Abstract
Unfavorable environmental conditions such as heat, cold, drought, metal/metalloid toxicity, and pathogens enhance production of intra-and inter-cellular levels of reactive oxygen species (ROS) in plants. ROS, acting as signaling molecules, activate signal transduction pathways in response to various stresses. Alternatively, ROS cause irreversible cellular damage due to lipid peroxidation, oxidation of protein, inactivation of enzymes, DNA damage, and interact with other vital constituents of plant cells through their strong oxidative properties, which drastically alter plant morphological structures, becoming disadvantageous for survival and productivity. Higher plants have complex defense systems to scavenge ROS. Being a central molecule of the defense system, gamma-aminobutyric acid (GABA) is ubiquitous from prokaryotes to eukaryotes cells. GABA helps mitigate ROS in plants and GABA shunt pathway plays a key role either as metabolites or endogenous signaling molecules in several regulatory mechanisms under stress conditions. The GABA transporters (GATs) being activated with the attachment of GABA under environmental stress stimuli facilitate high content of Ca2+ into the cytosol. Ca2+ combines with calmodulin (CaM) -binding domain that activates the glutamate decarboxylase (GAD) enzyme for the conversion of glutamate into GABA. This synchronized process regulates GABA shunt gene expressions under stress conditions and improves defense mechanisms in plants. This review highlights the regulatory aspects of GABA shunt pathway for ROS production as well as in the defense mechanism of plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotic and abiotic stresses hinder developmental mechanism of plants (Waqas et al. 2019; Jalil and Ansari 2020a, b). Amongst them, abiotic stresses such as drought, salinity, waterlogging, soil alkalinity, metal/metalloid toxicity, nutrient deficiency, and cold significantly eclipse crop productivity on a global scale (Jalil and Ansari 2008, 2020a, b; Cramer et al. 2011). Effects of environmental stresses diminish several physiological processes (Matysik et al. 2002; Mittler 2006) that the plants tend to deal with proficiently and effectively through diverse array of signal systems and regulatory mechanisms. The adverse environmental conditions exceeding beyond the tolerance limit, cause stress to plants and lead to lower reproduction rate, declined growth rate along with premature leaf senescence (Jalil et al. 2017, 2019a, b) due to the imbalanced production of reactive oxygen species (ROS) (Sachdev et al. 2021).
Plants have a plethora of direct and indirect stress-specific responses as well as an intricate network of an antioxidant system for scavenging ROS to mitigate oxidative stresses (Dietz 2003; Apel and Hirt 2004). Direct responses involve deceleration and eradication of ROS production. Indirect responses are upregulation of primary defense genes by hormone-mediated signaling pathways that help to express genes for secondary defense (Kwak et al. 2006; Jalil and Ansari 2020a). Plants also operate several mechanisms triggering production of signaling biomolecules that confer tolerance against stress conditions. Of these, gamma-aminobutyric acid (GABA) is a biomolecule of special interest among stress-responsive metabolites and ubiquitous in all prokaryotic and eukaryotic organisms including plants. The molecule was first detected in plants (potato tuber) over half a century ago (Steward et al. 1949; Satyanarayan and Nair 1990; Shelp et al. 1999). It is a non-protein amino acid and reportedly functions as an inhibitor to neurotransmitter in mammalian brain (Reimer et al. 2001). In plants system, GABA plays a key role as metabolites or endogenous signaling molecule in several regulatory mechanisms and accumulates in plant cells under hostile environmental conditions (Kenersley and Turano 2000; Bouche and Fromm 2004; Ansari and Chen 2009; Clark et al. 2009). In cytosol, there exists GABA shunt pathway that engages glutamate decarboxylase (GAD) for decarboxylation of glutamate into GABA (Baum et al. 1993; Breitbreuz and Shelp 1995). GABA shunt is a short metabolic pathway that involves three enzymes: GAD, GABA transaminase (GABA-T), and succinic semialdehyde dehydrogenase (SSADH) (Shelp et al. 1999; Ansari et al. 2014). The shunt pathway regulates GABA metabolism under stress conditions, strikes a balance of carbon/nitrogen metabolism and influences other physiological processes including plant growth and development (Bouché et al. 2003; Fait et al. 2008; Ansari et al. 2014; Al-Quraan and Al-Share 2016; Jalil et al. 2017, 2019a, b). The production of GABA in plants is significantly increased in response to hostile environmental conditions such as hypoxia, low temperature, mechanical stimulation, γ radiation, and low pH (Lane and Stiller 1970; Wallace et al. 1984; Bown and Shelp 1997; Kenersley and Turano 2000). These stresses trigger signal transduction pathway in which enhanced cytosolic Ca2+ stimulates Ca2+/CaM activity of GAD. GABA and other GABA shunt components play a crucial role in scavenging ROS during stress conditions (Bouché et al. 2003; Ansari et al. 2014; Jalil et al. 2017; Che-Othman et al. 2019; Jalil and Ansari 2020b). However, the specificity of the response and the mechanism of GABA and the GABA shunt during stress conditions is still unclear. In this review, we have focused on molecular, physiological, and metabolic aspects of GABA and its complex relationship with other GABA shunt component in improving stress tolerance in plants by scavenging ROS.
Generation and consequences of ROS in plant cell during stress conditions
Several metabolic pathways in plant cells produce ROS, i.e., superoxide radical (O2•−), hydroxyl radical (OH•), hydroperoxyl radical (HO2•), alkoxy radical (RO•), peroxy radical (ROO•), peroxynitrite (ONOO•), excited carbonyl (RO•) and some non- radicals such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) (Dismukes et al. 2001; Vellosillo et al. 2010; Karuppanapandian et al. 2011). ROS also plays a key role as a signal transducer in various physiological and developmental processes of plants, such as growth, development, stress tolerance, and program cell death (Sachdev et al. 2021; Bailey-Serres and Mittler 2006). Each type of ROS has a different oxidative capacity and affects different physiological and biochemical reactions controlled by different genes in plants. As an excited oxygen, 1O2 is usually produced in chlorophyll photosystem II (PSII) and has intense oxidation potential. Although 1O2 exists for a very short time and is very unstable in cells once produced, it has a profound effect on photosynthesis. O2•−, a different ROS is a priority because of its instability and strong oxidation/reduction. O2•− in plant can maintain the stability of stem cells (Zheng et al. 2017). Rice, roots and stalks seem to be the main sites of O2•− production, which may be related to their adaptation to the aquatic environment (Yamuchi et al. 2017). O2•− in photosynthesis can be produced by electron transport chains and membrane-based NADPH oxidase systems to form H2O2 by reacting with hydrogen ions to form oxygen molecules or with superoxide dismutase (Mhamdy and Van Bruzem 2018). Among these, H2O2 is considered an important redox molecule, given its specific physical and chemical properties, including significant stability (10 – s half-life) within cells and rapid and reversible oxidation of target proteins (Mittler 2017; Mhamdi and Van Breusegem 2018). H2O2 acts as the second messenger modulating the activities of redox-sensitive proteins or expression of genes by changing the redox balance in the cell (Swanson and Gilroy 2010) resulting in different stress tolerance responses and programmed cell death (Bhattacharjee 2005). H2O2 also involves in many regulatory activities like ion channel gating and modulating cell wall polymer structure (Swanson and Gilroy 2010). Additionally, H2O2 interferes with hormones to control the plant growth process and stress response. The cleavage of O–O double bond in H2O2 produces OH• which is active and usually works very close to its production site. Therefore, OH• is the most reactive ROS that reacts with all biological molecules. It can oxidize cell wall polysaccharides, resulting in cell wall lining (Kärkönen and Kuchitsu 2015).
In plants, ROS are very obnoxious as they hostilely impact on the structures and activities of the biomolecules inside the cells. ROS become toxic when their levels exceed the carrying capacity of defense mechanisms and cause oxidative damage to plant cells (Sachdev et al. 2021; Bhattacharjee 2005). Under normal conditions, excessive ROS can be scavenged by various antioxidative defense mechanisms. On the Contrary, the equilibrium between production and scavenging of ROS may be perturbed by various biotic and abiotic stresses (Shah et al. 2001; Mittler 2002; Hu et al. 2008; Ansari and Chen 2009; Maheshwari and Dubey 2009). The excessive generation of ROS causes destructive activities in the plant cell such as lipid peroxidation, degradation of protein and nucleic acids, inhibition of enzymes, and finally results in cell death (Shah et al. 2001; Mittler 2002; Meriga et al. 2004; Tanou et al. 2009; Sharma et al. 2012). ROS are produced in different cellular organelles, including mitochondria, chloroplast, peroxisomes, and endoplasmic reticulum as shown in Fig. 1 (Corpas et al. 2002; Asada 2006; Navrot et al. 2007; Sharma et al. 2012). The production of O2•− and H2O2 occurs in mitochondria by over reduction of the electron transport chain (ETC). The reaction centers of PSI and PSII in chloroplast thylakoids are the major generation site for ROS (Murphy 2009). Chloroplast ETC photoreduces O2 to H2O2 via a series of PSI reducing acceptor such as Fe–S centers, reduced thioredoxin (TRX), and ferredoxin. These are auto-oxidizable. However, O2•− is formed under conditions limiting NADP. Subsequently, its disproportionation produces H2O2 and O2•− by Mehler reaction (Noctor et al. 2007). In fact, peroxisomal matrix and membrane are major sites for H2O2 and O2•− production. Xanthine oxidase (Fig. 1) produces O2•−, while oxidizing xanthine and hypoxanthine into uric acid in peroxisomal matrix that degrades biomolecules of the cells and ultimately cell death (Verma and Dubey 2003). Plants are indebted to manage with excessive ROS production in order to regulate the cellular redox homeostasis. Therefore, the increased ROS levels are recognized and restrictively controlled by ROS-scavenging systems (Sachdev et al. 2021).
