Abstract
This study aimed to investigate and compare the effects of exogenous glycinebetaine (GB) and trehalose (TR) on the biological responses of duckweed (Lemna gibba L.) against cadmium (Cd) accumulation. Duckweed samples were exposed to 0.5, 1, and 3 mM of Cd for 6 days in the presence and absence of GB (0.5, 1, 2, and 5 mM) or TR (0.5, 1, 2, and 5 mM). The accumulation of Cd, GB, and TR were investigated, and their influence on the rates of lipid peroxidation, photosynthetic activity, proline content and enzymatic antioxidant performance was examined. Two-way ANOVA showed that exposure to Cd and/or GB or TR caused an increase in Cd accumulation concentration dependently. TR had significant effects on Cd accumulation. The application of 0.5 mM TR increased Cd accumulation, whereas 5 mM decreased Cd accumulation. However, Cd accumulation was not significantly affected by the presence of GB. Cd concentration alone or in combination with GB or TR had a significant effect on lipid peroxidation, photosynthetic activity, proline content, and antioxidant enzyme activities. In addition, statistically significant GB–Cd and TR–Cd interactions were observed. We conclude that both GB and TR play protective roles against Cd stress in aquatic plants. The use of a low level of TR (i.e., 0.5 mM) may be more useful than GB in phytoremediation studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The contamination of water with heavy metals is a critical problem that presently causes numerous problems all over the world. Cadmium (Cd) is one of the major environmental pollutants present in raw wastewater due to industries, such as the manufacture of pigments, stabilizers, alloys, electronic compounds and, especially, rechargeable nickel-cadmium batteries (Cannino et al. 2009). Cd is a non-essential heavy metal that is toxic to organisms (Prasad 1995; Mohan and Hosetti 1997; Megateli et al. 2009). Previous studies have shown that the Cd accumulation potential for aquatic macrophytes is very high (Cardwell et al. 2002; Singh et al. 2006; Duman et al. 2009). Some researchers recommend the use of Lemna gibba in phytoremediation to remove metallic pollutants from water (Megateli et al. 2009; Sasmaz and Obek 2009). However, the accumulation of Cd in plants causes physiological and biochemical changes. Cd is phytotoxic due to the generation of reactive oxygen species (ROS). Organisms can adapt to increased ROS production by upregulating their antioxidant defenses (Singh et al. 2006). These protective mechanisms include a variety of antioxidant enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT). However, under severe stress conditions, the antioxidant capacity of an organism may not be sufficient to prevent the harmful effects of the stressor. In such situations, plants lower their osmotic potential by accumulating osmolytes, such as proline and glycinebetaine (GB). Exogenous application of GB improves stress tolerance in different plant species, including both GB accumulators and non-accumulators (Ashraf and Foolad 2007). GB is environmentally safe, non-toxic, and water-soluble (Mäkelä et al. 1998). It can easily be procured as a relatively inexpensive by-product from sugar beets. A number of reports have demonstrated that the exogenous application of GB improves stress tolerance by reducing metal accumulation (Islam et al. 2009a), reducing lipid peroxidation (Banu et al. 2009), preventing photoinhibition (Ma et al. 2006), and upregulating antioxidant enzymes (Islam et al. 2009b). Trehalose (TR) is a non-reducing disaccharide of glucose. Exposure to TR has been correlated with tolerance to different stress conditions (Cortina and Culianez-Macia 2005). TR can be found in yeasts, fungi, and plants (Gancedo and Flores 2004). Luo et al. (2008) summarized that TR displays protective effects in two ways: by the stabilization of membranes and by the stabilization of biological macromolecules, such as proteins. Li et al. (2008a) determined those both intracellular and extracellular trehaloses are important for stress tolerance in yeast cells.
Previously, a large number of studies were performed in various plant species to investigate the physiological role (Demiral and Turkan 2006; Hoque et al. 2008) and biosynthetic pathway of GB (Hattori et al. 2009). However, little attention was given to the effects of GB on metal accumulation and biological responses. There are also no sufficient data relevant to the effects of exogenous application of TR on metal stress in the literature. In the present study, the duckweed species L. gibba was used as a model species of aquatic vascular plants to evaluate the impact of exogenous GB and TR on Cd accumulation and biological responses. Advancement of our knowledge on the effects of GB and TR on metal accumulation, photosynthetic activity, and biochemical alterations of aquatic plants will be useful for examining the phytoremediation of polluted water using aquatic plants.
