Physiol Plant 119:5–18
Storey R, Ahmad N, Wyn Jones RG (1977) Taxonomic and ecological aspects of the distribution of glycinebetaine and related compounds in plants. Oecologia 27:319–332
Sulpice R, Tsukaya H, Nonaka H, Mustardy L, Chen TH, Murata N (2003) Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J 36:165–176
Takahashi S, Murata N (2006) Glycerate-3-phosphate, produced by CO2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta 1757:198–205
Takhtajan AL (1980) Outline of the classification of flowering plants (magnoliophyta). Bot Rev 46:225–359
Tan YF, O’Toole N, Taylor NL, Millar AH (2010) Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiol 152:747–761
Tuteja N, Gill SS, Tuteja R (2011) Plant responses to abiotic stresses: shedding light on salt drought cold heavy metal stress. In: Tuteja N, Gill SS, Tuteja R (eds) Omics and plant abiotic stress tolerance. Bentham Science Publishers Ltd., Beijing, pp 39–64
Ueda A, Shi W, Sanmiya K, Shono M, Takabe T (2001) Functional analysis of salt-inducible proline transporter of barley roots. Plant Cell Physiol 42:1282–1289
Vahisalu T, Puzorjova I, Brosche M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojarvi J, Loog M, Kangasjärvi J, Kollist H (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62:442–453
Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W (2010) Over-accumulation of glycinebetaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 48:30–41
Wani SH, Singh NB, Haribhushan A, Mir JI (2013) Compatible solute engineering in plants for abiotic stress tolerance-role of glycinebetaine. Curr Genomics 14:157–165
Wise RR (1995) Chilling-enhanced photooxidation—the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res 45:79–97
Wood KV, Stringham KJ, Smith DL, Volenec JJ, Hendershot KL, Jackson KA, Rich PJ, Yang WJ, Rhodes D (1991) Betaines of alfalfa. Characterization by fast atom bombardment and desorption chemical ionization mass spectrometry. Plant Physiol 96:892–897
Yamada N, Promden W, Yamane K, Tamagake H, Hibino T, Tanaka Y, Takabe T (2009) Preferential accumulation of betaine uncoupled to choline monooxygenase in young leaves of sugar beet—importance of long-distance translocation of betaine under normal and salt-stressed conditions. J Plant Physiol 166:2058–2070
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Yang X, Wen X, Gong H, Lu Q, Yang Z, Tang Y, Liang Z, Lu C (2007) Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants. Planta 225:719–733
Yildirim E, Ekinci M, Turan M, Dursun A, Kul R, Parlakova F (2015) Roles of glycinebetaine in mitigating deleterious effect of salt stress on lettuce (Lactuca sativa L.). Arch Agron Soil Sci 61(12):1673–1689. doi:10.1080/03650340.2015.1030611
Zhang K, Guo N, Lian L, Wang J, Lv A, Zhang J (2011) Improved salt tolerance and seed cotton yield in cotton (Gossypium hirsutum L.) by transformation with betA gene for glycinebetaine synthesis. Euphytica 181:1–16
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgements
The financial support from the Science and Engineering Research Board, Government of India [grant number SR/FT/LS-93/2011] to V.K. as a Young Scientist Project is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kumar, V., Shriram, V., Hoque, T.S., Hasan, M.M., Burritt, D.J., Hossain, M.A. (2017). Glycinebetaine-Mediated Abiotic Oxidative-Stress Tolerance in Plants: Physiological and Biochemical Mechanisms. In: Sarwat, M., Ahmad, A., Abdin, M., Ibrahim, M. (eds) Stress Signaling in Plants: Genomics and Proteomics Perspective, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-319-42183-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-42183-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42182-7
Online ISBN: 978-3-319-42183-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)