Production of ROS in a different organelles of plant cells due to the exposure of abiotic stresses. The generation of superoxide radical (O2•−) and hydrogen peroxide (H2O2) occurs in mitochondria by over reduction of the electron transport chain (ETC) by the superoxide dismutase (SOD) enzyme further H2O2 produces OH˙ via Fenton reaction. In hypoxia condition, nitric oxide (NO) and O2•− levels increases in mitochondria, and their reaction produces peroxynitrite (ONOO−). In chloroplasts, the generation of O2•− and H2O2 occurs due to elevated oxygen pressure and condensed molecular oxygen in comparison to other organelles in the ETC within PSI by Mehler reaction. In peroxisome, the O2•− radicals produced in the peroxisomal matrix and the peroxisomal membrane by the oxidation of xanthine and hypoxanthine into uric acid by the enzyme xanthine oxidase (XO) lead to membrane leakage and cell death
Synthesis, signaling and transportation of GABA via GABA shunt pathway in plants
GABA is metabolized in plants via GABA shunt pathway (Fig. 2). Nevertheless, some polyamines (PAs) such as spermidine and putrescine together with proline (Pro) are also involved in the synthesis of GABA (Bouchereau et al. 1999; Shelp et al. 2012) probably by a non-enzymatic reaction under oxidative stress (Signorelli et al. 2015). Proline is biosynthetically derived from the amino acid L-glutamate. Glutamate-5-semialdehyde is first formed by glutamate 5-kinase and glutamate-5-semialdehyde dehydrogenase from L-glutamate. This can then either spontaneously cyclize to form 1-pyrroline-5-carboxylic acid, which is reduced to Pro by pyrroline-5-carboxylate reductase, or turned into ornithine by ornithine aminotransferase, followed by cyclization by ornithine cyclodeaminase to form Pro (Shelp et al. 2012). The catabolism of PAs in plants is mainly dependent on the action of amine oxidases such as diamine oxidase (DAO) and PA oxidase (PAO). DAO with Cu2+ and pyridoxal phosphate as its cofactors catalyzes the formation of H2O2, ammonia, and 4-aminobutanal from putrescine. Then, 4-aminobutanal undergoes cyclization to form pyrroline (PYRR), which is converted to GABA by the action of pyrroline dehydrogenase (PYRR-DH). Then, GABA is further converted into succinate, which enters the Krebs cycle (Shelp et al. 2012). Stressful conditions (hypoxia, salt stress, heat or cold shock, drought, mechanical injury, etc.) can strongly promote GAD and DAO activity for GABA formation (Bouché et al. 2003; Bouche and Fromm 2004). Glutamate is decarboxylated into GABA by catalytic enzyme GAD in the cytoplasm whose activity is regulated post-translationally by binding with calmodulin (CaM) in plants (Baum et al. 1993, 1996).
GABA shunt pathways. GABA is synthesized from glutamate either directly (enzymatically) via glutamate decarboxylase (GAD) with calmodulin (CaM)-binding domain or indirectly via proline non-enzymatically in the cytosol. In mitochondria, GABA transaminase (GABA-T) catalyzes the conversion of GABA into succinic semialdehyde (SSA), which in turn produces succinate concurrently along with NADH production in mitochondria by NAD+-dependent enzyme succinic semialdehyde dehydrogenase (SSADH). Alternatively, SSA can also be catabolized to γ-hydroxybutyric acid (GHB) via succinic semialdehyde reductase (SSR) and enters Krebs cycle. GABA is also synthesized in plants by some (PAs) such as spermidine and putrescine
GABA shunt is a metabolic pathway (Shi et al. 2010). It involves in nitrogen assimilation of amino acids during plant growth and leaf senescence process (Ansari et al. 2005, 2014). During the leaf senescence, the amino group from most of the amino acids can be transferred to α- ketoglutarate to form glutamate. In GABA shunt, the metabolism of GABA occurs at two sites of cell; synthesis of GABA in cytosol and degradation in mitochondria (Fig. 3). The GABA shunt pathway comprises three reactions: First reaction converts glutamate into GABA via action of GAD (EC 4.1.1.15), i.e. glutamate specific enzyme with an autoinhibitory (CaM-binding) domain in the cytosol (Breitbreuz and Shelp 1995). Second reaction produces succinic semialdehyde (SSA) from GABA that converts to succinate concurrently with NADH production in mitochondria by NAD+-dependent enzyme SSADH (EC 1.2.1.16). Alternatively, the third reaction catalyzes SSA into γ-hydroxybutyric acid (GHB) via succinic semialdehyde reductase (SSR) enzyme (Breitkreuz et al. 2003; Hoover et al. 2007; Simpson et al. 2008) and goes to Krebs cycle. GABA-T with pH optima of 8 to 10 and SSADH with pH optima of 8 are mitochondrial enzymes (Breitbreuz and Shelp 1995). GABA shunt within the mitochondria may provide a carbon skeleton to replenish carboxylic acids of the Krebs cycle (Ansari et al. 2005; Gregersen et al. 2013) as shown in Fig. 2.
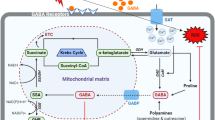
GABA shunt and associated pathways under stress conditions. The exogenous GABA acts as signaling molecule in stress conditions and attaches to surface binding cell receptors, GABA transporters (GATs) that enhance Ca2+ level. Ca2+ along with CaM domain activate glutamate decarboxylase (GAD) enzyme which regulates GABA shunt pathway by increasing the endogenous GABA content in the cytosol. GABA enters mitochondria by GABA permease (GABP) for catabolism by the enzymes GABA transaminase (GABA-T) and succinic semialdehyde dehydrogenase (SSADH) to form succinate supplied to Krebs cycle and electron transport chain (ETC). GABA elevates the photosynthetic activity and antioxidant enzyme activity, decreases ROS accumulation inside the cell and improves stress tolerance in plants
GABA elevation in plants has been found as a common response in concomitance with restriction of glutamine synthesis, reduction of protein synthesis and an increase in protein degradation (Bouche and Fromm 2004). During various stress conditions, GABA level can exceed that of amino acids involved in protein synthesis (Kenersley and Turano 2000). Therefore, many speculative functions in stress abiotic mitigation have been proposed. A GABA shunt would supply NADH and/or succinate to Krebs cycle (Bouche and Fromm 2004). It has been observed that GABA-T activity would be limited under stress conditions, determining an accumulation of GABA useful for the provision of anaplerotic succinate for the Krebs cycle upon stress relief (Shelp et al. 2012). When the condition changes, GABA is subsequently transported to the mitochondria where it is catabolized by GABA- T to SSA. SSA can be oxidized by the mitochondrial succinic SSADH to produce succinate or reduced to GHB by the cytosolic γ-hydroxybutyrate dehydrogenase (GHBDH) (Breitkreuz et al. 2003; Hoover et al. 2007; Simpson et al. 2008). Under stress GAD activity undergoes changes of catalytic properties mediated either by Ca2+/CaM in rapid responses (Bouche and Fromm 2004), or by pH when cytosolic acidification occurs (Breitkreuz et al. 2003). Since the deficiency of GAD determines the presence of necrotic regions under standard light conditions and ROS accumulation in Arabidopsis thaliana, it is suggested that GABA level could be mainly controlled by the rate of its synthesis (Bouché et al. 2003; Fait et al. 2008).
GABA is a signaling molecule that regulates cellular processes by binding to-and changing-the activities of protein receptors (Ramesh et al. 2015). Abiotic stresses regulate the expression of GABA shunt-associated genes. Theses stresses further increase the level of GABA, facilitating its attachment to cell-surface binding sites that generate the transient Ca2+. As a result, high affinity GABA transporters (GAT1) with Ca2+ are activated and facilitate GABA transport into cells (Meyer et al. 2006). Certainly, GAD gets activated via a Ca2+/CaM (Baum et al. 1993), increasing intracellular GABA level that helps to express various signaling and metabolism-associated genes. At the same time, GABA also suppresses expression of genes associated with cell wall-modifications. Depending on the environmental conditions, a major proportion of cytosolic GABA enters the mitochondria via GABA permease in A. thaliana, AtGABP (Michaeli et al. 2011), for catabolism by GABA-T and SSADH, ensuing in succinate formation and supply to Krebs cycle and mitochondrial ETC. Alternatively, the toxic intermediate, SSA gets transported out of the mitochondria to form GHB via the enzyme SSR (Fig. 2). Reportedly, a tonoplast glutamate/aspartate/GABA transporter in tomato fruit exports GABA content from the vacuole in exchange for import of glutamate/aspartate during ripening of fruits (Snowden et al. 2015).
Relationships of GABA shunt and ROS during stress conditions
As a result of environmental stress to in plant systems, the level of ROS dramatically increases to the extent of cell damage (Sachdev et al. 2021; Zimmermann and Zentgraf 2005; Van Breusegem and Dat 2006). ROS level increases because of their excessive production and inhibition of antioxidative enzymes (Halliwell 2006). Plants have complex antioxidative defense mechanisms including enzymatic and non-enzymatic components, to reduce the production of ROS (Fig. 3) (Jalil and Ansari 2020a). Major enzymes involved in this system are superoxide dismutase, glutathione reductase, peroxidase, and catalase. Beside these enzymes, certain carotenoids and glutathione can also play part in the antioxidant system as non-enzymatic components (Fahad et al. 2017). GABA shunt is a metabolic pathway that involves in carbon/ nitrogen metabolism and nitrogen assimilation in amino acids (Ansari et al. 2005, 2014) and mitigates ROS production during various stress conditions (Fig. 3) (Bouché et al. 2003; Al-Quraan and Al-Share 2016; Jalil et al. 2019a, b).
Several studies uncover the function of GABA shunt in the development of plants, possibly by suppressing the accumulation of ROS. GABA shunt provides NADH and/or succinate under stress conditions that inhibits the Krebs cycle, impairs respiration, and enhances the production of ROS (Bouché et al. 2003). This proposed mechanism is supported by a study in mammalian brain nerve terminals where H2O2 inhibits the Krebs cycle enzyme “aconitase” though inhibition of α-ketoglutarate dehydrogenase limiting the amount of NADH available for the respiratory chain and impairment of mitochondrial function under oxidative stress (Tretter et al. 2000). The inhibition reduces the content of glutamate (Tretter et al. 2000), because of the increased GABA shunt activities. Furthermore, GABA shunt reportedly protects yeast against oxidative stress, for its knockout mutants for GABA shunt genes become susceptible toward the exogenous treatment of H2O2. On the contrary, the overexpression of the consequent genes improves resistance (Coleman et al. 2001). Alternatively, the evident function of the GABA shunt in supports to the development of plant and stress tolerance may possibly be related to a condition to maintain adequate level of other metabolites, either directly or indirectly acquisition of the GABA shunt pathway (Fig. 3).