2 Material and Methods
2.1 Sample Collection and Cultivation
In this study, duckweed was collected from Dipsiz spring in Kayseri, Turkey and brought to the laboratory within 3 h. Samples were taxonomically classified as L. gibba L. (Davis 1984). Prior to the experiment, containers were disinfected by immersion in 1% (v/v) NaClO for 3 to 5 min. Collected samples were washed in distilled water and acclimatized for 5 days in a climate chamber (23°C and 14-h photoperiod, 350 μmol m−2 s−1).
2.2 Experimental Design
Experiments were designed as two series. In the series 1 experiment, L. gibba fronds (approximately 4 g) were exposed to nutrient solutions (10% Hoagland’s solution) with initial Cd (CdSO4.7H2O) concentrations of 0.5, 1, and 3 mM without the addition of GB. In the series 2 experiment, fronds were exposed to a nutrient solution containing GB (0.5, 1, 2, and 5 mM) and Cd (0.5, 1, and 3 mM) for a total of 12 treatments. The pH of the solutions was initially adjusted to 6.0 using a 0.1 M KOH or HCl solution, and no pH adjustment was made during the experiments. All experiments were also repeated for TR. Each treatment was conducted in triplicate. The experiments were performed in a climate chamber under the aforementioned conditions for a period of 6 days. Solutions were replaced after 3 days. The change in volume within the flasks due to evapotranspiration was compensated for by the addition of double distilled water. At the end of the exposure experiment, fronds were collected and sieved with a plastic griddle. Plants were rinsed with double distilled water, drained, and then blotted on paper towels for 2 min.
2.3 Quantification of Cd, GB, and TR
An aliquot of each plant sample was dried at 70°C. Each sample was then digested using a CEM Mars 5 (CEM Corporation Matthews, NC, USA) microwave digestion system. The digestion conditions were as follows: maximum power was 1,200 W, power was 100%, ramp was 20 min, pressure was 180 psi, temperature was 200°C, and hold time was 10 min. After digestion, the volume of each sample was adjusted to 25 mL using double deionized water. Cd concentrations were determined by inductively coupled plasma optical emission spectroscopy (Varian-Liberty II, ICP-OES). The stability of the device was evaluated every ten samples by examining the internal standard. Reagent blanks were also prepared to detect potential contamination during the digestion and analytical procedure. Peach leaves (NIST, SRM-1547) were used as a reference material, and all analytical procedures were also performed on this reference material. The samples were analyzed in triplicate.
GB determination was done by high-performance liquid chromatography (HPLC; YL 9100 HPLC, Korea) using modified procedures of Demiral and Turkan (2004). Samples (1 g) were homogenized with 10 ml of a mixture of methanol/chloroform/water (ultra pure; 12:5:3 v/v). The extraction mixture was kept at +4°C overnight; 1 ml of the upper methanol phase was taken and passed through the Spherisorb ODS-2 (4.6 × 100 mm, 3 μM) column. GB was detected by an UV detector at 195 nm and quantification was done by comparing the peak surface areas with those obtained with pure GB standards.
For TR determination, extracts were obtained following Rodriguez et al. (2010): 1 g fresh weight sample was ground to a fine powder with liquid nitrogen and homogenized, suspended in 2 mL ethanol 80%, and kept at 80°C for 20 min. The extract was centrifuged at 12,000×g for 10 min and extracted three times with hot ethanol. Ethanol was evaporated to 60°C overnight and re-suspended in 0.5 mL distilled H2O. Samples passed through LKB, Bromma (TSK ODS-1207; 4.6 × 250 mm, 5 μM) ultrapac column. Trehalose was determined by HPLC using conductivity detector (CDD-10A VP). Flow rate was 0.8 mL min−1, at 30°C. Quantification was done by comparing the peak surface areas with those obtained with pure TR standards.
All chemicals used in this study were analytical reagent grade. All chemicals used in this study were analytical reagent grade (Merck, Darmstadt, Germany). GB and TR were provided by Chem. Service, Inc. All chemicals had a purity of more than 98%.
2.4 Determination of Lipid Peroxidation
For determination of lipid peroxidation, plant material (500 mg fw) was homogenized with 3 mL of 0.5% TBA in 20% TCA (w/v). The homogenate was incubated at 95°C for 30 min, and ice was used to stop the reaction. The samples were centrifuged at 10,000×g for 10 min, and the absorbance of the resulting supernatant was recorded at 532 and 600 nm. The amount of malondialdehyde (MDA; extinction coefficient of 155 mM−1 cm−1) was calculated by subtracting the non-specific absorbance at 600 nm from the absorbance at 532 nm (Heath and Packer 1968).
2.5 Determination of Photosynthetic Pigment and Proline Contents
For determination of chlorophyll content, a modified method of Arnon (1949) was used. Plant samples (100 mg) were extracted in 10 mL of chilled acetone solution in the dark. After centrifugation at 4,000×g for 10 min, the absorbance of the supernatant was recorded at 480, 510, 645, and 663 nm. The total chlorophyll content was calculated using the formula given by MacKinney (1941). The amount of proline was determined according to a modified method of Bates (1973). Free proline was extracted from 0.3 g of plant samples in 5 mL of 3% (w/v) aqueous sulfosalicylic acid, and the proline content was estimated by the use of ninhydrin reagent. The absorbance of the upper phase was recorded at 520 nm against a toluene blank.
2.6 Antioxidant Enzyme Extraction and Assay
For the SOD and CAT extraction, a modified method of Demiral and Turkan (2004) was used. Plant samples (500 mg) were homogenized in 50 mM sodium phosphate buffer (pH 6.8) containing 1 mM ethylenediaminetetraaceticacid (EDTA), 2% polyvinyl pyrrolidone (w/v) and 1 mM phenylmethylsulfonylfloride at 4°C. The homogenate was centrifuged at 15,000×g for 15 min at 4°C. The supernatant was extracted and used for measurement of the SOD and CAT activities (Demiral and Turkan 2004). The method developed by Tomassi (2001) was used for the extraction of the APX enzyme. Plant samples were homogenized in 50 mM Tris HCl (pH 7.8), 0.3 M mannitol, 1 mM EDTA, 1% bovine serum albumin and 0.05% cysteine at 4°C. The homogenate was centrifuged at 20,000×g for 20 min at 4°C.
SOD activity was assayed by the photochemical method described by Grannopolitis and Ries (1977) with some modifications. The reaction mixture contained 20 mM sodium phosphate (pH 7.5), 10 mM methionine, 0.1 mM EDTA, 0.1 mM nitro-blue-tetrazolium (NBT), 5 μM riboflavin, and 50 μg mL−1 crude enzyme extract in a total volume of 3 mL. One unit of SOD activity was defined as the amount of enzyme required for 50% inhibition of the rate of ρ-NBT chloride reduction that was measured at 560 nm (Liang et al. 2003). To measure CAT activity, the reaction mixture used consisted of 2.5 mL of 20 mM sodium phosphate buffer (pH 7.5), 300 μL of 15 mM H2O2 and 50 μg protein extract. The change in absorbance was measured at 240 nm (extinction coefficient 40 mM−1 cm−1). Enzyme activity was expressed in units of protein mg−1. The activity of APX was measured by estimating the rate of ascorbate oxidation (extinction coefficient 2.8 mM−1 cm−1). The reaction mixture contained 3 mL of 50 mM phosphate buffer (pH 6.6), 1 mM H2O2, 0.5 mM sodium ascorbate, 0.1 mM EDTA, and 100 μg enzyme extract. The change in absorbance was monitored at 290 nm (Nakano and Asada 1981), and the enzyme activity was expressed in units of protein mg−1.
2.7 Statistical Analysis
The data were expressed as mean values with standard errors. The Kolmogorov–Simirnov test and Levene’s test were used to ensure the normality assumption and the homogeneity of variances, respectively. Two-way analysis of variance (two-way analysis of variance (ANOVA)) was used to assess the significance of the effects of Cd exposure and GB or TR exposure, as well as of their interaction, on studied parameters. All pairwise mean comparisons were made using post hoc analyses (Tukey’s test). Additionally, we addressed the effect size of association of each factor to the ANOVA model by calculating the partial Eta-squared value. We used 0.05 as the statistical significance threshold. All statistical analyses were performed with the SPSS 15.0 software package.
3 Results
3.1 Cd, GB, and TR Accumulation
As shown at Tables 1 and 2 , exposure to Cd and/or GB caused an increase in Cd accumulation concentration dependently. The highest Cd accumulation (12,772 μg g−1 dw) was observed in plants exposed to 3 mM Cd +5 mM GB. With the application of GB, Cd accumulation was strongly affected by the Cd concentration in the solution (η2 = 0.950, p < 0.001), and GB did not show any effect on Cd accumulation (η2 = 0.245, p > 0.05). Two-way ANOVA revealed a significant GB–Cd interaction (η2 = 0.398, p < 0.05). Similarly, with the application of TR, Cd accumulation was strongly affected by the Cd concentration (η2 = 0.947, p < 0.001) and by the presence of TR in the solution (η2 = 0.454, p < 0.01). At 0.5 mM TR, Cd accumulation significantly increased, and at 5 mM TR, Cd accumulation significantly decreased compared with the control (p < 0.05). The interaction between TR and Cd was not determined to be significant.