Other GABA shunt components show scavenging activity for ROS during stress conditions. The GAD activity regulated in response to cytosolic Ca2+ levels induces ability in plant cells to confer tolerance against different stress conditions by regulating the synthesis of GABA indicating involvement of GABA-shunt in stress signaling in plant system (Lee et al. 2010; Al-Quraan et al. 2013; Mazzucotelli et al. 2006). A homologue of GAD also protects yeast from oxidative stress (Coleman et al. 2001). In GABA shunt, Ca2+/CaM plays a pivotal role in reducing oxidative stress in CaM mutant of A. thaliana as they bind to the GAD and induces GABA production and accumulation during stress conditions (Al-Quraan et al. 2011). SSADH knockout mutant, GABA shunt deficient, shows the accumulation of GHB, the byproduct of GABA shunt that causes the synthesis of ROS in A. thaliana (Bouché et al. 2003; Fait et al. 2005). Further, the detoxification of SSA involves its reduction to GHB. Under oxygen deficiency, SSA is diverted to the production of GHB, a reaction catalyzed via the enzyme SSA reductase, which is localized in the cytosol and uses NADPH as a cofactor. GHB, a short chain fatty acid similar in structure to GABA, is normally present at about 1% of the GABA level (Breitkreuz et al. 2003; Simpson et al. 2008). Oxygen deficiency increases GHB level in soybean sprouts, and in green tea leaves. Furthermore, the GHB and GABA concentrations get increased in A. thaliana plants under hypoxia conditions. Simultaneously, the cellular NADH: NAD+ ratio also increases and the adenylate energy charge decreases, thus inhibiting SSADH activity and diverting carbon from succinate. Other study reveals that (i) ssadh mutant of A. thaliana plants, grown under high UV light, have five times the normal level of GHB and high levels of ROS (Fait et al. 2005), and (ii) the pattern of GHB in cold-acclimated A. thaliana plants is consistent with the rise and fall of GABA (Simpson et al. 2008). GHB also accumulates in response to salinity, drought and heat stress, confirming its accumulation under hypoxia condition and cold stress (Bouché et al. 2003; Fait et al. 2005; Simpson et al. 2008). Thus, the data indicate that GHB accumulation is a general response to abiotic stress. Moreover, GABA-T during stress conditions in plants act as a key factor that reduces oxidative damage and confers stress tolerance (Park et al. 2010; Renault et al. 2010, 2013; Cao et al. 2013; Al-Quraan and Al-Share 2016; Jalil et al. 2017).
GABA shunt metabolites significantly involve in the osmoregulation, nitrogen/carbon metabolism, and signaling in response to salt and oxidative stresses (Jalil and Ansari 2020b). It has been studied that high accumulation of GABA and Pro acts as osmoprotectant during oxidative and osmotic stress conditions and can lead to improve synthesis of cells and reduce their degradation (Al-Quraan and Al-Share 2016; Jalil et al. 2017). Further, GABA and Pro play an important role in reducing ROS production in plants during various environmental stress conditions; they are synthesized from the common precursor of glutamic acid (Liu et al. 2011). Similarly, the GABA and Pro have quenching ability on ROS production in Nicotiana tabacum leaves under water stress (Liu et al. 2011). Exogenous application of GABA also plays a regulatory role for free radical and ethylene production against salt stress (Shi et al. 2010). Other studies have also reported the defensive function of Pro in transgenic crops, in which Pro overproduction enhances tolerance to osmotic stress. Along with Pro, the response to osmotic stress additionally includes other amino acids. Glutamine synthase overexpression improves the tolerance to osmotic stress in Oryza sativa. These investigations conclude that changes in osmotic pressure enhances amino acid content due to modified gene expression encoding the enzymes involved in their metabolism (Ashraf and Foolad 2007).
The response of GABA under abiotic stress conditions
GABA signaling pattern regulates GABA shunt and connected pathways during stress conditions. The exogenous application of GABA under stress conditions binds on to the cell surface that increases Ca2+ level in the cytosol. Ca2+ via GAD regulates the GABA shunt pathway that increases the endogenous GABA content, elevates the photosynthetic activity and antioxidant enzyme activity and decreases malondialdehyde (MDA) and ROS accumulation, maintains membrane integrity and improves stress tolerance in plants (Fig. 3) (Liu et al. 2011; Jalil et al. 2017) (Bao and Li 2015; Jia et al. 2017). Table 1 enlists amelioration of ill effects of abiotic stresses in various plants by exogenous application of GABA. It has been observed that the enzymes which are diverted by the GABA shunt circumvents susceptible enzymes to oxidative stress. This susceptibility can decrease the efficiency of the Krebs cycle for which the GABA shunt can reimburse in stressed plants (Michaeli et al. 2011). GABA shunt also involves in the leaf senescence process. Exposure of abiotic stresses causes precocious leaf senescence followed by the breakdown of macromolecules. The breakdown products are further remobilized to developing parts of the plants (Ansari et al. 2014). The degradation of proteins produces amino acids which are utilized for the conversion of glutamate and ultimately to GABA. The C and N skeletons are accessible to the metabolism of the plant by the improved activity of the GABA shunt, specifically GABA-T in knockout mutants of A. thaliana (Jalil et al. 2016, 2017). The proper functioning of GABA shunt seems to be required for the restriction of ROS accumulation.
GABA in cold stress tolerance
Cold stress is a serious threat to the sustainability of harvest yields (Yadav 2010). Low temperature during germination and early seedling growth is one of the most significant limiting factors in the productivity of plants and induces considerable changes in biochemistry and physiology of plants such as damage to membranes, generation of ROS, protein denaturation and accumulation of toxic compounds at various cellular organizational levels (Nayyar and Chander 2004). Few studies vividly indicate a positive role of GABA in cold stress tolerance in different crops (Table 1). GABA enhancement in mulberry leaves is generally caused by the increase of GAD activity and the decline in GABA-T activity in cold stress (Li et al. 2018). Similarly, Exogenous GABA treatment increases chilling tolerance through enhancing its enzymatic antioxidant system by enhancing activities of antioxidant enzymes, such as superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, glutathione S-transferase, monodehydroascorbate reductase and dehydroascorbate reductase and maintaining energy status in peach fruit (Yang et al. 2011). Furthermore, GABA treatment protects wheat by alleviating oxidative damage and tomato seedlings from chilling stress by increasing antioxidant enzyme activity of catalase, ascorbate peroxidase and superoxide dismutase and reducing MDA content which results in maintaining membrane integrity (Malekzadeh et al. 2014). Additionally, proteomic analysis of tea plants under cold stress reportedly exhibits tolerance mechanisms as well as carbon and nitrogen metabolism under cold stress significantly related to exogenous GABA application (Zhu et al. 2019).
GABA in heat stress tolerance
Heat stress is also a key issue for plants and other photosynthetic organisms, which debilitates plant growth and development by changing phenology, physiology, biochemical and genetic expression (Prasad et al. 2017). Many physiological factors could be involved in heat stress injury as heat stress damags cell membranes leading to cell death. The adverse effects of heat stress may also be related to oxidative damage to cell membranes by the production of ROS that ultimately hinder the productivity of stressed plants (Liu and Huang 2000). GABA can induce tolerance in plants against heat stress (Table 1). A relationship between GABA metabolism and regulation of oxidative stress has been reported in A. thaliana SSADH mutant indicating that a functional GABA shunt is required to limit ROS intermediates during stress conditions such as heat and UV exposure by supplies of NADH and succinate under stressful conditions to maintain cellular oxidative balance, and allows to bypass steps of Krebs cycle involving enzymes sensitive to oxidative stress such as aconitase (Bouche et al. 2003; Fait et al. 2005). Further investigations in this direction suggest that Ca2+–dependent activation of the GABA shunt is important for ROS scavenging under UV-light stress in A. thaliana (Al-Quraan 2015). The exogenous treatment of GABA improves heat stress tolerance in creeping bentgrass, by maintaining cell membrane stability, enhancement of photosynthesis and ascorbate–glutathione cycle, the maintenance of osmotic adjustment, and the increase in GABA shunt. The increased GABA shunt activity appears to supply intermediates to the respiratory tricarboxylic acid cycle metabolism during a long-term heat stress, thereby maintaining metabolic homeostasis. Exogenous application of GABA also increases the accumulations of amino acids (glutamic acid, aspartic acid, alanine, threonine, serine, and valine), organic acids (aconitic acid, malic acid, succinic acid, oxalic acid, and threonic acid), sugars (sucrose, fructose, glucose, galactose, and maltose), and sugar alcohols (mannitol and myo-inositol) in heat stressed plant (Li et al. 2016a, b). Heat stress enhances GABA content in lichens. However, GAD and GDH activities get decreased, preventing degradation of chlorophyll and lipid peroxidation and improving heat stress tolerance in lichens (Cekic et al. 2018). GABA involves in the partial protection of rice seedlings (Nayyar et al. 2014) and reproductive function in mungbean (Priya et al. 2019) from heat stress by elevating leaf turgor due to increased accumulation of proline and trehalose and reduction of oxidative damage by stimulation of the activities of antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase and nonenzymatic antioxidants like ascorbate and glutathione. In mungbean, GABA also improves carbon fixation and assimilation processes. As a result, the lipid peroxidation and H2O2 production diminish. As a measure of heat stress regulation and tolerance, A. thaliana roots elevate Ca2+ level in the cytosol, which activates GAD in association with calmodulin, facilitating production and accumulation of GABA (Locy et al. 2000).