The highest GB accumulation (48.73 μmol g−1 fw) was observed in plants exposed to 0 mM Cd + 2 mM GB (Tables 3 and 4). GB accumulation was strongly affected by the GB concentration (η2 = 0.753, p < 0.001) and by the presence of Cd in the solution (η2 = 0.322, p < 0.001). The application of 1 mM Cd increased significantly GB accumulation compared to control (p < 0.05). The strong interaction between GB and Cd was observed on GB accumulation (η2 = 0.717, p < 0.001). Similarly, TR accumulation also was strongly affected by Cd concentration (η2 = 0.815, p < 0.001) and by the presence of TR in the solution (η2 = 0.742, p < 0.001). The highest TR accumulation (45.8 μmol g−1 fw) was observed in plants exposed to 1 mM Cd + 2 mM TR. It was determined that Cd exposure significantly increased the TR content compared with the control. Interaction between TR and Cd (η2 = 0.885, p < 0.01) was determined to be significant with respect to TR content.
3.2 Effect of Exogenous GB and TR on MDA Content
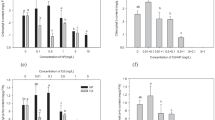
As shown at Table 5, MDA content was strongly affected by Cd concentration (η2 = 0.973, p < 0.01) and by the presence of GB in the solution (η2 = 0.258, p < 0.05). Exposure to Cd significantly increased the MDA content compared with the control. Application of 0.5 mM GB significantly decreased the MDA content compared to the control. TR had a significant effect on the content of MDA (η2 = 0.876, p < 0.01). At 0.5 and 1 mM TR, the MDA content significantly increased compared to the control. The highest MDA level (4.72 μmol g−1 fw) was observed at 3 mM Cd + 0.5 mM TR (Fig. 1). At 2 and 5 mM TR, Cd accumulation significantly decreased (p < 0.05). Significant GB-Cd and TR-Cd interactions were observed with respect to MDA content. However, the application of exogenous GB was found to produce a greater effect than that of exogenous TR.
3.3 Effect of Exogenous GB and TR on Total Chlorophyll Content
Two-way ANOVA showed that Cd alone or in combination with GB or TR had a significant effect on the total chlorophyll content (Table 5). Post hoc comparisons demonstrated that at 0.5 and 1 mM Cd, the total chlorophyll content significantly decreased compared to the control (p < 0.05). However, the application of GB resulted in an increase in the total chlorophyll content compared to the control. An increase in Cd concentration in the solution resulted in a decrease in the total chlorophyll content, but exposure to 0.5 and 1 mM TR resulted in an increase the total chlorophyll content. The highest chlorophyll content (1.27 mg g−1 fw) was observed at 3 mM Cd + 2 mM GB (Fig. 1).
3.4 Effect of Exogenous GB and TR on Proline Content
Proline content was strongly affected by Cd exposure (η2 = 0.684, p < 0.01), by the presence of GB in the solution (η2 = 0.945, p < 0.01) and by the presence of TR in the solution (η2 = 0.688, p < 0.01). In this study, the interaction between GB and Cd (η2 = 0.950, p < 0.01) and between TR and Cd (η2 = 0.920, p < 0.01) were determined to be significant with respect to proline content. Application of exogenous GB was found to be more effective than the application of exogenous TR in terms of altering proline content. According to post-hoc comparisons, with the application of GB, the proline content increased with exposure to 0.5 mM Cd. However, exposure to higher concentrations of Cd (1–3 mM) resulted in a decrease in the level of proline. Exposure to 0.5 mM GB reduced the proline content, but an increase was observed for 2 and 5 mM GB. With the application of TR, an increase in Cd concentration in the solution resulted in a decrease in the proline content. Exposure to 1 and 5 mM TR decreased the proline content compared with the control sample (p < 0.05).
3.5 Effect of Exogenous GB and TR on Antioxidant Enzyme Activities
All of the studied enzymes were strongly affected by Cd concentration, by the presence of GB in the solution and by the presence of TR in the solution (Table 5). In addition, strong synergetic interactions (Cd–GB and Cd-TR) on antioxidant enzyme activities were observed. Post hoc comparisons demonstrated that both the application of GB and TR significantly increased the SOD activity. For 0.5 and 3 mM Cd, an increase was observed compared with the control (Fig. 2).
An increase in GB concentration in the solution resulted in an increase in CAT activity. With the application of GB, 3 mM Cd reduced the CAT activity, but an increase was observed for 0.5 and 1 mM Cd. With the application of TR, 0.5, 1 and 2 mM TR resulted in an increase in CAT activity. With exposure to 1 and 3 mM Cd, a decrease in CAT activity was observed compared to the control.
An increase in GB concentration in the solution also resulted in an increase in APX activity. With the application of GB, 1 and 3 mM Cd increased the APX activity. At 0.5, 1, and 2 mM TR and 3 mM Cd, an increase in APX activity was observed compared to the control.
4 Discussion
An increase in the accumulation of Cd with an increase in the initial metal concentration has been reported in Lemna trisulca (Prasad et al. 2001) and Lemna minor (Razinger et al. 2008). Our results are compatible with these findings. Megateli et al. (2009) stated that L. gibba can accumulate a large amount of Cd and has great potential for phytoremediation. Our results support these findings as well. Regarding tolerance to metals, plants develop a variety of mechanisms to maintain and regulate cellular metal homeostasis (Hall 2002). Most of these mechanisms are related to lower uptake caused by altered adsorption. GB is one of the major organic osmolytes produced in a variety of plant species in response to environmental stresses caused by conditions, such as drought, increased salinity and the presence of heavy metals (Ashraf and Foolad 2007). Islam et al. (2009a) determined that exogenous GB significantly contributed to the suppression of Cd accumulation in cultured tobacco cells. However, in this study, Cd accumulation was not significantly affected by the application of exogenous GB. The application of TR, instead, significantly affected Cd accumulation, with Cd accumulation increasing at low (0.5 mM) TR concentrations. Therefore, it can be concluded that a low level of TR increases the uptake of Cd. Thus, the application of trehalose might be useful for the phytoextraction of metals. However, it was demonstrated that the presence of a higher level of TR in the growth medium reduced the accumulation of Cd. Haas (1986) determined that sugars with carboxylate groups formed strong complexes with Ca(II) and Cu(II) ions. Similarly, Cd ions might be captured by TR molecules in solution and might subsequently interrupt the accumulation of Cd ions by the plant. In addition, the use of TR appeared to be useful in reducing the accumulation of Cd.
GB is compatible solute which is highly soluble and protects plants from stress through different courses, including contribution to cellular osmotic adjustment, detoxification of reactive oxygen species and protection of membrane integrity (Ashraf and Foolad 2007). Externally applied GB can rapidly penetrate through leaves and be transported to other organs, where it would contribute to improved stress tolerance (Mäkelä et al. 1998). Our results showed that exogenous GB application increase the intracellular GB levels according to control. Similarly, Harinasut et al. (1996) determined that exogenous application of 15 mM GB to rice seedlings through their roots caused them to accumulate it in their leaves. Effective and efficient doses of GB may vary with plant species (Ashraf and Foolad 2007). In the present study, the highest accumulation of GB in L. minor was observed at 2 mM GB application. However, GB accumulation decreased at 5 mM GB application compared to 2 mM GB application. Heuer (2003) stated that GB has deleterious effects such as inhibition of ion accumulation and osmotic regulation. We can conclude from this situation that L. minor prevents the uptake of GB anymore.
Trehalose is a high soluble sugar capable of protecting biomolecules against environmental stress. In this study, we observed that exogenous TR application increase the intracellular TR levels according to control. Similarly, earlier studies showed that yeasts and Arabidopsis thaliana can uptake and accumulate the trehalose (Li et al. 2008a; Bae et al. 2005). However, it was seen that 5 mM application decreased TR content compared to the 2 mM TR application. The reason of the decrease may be provide the osmotic balance.
The oxidative stress produced by metals is evident from an enhancement in lipid peroxidation. A common product of lipid peroxidation is MDA, which is used to determine the oxidative stress level of plant cells (Khan et al. 2009). Plant cell membranes are generally considered as primary sites of metal injury. In the present study, MDA levels increased significantly in accordance with increasing Cd concentration in the solution, which is in agreement with previous reports from other laboratories (Singh et al. 2006; Razinger et al. 2008). In our study, it was found that at low (0.5 mM) GB concentrations, the MDA content decreased suggesting that exogenous GB alleviates oxidative damage. Similarly, Islam et al. (2009b) suggested that exogenous GB provides efficient protection against Cd-induced oxidative damage. TR is known to maintain the stability of membranes and proteins and to reduce the aggregation of denatured proteins (Singer and Lindquist 1998). In agreement with Singer and Lindquist (1998), 2 and 5 mM TR decreased lipid peroxidation in this work. These results suggest that both GB and TR are effective against Cd-induced oxidative damage.