GABA in drought stress tolerance
Drought stress is one of the most serious restricting factors that adversely influences growth and development in perennial forage crops around the globe (Annicchiarico and Piano 2004). The insufficiency of accessible water controls plant productivity by damaging cell membrane, nutritional imbalance, and metabolic disorders in drought affected plants. Exposure of plants to drought stresses initially causes oxidative damage by the formation of ROS. These ROS pose serious threat to the cell functioning by damaging lipids and proteins (Fahad et al. 2017). Plants have developed physiological, biochemical, and metabolic response to cope with drought stress. It has been reported that the expression of GABA receptor genes is very high in drought-tolerant mutants of barley that play significant roles in enhancing drought tolerance through controlling stomatal closure via carbon metabolism, synthesizing the osmoprotectant glycine-betaine, generating protectants for ROS scavenging, and stabilizing membranes and heat shock proteins (Guo et al. 2009). Similarly, GABA content exceeds in hybrid bermudagrass (Cynodon dactylon × C. transvaalensis ‘Tifdwarf’) in comparison with the controls under drought stress (Du et al. 2012). The result vividly indicates that GABA plays a very important role in tolerance to drought stress by maintaining adequate cellular turgor. Further investigation reveals that exogenous GABA treatment at 50 mM alleviates drought stress damage in perennial ryegrass by regulating higher relative water content, membrane stability and limiting oxidative damage by showing higher relative water content (RWC), turf quality, and peroxidase activity and lower wilt rating, electrolyte leakage, and lipid peroxidation compared with control plants (Krishnan et al. 2013). Exogenous application of GABA elevates endogenous GABA content that improves drought tolerance possibly through regulation of GABA-shunt, PAs and Pro metabolism in white clover (Yong et al. 2017). Moreover, exogenous application of GABA improves growth and productivity of black cumin under water deficit stress conditions through maintaining osmoregulation and antioxidant defense by increasing catalase, peroxidase, and superoxide dismutase activity including increased soluble sugars and proline content (Rezaei-Chiyaneh et al. 2018).
GABA in waterlogging/hypoxia tolerance
Insufficiency of molecular oxygen prompts hypoxia in plants, which brings about an irregularity in metabolism and reduction in crop productivity. Oxygen is significant for plants as it participates in ROS production, which acts as a signaling molecule and electron acceptor in electron transport chain (Fukao and Bailey-Serres 2004). Reduced level of oxygen in plant cells brings to lower ATP production that finally declines the metabolic rate (Drew et al. 1997). Plants experience different physiological changes including elongation of petioles, development of root aerenchyma, and modification in root morphology to adapt in hypoxia condition. The cells respond to initial hypoxia by metabolizing pyruvate under anaerobic condition (Fukao and Bailey-Serres 2004). The protective role of GABA in plants towards hypoxia encompasses by regulating cytosolic pH, maintaining Krebs cycle, and supporting carbon/nitrogen metabolism (Fait et al. 2008). Exogenous application of GABA promotes nitrate uptake in melons in order to alleviate hypoxia stress (Song et al. 2012). GABA also promotes PAs synthesis under hypoxia conditions (Wang et al. 2014a). During hypoxia stress, activity of malate dehydrogenase (MDH) declines while the protein content increases. Exogenous application of GABA further reduces MDH level under oxygen-deficient condition, thereby acting as an anaplerotic molecule to replenish the intermediate of Krebs cycle (Fan et al. 2015). When gad1 mutant of A. thaliana having been exposed to hypoxia stress, GAD activity decreases in root, but no significant difference being observed in shoot as compared to the wildtype plants. However, under normal conditions, no significant difference has been observed in the mutant plants. The role of GABA shunt in alanine accumulation under hypoxia stress has also been recorded in mutant plants (Miyashita and Good 2008). Moreover, the exogenous treatment of GABA promotes growth of maize seedlings during waterlogging conditions by promoting photosynthetic rate and chlorophyll content, reducing ROS production such as MDA, H2O2, and O2−, down-regulation of the ROS-generating enzymes, activating antioxidant defense systems by fortifying antioxidants enzyme activities of superoxide dismutase, peroxidase, ascorbate peroxidase, catalase and glutathione reductase and improving chloroplast ultrastructure and photosynthetic system (Salah et al. 2019).
GABA in salt stress tolerance
Salt stress influences physiological process of plants, including seed germination, plant growth, and productivity. Several studies have reported GABA to have protective role against salinity stress tolerance (Table 1). Further, GABA shunt gets activated in stress conditions, regulating Ca2+ channel by GAD enzyme (Bai et al. 2013). The elevated level of GAD expression accumulates GABA content that improves tolerance in wheat against salinity stress (Al-Quraan et al. 2013). The seeds of wheat (Triticum aestiveum L.) and barley (Hordeum vulgare L.) cultivars in salinity stress condition show enhanced GAD expression that converts glutamate into GABA. Production of the latter opens another metabolic route to maintain ETC, balanced C/N metabolism and synthesize osmolytes that support seed germination of wheat and barley during salinity stress (Al-Quraan et al. 2019). The same has been testified with the exogenous application of GABA to wheat seedlings that improves photosynthetic and antioxidant enzyme activities such as superoxide dismutase and catalase as well as growth and decreases MDA content and electrolyte conductivity during ROS induced salinity stress (Li et al. 2016a, b). In consonant, NaCl derived stress tolerance has been improved in barley seedlings by Ca2+ that activates GABA signal transduction for accumulation and production of phenolics and promotion of antioxidant activities by regulating the peroxidase, catalase, superoxide dismutase, ascorbate peroxidase, glutathione reductase and glutathione S-transferase (Ma et al. 2018). GABA also helps express abscisic acid (ABA) and ethylene metabolic genes and signal mechanism in poplar during salinity stress that indicates the possible function of GABA in signal pathways of ABA and ethylene (Ji et al. 2018). Polyamine mediated GABA production in A. thaliana elevates aldehyde dehydrogenase (ALDH) and confers salinity tolerance (Zarei et al. 2016). GABA also improves germination, photosynthetic activity, and reduced oxidative damages by increasing catalase, ascorbate peroxidase, and superoxide dismutase activities that resulted in regulated hydrogen peroxide level in lettuce under salinity stress (Kalhor et al. 2018). Similarly, GABA metabolism has been involved in improved performance of Nicotiana sylvestris cytoplasmic male sterile CMSII mutant plants due to the operation of alternative dehydrogenases in CMSII that avoiding oxidative stress in salinity conditions (Akçay et al. 2012).
GABA in metal/metalloid stress tolerance
Metal/metalloid stress has become a foremost concern in different ecosystems around the world. Nowadays wide-ranging industrialization confers inconvenient impacts on soil as well as on crop productivity by accumulating toxic metals that directly and/or indirectly reduced plant growth by antagonistically influencing different physiological and molecular responses of plants (Tiwari and Lata 2018). Several studies indicate an augmenting role of GABA to confer tolerance in plants against toxic metals (Table 1). GABA reduces absorption/ accumulation of cadmium ions (Cd2+) in microalgae (Monoraphidium spp.) and promotes lipid biosynthesis and uptake of mineral nutrients via ROS signaling transduction under cadmium stress (Zhao et al. 2020). GABA also alleviates oxidative damage caused by proton (H+) and aluminum (Al3+) toxicities in barley seedlings by activating antioxidant defense responses by increasing the activity of antioxidant enzymes such as superoxide dismutase, catalase and peroxidase along with reducing the elevated levels of carbonylated proteins caused by ROS (Song et al. 2010). Moreover, GABA improves the physiological mechanisms of mustard seedlings in response to chromium (Cr) stress by reducing Cr uptake and upregulating the non-enzymatic(ascorbate and glutathione) and enzymatic antioxidants (ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, glutathione peroxidase, superoxide dismutase, catalase, glyoxalase I, and glyoxalase II) and ultimately reducing oxidative damage (Mahmud et al. 2017).
Role of GABA has also been reported in improving tolerance against other abiotic stresses in different crops as listed in Table 1. It has been suggested that the accumulation of GABA under dark and anaerobic conditions provides succinate to the Krebs cycle for the seed germination of rice (Lee et al. 2020). GABA also improves photosynthesis, antioxidant system including increased activities of superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, ascorbate and glutathione and exhibited lower MDA, O2•− and H2O2 production in Capsicum annuum under low light stress (Li et al. 2017).
GABA function in biotic stress conditions
GABA acts as a signaling molecule and is utilized as a carbon and nitrogen source by bacteria. For example, GABA content in the apoplast facilitates Cladosporium fulvum infection in tomato (Solomon and Oliver 2002). Pseudomonas syringae pv. Tomato DC3000 strain utilizes tomato apoplast derived GABA as a sole source of carbon or nitrogen (Rico and Preston 2008; Park et al. 2010). Similarly, K12 mutant strains of E. coli, with high GABA-T activity utilize GABA as a sole source of carbon and nitrogen (Dover and Halpern 1972). Conversely, there is a report indicating improvement of plant tolerance by high content of GABA due to repression of the hrp genes in the bacteria (Park et al. 2010).
GABA also induces resistance in crops against pathogenic fungi, it activates the defense machinery of plants against harmful insects, for plants with deterrence for insect accumulate GABA (Morse et al. 1979). Mechanical damage/injury, even crawling on leaves by insects also induces accumulation of GABA. It is believed that the ingested GABA interrupts the development process of insects (Bown et al. 2002). Moreover, transgenic tobacco plants with increased GABA content exhibit resistance against root-knot nematode and tobacco budworm larvae (MacGregor et al. 2003; McLean et al. 2003). Similarly, GABA level enhances in Asparagus cells on exposure to pathogen-induced oxidative stress (Shelp et al. 2003, 2006; Bown et al. 2006). Besides, the growth media supplemented with GABA directly inhibits larvae of Spodoptera littoralis and Choristoneura rosaceana in A. thaliana mutants. High GABA content inhibits growth rate of the larvae and their survival rate as well (Ramputh et al. 1996; Bown et al. 2002, 2006; Scholz et al. 2015). These findings confirm that GABA not only has an immediate and prompt impact on insects but also involves in activating secondary defense responses in the infected plants vis-à-vis accumulation of GABA.