Our results indicate that exposure to Cd decreased the total chlorophyll content in Lemna plants. A decrease in pigment concentration under Cd stress was previously reported (Sivaci et al. 2004; Megateli et al. 2009; Ngayila et al. 2009). However, the application of GB and TR affected the total chlorophyll content in a positive manner. Similarly, Demiral and Turkan (2006) determined that the application of exogenous GB partially preserved net photosystem-II efficiency in a salt-sensitive rice cultivar. Bae et al. (2005) determined that in A. thaliana exposed to 30 mM of exogenous TR, the chlorophyll content was not affected. However, in this study, we observed that 0.5 and 1 mM TR increased the total chlorophyll content. Our results indicate that both GB and TR are efficient to mitigate the deleterious consequences of Cd stress on the photosynthetic activity of L. gibba.
Proline is known as an important organic solute that accumulates in many plant species under conditions of abiotic stress (Ozden et al. 2009). In the experiments conducted in the present study, the proline level was affected by presence of Cd in solution. Siripornadulsil et al. (2002) stated that higher proline production has been correlated with increased metal tolerance in a transgenic alga. In this study, we observed that exposure to 0.5 mM Cd increased the proline level. Chen et al. (2003) studied the physiological effects of Cd on radish and carrot roots. The Chen group determined that the concentration of proline in the roots reached a maximum level when the application of cadmium reached 20 mg l−1 in the liquid culture and then declined slowly with increasing concentrations of cadmium. Similarly, in this study, we observed that 0.5 mM Cd increased the proline level, and a decrease was observed at high Cd concentrations. The application of 2 and 5 mM GB resulted in an increase in the proline level. Similarly, Islam et al. (2009a) determined that exogenous GB intensified the accumulation of proline in Cd-stressed tobacco cells. The accumulation of both betaine and proline is a widespread response that may protect plants against environmental stress. Our results show that both GB and TR affect the accumulation of intracellular proline.
Plants are equipped with a defense mechanism for repairing the ROS-induced damage. Antioxidant enzymes, such as SOD, CAT, and APX, play an important role in this process. SOD is considered to be the key enzyme of the cellular defense system that is formed against ROS. It has previously been reported that antioxidant enzyme activity may increase, decrease or remain unchanged in response to heavy metal exposure (Zhang et al. 2007). Similar to our findings, Li et al. (2008b) determined that Jussiaea rapens exposed to Cd had increased SOD activity. However, the opposite results were observed in pea plants (Dixit et al. 2001). Hoque et al. (2007) determined that exogenous GB increased antioxidant enzyme activity. Nery et al. (2008) determined that TR plays an important protection role against ROS. Our study, together with the findings of the aforementioned studies, suggests that GB and TR could contribute to the detoxification of \( {{\hbox{O}}_2}^{. - } \) radicals by increasing the activity of SOD under Cd stress.
Hydrogen peroxide (H2O2), a product of SOD activity, is toxic to cells and must be converted into H2O in subsequent reactions. As a result of CAT and APX activity, H2O2 is broken down to form water and oxygen (Cao et al. 2004; Zhang et al. 2007). In this study, CAT activity was found to increase in response to an initial increase in Cd exposure, but then decreased. This initial increase could be due to an increase in the amount of H2O2. The later decrease in activity of these enzymes may be caused by inactivation or degradation by peroxisomal protease (Cakmak 2000). CAT activity was significantly increased by the application of exogenous GB and corroborate with the results of Islam et al. (2009a). Luo et al. (2008) stated that TR has an adverse effect on CAT activity. However, in this study, we observed that 0.5, 1, and 2 mM of exogenous TR increased the CAT activity. Also, a significant increase in APX activity was observed in response to exposure with 1 and 3 mM Cd. Similar responses were observed in previous studies (Mishra et al. 2006; Aravind and Prasad 2003). We determined that the application of GB increased the APX activity. Our results are also supported by the results of Demiral and Turkan (2004). Additionally, we observed that 0.5, 1, and 2 mM TR increased the APX activity. According to our results, both GB and TR play an important role in the detoxification of H2O2.
5 Conclusions
In conclusion, our results suggest that the use of exogenous GB and TR reduced the deleterious effects of Cd stress. However, TR seemed to be more effective than GB on Cd accumulation. These results support the use of L. gibba as a phytoremediator of Cd polluted waters, and the findings of the present study can be used in further phytoremediation studies.
References
Aravind, P., & Prasad, M. N. V. (2003). Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: a free floating freshwater macrophyte. Plant Physiology and Biochemistry, 41, 391–397.
Arnon, D. I. (1949). Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiology, 24, 1–15.
Ashraf, M., & Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59, 206–216.
Bae, H., Herman, E., & Sicher, R. (2005). Exogenous trehalose promotes non-structural carbohydrate accumulation and induces chemical detoxification and stress response proteins in Arabidopsis thaliana grown in liquid culture. Plant Science, 168, 1293–1301.
Banu, M. N. A., Hoque, M. A., Watanabe-Sugimoto, M., Matsuoka, K., Nakamura, Y., & Shimoishi, Y. (2009). Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. Journal of Plant Physiology, 166, 146–156.
Bates, L. S. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Cakmak, I. (2000). Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. The New Phytologist, 146, 185–205.
Cannino, G., Ferruggia, E., Luparello, C., & Rinaldi, A. M. (2009). Cadmium and mitochondria. Mitochondrion, 9, 377–384.
Cao, X., Ma, L. Q., & Tu, C. (2004). Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.). Environmental Pollution, 128, 317–325.
Cardwell, A. J., Hawker, D. W., & Greenway, M. (2002). Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere, 48, 653–663.
Chen, Y. X., He, Y. F., Luo, Y. M., Yu, Y. L., Lin, Q., & Wong, M. H. (2003). Physiological mechanism of plant roots exposed to cadmium. Chemosphere, 50, 789–793.
Cortina, C., & Culianez-Macia, F. A. (2005). Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Science, 169, 75–82.
Davis, P. H. (1984). Flora of Turkey and East Aegean Islands vol. 8. Edinburg: Edinburgh University Press.
Demiral, T., & Turkan, I. (2004). Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? Journal of Plant Physiology, 161, 1089–1100.
Demiral, T., & Turkan, I. (2006). Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stres. Environmental and Experimental Botany, 56, 72–79.
Dixit, V., Pandey, V., & Shyam, R. (2001). Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv.Azad). Journal of Experimental Botany, 52, 1101–1109.
Duman, F., Leblebici, Z., & Aksoy, A. (2009). Growth and bioaccumulation characteristics of watercress (Nasturtium officinale R. Br.) exposed to cadmium, cobalt and chromium. Chemical Speciation and Bioavailability, 21, 257–265.
Gancedo, C., & Flores, C. L. (2004). The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Research, 4, 351–359.
Grannopolitis, N., & Ries, K. (1977). SOD occurence in higher plants. Plant Physiology, 59, 309–314.
Haas, J. W. (1986). Complexation of calcium and copper with carbohydrates: implications for seawater speciation. Marine Chemistry, 19, 299–304.
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53, 1–11.
Harinasut, P., Tsutsui, K., Takabe, T., Nomura, M., Takabe, T., & Kishitani, S. (1996). Exogenous glycinebetaine accumulation and increased salt-tolerance in rice seedlings. Bioscience, Biotechnology, and Biochemistry, 60, 366–368.
Hattori, T., Mitsuya, S., Fujiwara, T., Jagendorf, A. T., & Takabe, T. (2009). Tissue specificity of glycinebetaine synthesis in barley. Plant Science, 176, 112–118.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Heuer, B. (2003). Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Science, 165, 693–699.
Hoque, M. A., Okuma, E., Banu, M. N. A., Nakamura, Y., Shimoishi, Y., & Murata, Y. (2007). Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. Journal of Plant Physiology, 164, 553–561.
Hoque, M. A., Banu, M. N. A., Nakamura, Y., Shimoishi, Y., & Murata, Y. (2008). Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. Journal of Plant Physiology, 165, 813–824.
Islam, M. M., Hoque, M. A., Okuma, E., Banu, M. N. A., Shimoishi, Y., Nakamura, Y., et al. (2009). Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. Journal of Plant Physiology, 166, 1587–1597.
Islam, M. M., Hoque, M. A., Okuma, E., Jannat, R., Banu, M. N. A., Jahan, S., et al. (2009). Proline and glycinebetaine confer cadmium tolerance on tobacco bright yellow-2 cells by increasing ascorbate-glutathione cycle enzyme activities. Bioscience, Biotechnology, and Biochemistry, 73, 2320–2323.
Khan, I., Ahmad, A., & Iqbal, M. (2009). Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicology and Environmental Safety, 72, 626–634.
Li, B. Q., Zhou, Z. W., & Tian, S. P. (2008a). Combined effects of endo- and exogenous trehalose on stress tolerance and biocontrol efficacy of two antagonistic yeasts. Biological Control, 46, 187–193.
Li, M., Zhang, L. J., Tao, L., & Li, W. (2008b). Ecophysiological responses of Jussiaea rapens to cadmium exposure. Aquatic Botany, 88, 347–352.
Liang, Y. C., Chen, Q., Liu, Q., Zhang, W. H., & Ding, R. X. (2003). Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). Journal of Plant Physiology, 160, 1157–1164.
Luo, Y., Li, W. M., & Wang, W. (2008). Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environmental and Experimental Botany, 63, 378–384.