The biphasic defense mechanism of GABA has been demonstrated in tomato mutants against necrotrophic fungus Botrtytis cinerea. Exogenous application of GABA inhibits fungal infection by formation of the chlorotic rings around the infected tissue and triggers over-activation of GABA shunt that limits the cell death caused by the H2O2 mediated defense response at the penetration site of Botrtytis cinerea (Seifi et al. 2013). GABA shunt regulates the redox reaction through the production of NADH by SSADH and by the supply of energy and carbon skeletons to Krebs cycle under oxidative stress. GABA shunt restricts cell death after infection of fungus Magnaporte oryzae in rice crops. Moreover, the application of exogenous GABA in tomatoes and pears (Seifi et al. 2013; Yu et al. 2014; Fu et al. 2017; Yang et al. 2017) prompts tolerance against fungal pathogen mainly through the induction of both transcript and protein activity levels of antioxidant and defense related enzymes including chitinase, β-1,3-glucanase, phenylalnine ammonialyase, peroxidase and polyphenol oxidase (Yang et al. 2017; Yu et al. 2014).
The concurrent elevation of antioxidant enzymes in the GABA treated plants proposes that GABA improves tolerance against biotic stress by restricting the ROS mediated cell death in plants. The accumulated GABA can interfere with quorum sensing (QS) in response to bacterial pathogens; however, some of them are specialized in using GABA as preferred nutrient source. Moreover, GABA uptake by herbivorous insects can result in inhibition of GABA neuronal receptors, provoking neuromuscular disorders and abnormal development. Furthermore, GABA boosts endurance as its metabolism sustains host cells against infections by fueling the Krebs cycle and contrasting oxidative damage derived from ROS burst (Tarkowski et al. 2020). The development of tolerance in plants against pathogens is uncertain as GABA is a constituent of plant metabolism and the metabolization of exogenous and endogenous GABA occurs in a similar manner.
PAs pathway for GABA to affect ROS homeostasis during stress conditions
PAs act along with GABA to indirectly influence ROS homeostasis. PAs catabolism prompted by DAO activity leads to GABA synthesis (Shelp et al. 2012). Stressful conditions enhance GAD and DAO activities for GABA formation (Bouché et al. 2003; Bouche and Form 2004); this action also produces H2O2, which acts as a signal to activate antioxidant responses (Gupta et al. 2016). The role of PAs to increase antioxidant defenses under various stresses has been extensively described (Minocha et al. 2014). Degradation pathways of PAs feed into the GABA biosynthesis pathway, and endogenous levels of PAs and GABA are closely linked in plant cells (Wang et al. 2014a). PAs content is closely coordinated with environmental factors, and rapidly changes on exposure to abiotic stress. PAs and GABA accumulate under hypoxia stress and when stress is removed, the contents of PAs and GABA decrease significantly, indicating their dependent interrelationship (Song et al. 2012). Spermidine, spermine, and putrescine, are the major PAs in plants. These metabolites act as second messengers and mediate plant responses to several environmental stresses. Moreover, exogenous GABA plays an important role in alleviating Ca(NO3)2 induced injury to muskmelon seedlings by improving/ preventing PAs biosynthesis/degradation (Hu et al. 2015).
PA degradation via DAO and PAO produces GABA, which is converted via transamination and oxidation to succinate, then introduced into the Krebs cycle (Duan et al. 2008). However, only about 30% of GABA is formed at the expense of PAs degradation in germinating fava beans (Vicia faba) during hypoxic conditions (Yang et al. 2013). Moreover, exogenous GABA application suppresses the gene expression and activities of cell wall bound DAO and PAO; however, the addition of GABA alters the enzyme activity of PAs degradation more than those of PAs biosynthesis in the control plants of melon (Wang et al. 2014a, b). This suggests that higher levels of endogenous GABA, a product of polyamine oxidation, may inhibit its own formation from the potential negative feedback mechanism in melon (Wang et al. 2014a). Concurrently, GABA protects and maintains high level of PAs and reduces injury from hypoxia stress (Wang et al. 2014a). Exogenous application of GABA elevates endogenous GABA content that improves drought tolerance possibly through regulation of GABA-shunt, PAs and Pro metabolism in white clover by significantly increasing relative water content, lowering electrolyte leakage, lipid peroxidation, and leaf wilt and further promotes drought-induced increases in GABA transaminase and alpha ketone glutamate dehydrogenase activities, but it inhibits glutamate decarboxylase activity, resulting in an increase in endogenous glutamate and GABA content (Yong et al. 2017). In addition, exogenous GABA enhances PAs biosynthesis and represses PAs catabolism. Concomitantly, there is tremendous increase in different types of PAs content such as putrescine and spermidine along with the activation of drought-induced Δ1-pyrroline-5-carboxylate synthetase and proline dehydrogenase activities, but represses ornithine-δ-amino transferase activities, leading to a higher Pro accumulation and metabolism in GABA-treated plants under drought stress (Yong et al. 2017).
PAs directly control the genetic expression of catalase in green microalga Ulva fasciata, suggesting their potential as signaling molecules (Sung et al. 2011). Similarly, plant interactions with pathogens engage PAs in regulation of ROS accumulation (Lou et al. 2016; Seifi and Shelp 2019). Catabolism of polyamine spermine confers hypersentivity response to tobacco (Nicotiana tabacum) and cotton (Gossipium hirsutum L.). On the other hand, the exogenous spermine treatment induces H2O2 to involve in signal transduction in Arabidopsis cucumber mosaic virus pathosystem (Mo et al. 2015; Yoda et al. 2003). In line with this, inhibition of putrescine catabolism through putrescine acetylation reduces H2O2 production during an overactive response to A. thaliana, leading to a suppression of antibodies against P. syringae DC3000 (Lou et al. 2016). Nevertheless, GABA appears to be critical in regulating antioxidant mechanisms during stressful situations (Seifi et al. 2013; Song et al. 2010; Wang et al. 2014a). PAs catabolism-dependent production of both H2O2 and GABA offers additional regulation of ROS production under stress conditions (Gupta et al. 2016). Because of such considerations and the strong links between GABA and PAs during abiotic and biotic stress (Hatmi et al. 2015; Podlešáková et al. 2019; Wang et al. 2014a), a strategic modulation of fluxes between GABA and PA metabolic pathways to control generation and signaling of ROS offers fascinating novel circumvention to biotic and abiotic stress conditions.
Role of GABA transporters in improving stress tolerance
The amino acids are transported across the membranes via amino acid transporters (AATs) that are the key supplier in the mineral nutrient distribution mechanism, which helps in plant growth, development, and regulation of defense machinery (Tegeder 2012). The membrane bound GABA transporter (GATs) facilitates transport of high content of Ca2+ into the cells. GATs regenerate and get activated with the attachment of GABA that possibly arises from expression of GABA shunt genes under stress conditions (Fig. 3) (Meyer et al. 2006).
GATs serve as conduit for pervasive amino acid GABA (Satyanarayan and Nair 1990) which involves in signaling mechanism influencing C and N balance, pH regulation, nitrogen storage, and stress resistance (Shelp et al. 1999; Bouche and Fromm 2004). GABA accumulation regulates the activities of plant-specific anion transporter that helps in root development and enhanced stress tolerance (Bouche and Fromm 2004). GAT1 transporter of A. thaliana can’t transport Pro into the cell, but it has a high affinity for GABA to intercede association with carbon and nitrogen (Breitkreuz et al. 1999; Schwacke et al. 1999; Batushansky et al. 2015). Furthermore, overexpression of GATs helps in expression of PeuGAT3 gene that is responsible for growth of xylem tissues in A. thaliana and Populus euphratica and increases lignin content of xylem tissues and the Pro accumulation in P. euphratica leaves. As result, P. euphratica acquires tolerance against salt and drought stresses (Bai et al. 2019). The transportation of GABA also occurs through Pro transporters (ProTs) from Pro transporter1, 2 (AtProT1, 2) of A. thaliana, and Pro transporter1 (LeProT1) of Solanum lycopersicum. It has been reported that AtProT2 is highly activated under stress conditions. Pro-GATs also serve as conduit for osmolytes in cells for protecting plants from osmotic stress conditions (Schwacke et al. 1999; Batushansky et al. 2015).
GABA permease of A. thaliana (AtGABP) is responsible for the transportation of major content of cytosolic GABA into mitochondria for catabolism by the enzymes, GABA-T and SSADH to form succinate that gets passage to Krebs cycle and ETC (Michaeli et al. 2011). On the other hand, the noxious SSA may be mobilized from the mitochondria into the cytosol for the formation of GHB by the enzyme SSR. Furthermore, mitochondrial GAT knockout mutant of A. thaliana, showing a minimal consumption of GABA elevates the activity of the Krebs cycle (Michaeli et al. 2011). It has been studied that GABA inhibits the aluminum-activated malate transporter (ALMT), which plays a key role as GABA receptor in plant tissue. Furthermore, ALMT regulation by GABA represents a signaling pathway possibly modifies membrane potentials responsible for immediate physiological changes in the plant system (Ramesh et al. 2015; Gilliham and Tyerman 2016). These studies suggest that somehow GATs directly or indirectly regulate GABA metabolism that involves in the mitigation of ROS for alleviating stress tolerance in plant system.
Conclusion and future prospects
ROS are generated by various plant metabolic pathways in the cells. Their production and eradication are balanced under normal conditions. However, under hostile environmental conditions, this balance gets disturbed via escalation of ROS levels. The disproportionate production of ROS causes destructive activities and oxidative damage in plant cells. Plants have various stress-specific responses and have complex networking of antioxidant system for scavenging ROS. GABA shunt is a signaling and metabolic pathway involved in molecular mechanisms that are important for plant development and proficient in regulating the stress-accumulated signaling molecule GABA content in the cytosol. GABA and other components of the GABA shunt play a crucial role in scavenging ROS and protecting the plant system from oxidative damage during various stress conditions. GABA shunt copiously involves in oxidative stress tolerance. The review elucidates the free radical scavenging mechanism of GABA during stress conditions for the agroeconomic improvement. Most of the researches has been carried out under abiotic stress, and the role of the GABA shunt with its metabolites is still an important issue to be addressed with further investigations. Future studies are ought to illuminate the mechanism through which physiological enhancement in GABA contents during stress condition occurs and encompasses GAD stimulation, suppression of catabolism or expanded flux within the route of the GABA shunt, influence plant development and activation of defense machinery in plants. Characterization of the surface-bind GABA receptors, GATs, and other components involved in GABA-mediated gene regulation for stress tolerance is indispensable to fully exploit the system for crop productivity and quality improvement in prevalent diverse array of abiotic and biotic stresses for food security to burgeoning human population.