Ma, Q. Q., Wang, W., Li, Y. H., Li, D. Q., & Zou, Q. (2006). Alleviation of photoinhibition in drought stressed wheat (Triticum aestivum) by foliar applied glycinebetaine. Journal of Plant Physiology, 163, 165–175.
MacKinney, G. (1941). Absorption of light by chlorophyll solutions. The Journal of Biological Chemistry, 140, 315–322.
Mäkelä, P., Jokinen, K., Kontturi, M., Peltonen-Sainio, P., Pehu, E., & Somersalo, S. (1998). Foliar application of glycinebetaine a novel product from sugar beet as an approach to increase tomato yield. Indian Crop Production, 7, 139–148.
Megateli, S., Semsari, S., & Couderchet, M. (2009). Toxicity and removal of heavy metals (cadmium, copper, and zinc) by Lemna gibba. Ecotoxicology and Environmental Safety, 72, 1774–1780.
Mishra, S., Srivastava, S., Tripathi, R. D., Govindarajan, R., Kuriakose, S. V., & Prasad, M. N. V. (2006). Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiology and Biochemistry, 44, 25–37.
Mohan, B. S., & Hosetti, B. B. (1997). Potential phytotoxicity of lead and cadmium to Lemna minor grown in sewage stabilization ponds. Environmental Pollution, 98, 233–238.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant & Cell Physiology, 22, 867–880.
Nery, D. C. M., da Silva, C. G., Mariani, D., Fernandes, P. N., Marcos Pereira, D., Panek, A. D., et al. (2008). The role of trehalose and its transporter in protection against reactive oxygen species. Biochimica et Biophysica Acta, 1780, 1408–1411.
Ngayila, N., Botineau, M., Baudu, M., & Basly, J. P. (2009). Myriophyllum alterniflorum DC. Effect of low concentrations of copper and cadmium on somatic and photosynthetic endpoints: a chemometric approach. Ecological Indicators, 9, 307–312.
Ozden, M., Demirel, U., & Kahraman, A. (2009). Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Scientia Horticulturae, 119, 163–168.
Prasad, M. N. V. (1995). Cadmium toxicity and tolerance in vascular plants. Environmental and Experimental Botany, 35, 525–545.
Prasad, M. N. V., Malec, P., Waloszek, A., Bojko, M., & Strzaka, K. (2001). Physiological responses of Lemna trisulca L. (duckweed) to cadmium and copper bioaccumulation. Plant Science, 161, 881–889.
Razinger, J., Dermastia, M., Koce, J. D., & Zrimec, A. (2008). Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environmental Pollution, 153, 687–694.
Rodriguez, M., Taleisnik, E., Lenardon, S., & Lascano, R. (2010). Are Sunflower chlorotic mottle virus infection symptoms modulated by early increases in leaf sugar concentration? Journal of Plant Physiology, 167, 1137–1144.
Sasmaz, A., & Obek, E. (2009). The accumulation of arsenic, uranium, and boron in Lemna gibba L. exposed to secondary effluents. Ecological Engineering, 35, 1564–1567.
Singer, M. A., & Lindquist, S. (1998). Multiple effects of trehalose on protein folding in vitro and in vivo. Molecular Cell, 1, 639–648.
Singh, N., Ma, L. Q., Srivastava, M., & Rathinasabapathi, B. (2006). Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Science, 170, 274–282.
Siripornadulsil, S., Traina, S., Verma, D. P. S., & Sayre, R. T. (2002). Molecular mechanisms of proline mediated tolerance to toxic heavy metals in transgenic microalgae. The Plant Cell, 14, 2837–2847.
Sivaci, E. R., Sivaci, A., & Sökmen, M. (2004). Biosorption of cadmium by Myriophyllum spicatum L. and Myriophyllum triphyllum orchard. Chemosphere, 56, 1043–1048.
Tomassi, F. (2001). A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. Journal of Experimental Botany, 52, 1647–1654.
Zhang, F. Q., Wang, Y. S., Lou, Z. P., & Dong, J. D. (2007). Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere, 67, 44–50.
Acknowledgements
The authors are deeply grateful for the technical assistance of Fatih Dogan Koca and Musa Kar. This study was supported by Erciyes University Scientific Research Project Fund (FBA 07-32).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duman, F., Aksoy, A., Aydin, Z. et al. Effects of Exogenous Glycinebetaine and Trehalose on Cadmium Accumulation and Biological Responses of an Aquatic Plant (Lemna gibba L.). Water Air Soil Pollut 217, 545–556 (2011). https://doi.org/10.1007/s11270-010-0608-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0608-5