Abbreviations
- AATs:
-
Amino acid transporters
- ABA:
-
Abscisic acid
- ALDH:
-
Aldehyde dehydrogenase
- ALMT:
-
Aluminum-activated malate transporter
- Al:
-
Aluminium
- AtGABP:
-
Arabidopsis thaliana GABA permease
- ATP:
-
Adenosine triphosphate
- AtProT:
-
Arabidopsis thaliana proline transporter
- C:
-
Carbon
- Ca:
-
Calcium
- Ca(NO3)2 :
-
Calcium nitrate
- CaM:
-
Calmodulin or calcium-modulated
- Cd:
-
Cadmium
- Cr:
-
Cromium
- DAO:
-
Diamine oxidase
- ETC:
-
Electron transport chain
- GABA:
-
Gamma-aminobutyric acid
- GABA-T:
-
GABA transaminase
- GAD:
-
Glutamate decarboxylase
- GATs:
-
GABA transporters
- GHB:
-
γ-Hydroxybutyric acid
- H+ :
-
Proton
- HO2 • :
-
Hydroperoxyl radical
- H2O2 :
-
Hydrogen peroxide
- LeProT:
-
Solanum lycopersicum proline transporter
- MDA:
-
Malondialdehyde
- MDH:
-
Malate dehydrogenase
- N:
-
Nitrogen
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADH:
-
Nicotinamide adenine dinucleotide + hydrogen
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate + hydrogen
- O2 •− :
-
Superoxide radical
- 1O2 :
-
Singlet oxygen
- OH• :
-
Hydroxyl radical
- ONOO• :
-
Peroxynitrite
- PAs:
-
Polyamines
- PAO:
-
Polyamine oxidase
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- Pro:
-
Proline
- ProTs:
-
Proline transporters
- PYRR:
-
Pyrroline
- PYRRDH:
-
Pyrroline dehydrogenase
- ROS:
-
Reactive oxygen species
- RO• :
-
Alkoxy radical
- ROO• :
-
Peroxy radical
- SSA:
-
Succinic semialdehyde
- SSADH:
-
Semialdehyde dehydrogenase
- SSR:
-
Succinic semialdehyde reductase
- TRX:
-
Thioredoxin
- UV:
-
Ultra violet
References
Aghdam MS, Naderi R, Jannatizadeh A, Sarcheshmeh MAA, Babalar M (2016) Enhancement of postharvest chilling tolerance of Anthurium cut flowers by gamma-aminobutyric acid (GABA) treatments. Sci Hortic 198:52–60
Akçay N, Bor M, Karabudak T, Özdemir F, Türkan I (2012) Contribution of gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J Plant Physiol 169:452–458
Alqarawi AA, Hashem A, Abd-Allah EF, Al-Huqal AA, Alshahrani TS, Alshalawi SR (2016) Protective role of γ- aminobutyric acid on Cassia italica Mill under salt stress. Legume Res 39:396–404
Al-Quraan NA (2015) GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol Plant 37:86
Al-QuraanAL-AjlouniObedat NZD (2019) The GABA shunt pathway in germinating seeds of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) under salt stress. Seed Sci Res 29:250–260
Al-Quraan NA, Al-Share AT (2016) Characterization of the γ-aminobutyric acid shunt pathway and oxidative damage in Arabidopsis thaliana pop 2 mutants under various abiotic stresses. Biol Plant 60:132–138
Al-Quraan NA, Locy RD, Singh NK (2011) Implications of paraquat and hydrogen peroxide-induced oxidative stress treatments on the GABA shunt pathway in Arabidopsis thaliana calmodulin mutants. Plant Biotechnol Rep 5:225–234
Al-Quraan NA, Sartawe FA, Qaryouti MM (2013) Characterization of γ-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J Plant Physiol 170:1003–1009
Annicchiarico P, Piano E (2004) Indirect selection for root development of white clover and implications for drought tolerance. J Agron Crop Sci 190:28–34
Ansari MI, Chen SCG (2009) Biochemical characterization of gamma-aminobutyric acid (GABA): pyruvate transaminase during rice leaf senescence. Int J Integr Biol 16:27–32
Ansari MI, Lee RH, Chen SCG (2005) A novel senescence- associated gene encoding γ-aminobutyric acid (GABA): pyruvate transaminase is upregulated during rice leaf senescence. Physiol Plant 123:1–8
Ansari MI, Hasan S, Jalil SU (2014) Leaf senescence and GABA shunt. Bioinformation 10:730–732
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Ashraf MFMR, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bai Q, Yang R, Zhang L, Gu Z (2013) Salt stress induces accumulation of γ-aminobutyric acid in germinated foxtail millet (Setaria italica L.). Cereal Chem 90:145–149
Bai X, Xu J, Shao X, Luo W, Niu Z, Gao C, Wan D (2019) A novel gene coding γ-aminobutyric acid transporter may improve the tolerance of Populus euphratica to adverse environments. Front Plant Sci 10:123–131
Bailey-Serres J, Mittler R (2006) The roles of reactive oxygen species in plant cells. Plant Physiol 141:311. https://doi.org/10.1104/pp.104.900191
Bao H, Li Y (2015) Virus-induced gene silencing reveals control of reactive oxygen species accumulation and salt tolerance in tomato by γ- aminobutyric acid metabolic pathway. Plant Cell Environ 38:600–613
Batushansky A, Kirma M, Grillich N, Pham PA, Rentsch D, Galili G, Fernie AR, Fait A (2015) The transporter GAT1 plays an important role in GABA-mediated carbon–nitrogen interactions in Arabidopsis. Front Plant Sci 6:785. https://doi.org/10.3389/fpls.2015.00785
Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H (1993) A plant glutamate decarboxylase containing a calmodulin binding domain. J Biol Chem 268:19610–19617
Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmudulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. Embo J 15:2988–2996
Bhattacharjee S (2005) Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plants. Curr Sci 89:1113–1121
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Bouché N, Fait A, Bouché D, Møller SG, Fromm H (2003) Mitochondrial succinate-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt in required to restricts levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci USA 100:6843–6848
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bown AW, Shelp BJ (1997) The metabolism and function of gamma aminobutyric acid. Plant Physiol 115:1–5
Bown AW, Hall DE, MacGregor KB (2002) Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol 129:1430–1440
Bown AW, MacGregor KB, Shelp BJ (2006) γ- aminobutyrate: defense against invertebrate pests? Trends Plant Sci 11:424–427
Breitbreuz KE, Shelp BJ (1995) Subcellular compartmentation of the 4-aminobutyrate shunt in protoplast from developing soybean cotelydons. Plant Physiol 108:99–103
Breitkreuz KE, Shelp BJ, Fischer WN, Rentsch D (1999) Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett 450:280–284
Breitkreuz KE, Allan WL, Van Cauwenberghe OR, Jakobs C, Talibi D, Andre B, Shelp BJ (2003) A novel gamma-hydroxybutyrate dehydrogenase: identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. J Biol Chem 278:41552–41556
Cao J, Barbosa JM, Singh NK, Locy RD (2013) GABA shunt mediates thermotolerance in Saccharomyces cerevisiae by reducing the production of reactive oxygen species. Yeast 30:129–144
Cekic FÖ (2018) Exogenous GABA stimulates endogenous GABA and phenolic acid contents in tomato plants under salt stress. Celal Bayar Uni J Sci 14:61–64
Che-Othman MH, Jacoby RP, Millar AH, Taylor NL (2019) Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol 225:1166–1180
Clark SM, Di Leo R, Dhanoa PK, Van Cauwenberghe OR, Mullen RT, Shelp BJ (2009) Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis γ-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J Exp Bot 60:1743–1757
Coleman ST, Fang TK, Rovinsky SA, Turano FJ, Moye- Rowley WS (2001) Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J Biol Chem 276:244–250
Corpas FJ, Barroso JB, del Río LA (2002) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6:145–150
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:163. https://doi.org/10.1186/1471-2229-11-163
Dietz KJ (2003) Plant peroxiredoxins. Annu Rev Plant Biol 54:93–107
Dismukes GC, Klimov VV, Baranov SV, Kozlov YN, Das Gupta J, Tyryshkin A (2001) The origin of atmospheric oxygen on earth: the innovation of oxygenic photosynthesis. Proc Natl Acad Sci USA 98:2170–2175
Dover S, Halpern YS (1972) Utilization of γ-aminobutyric acid as the sole carbon and nitrogen source by Escherichia coli K-12 mutants. J Bacteriol 109(2):835–843
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Ann Rev Plant Physiol Plant Mol Biol 48:223–250
Duan JJ, Li J, Guo SR, Kang YY (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620e1635. https://doi.org/10.1016/j.jplph.2007.11.006
Du H, Wang Z, Yu W, Huang B (2012) Metabolic responses of hybrid bermudagrass to short-term and long-term drought stress. J Am Soc Hortic Sci. https://doi.org/10.21273/JASHS.137.6.411
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Fait A, Yellin A, Fromm H (2005) GABA shunt deficiencies and accumulation of reactive oxygen intermediates: insight from Arabidopsis mutants. FEBS Lett 579:415–420
Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci 13:14–19
Fan L, Wu X, Tian Z, Jia K, Pan Y, Li J, Gao H (2015) Comparative proteomic analysis of gamma-aminobutyric acid responses in hypoxia-treated and untreated melon roots. Phytochem 116:28–37
Fu D, Sun Y, Yu C, Zheng X, Yu T, Lu H (2017) Comparison of the effects of three types of aminobutyric acids on the control of Penicillium expansum infection in pear fruit. J Sci Food Agric 97:1497–1501
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia – is survival a balancing act? Trends Plant Sci 9:449–456
Gilliham M, Tyerman SD (2016) Linking metabolism to membrane signaling: the GABA-malate connection. Trends Plant Sci 21:295–301
Gregersen PL, Culetic A, Boschian L, Krupnska K (2013) Plant senescence and crop productivity. Plant Mol Biol 82:603–622
Guo P, Baum M, Grando S, Ceccarelli S, Bai G, Li R, Korff MV, Varshney RK, Graner A, Valkoun J (2009) Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J Exp Bot 60:3531–3544
Gupta K, Sengupta A, Chakraborty M, Gupta B (2016) Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front Plant Sci 7(9):1343. https://doi.org/10.3389/fpls.2016.01343
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hatmi S, Gruau C, Trotel-Aziz P, Villaume S, Rabenoelina F, Baillieul F, Aziz A (2015) Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J Exp Bot 66(3):775–787
Hoover GJ, Van Cauwenberghe OR, Breitkreuz KE, Clark SM, Merrill AR, Shelp BJ (2007) Characteristics of an Arabidopsis glyoxylate reductase: general biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta. Can J Bot 85:883–895
Hu WH, Song XS, Shi K, Xia XJ, Zhou YH, Yu JQ (2008) Changes in electron transport, superoxide dismutase and ascorbate peroxidase isoenzymes in chloroplasts and mitochondria of cucumber leaves as influenced by chilling. Photosynthetica 46:581–588
Hu X, Xu Z, Xu W, Li J, Zhao N, Zhou Y (2015) Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol Biochem 92:1–10
Jalil SU, Ansari MI (2008) Plant microbiome and its functional mechanism in response to environmental stress. Int J Green Pharm 12:S81. https://doi.org/10.22377/ijgp.v12i01.1603
Jalil SU, Ansari MI (2020a) Stress implication and crop productivity. In: Hasanuzzaman M (ed) Plant ecophysiology and adaptation under climate change: mechanism and perspectives. Springer, Singapore, pp 73–86
Jalil SU, Ansari MI (2020b) Physiological role of gamma aminobutyric acid in salt stress tolerance. In: Hasanuzzaman M, Tanveer M (eds) Salt and drought stress tolerance in plants, signaling and communication in plants. Springer, Switzerland, pp 337–350
Jalil SU, Ahmad I, Ansari MI (2016) Phenotypic characterization of GABA-transaminase mutants of Arabidopsis thaliana. Adv Life Sci 5:10005–10008
Jalil SU, Ahmad I, Ansari MI (2017) Functional loss of GABA transaminase (GABA-T) expressed early leaf senescence under various stress conditions in Arabidopsis thaliana. Curr Plant Biol 9–10:11–22
Jalil SU, Khan MAA, Ansari MI (2019a) Tremendous role of GABA shunt metabolic pathway in plant system. J Biol Chem Res 36:188–196
Jalil SU, Khan MQR, Ansari MI (2019b) Role of GABA transaminase in the regulation of development and senescence in Arabidopsis thaliana. Curr Plant Biol 19:100119. https://doi.org/10.1016/j.cpb.2019.100119
Ji J, Yue J, XieChenDuChangChenJiangShi TWCELZS (2018) Roles of γ-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: involving in abscisic acid and ethylene-signalling pathways. Planta 248:675–690
Jia Y, Zou D, Wang J, Sha H, Liu H, Inayat MA, Sun J, Zheng H, Xia N, Zhao H (2017) Effects of γ- aminobutyric acid, glutamic acid, and calcium chloride on rice (Oryza sativa L.) under cold stress during the early vegetative stage. J Plant Growth Regul 36:240–253
Jin X, Liu T, Xu J, Gao Z, Hu X (2019) Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol 19:48. https://doi.org/10.1186/s12870-019-1660-y
Kalhor MS, Aliniaeifard S, Seif M, Asayesh EJ, Bernard F, Hassani B, Li T (2018) Enhanced salt tolerance and photosynthetic performance: implication of γ-aminobutyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol Biochem 130:157–172
Kalhor MS, Aliniaeifard S, Bernard F, Seif M, Latifi M, Hassani B, Didaran F, Bossachi M, Rezadoost H, Li T (2020) γ-aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems. Sci Rep 10:3356. https://doi.org/10.1038/s41598-020-59592-1
Kärkönen A, Kuchitsu K (2015) Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112:22–32
Karuppanapandian T, Moon JC, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725
Kaur R, Zhawar VK (2021) Regulation of secondary antioxidants and carbohydrates by gamma-aminobutyric acid under salinity–alkalinity stress in rice (Oryza sativa L.). Biol Fut. https://doi.org/10.1007/s42977-020-00055-z
Kenersley AM, Turano FJ (2000) Gamma-aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Krishnan S, Laskowski K, Shukla V, Merewitz EB (2013) Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ-aminobutyric acid on perennial ryegrass. J Am Soc Hortic Sci 138:358–366
Kumar N, Dubey AK, Upadhyay AK, Gautam A, Ranjan R, Srikishna S, Sahu N, Behera SK, Mallick S (2017) GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci Rep 7:1. https://doi.org/10.1038/s41598-017-09428-2
Kwak J, Nguyen V, Schroeder J (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141:323–329
Lane TR, Stiller M (1970) Glutamic acid decarboxylation in Chlorella. Plant Physiol 45:558–562
Lee JH, Kim YJ, Jeong DY, Sathiyaraj G, Pulla RK, Shim JS, In JG, Yang DC (2010) Isolation and characterization of a glutamate decarboxylase (GAD) gene and their differential expression in response to abiotic stresses from Panax ginseng C. A. Meyer. Mol Biol Rep 37:3455–3463
Lee J, Kim S, Kim S, Shim IS (2020) Production of γ-aminobutyric acid and its supplementary role in the TCA cycle in rice (Oryza sativa L.) seedlings. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10066-8
Li MF, Guo SJ, Yang XH, Meng QW, Wei XJ (2016a) Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol Plant 60:123–131
Li Z, Yu J, Peng Y, Huang B (2016b) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostissto lonifera). Sci Rep 6:30338. https://doi.org/10.1038/srep30338
Li Y, Fan Y, Ma Y, Zhang Z, Yue H, Wang L, Li J, Jiao Y (2017) Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J Plant Growth Regul 36:436–449
Li E, Luo X, Liao S, Shen W, Li Q, Liu F, Zou Y (2018) Accumulation of γ-aminobutyric acid during cold storage in mulberry leaves. Int J Food Sci Technol 53:2664–2672
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bent grass. Crop Sci 40:503–510
Liu C, Zhao L, Yu G (2011) The dominant glutamic acid metabolic flux to produce γ-aminobutyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J Integr Plant Biol 53:608–618
Locy RD, Wu SJ, Bisnette J, Barger TW, McNabb D, Zik M, Fromm H, Singh NK, Cherry JH (2000) The regulation of GABA accumulation by heat stress in Arabidopsis. In: Cherry JH, Locy RD, Rychter (eds) Plant tolerance to abiotic stresses in agriculture: Role of genetic engineering. NATO Science Series (Series 3: High Technology) Springer: Dordrecht. pp. 39-52
Lou YR, Bor M, Yan J, Preuss AS, Jander G (2016) Arabidopsis NATA1 acetylates putrescine and decreases defense-related hydrogen peroxide accumulation. Plant Physiol 171(2):1443–1455
Ma Y, Wang P, Wang M, Sun M, Gu Z, Yang R (2018) GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem 270:593–601
MacGregor KB, Shelp BJ, Peiris SE, Bown AW (2003) Overexpression of glutamate decarboxylase in transgenic tobacco deters feeding by phytophagous insect larvae. J Chem Ecol 29:2177–2182
Maheshwari R, Dubey RS (2009) Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul 59:37–49
Mahmud JA, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M (2017) γ-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology 26:675–690
Malekzadeh P, Khara J, Heydari R (2014) Alleviating effects of exogenous gamma-aminobutyric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants 20:133–137
Matysik J, Alia A, Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Mazzucotelli E, Tartari A, Cattivelli L, Forlani G (2006) Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot 57:3755–3766
McLean MD, Yevtushenko DP, Deschene D, Van Cauwenberghe OR, Makhmoudova A, Potter JW, Bown AW, Shelp BJ (2003) Overexpression of glutamate decarboxylase in transgenic tobacco plants confers resistance to the northern root-knot nematode. Mol Breed 11:277–285
Meriga B, Reddy BK, Rao KR, Reddy LA, Kishor PBK (2004) Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). J Plant Physiol 161:63–68
Meyer A, Eskandari S, Grallath S, Rentsch D (2006) AtGAT1, a high affinity transporter for γ-aminobutyric acid in Arabidopsis thaliana. J Biol Chem 281:7197–7204
Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145:dev164376. https://doi.org/10.1242/dev.164376
Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich A, Yellin A, Bar D, Khan M, Fernie AR, Turano FJ, Fromm H (2011) A mitochondrial GABA permease connects the GABA shunt and the TCA cycle and is essential for normal carbon metabolism. Plant J Cell Mol Biol 67:485–498
Minocha R, Majumdar R, Minocha SC (2014) Polyamines and abiotic stress in plants: a complex relationship. Front Plant Sci 5(5):175. https://doi.org/10.3389/fpls.2014.00175
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Miyashita Y, Good A (2008) Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol 49:92–102
Mo H, Wang X, Zhang Y, Zhang G, Zhang J, Ma Z (2015) Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to Verticillium dahliae. Plant J 83:962–975
Morse DE, Hooker N, Duncan H, Jensen L (1979) Gamma aminobutyric acid, a neurotransmitter, induces planktonic abalone larvae to settle and begin metamorphosis. Science 204:407–410
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Navrot N, Roubier N, Gelbaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365
Nayyar H, Kaur R, Kaur S, Singh R (2014) Υ- aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul 33:408–419
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134
Palma F, Carvajal F, Jiménez-Muñoz R, Pulido A, Jamilena M, Garrido D (2019) Exogenous γ-aminobutyric acid treatment improves cold tolerance of zucchini fruit during postharvest storage. Plant Physiol Biochem 136:188–195
Park DH, Mirabella R, Bronstein PA, Preston GM, Haring MA, Lim CK, Collmer A, Schuurink RC (2010) Mutations in γ-aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence. Plant J 64:318–330
Podlešáková K, Ugena L, Spíchal L, Dole K, De Diego N (2019) Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol 48(1):53–65
Prasad PVV, Bheemanahalli R, Jagadish SVK (2017) Field crops and the fear of heat stress-opportunities, challenges and future directions. Field Crops Res 200:114–121
Priya M, Sharma L, Kaur R, Bindumadhava H, Nair RM, Siddique KHM, Nayyar H (2019) GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci Rep 9:7788. https://doi.org/10.1038/s41598-019-44163-w
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 29:7879. https://doi.org/10.1038/ncomms8879
Ramputh AI, Bown AW (1996) Rapid γ- aminobutyric acid synthesis and the inhibition of the growth and development of oblique-banded leaf roller larvae. Plant Physiol 111:1349–1352
Reimer RJ, Fremeau RT, Bellocchio EE, Edwards RH (2001) The essence of excitation. Curr Opin Cell Biol 13:417–420
Renault H, Roussel V, El-Amrani A, Arzel M, Renault D, Bouchéreau A, Deleu C (2010) The Arabidopsis pop 2–1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10:20. https://doi.org/10.1186/1471-2229-10-20
Renault H, El-Amrani A, Berger A, Mouille G, Taconnat LS, Bouchereau A, Deleu C (2013) γ-aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ 36:1009–1018
Rezaei-Chiyaneh E, Seyyedi SM, Ebrahimian E, Moghaddam SS, Damalas CA (2018) Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind Crops Prod 112:741–748
Rico A, Preston GM (2008) Pseudomonas syringae pv. Tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol Plant-Microbe Interact 21:269–282
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10(2):277. https://doi.org/10.3390/antiox10020277
Salah A, Zhan M, Cao C, Han Y, Ling L, Liu Z, Li P, Ye M, Jiang Y (2019) γ-aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci Rep 9:484. https://doi.org/10.1038/s41598-018-36334-y
Satyanarayan V, Nair PM (1990) Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochem 29:367–375
Scholz SS, Reichelt M, Mekonnen DW, Ludewig F, Mithöfer A (2015) Insect herbivory-elicited GABA accumulation in plants in wound-induced, direct, systemic and jasmonate-dependent defense response. Front Plant Sci. https://doi.org/10.3389/fpls.2015.01128
Schwacke R, Gralath S, Breitkreuz KE, Stransky E, Stransky H, Frommer WB, Rentsch D (1999) LeProT1, a transporter for proline, glycine betaine, and gamma-aminobutyric acid in tomato pollen. Plant Cell 11:377–392
Seifi HS, Shelp BJ (2019) Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front Plant Sci 10(2):117. https://doi.org/10.3389/fpls.2019.00117
Seifi HS, Curvers K, De Vleesschauwer D, Deleare I, Aziz A, Hofte M (2013) Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant tomato leads to resistance against Botrytis cinerea. New Phytol 199:490–550
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shang H, Shifeng C, Zhenfeng Y, Yuting C, Yonghua Z (2011) Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem 59:1264–1268
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Shelp BJ, Bown AW, Mclean MD (1999) Metabolism and function of gamma aminobutyric acid. Trends Plant Sci 4:446–452
Shelp BJ, Van Cauwenberghe OR, Bown AW (2003) Gamma aminobutyrate: from intellectual curiosity to practical pest control. Can J Bot 81:1045–1048
Shelp BJ, Bown AW, Faure D (2006) Extracellular γ-aminobutyrate mediates communication between plants and other organisms. Plant Physiol 142:1350–1352
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 193–194:130–135
Shi SQ, Shi Z, Jiang ZP, Qi LW, Sun XM, Li CX, Liu JF, Xiao WF, Zhang SG (2010) Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ 33:149–162
Signorelli S, Dans PD, Coitiño EL, Borsani O, Monza J (2015) Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 10:e0115349. https://doi.org/10.1371/journal.pone.0115349
Simpson JP, Di Leo R, Dhanoa PK, Allan WL, Makhmoudova A, Clark SM, Hoover GJ, Mollen RT, Shelp BJ (2008) Identification and characterization of a plastid localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification. J Exp Bot 59:2545–2554
Snowden CJ, Thomas B, Baxter CJ, Smith JAC, Sweetlove LJ (2015) A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J 81:651–660
Solomon PS, Oliver RP (2002) Evidence that γ-aminobutyric acid is a major nitrogen source during Cladosporium fulvum infection of tomato. Planta 214:414–420
Song H, Wang XX, Wang H, Taoa YH (2010) Exogenous γ-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J Sci Food Agri 90:1410–1416
Song S, Li J, Gao H, Li QY, Yang LW, Gong RJ (2012) Effect of exogenous γ-aminobutyric acid on inorganic nitrogen metabolism and mineral elements contents of melon seedling under hypoxia stress. Acta Hort Sin 39:695–704
Steward FC, Thompson JF, Dent CE (1949) γ-aminobutyric acid a constituent of potato tubers? Sci 110:439–440
Sung MS, Chow TJ, Lee TM (2011) Polyamine acclimation alleviates hyper salinity-induced oxidative stress in a marine green microalga, Ulva fasciata, by modulation of antioxidative enzyme gene expression. J Phycol 47(3):538–547
Swanson S, Gilroy S (2010) ROS in plant development. Physiol Plant 138:384–392
Tanou G, Molassiotis A, Diamantidis G (2009) Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environ Exp Bot 65:270–281
Tarkowski ŁP, Signorelli S, Höfte M (2020) γ-Aminobutyric acid and related amino acids in plant immune responses: emerging mechanisms of action. Plant Cell Environ 43:1103–1116
Tegeder MWJM (2012) Molecular evolution of plant AAP and LHT amino acid transporters. Front Plant Physiol 3:1–11. https://doi.org/10.3389/fpls.2012.00021
Tian XL, Wu XL, Li Y, Zhang SQ (2005) The effect of gamma-aminobutyric acid in superoxide dismutase, peroxidase and catalase activity response to salt stress in maize seedling. Shi Yan Sheng Wu Xue Bao 38:75–79
Tiwari S, Lata C (2018) Heavy metal stress, signaling, and tolerance due to plant-associated microbes: an overview. Front Plant Sci 9:452. https://doi.org/10.3389/fpls.2018.00452
Tretter YP, Hertel M, Munz B, Bruggencate G, Werner S, Alzheimer C (2000) Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat Med 6:812–815
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C (2010) Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiol 154:444–448
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Vijayakumari K, Puthur JT (2015) γ-aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul 78:57–67
Wallace W, Secor J, Schrader LE (1984) Rapid accumulation of gamma-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol 75:170–175
Wang CY, Li JR, Xia QP, Wu XL, Gao HB (2014a) Influence of exogenous γ-aminobutyric acid (GABA) on GABA metabolism and amino acid contents in roots of melon seedling under hypoxia stress. J Appl Ecol 25:2011–2018
Wang Y, Luo Z, Huang X, Yang K, Gao S, Du R (2014b) Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci Hortic 168:132–137
Wang Y, Gu W, Meng Y, Xie T, Li L, Li J (2017) γ-aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep 7:43609. https://doi.org/10.1038/srep43609
Waqas MA, Kaya C, Riaz A, Farooq M, Nawaz I, Wilkes A, Li Y (2019) Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front Plant Sci 10:1336. https://doi.org/10.3389/fpls.2019.01336
Xia QP, Goa HB, Li JR (2011) Effects of γ– aminobutyric acid on the photosynthesis and chlorophyll fluorescence parameters of muskmelon seedlings under hypoxia stress. Chin J Appl Ecol 22:999–1006
Yadav SK (2010) Cold stress tolerance mechanisms in plants. A review. Agron Sustain Dev 30:515–527
Yamauchi T, Fukazawa A, Nakazono M (2017) METALLOTHIONEIN genes encoding ROS scavenging enzymes are down-regulated in the root cortex during inducible aerenchyma formation in rice. Plant Signal Behav 12(11):e1388976. https://doi.org/10.1080/15592324.2017.1388976
Yang A, Cao S, Yang Z (2011) γ-aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem 129:1619–1622
Yang R, Guo Q, Gu Z (2013) GABA shunt and polyamine degradation pathway on g-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem 136:152159. https://doi.org/10.1016/j.foodchem.2012.08.008
Yang J, Sun C, Zhang Y, Fu D, Zheng X, Yu T (2017) Induced resistance in tomato fruit by γ- aminobutyric acid for the control of Alternaria rot caused by Alternaria alternata. Food Chem 22:1014–1020
Yoda H, Yamaguchi Y, Sano H (2003) Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation. Plant Physiol 132(4):1973–1981
Yong B, Xie H, Li Z, Li YP, Zhang Y, Nie G, Zhang XQ, Ma X, Huang LK, Yan YH (2017) Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol 8:1107. https://doi.org/10.3389/fphys.2017.01107
Yu C, Zeng L, Sheng K, Chen F, Yu T (2014) Υ- aminobutyric acid induces resistance against Penicillium expansum by priming of defense responses in pear fruit. Food Chem 159:29–37
Zarei A, Christophe P, Trobacher Shelp J (2016) Arabidopsis aldehyde dehydrogenase 10 family members confer salt tolerance through putrescine-derived 4-aminobutyrate (GABA) production. Sci Rep 6:35115. https://doi.org/10.1038/srep35115
Zeng J, Dong Z, Wu H, Tian Z, Zhao Z (2017) Redox regulation of plant stem cell fate. EMBO J 36:2844–2855
Zhao Y, Song X, Zhong DB, Yu L, Yu X (2020) γ-aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Biores Technol 297:122500. https://doi.org/10.1016/j.biortech.2019.122500
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Wen B, Ma Y, Wang Y, Fang W (2019) Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol 19:43. https://doi.org/10.1186/s12870-019-1646-9
Zimmermann P, Zentgraf U (2005) The correlation between oxidative stress and leaf senescence during plant development. Cell Mol Biol Lett 10:515–534
Acknowledgements
We acknowledge very gratefully the past and present candidates of my laboratory as well as my scientific collaborator. Thanks are also due to Department of Science and Technology (Science and Engineering Research Board), Government of India, for their financial support to Mohammad Israil Ansari (Grant No. CRG/2018/000267). We acknowledge Md. Mahabub Alam, Department of Agronomy, Sher-e-Bangla Agricultural University, for his critical readings of the manuscript draft.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.I.A. and M.H.; writing—original draft preparation, M.I.A., S.U.J; writing—review and editing, M.H., SAA, MIA; visualization, S.U.J., M.H.; supervision, M.I.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ansari, M.I., Jalil, S.U., Ansari, S.A. et al. GABA shunt: a key-player in mitigation of ROS during stress. Plant Growth Regul 94, 131–149 (2021). https://doi.org/10.1007/s10725-021-00710-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-021-00710-y